Highlights
-
•
Head and neck squamous cell carcinoma is an immune suppressive malignancy.
-
•
Conventional intensification strategies didn’t provide any incremental benefit.
-
•
Inhibiting the PD-1/PD-L1 checkpoint may synergize with cetuximab and radiotherapy.
Keywords: Radiotherapy, Head and neck cancer, Cetuximab, Durvalumab, Immunotherapy
Abstract
Introduction and background
Head and neck squamous cell carcinoma (HNSCC) has been increasingly recognized as an immune suppressive malignancy. The efficacy of immune checkpoint inhibitors (ICI’s) in the context of recurrent/metastatic (R/M) setting anticipates the possible integration of immunotherapy into the therapeutic armamentarium of locally advanced disease. Durvalumab (DUR) is a humanized monoclonal IgG1, anti-PD-L1 antibody with promising data in R/M HNSCC. The aim of our study is to test the antitumor activity of a combined regimen incorporating an immune checkpoint inhibitor into a conventional bio-radiation strategy for the cure of unfavorable locally advanced HNSCC.
Methods/design
In this open label, multi-center, single-arm, phase I/II study, enrolled patients will receive Radiotherapy (RT) (69.9 Gy/2.12 Gy in 33 fractions) with concurrent Cetuximab (CTX) (400 mg/m2 1 week before RT start followed by 250 mg/m2 weekly) and DUR (fixed dose of 1500 mg every 4 weeks starting from RT-CTX week 1) followed by adjuvant DUR (to a maximum of 6 months after completion of RT-CTX). Primary endpoint of the study is 2-year progression-free survival (PFS). A safety run-in is planned after the enrollment of first 12, 24 and 36 patients. Patients affected by high-risk (≥N2a or ≥T3, any N) larynx, hypopharynx and HPV negative oropharynx or HPV-positive oropharynx (≥T2, ≥N2b, ≥10 pack/years) will be eligible.
Discussion
Conventional intensification strategies failed to provide any benefit for the cure of locally advanced HNSCC. For the still prevalent HPV-negative population and the high risk-HPV positive disease, there is an unmet need for alternative treatment paradigms. Potentially, the inhibition of the PD-1/PD-L1 checkpoint may synergize with both CTX and RT through immunologic interplay, ultimately aiming to reverse the HNSCC-induced immune suppression. The DUCRO study will seek to demonstrate if such a strategy may be safe and active.
Trial registration
NCT number: NCT03051906
Eudract number: 2016-004668-20
Introduction
Loco-regionally advanced head and neck squamous cell carcinoma (HNSCC) is amenable to curative treatment but its management poses a significant challenge to the multidisciplinary team. In both primary [1], [2] and high-risk post-operative settings [3], [4], the combination of radiotherapy (RT) with cisplatin (100 mg/m2 every 3 weeks) is the standard non-surgical approach. However, this treatment is associated with poor compliance and high rates of acute and late side effects [5].
In 2006, the landmark IMCL 9815 phase 3 trial [6] demonstrated that the combination of RT with Cetuximab (CTX), a chimeric mouse IgG1 monoclonal anti-EGFR antibody, led to improved survival compared with RT alone without an increased rate of ≥G3 acute toxicity or a detrimental effect on compliance and quality of life [7], [8]. In current practice, this effective regimen is an option for patients with locally advanced HNSCC who are deemed ineligible to cisplatin, still pending the results of RT0G 1016 (NCT01302834), the only large phase 3 randomized trial ever designed to directly compare RT-CTX with chemo-radiation with overall survival (OS) as primary endpoint. A series of clinical trials conducted in last 10 years exploring other anti-EGFR targeted strategies consistently failed [9], [10], [11], [12] to replicate the magnitude of benefit observed with CTX, both in the locally advanced and recurrent/metastatic (R/M) setting. The hallmark of an unsuccessful intensification approach in biomarker-unselected patients is represented by the phase III RTOG 0522 study [13], which did not show any benefit by adding CTX to cisplatin-based chemoradiation, but only led to more ≥G3 toxicity and RT interruptions. The negative results of the trial generated the hypothesis [14] that platin-compounds and CTX may exert overlapping, but not supra-additive, effects of radiosensitization, therefore resulting in no additional benefit when administered together. The observation that the efficacy of anti-EGFR treatment in HNSCC is mainly restricted to CTX can justify the hypothesis that other factors play a role in favoring its anticancer effect, namely immunologic mechanisms. Other than inducing pro-apoptotic signals and inhibiting DNA double strand break repair mechanisms, the interplay of CTX with both innate and adaptive immunity has been described by several investigators [15], [16], [17], [18]. In light of its chimeric antibody composition and IgG1 isotype, it has been shown that CTX can rapidly elicit a process of antibody-dependent-cellular cytoxicity (ADCC) by natural killer (NK) cells. In addition, CTX is able to enhance the antigenic cross-talk between dendritic and NK cells, which in turn may favor a sustained recruitment of EGFR-specific T cells [19], [20].
Despite the fact that multimodality treatment is standard of care in locally-advanced HNSCC, the overall prognosis has not changed appreciably in last decades, with the only notable exception represented by the 60% reduction in risk of death observed in the growing population with Human Papilloma Virus (HPV) – driven oropharyngeal cancer.
It is growingly recognized that HNSCC is an immune suppressive malignancy [21], [22]. Among other mechanisms of immune evasion, both HPV negative and positive tumors are able to induce a marked anergy in tumor-infiltrating lymphocytes (TIL’s) by upregulating co-inhibitory signals at the tumor cell – T cell interface. In particular, as one of main immune system’s mechanisms involved in preventing excessive inflammatory responses, the programmed death ligand 1 (PD-L1)/PD-1 axis is commonly exploited in HNSCC to promote immune escape. Over 60% of both HPV positive and negative tumors overexpress PD-L1, thereby exhausting PD-1 positive T cells and preventing immune elimination. Given these observations, it has been postulated that HNSCC may benefit from immunotherapeutic strategies, primarily aimed at PD-L1/PD1 checkpoint blockade. In analogy with two other anti-PD1 antibodies [23], [24], Durvalumab (DUR) was the first humanized monoclonal IgG1 anti PD-L1 agent to yield promising anti-tumor response in heavily pre-treated, PD-L1 positive HNSCC patients with R/M disease [25]. The efficacy of immune checkpoint inhibitors (ICI’s) in the context of R/M setting anticipates the potential implications of integrating immunotherapy into the therapeutic armamentarium of locally advanced disease.
For the still predominant patients’ population with HPV – negative disease, disease-free survival has not improved beyond the historical 50% rate notwithstanding intensification approaches. In light of the unacceptable toxicity observed with conventional strategies, an unmet clinical need is to look for alternative therapeutic paradigms.
For HPV-positive, low-risk oropharyngeal cancer, ongoing de-escalation trials will seek to demonstrate if a reduction of treatment intensity can be safely employed, both in terms of non-inferior outcome and reduced morbidity compared with standard concurrent chemo-radiation. However, novel treatments have to be explored for the high-risk HPV-positive subgroup, where advanced disease and significant tobacco exposure are associated with suboptimal long-term disease control, particularly due to distant failure [26].
With this background in mind, the aim of our study is to test the antitumor activity of a combined regimen incorporating an immune checkpoint inhibitor into a conventional bio-radiation strategy for the cure of unfavorable locally advanced HNSCC.
Materials and methods
Study design and patient population
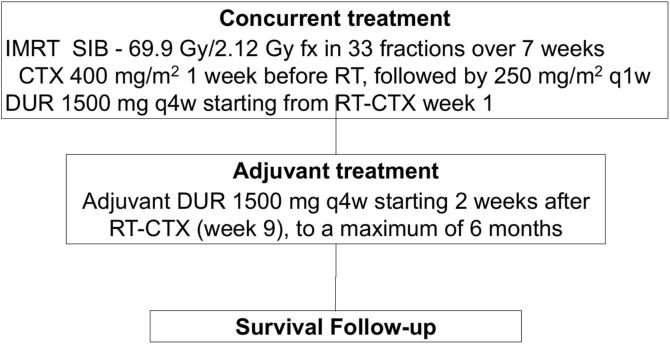
In this open label, multi-center, single-arm, phase I/II study, enrolled patients will receive RT (69.9 Gy/2.12 Gy fx in 33 fractions over 7 weeks) with concurrent CTX (400 mg/m2 1 week before RT start followed by 250 mg/m2 weekly) and DUR (fixed dose of 1500 mg delivered every 4 weeks starting from RT-CTX week 1) followed by adjuvant DUR with the same schedule to a maximum of 6 months after completion of RT-CTX (flow chart shown in Fig. 1). The total treatment time will be of 8 months. After the last administration of DUR, the follow-up time will consist of 36 months of observation.
Fig. 1.
Study schema.
Patients will undergo a centralized assessment of their tumor tissue sample to determine PD-L1 status through Ventana SP263 assay. A pre-specified cut-off level of ≥25% of tumoral PD-L1 expression will categorize the samples into PD-L1 positive or negative.
Patients eligible for curative treatment not considered for primary surgery based on multidisciplinary decision will be enrolled. The main inclusion criteria are as follows:
-
-
histologically proven diagnosis of squamous cell carcinoma of the oropharynx, hypopharynx and larynx
-
-
for patients with oropharyngeal cancer: confirmed HPV status by HPV-DNA in-situ hybridization prior to registration
-
-
clinical stage of HPV-negative oropharynx and all hypopharynx and larynx: T1-2, N2a-N3 or T3-4, any N (AJCC, 7th ed.)
-
-
clinical stage of HPV-positive oropharynx: T2-4, N2b-N3 (AJCC, 7th ed), with smoking history of ≥10 pack/years
-
-
adequate normal organ and marrow function
-
-
Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1.
Subjects should not enter the study if any of the following exclusion criteria are fulfilled:
-
-
head and neck cancer of any other primary anatomic location not specified in the inclusion criteria including patients with HNSCC of unknown primary
-
-
gross total excision of both primary and nodal disease
-
-
induction chemotherapy
-
-
current or prior use of immunosuppressive medication within 28 days before the first dose of DUR, with the exceptions of intranasal and inhaled corticosteroids or systemic corticosteroids at physiological doses, which are not to exceed 10 mg/day of prednisone
-
-
active or prior documented autoimmune disease (within the past 2 years) or inflammatory bowel disease.
Study objectives
The primary objective of the study is to evaluate the antitumor activity of the combined regimen of Durvalumab, Cetuximab and Radiotherapy (DUCRO) in terms of 2-year progression free-survival (PFS) according to RECIST 1.1. The secondary objectives of the study are to assess:
-
-
the safety of DUCRO in terms of acute and late toxicities (according to CTCAE v.4)
-
-
the tolerability of DUCRO in terms of relative dose intensity (RDI) of each component
-
-
the efficacy of DUCRO in terms of 2-year locoregional control and 2-, 5-year overall survival (OS)
-
-
disease-related symptoms and health-related quality of life with validated questionnaires (EORTC QLQ-C30 and EORTC QLQ-H&N35).
The exploratory objectives of the study are to assess the role of immune-related markers in tumor and blood samples predicting clinical benefit (Table 1).
Table 1.
Translation research overview.
| Material/Timing | T1 (pre-treatment) | T2 (+15 days from RT start) | T3 (+90 days from RT last day) |
|---|---|---|---|
| Tumor | TIL’s (CD3+,CD4+, CD8+; Foxp3+); PD-L1; HPV-ISH; PTEN; HER3 |
(Optional re-biopsy) TIL’s (CD3+,CD4+, CD8+; Foxp3+); PD-L1 |
None (optional in case of salvage surgery) |
| Plasma | PBMCs; IL-6, IL-10, TGFb, Galectin-1, IFN-gamma |
IL-6, IL-10, TGFb, Galectin-1, IFN-gamma | IL-6, IL-10, TGFb, Galectin-1, IFN-gamma |
RT: radiotherapy; PBMCs: peripheral blood mononuclear cells
Methodology
A fixed dose of 1500 mg DUR will be administered every 4 weeks. In line with standard practice, CTX will be given weekly at a dose of 250 mg/m2 following a loading dose of 400 mg/m2 7 days before the start of RT. DUR and CTX will be delivered with a minimum of 48 h apart. Intensity modulated radiation therapy (IMRT) is deemed mandatory for this study. IMRT will be delivered in 33 fractions over 7 weeks, 5 fractions weekly with a simultaneous integrated boost (SIB) technique. The dose will be prescribed to the median of Planning Target Volumes (PTV’s) as follows:
-
•
PTV1 will be prescribed 2.12 Gy per fraction up to a total dose of 69.9 Gy
-
•
PTV2 will be prescribed 1.8 Gy per fraction up to a total dose of 59.4 Gy
-
•
PTV 3 will be prescribed 1.6 Gy per fraction up to a total dose of 52.8 Gy.
By definition, the PTV will consist of a 5-mm expansion margin given to the corresponding Clinical Target Volume (CTV). A margin expansion of 3 mm will be allowed in case of daily Image Guided Radiation Therapy (IGRT). In order to exploit the positive immune modulatory effects of RT on tumoral micromilieu, but also to to avoid a potential RT-induced chronic loco-regional immunosuppression, a dose-painting technique will be employed to spare lymph node basins deemed at very low risk of disease spread. As a low-planning priority, the objective is to keep a median dose ≤40 Gy to these areas. Nodal target volume selection recommendations are described in Table 2. In addition, planning efforts are encouraged to avoid skin hot spots and snap the dose off the superficial layers. If judged clinically acceptable, it is recommended that the PTV’s are cropped within 3 mm of the skin surface. Preliminary credentialing of participating centres focused on their technological assessment is planned. The execution of a dummy-run procedure before the start of the clinical activity will be required. The quality assurance procedures will be performed by the coordinating center.
Table 2.
Recommendations on nodal target volume selection.
| Site | CTV2 | CTV3 | Spared level(s) | Caveats |
|---|---|---|---|---|
|
Oropharynx T1-T3 N0-2a (lateralized T, mainly tonsillar fossa/pillars: BOT not involved, T < 1 cm soft palate) |
ipsilateral II, III, IV | controlateral II | bilateral IB and V, controlateral III, IV, RF | if pathologic node is IIA, ipsilateral IB should be in CTV2 |
|
Oropharynx T1-T3 N2a (BOT, soft palate, median tumors) or T1-T3 N2b |
ipsilateral II, III, IV and V | controlateral II and III | bilateral IB, controlateral IV and V, RF |
|
|
Oropharynx T4N0 (median or bulky tumors) |
bilateral II | bilateral III | bilateral IB, IV and V, RF | if posterior pharyngeal wall is infiltrated, RF should be in CTV2 |
|
Oropharynx T4, N1-N2b |
ipsilateral II, III, IV and V and controlateral II | controlateral III, IV and V | bilateral IB and RF | (See above caveats) |
|
Oropharynx Any T, N2c or N3 |
bilateral II, III, IV and V | consider avoiding CTV3 | bilateral IB and RF | (See above caveats) |
|
Hypopharynx T1/T2 N2a (lateral wall of pyriform sinus) |
ipsilateral II, III, IV | controlateral II and III | bilateral IB and V, controlateral IV, RF | if pathologic node is IIA, ipsilateral IB should be in CTV2 |
|
Hypopharynx T1/T2 N2b (lateral wall of pyriform sinus) |
ipsilateral II, III, IV and V | controlateral II and III | bilateral IB, controlateral IV and V, RF | (See above caveats) |
|
Hypopharynx T3/T4N0 |
bilateral II and III | bilateral IV | bilateral IB and V, RF | if posterior pharyngeal wall is infiltrated, RF should be in CTV2 |
|
Hypopharynx T4, N1-N2b |
ipsilateral II, III, IV and V and controlateral II, III | controlateral IV and V | bilateral IB and RF | (See above caveats) |
|
Hypopharynx Any T, N2c or N3 |
bilateral II, III, IV and V | consider avoiding CTV3 | bilateral IB and RF | (See above caveats) |
|
Larynx T3/T4N0 |
bilateral II and III | bilateral IV | bilateral IB and V, RF | if posterior pharyngeal wall is infiltrated, RF should be in CTV2 |
|
Larynx Any T, N1-N2b |
ipsilateral II, III, IV and V and controlateral II, III | controlateral IV and V | bilateral IB and RF | (See above caveats) |
|
Larynx Any T, N2c or N3 |
bilateral II, III, IV and V | consider avoiding CTV3 | bilateral IB and RF | (See above caveats) |
BOT: base of tongue; CTV: clinical target volume; RF: retropharyngeal nodes.
Statistical analysis
Assuming a 2-year PFS of 66% based on historical data from RTOG study 0129 [27] in patients treated with concomitant chemoradiotherapy, the combined regimen of DUR, CTX and RT is hypothesized to yield a 12% absolute increase in anti-tumor activity at 2 years, corresponding to a hazard ratio of 0.6. An A’Hern design will be used, assuming the null hypothesis (H0) that the 2-year PFS is 66% versus the alternative hypothesis (Ha) that the 2-year PFS is 78%. The significance level is alfa = 0.1 and the power is 0.80 when the 2-year PFS is 78%. The required sample size with this design is 66. The proportion of patients progression-free at 24 months will be summarized with corresponding 80% 2-sided confidence intervals. If at least 49/66 patients are progression-free at 24 months the combination therapy will be declared worthy of further investigation, corresponding to the lower limit of the confidence interval excluding 66%. In order to account for withdrawal and drop out of patients from the trial, a 5% increase in accrual is warranted, to a total of 69 patients to be enrolled. Patient accrual is expected to be completed within 24 months. PFS, locoregional control and OS will be estimated by the Kaplan Meyer method.
The safety of DUCRO will be specifically evaluated by the investigators after the enrolment of first 12, 24 and 36 patients (phase I part of the study). In particular, the assessment period of 3 consecutive cohorts of 12 patients each will start from the beginning of the concurrent phase until 8 weeks after its completion. By definition:
-
-
the incidence of dose limiting toxicity (DLT) should be <33% of each consecutive cohort
-
-
the incidence of serious adverse event (SAE’s) should be <10% of each consecutive cohort
-
-
the incidence of DUR-related, out-of field >G3 rash (as AESI) should be <10% of each consecutive cohort.
A DLT is defined as follows:
-
-
onset of ≥G3 bioradiation dermatitis during the first two weeks of combined treatment (before week 3, within 21.2 Gy)
-
-
onset of >G4 bioradiation dermatitis during the first three weeks of combined treatment (before week 4, within 31.8 Gy)
-
-
onset of ≥G3 mucositis during the first two weeks of combined treatment (before week 3, within 21.2 Gy)
-
-
onset of G4 mucositis during the first three weeks of combined treatment (before week 4, within 31.8 Gy)
-
-
G4 bioradiation dermatitis and/or G4 mucositis lasting >14 days
-
-
any toxicity leading to an interruption of RT longer than 1 week
-
-
persistence of > G3 mucositis and/or bioradiation dermatitis for >4 weeks after completion of the combined regimen.
A formal assessment of tolerability will be performed by the investigators as part of the safety run-in after the enrolment of the 36th patient. The study may be discontinued if, in the judgement of the investigators and the institutional review board, study patients are placed at undue risk because of clinically significant findings based on the aforementioned criteria analyzed in the phase I part of the trial.
Funding
This is an investigator-initiated study designed by the coordinating center. The study sponsor is Azienda Ospedaliero-Universitaria Careggi, University of Florence, Italy. Partial financial support for the conduct of the research and disposal of DUR will be provided by Astrazeneca, who had no role in study design and in the preparation of this article. It will also have no role in the collection, analysis and interpretation of the data as in the writing of the final manuscript. Funding is also ensured by the study sponsor and by a no-profit charity organization (Fondazione Firenze Radioterapia Oncologica). Eight Italian centers will participate in the trial.
Discussion
Recent trials [18], [23], [24], [25] showed that ICI’s have promising efficacy in HNSCC. Both in R/M and locally advanced disease, combination strategies are currently being explored to enhance the antitumor activity achieved with single-agent PD1/PD-L1 blockers. In the curative setting, when prioritizing how to exploit and potentially boost the efficacy of ICI’s, two main strategies can be envisioned. In light of its ability to induce ADCC, some investigators referred to CTX as the first immunotherapy available in head and neck cancer. Recently, the biologic mechanisms supporting a potential synergy between CTX and ICI’s were further unravelled. The EGFR pathway is recognized to mediate several immune-suppressive signals [28]. After analyzing PD-L1 expression in tissue samples from 134 patients, Concha Benavente et al. [29] investigated in a panel of 8 HNSCC cell lines the molecular pathways involved in PD-L1 upregulation. As already demonstrated in lung cancer [30], the authors showed that PD-L1 is upregulated at mRNA and protein levels as a result of EGFR downstream pathways, such as the JAK2/STAT1 signaling. In addition, Jie et al. [31] showed that in patients receiving CTX in a prospective phase 2 study (NCT01218048), the increased frequency of PD-1 on CD8+ TIL’s was inversely correlated with response to treatment. Overall, translational data and early preclinical evidence warrant further investigations on the combination of CTX and ICI’s. Another active area of research is focused on the possible interplay between RT and immune blockers. There is accumulating evidence that RT is able to induce an immunogenic cell death mainly through enhanced antigen presentation, activation of dendritic cells, priming of tumor-specific T cells and increased density of TIL’s [32]. In addition, PD-L1 is commonly upregulated in surviving tumor cells after radiation, thus representing a possible counterbalancing mechanism of radioresistance [33], [34]. In a murine model, Deng et al. [35] showed that a significant growth delay was obtained when anti-PD-L1 blockade was delivered together with radiation through a CD8+ T-cell dependent manner. The induction of stimulatory effects by RT can be accompanied by immune suppressive mechanisms such as an increase in regulatory T cells and myeloid-derived suppressor cells, as showed by a longitudinal analysis of peripheral blood samples in a pilot cohort of 20 patients [36]. In addition, it was hypothesized [32], [37] that an extensive irradiation of elective lymph node basins may prevent the generation of favorable radiation-induced immune responses through the elimination of tumor-specific CD8+ T cells and effectory memory cells. Taking altogether, a sound rationale may support the hypothesis that a restored PD-L1/PD-1 axis through the use of ICI’s may synergize with a combined CTX - RT approach, ultimately aiming to reverse the HNSCC-induced immune suppression. Preliminary data on the safety of ICI’s in the context of locally advanced HNSCC have recently been disclosed. A dose finding study [38] on 18 patients showed that the combination of CTX, IMRT and 1 mg/kg of Ipilimumab is feasible. In a cohort of 27 patients [39], the addition of Pembrolizumab to cisplatin-based chemo-radiation was well tolerated, not compromising the delivery of standard treatment. Large phase 3 trials testing the integration of ICI’s with standard chemo- (NCT02764593; NCT03040999; NCT02952586) or bio-radiation (NCT02999087) are ongoing or soon due to start accrual. The potential breakthrough of immunotherapy and its combination with standard treatments in the context of locally advanced HNSCC will be dependent on the evidence produced by such trials in upcoming years.
References
- 1.Pignon J.P., le Maître A., Maillard E., Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Adelstein B.D.J., Li Y., Adams G.L., Wagner H., Kish J.A., Ensley J.F. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21(1):92–98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Cooper J.S., Pajak T.F., Forastiere A.A., Jacobs J., Campbell B.H., Saxman S.B. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 4.Bernier J., Domenge C., Ozsahin M., Matuszewska K., Lefèbvre J.L., Greiner R.H. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 5.Bentzen S.M., Trotti A. Evaluation of early and late toxicities in chemoradiation trials. J Clin Oncol. 2007;25:4096–4103. doi: 10.1200/JCO.2007.13.3983. [DOI] [PubMed] [Google Scholar]
- 6.Bonner J.A., Harari P.M., Giralt J., Azarnia N., Shin D.M., Cohen R.B. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. NEJM. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 7.Curran D., Giralt J., Harari P.M., Ang K.K., Cohen R.B., Kies M.S. Quality of life in head and neck cancer patients after treatment with high-dose radiotherapy alone or in combination with cetuximab. J Clin Oncol. 2007;25:2191–2197. doi: 10.1200/JCO.2006.08.8005. [DOI] [PubMed] [Google Scholar]
- 8.Bonner J.A., Harari P.M., Giralt J., Cohen R.B., Jones C.U., Sur R.K. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 9.Mesía R., Henke M., Fortin A., Minn H., Yunes Ancona A.C., Cmelak A. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-1): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015;16:208–220. doi: 10.1016/S1470-2045(14)71198-2. [DOI] [PubMed] [Google Scholar]
- 10.Giralt J., Trigo J., Nuyts S., Ozsahin M., Skladowski K., Hatoum G. Panitumumab plus radiotherapy versus chemoradiotherapy in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-2): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015;16:221–232. doi: 10.1016/S1470-2045(14)71200-8. [DOI] [PubMed] [Google Scholar]
- 11.Vermorken J.B., Stöhlmacher-Williams J., Davidenko I., Licitra L., Winquist E., Villanueva C. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 2013;14:697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 12.Machiels J.P., Subramanian S., Ruzsa A., Repassy G., Lifirenko I., Flygare A. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: an open-label, randomised phase 3 trial. Lancet Oncol. 2011;12(4):333–343. doi: 10.1016/S1470-2045(11)70034-1. [DOI] [PubMed] [Google Scholar]
- 13.Ang K.K., Zhang Q., Rosenthal D.I., Nguyen-Tan P.F., Sherman E.J., Weber R.S. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Psyrri A., Dafni U. Combining cetuximab with chemoradiotherapy in locally advanced head and neck squamous cell carcinoma: is more better? J Clin Oncol. 2014;32:2929–2932. doi: 10.1200/JCO.2014.56.1902. [DOI] [PubMed] [Google Scholar]
- 15.Taylor R.J., Chan S.L., Wood A., Voskens C.J., Wolf J.S., Lin W. FcγRIIIa polymorphisms and cetuximab induced cytotoxicity in squamous cell carcinoma of the head and neck. Cancer Immunol Immunother. 2009;58:997–1006. doi: 10.1007/s00262-008-0613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López-Albaitero A., Lee S.C., Morgan S., Grandis J.R., Gooding W.E., Ferrone S. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother. 2009;58:1855–1864. doi: 10.1007/s00262-009-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferris R.L., Jaffee E.M., Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28:4390–4399. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Argiris A., Harrington K.J., Tahara M., Schulten J., Chomette P., Ferreira Castro A. Evidence-based treatment options in recurrent and/or metastatic squamous cell carcinoma of the head and neck. Fron Oncol. 2017;7:1–14. doi: 10.3389/fonc.2017.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S.C., Srivastava R.M., López-Albaitero A., Ferrone S., Ferris R.L. Natural killer (NK): dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunol Res. 2011:248–254. doi: 10.1007/s12026-011-8231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lattanzio L., Denaro N., Vivenza D., Varamo C., Strola G., Fortunato M. Elevated basal antibody-dependent cell-mediated cytotoxicity (ADCC) and high epidermal growth factor receptor (EGFR) expression predict favourable outcome in patients with locally advanced head and neck cancer treated with cetuximab and radiotherapy. Cancer Immunol Immunother. 2017;66:573–579. doi: 10.1007/s00262-017-1960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauman J.E., Ferris R.L. Integrating novel therapeutic monoclonal antibodies into the management of head and neck cancer. Cancer. 2014;120(5):624–632. doi: 10.1002/cncr.28380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferris R.L., Ferris R.L. Immunology and immunotherapy of head and neck cancer. J Clin Oncol. 2015;33(29):3293–3304. doi: 10.1200/JCO.2015.61.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferris R.L., Blumenschein G., Jr, Fayette J., Guigay J., Colevas A.D., Licitra L. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016 doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seiwert T.Y., Burtness B., Mehra R., Weiss J., Berger R., Eder J.P. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;2045:1–10. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 25.Zandberg D., Algazi A., Jimeno A., Good J.S., Fayette H., Bouganim N. Durvalumab for recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): preliminary results from a single-arm, phase 2 study. Ann Oncol. 2017;28(5):v372–v394. [Google Scholar]
- 26.Huang S.H., Xu W., Waldron J., Siu L., Shen X., Tong L. Refining American joint committee on cancer/union for international cancer control TNM stage and prognostic groups for human papillomavirus – related oropharyngeal carcinomas. J Clin Oncol. 2015;33(8):836–845. doi: 10.1200/JCO.2014.58.6412. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen-tan P.F., Zhang Q., Ang K.K., Weber R.S., Rosenthal D.I., Soulieres D. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the radiation therapy oncology group 0129 trial: long-term report of efficacy and toxicity. J Clin Oncol. 2014;32(34):3858–3866. doi: 10.1200/JCO.2014.55.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Concha-Benavente F., Srivastava R., Trivedi S., Lei Y., Chandran U., Seethala R.R. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNγ that induce PD-L1 expression in head and neck cancer. Cancer Res. 2016;76(5):1031–1043. doi: 10.1158/0008-5472.CAN-15-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Concha-Benavente F., Ferris R.L. Reversing EGFR mediated immunoescape by targeted monoclonal antibody therapy. Front Pharmacol. 2017;8:332. doi: 10.3389/fphar.2017.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akbay E.A., Koyama S., Carretero J., Altabef A., Tchaicha J.H., Christensen C.L. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jie H., Srivastava R.M., Argiris A., Bauman J.E., Kane L.P., Ferris R.L. Increased PD-1 þ and TIM-3 þ TILs during cetuximab therapy inversely correlate with response in head and neck cancer patients. Cancer Immunol Res. 2017;5(5):408–416. doi: 10.1158/2326-6066.CIR-16-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharabi A.B., Lim M., DeWeese T.L., Drake C.G. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–e509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 33.Dovedi S.J., Adlard A.L., Lipowska-Bhalla G., McKenna C., Jones S., Cheadle E.J. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava R.M., Trivedi S., Concha-Benavente F., Gibson S.P., Reeder C., Ferrone S. CD137 stimulation enhances cetuximab-induced natural killer: dendritic cell priming of antitumor T-cell immunity in patients with head and neck cancer. Clin Cancer Res. 2017;23(3):707–716. doi: 10.1158/1078-0432.CCR-16-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng L., Liang H., Burnette B., Beckett M., Darga T., Weichselbaum R.R. Irradiation and anti – PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sridharan V., Margalit D.N., Lynch S.A., Severgnini M., Zhou J., Chau N.G. Definitive chemoradiation alters the immunologic landscape and immune checkpoints in head and neck cancer. Br J Cancer. 2016;115:252–260. doi: 10.1038/bjc.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharabi A.B., Nirschl C.J., Kochel C.M., Nirschl T.R., Francica B.J., Velarde E. Stereotactic radiation therapy augments antigen-specific PD-1 mediated anti-tumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015;3:345–355. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauman J., Ferris R.L., Clump D.A., Ohr J., Gooding W., Kim S. Phase I trial of cetuximab, intensity modulated radiotherapy (IMRT), and ipilimumab in previously untreated, locally advanced head and neck squamous cell carcinoma (PULA HNSCC) Ann Oncol. 2016;27(6):1–36. [Google Scholar]
- 39.Powell SF, Gitau MM, Sumey CJ, Reynolds TJ, Lohr M, McGraw S, et al. Safety of pembrolizumab with chemoradiation (CRT) in locally advanced squamous cell carcinoma of the head and neck (LA-SCCHN). J Clin Oncol 2017;(suppl; abstr 6011):35.