Highlights
-
•
In a six-year follow-up the patient is alive and well with no evidence of disease.
-
•
This case reports successful treatment of a patient with advanced NSCLC using LRT.
-
•
LRT is frequently referred to as spatially fractionated GRID radiotherapy or SFGFT.
-
•
Lattice Radiotherapy (LRT) is a technical extension of 2D Grid Technique.
Keywords: Voluminous tumors, IMRT, Lung cancer, Advanced stage, Lattice, Radiation therapy
Abstract
The Lattice Radiotherapy (LRT) technique is mainly based on the GRID technology with the improved feature of the 3D treatment delivery. A 72 year old male presented with left shoulder pain due to a 6 cm pulmonary mass in the left upper lobe (LUL) histologically proven Non-Small Cell Lung Cancer (NSCLC) stage IIIA. In July 2011 he was treated in our center with LRT followed by conventional fractionated Volumetric Modulated Arc Therapy (VMAT) combined with chemotherapy. Clinical and imaging follow up of 6 years demonstrated continued improvement and the patient is currently with no evidence of disease (NED). This outstanding result obtained in our first lung cancer patient treated with this approach corroborates its potential in the treatment of locally advanced lung cancer. In a period of 7 years we have treated more than 30 patients with LRT for different diagnosis and sites; 12 of them NSCLC patients, with markedly improved local control and minimal toxicity.
Introduction
Non-small cell lung carcinoma (NSCLC) represents at least 80% of all lung cancers and about 30% of these present with unresectable locally advanced disease of stage IIIA-IIIB [1]. The management of locally advanced NSCLC is challenging [1], [2], [3], [4], and surgery is usually not feasible for the majority of patients. External beam radiation therapy (EBRT) is the commonly used modality for non-metastatic but unresectable NSCLC. It can be delivered either alone, concurrently, or sequentially with systemic therapy [2]. Recommended doses of radiation are in the range of 60–66 Gy with conventional fractionation of 2 Gy daily over 6 weeks. However standard radiation therapy delivered to bulky tumors that require large fields combined with systemic chemotherapy causes high toxicity. In general, doses given are suboptimal due to the limited dose tolerance of the surrounding normal structures. The treatment for locally advanced lung cancer patients based on chemo-radiation regimens results in increased overall survival as compared to either modality alone [4]. Unfortunately the 5-year survival rate for stage IIIA NSCLC remains about 15% and for stage IIIB cancers is about 5% [3].
The concept of Lattice Radiotherapy (LRT) has been described by Wu et al. [5], as a technical extension of 2D GRID technique, frequently referred to as the spatially fractionated GRID radiotherapy or SFGRT [6]. Its basic principle is to create volumetrically distributed islands of very high dose of radiation (vertices) inside the tumor. One of the most important benefits is that this technique does not add any extra toxicity in the peripheral normal tissue, given that the vertices are strictly contained within the gross tumor volume.
Case presentation
A 72 year old man with history of heavy tobacco use originally presented with left shoulder pain and hemoptysis. A chest X-ray showed a left upper lobe (LUL) pulmonary mass and trans-thoracic core biopsy revealed squamous cell carcinoma. FDG PET/CT scan showed a 6 cm LUL mass extending into the superior aspect of the left pulmonary hilum with SUV of 15.5 and a left hilar adenopathy with SUV of 17.1. Metastatic workup including brain MRI was negative indicating a locally advanced tumor T3N1M0 (Stage IIIA) by virtue of involvement of parietal and mediastinal pleura.
He was started on chemotherapy using Cisplatin and Vinorelbine but increasing shoulder pain prompted radiation oncology consult. After multidisciplinary review, appropriate informed consent and internal review board approval were obtained. The patient started LRT using volumetric arc therapy (VMAT) with the Varian Trilogy™ linear accelerator followed by conventional radiotherapy (RT) with concomitant chemotherapy.
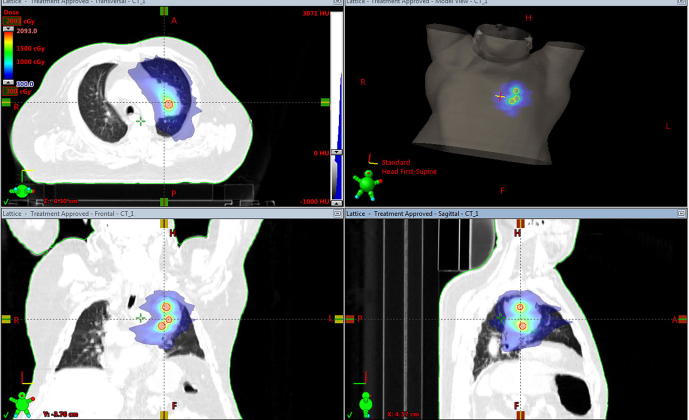
The gross tumor volume (GTV) included the large LUL mass and the hilar adenopathy that measured a combined volume of 218.5 cc, equivalent to a 7.5 cm spherical diameter. The planning target volume (PTV) was created as the gross tumor volume adding 1 cm margin. Three small spheres of 1.5 cm in diameter were used to create the lattice vertices inside the GTV providing inhomogeneity inside the tumor. The periphery of the PTV received 3 Gy and the vertices received 18 Gy (Fig. 1). The location of the spheres was determined by the size and shape of the tumor, and positioned away from vital structures to guarantee a significant drop of the dose to maintain dose constrains at the periphery. The distance between the spheres was 2 cm. Two partial, coplanar arcs with 6 MV photons were used. Total dose delivered was 61 Gy to the GTV and 76 Gy to the vertices with a BED of 120 Gy while the remaining of the tumor received a BED of 73.5 Gy or higher. Treatment was delivered in 30 fractions, 29 fractions of 2 Gy per fraction and one fraction with LRT over 44 elapsed days.
Fig. 1.
Treatment plan of the LRT fraction showing color wash dose distribution of 3 Gy in the periphery and 18 Gy in the three lattice vertices of 1.5 cm diameter.
The tumor position was aligned to the planned position before every daily session using Cone Beam CT (CBCT). After 15 conventional fractions, the patient underwent a second CT simulation due to 50% tumor reduction, requiring a new treatment plan to deliver the remaining 14 prescribed fractions (Fig. 2). The patient tolerated treatment well without any acute toxicity except for mild focal erythema in the skin of the anterior chest which eventually cleared with topical treatment.
Fig. 2.
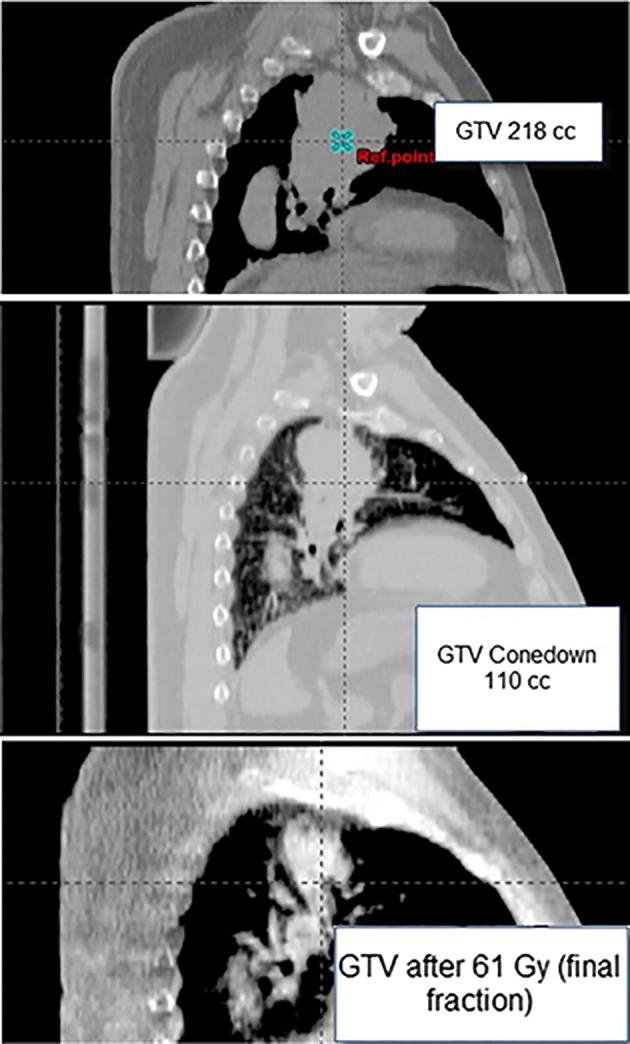
Top: CT-simulation showing a GTV of 218 cc equivalent to a 7.5 cm sphere diameter. Middle: CBCT demonstrating that volume of the lesion has decreased over 50% after 15 fractions. Bottom: CBCT on the last day of treatment.
In the first follow up visit at 3 months the patient only complained of intermittent low-intensity left shoulder pain and a FDG PET-CT demonstrated decrease in diameter of the LUL mass to 2.8 cm consistent with a partial response according to the RECIST 1.1 criteria with a maximal SUV of 4.8. There were no metabolically active mediastinal or hilar lymph nodes. In a 6-month follow-up, the patient was asymptomatic and at FDG PET-CT the size of the LUL mass was 1.4 cm in maximal diameter with SUV of 2.8. Further clinical and imaging follow up with PET-CT studies (Table 1) demonstrated continued improvement. Patient is NED after 6 years with only minor post-radiation changes as shown in the latest imaging follow-up (Fig. 3). In his most recent clinical follow-up visit on August 2017 patient had a Karnofsky Performance Status (KPS) of 90% due to occasional shortness of breath with exercise.
Table 1.
Six year imaging follow up with PET-CT after Lattice Radiotherapy of advanced lung carcinoma.
| PET-CT date | Time from Tx completion | Lesion diameter | SUV |
|---|---|---|---|
| 6/13/2011 | pre tx | LUL 6.0 cm/hilum 1.5 cm | 15.5/17.1 |
| 12/1/2011 | 3 months | LUL 2.8 cm/hilum 0 cm | 4.8 |
| 3/12/2012 | 6 months | LUL 1.4 cm/hilum 0 cm | 2.8 |
| 11/20/2012 | 10 months | LUL persists (does not report size) | 1.8 |
| 6/12/2013 | 21 months | LUL 2.1 cm | 2.3 |
| 12/26/2013 | 27 months | LUL 2.2 cm | 2.4 |
| 7/16/2014 | 34 months | LUL 2.3 cm | 2.8 |
| 12/26/2014 | 39 months | LUL 3.2 cm | 2.5 |
| 6/17/2015 | 45 months | Unchanged from previous study | Unchanged |
| 3/7/2016 | 54 months | Unchanged from previous study | Unchanged |
| 8/16/2016 | 60 months | Unchanged from previous study | 1.8 |
| 8/8/2017 | 72 months | LUL 2.8 cm | 2.9 |
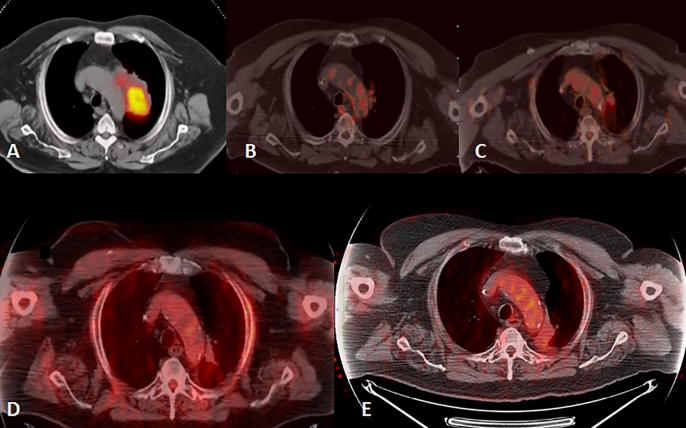
Fig. 3.
A: Initial FDG PET-CT showing metabolically active lesion in the left lung. B, C, D and E: serial follow up PET-CT images after 3, 9, 15, and 60 months of treatment completion with only minor post-radiation changes in the chest CT showing excellent response to treatment.
Discussion
For the last seven years, we have used LRT in 30 patients; most commonly in advanced gynecological cancer [7], [8]. The case presented herein was the first patient with locally advanced lung cancer treated in our clinic with this technique. In general, radiation therapy doses delivered to bulky tumors in large fields combined with systemic chemotherapy cause high toxicity. As such, radiation doses given (60 to 70 Gy) are usually inadequate to obtain an optimal treatment outcome. Stage III lung cancer is one of the prime examples for which standard options of care offer little advantage with the anticipated 5 year survival of 15% for stage IIIA and 3 to 7% for stage IIIB lesions [9], [10].
In the present case, we have observed a complete response in a patient with bulky NSCLC of stage IIIA after LRT followed by 29 fractions of conventional fractionation for a total of 61 Gy to the GTV and 76 Gy to the vertices. We attribute the favorable outcome in terms of response and local control to the addition of LRT.
Advances in the radiation delivery instrumentation and image-guided radiation therapy (IGRT) techniques coupled with the use of LRT may change the strategy for management of advanced tumors [5], [6]. In the case presented herein, partial GTV where the vertices were located received a BED of 120 Gy; while the peripheral margin of the tumor received at least a BED of 73.5 Gy. Aside from the fact that a higher dose can be safely delivered to a partial volume of a bulky tumor, additional effects, including bystander and immunogenic effects might contribute synergistically to the tumor control [11], [12].
Conclusions
In this case presentation, we describe the use of LRT using modern radiotherapy techniques combined with chemotherapy as a novel and highly effective approach to improve treatment results in patients with locally advanced lung cancer. We encountered minimal toxicity, and the patient demonstrated excellent clinical and imaging response after 6 years. We have used this technique successfully in the treatment of more than 30 patients with advanced bulky tumors in different body sites and diverse diagnoses, with favorable outcomes. We conjecture that the higher dose delivered to the vertices is not the only explanation for the remarkable results obtained. Resent research in Radiobiology of ablative doses has revealed the existence of radiation-induced effects, most notably the immunogenic, bystander and abscopal effects. However, all radiation delivery techniques are not equal in harvesting these effects synergistically toward a better clinical outcome. Our single-institution experience suggests that LRT could be one of the ways to achieve a significant improvement in the management of locally advanced lung cancer. If these encouraging results may be consistently reproduced, it may justify a formal clinical study to determine the effectiveness and the role of LRT.
Conflict of interest
None declared.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ctro.2018.01.003.
Appendix A. Supplementary data
References
- 1.Scorsetti M., Navarria P., Mancos P. Large volume unresectable locally advanced non-small cell lung cancer: acute toxicity and initial outcome results with rapid arc. Radiat Oncol. 2010;5:94. doi: 10.1186/1748-717X-5-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodrigues G., Choy H., Bradley J. Definitive radiotherapy in locally advanced non-small cell lung cancer: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based clinical practice guideline. Pract Radiat Oncol. 2015;5:141–148. doi: 10.1016/j.prro.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Non-Small Cell Lung Cancer (NSCLC) Survival Rates, by Stage. (2016). Accessed: March 1, 2017: https://www.cancer.org/cancer/non-small-cell-lung-cancer/detection-diagnosis-staging/survival-rates.html.
- 4.Aupérin A., Le Péchoux C., Rolland E. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 5.Wu X., Ahmed M., Wright J. On modern technical approaches of three-dimensional high-dose lattice radiotherapy. Cureus. 2010;2(3):e9. [Google Scholar]
- 6.Penagaricano J.A., Moros E.G., Ratanatharathorn V. Evaluation of spatially fractionated radiotherapy (GRID) and definitive chemoradiotherapy with curative intent for locally advanced squamous cell carcinoma of the head and neck: initial response rates and toxicity. Int J Rad Onc Biol Phys. 2010;76:1369–1375. doi: 10.1016/j.ijrobp.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Amendola B.E., Perez N., Amendola M.A. Lattice radiotherapy with rapid arc for treatment of gynecological tumors: dosimetric and early clinical evaluations. Cureus. 2010;2(9):e15. [Google Scholar]
- 8.Blanco Suarez J., Amendola B.E., Perez N. The use of Lattice Radiation Therapy (LRT) in the treatment of bulky tumors: a case report of a large metastatic mixed milesian ovarian tumor. Cureus. 2015;7(11):e389. doi: 10.7759/cureus.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975-2014, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017 Alberg AJ, Brock MV, Ford JC, et al. Epidemiology of Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013;143(5 Suppl):e1S–29S. [DOI] [PMC free article] [PubMed]
- 10.Twyman-Saint Victor C., Rech A.J., Maity A. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demaria S., Golden E.B., Formenti S.C. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1:1325–1332. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 12.Kanagavelu S., Gupta S., Wu X. In vivo effects of lattice radiation therapy on local and distant lung cancer: potential role of immunomodulation. Radiat Res. 2014;182(2):149–162. doi: 10.1667/RR3819.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.