Graphical abstract
Keywords: Cervix cancer, Brachytherapy, Adaptive radiotherapy, MRI guided radiotherapy, Local control, Morbidity
Highlights
-
•
Image guided adaptive brachytherapy (IGABT) is changing clinical practice.
-
•
The EMBRACE studies benchmark IGABT in cervix cancer.
-
•
A multi-parametric dose prescription protocol is being validated in EMBRACE II.
-
•
EMBRACE II is hypothesised to improve outcome: disease, morbidity, quality of life.
Abstract
The publication of the GEC-ESTRO recommendations one decade ago was a significant step forward for reaching international consensus on adaptive target definition and dose reporting in image guided adaptive brachytherapy (IGABT) in locally advanced cervical cancer. Since then, IGABT has been spreading, particularly in Europe, North America and Asia, and the guidelines have proved their broad acceptance and applicability in clinical practice. However, a unified approach to volume contouring and reporting does not imply a unified administration of treatment, and currently both external beam radiotherapy (EBRT) and IGABT are delivered using a large variety of techniques and prescription/fractionation schedules.
With IGABT, local control is excellent in limited and well-responding tumours. The major challenges are currently loco-regional control in advanced tumours, treatment-related morbidity, and distant metastatic disease. Emerging evidence from the RetroEMBRACE and EMBRACE I studies has demonstrated that clinical outcome is related to dose prescription and technique. The next logical step is to demonstrate excellent clinical outcome with the most advanced EBRT and brachytherapy techniques based on an evidence-based prospective dose and volume prescription protocol.
The EMBRACE II study is an interventional and observational multicentre study which aims to benchmark a high level of local, nodal and systemic control while limiting morbidity, using state of the art treatment including an advanced target volume selection and contouring protocol for EBRT and brachytherapy, a multi-parametric brachytherapy dose prescription protocol (clinical validation of dose constraints), and use of advanced EBRT (IMRT and IGRT) and brachytherapy (IC/IS) techniques (clinical validation). The study also incorporates translational research including imaging and tissue biomarkers.
Development of image guided adaptive brachytherapy: The GEC-ESTRO GYN working group and network
For many decades, technological developments in cervix cancer brachytherapy have been limited and treatment was based on 2D imaging and standard approaches originally developed by the classical brachytherapy schools in the early 20th century. In recent years, image guided adaptive brachytherapy (IGABT) has resulted in a major change of practice. The GEC-ESTRO GYN Working Group was established in 2000 (http://www.estro.org/about/governance-organisation/committees-activities/gec-estro-gynaecology) to support and shape the emerging field of gynaecological IGABT based on initial experience from a few pioneering European centres. The aim was to develop a common language for prescribing, recording and reporting of magnetic resonance image (MRI)-guided cervix cancer. Through regular group meetings and discussions concepts were developed and validated based on clinical examples from centres with different historical traditions (Paris, Leuven, Vienna). This culminated in the publication of two recommendations on MRI-based IGABT for cervix cancer: (1) concepts and terms for the adaptive target volume concept including assessment of initial and response adaptive GTV and CTV [1] and (2) concepts and terms for dose and volume reporting including biological modelling based on the linear quadratic model [2].
In 2005, the GEC-ESTRO GYN Working Group founded a network to promote collaboration between the increasing number of institutions with research and development activities in IGABT. The focus was on joint research and development as well as on education and dissemination. In 2010 and 2011, The GEC-ESTRO GYN network published recommendations III and IV on applicator reconstruction [3] and imaging [4] for MRI-based IGABT. The network stimulated several multi- and mono-centre studies on uncertainties related to gynaecological IGABT leading to the publication of a collection of papers on this topic in a special issue of Radiotherapy and Oncology (vol 107(1), 2013). In collaboration with the GEC-ESTRO GYN network, Radiotherapy and Oncology also published a special issue on gynaecological radiotherapy, with particular focus on cervix IGABT and outcome (vol 120(3), 2016). The GEC-ESTRO GYN network is currently working on several new guidelines: (1) CT contouring for brachytherapy in cervix cancer, (2) Treatment planning in cervix brachytherapy, (3) Image registration for brachytherapy, and (4) Target definition for brachytherapy in vaginal cancer.
IGABT (with repetitive MRI regarded as the gold standard) is increasingly replacing 2D brachytherapy throughout the world, especially in Europe [5] and North America [6], but also in many places in Asia [7], [8]. The Gyn GEC-ESTRO Recommendations I-IV have been used as the conceptual framework for the implementation of IGABT worldwide, and have been embedded and further developed in the new ICRU report 89 [9]. IGABT and image guided external beam radiotherapy (EBRT) will be the major pillars of the upcoming multidisciplinary ESGO-ESTRO-ESP recommendations for the radiotherapy management of cervical cancer in Europe.
In order to evaluate the outcome of IGABT in a multicentre setting, the GEC-ESTRO GYN network launched the “International study on MRI-based brachytherapy in cervical cancer” in 2008 [10]. The first EMBRACE study (EMBRACE I) was a prospective observational study including external beam (45–50 Gy) and MRI-based IGABT. The study closed in 2015 with the accrual of 1416 patients. The GEC-ESTRO GYN network also carried out a retrospective collection of data (retroEMBRACE) on 852 patients from 12 centres, treated with IGABT before the start of EMBRACE I [11]. In 2016, The EMBRACE II study was launched as a prospective interventional study with specific treatment interventions based on the outcome results of the retroEMBRACE and EMBRACE I studies.
This paper describes the rationale and evolution of the EMBRACE II study. The full study protocol is accessible at the EMBRACE website [10] as well as in Appendix A.
Current evidence from the EMBRACE studies and image guided adaptive brachytherapy
The excellent outcome of IGABT has been demonstrated in several mono-institutional reports as well as in the RetroEMBRACE study [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. The 3-year local and pelvic control rates reached 98–100% and 96%, respectively, for FIGO stage IB1 and IB2 disease, and 93–96% and 89–91%, respectively, for stage IIB disease [12], [22]. For stage III/IVA disease, the local and pelvic control rates between centres were more variable ranging from 73–86% [12], [13], [14], [15], [16], [17], [18], [19], [20]. In the RetroEMBRACE study, the improved local and pelvic control was associated with an overall survival benefit of around 10% compared to historical cohorts [22]; a similar benefit was also observed in several mono-institutional reports [12], [13], [17]. In parallel, overall major morbidity (G3-5) was limited after IGABT (3–6% per organ in RetroEMBRACE and EMBRACE I [22], [23], [24]). The prospective French STIC trial reported a 50% reduction of grade 3 and 4 morbidity for 3D compared to 2D brachytherapy [18] which is in agreement with mono-institutional cohorts [12], [13], [16], [17], [25]. While major morbidity is limited, mild morbidity reported by patients and physicians is still frequent [23], [24], [26], [27] and with impact on quality of life [28].
When the EMBRACE I study was initiated, there was insufficient evidence to recommend that all centres follow specific dose constraints for targets and organs at risk (OAR). A variety of institutional practices were therefore allowed regarding applicators, intracavitary/interstitial techniques, dose planning aims, prescribed dose, dose rate and fractionation. While dose delivery was therefore heterogeneous, uniform volume contouring [29], [30] and dose volume reporting were mandatory according to the GEC-ESTRO recommendations [1], [2]. The inter-institution and inter-patient dose variations of the EMBRACE I and RetroEMBRACE studies have provided a unique opportunity to investigate the effects of different dose levels and techniques. Furthermore, several mono-institutional studies as well as the STIC study also followed the GEC-ESTRO recommendations in terms of volume selection, contouring and reporting, which allowed for further comparisons between studies and institutions. Altogether, a vast amount of new knowledge on dose and volume effect relationships for targets and OARs has emerged from mono- and multi-centre IGABT studies [31].
The response-adapted target volume concept developed by GEC-ESTRO has been validated by analysing the patterns of local failure in the EMBRACE I study, which found that 98% of local failures were located within the CTVHR and the CTVIR [32]. A significant correlation between local control and dose, volume, and overall treatment time (OTT) was demonstrated in the retroEMBRACE data for all target volumes: GTVres, CTVHR and CTVIR [33]. A CTVHR dose of ≥85Gyα/β = 10 (D90) delivered in 7 weeks results in a 3-year local control rate of ≥94% in small targets (CTVHR <20 cm3), >93% in intermediate size targets (CTVHR 20–30 cm3) and >86% in large targets (CTVHR up to 70 cm3) at brachytherapy. For the CTVIR and GTVres, doses of ≥60 Gy and ≥ 95Gyα/β = 10 (D98), respectively, are required for similar levels of local control. The ability to achieve a CTVHR dose of 85Gyα/β = 10 (D90) depends on the brachytherapy technique used. RetroEMBRACE demonstrated that use of intracavitary/interstitial (IC/IS) brachytherapy in large tumours significantly increased local control without increasing morbidity [34]. In EMBRACE I, early results suggest that the use of combined IC/IS brachytherapy results in less OAR morbidity compared to IC brachytherapy alone in patients with parametrial infiltration [35]. The retroEMBRACE data also confirmed that prolonged OTT had a negative impact on local control [33]. This emphasises that it is still important to limit the OTT to ≤50 days in the era of IGABT combined with concomitant chemotherapy.
The IGABT experience has also provided descriptive evaluations of morbidity time patterns as well as analyses of risk factors including dose. Vaginal morbidity, particularly stenosis, is prevalent [36] and correlates with external beam dose (prescribed pelvic dose) as well as brachytherapy dose (ICRU recto-vaginal point dose) [37]. These findings challenge the dogma that the vagina is a radio-resistant organ, and evidence-based dosimetric guidance can be introduced with the aim of reducing vaginal dose and morbidity [38]. New recommendations on vaginal dose reporting for the mid and low vagina may furthermore contribute to increased sparing of vaginal tissue from unnecessary irradiation [39], [40]. Analysis of rectal morbidity in the EMBRACE I study demonstrated that G3 and G4 rectal morbidity is uncommon with IGABT [41]. Dose effect relationships have been demonstrated for rectal morbidity [25], [41], [42], [43], [44], [45], and limiting the rectal D2cm3 to ≤65 Gy reduces the incidence of G2 or greater bleeding and proctitis to ≤5.2% and ≤4.6%, respectively, while limiting the rectal to ≤75 Gy reduces the incidence of fistulae to ≤2.7% [41]. In addition, intermediate dose to larger volumes of the rectum (e.g. V55Gy) are predictive of rectal morbidity [46]. Dose-and-effect relationships for the bladder have been demonstrated in mono-institutional analyses [42], [44], and preliminary findings from EMBRACE I suggest an advantage in limiting the bladder to ≤80 Gy. Bowel and sigmoid D2cm3 may be associated with stenosis, strictures and fistulae (data under evaluation [23]).
The overall volume irradiated to 43 Gy during EBRT was shown to be associated with acute and late bowel morbidity [47], [48], [49]. There is evidence that intensity-modulated radiotherapy (IMRT) reduces the risk of acute [50], [51] and late [52], [53] morbidity [54]. Worldwide, many institutions still treat cervix cancer with 3D conformal radiotherapy (3D CRT) although IMRT has been available for many years. The use of IMRT is instrumental for reducing the incidence of bowel morbidity and may also reduce urinary morbidity. Furthermore, image guided radiotherapy (IGRT) allows tight treatment margins to be applied which has significant potential to reduce the overall volume irradiated with EBRT.
The advantage of radiochemotherapy over radiotherapy alone is well documented with several randomised studies published in 1999, and the benefit on overall survival, event-free survival and pelvic control was confirmed in meta-analyses [55], [56]. Several platinum-based and non-platinum chemotherapy regimens were studied, but there is insufficient evidence to recommend a specific regimen/schedule as superior [55]. The number of cycles received during treatment seems important for systemic control in high risk patients e.g. patients with positive nodes or advanced FIGO stage [57]; administration of 5–6 cycles of chemotherapy at full dose may reduce the risk of distant metastases. In line with these results, an early analysis from EMBRACE I shows significantly more systemic relapses in node-positive and advanced stage patients who received ≤4 chemotherapy cycles compared with the patients who received ≥5 cycles (data under evaluation [58]).
The overall rate of EMBRACE I patients developing nodal failure was low (crude 11%) with significantly less failures in patients without pathologic nodes at diagnosis compared to the node positive ones (7% versus 16%, respectively) (data under evaluation). Pathologic nodes at time of diagnoses were confined to the pelvis (common iliac, internal/external iliac including obturator region and parametrium) in 81% of node positive patients. Nodal failures were more often reported for the region of the cranial pelvic field border and the para-aortic region, crude 71% [59], compared to 58% in the pelvis. Failure outside the irradiated volume occurred in 36% of patients with nodal failure, in line with data from Beadle et al. showing that a high number of regional recurrences occurred marginal to the irradiated volumes (42%) and especially along the cranial edge of the elective pelvic fields [60].
The EMBRACE II study – rationale for change of practice
Based on the promising results of IGABT, the EMBRACE study and research group decided to initiate the EMBRACE II study with interventions derived from the evidence collected from the first two EMBRACE studies. The EMBRACE II study will combine and investigate the most advanced techniques currently available for EBRT and brachytherapy in cervix cancer with the delivery of concomitant chemotherapy to the highest standard. The aims of the EMBRACE II study are to prospectively validate the findings of the RetroEMBRACE and EMBRACE I studies and to benchmark an excellent overall survival, based on improved local, nodal and systemic control, as well as reduction of morbidity and improvement of quality of life.
Application of IC/IS brachytherapy
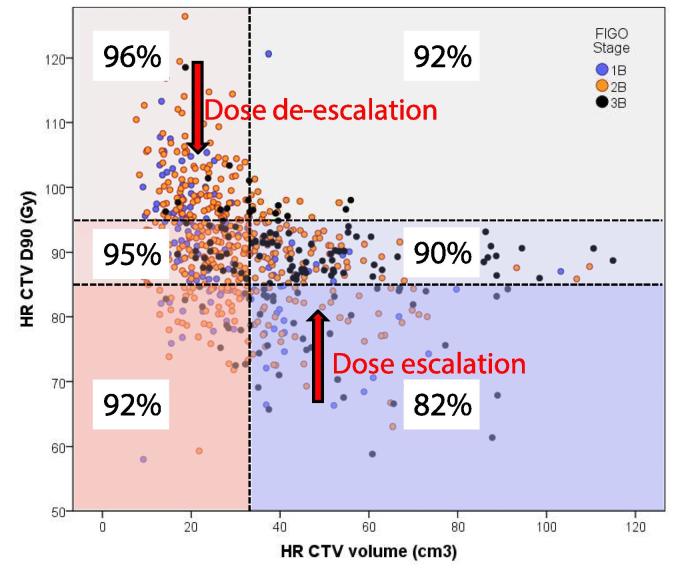
The dose-effect relationships described previously provide rationale for recommending a specific dose prescription protocol for the primary tumour targets (GTVres, CTVHR, CTVIR) and OARs, to balance the aim for high local control with acceptable morbidity. In EMBRACE institutions performing mainly IC brachytherapy half of the patients with CTVHR volume larger than 30 cm3 received D90 doses of less than 85 Gy. Fig. 1 shows that suboptimal local control is predicted for the patients not achieving the 85 Gy constraint [33]. The ability to reach dose constraints for both targets and OARs relies on a change of practice, which mainly involves increased use of IC/IS brachytherapy [34], [61], [62], [63]. Furthermore, a significant number of patients, mainly with small tumours at the time of brachytherapy and small CTVHR (<30 cm3), were treated to high doses which did not translate to higher local control (Fig. 1). There is therefore potential to de-escalate the dose in these patients to reduce OAR dose.
Fig. 1.
The figure demonstrates the principles for dose de-escalation and dose escalation in EMBRACE II. The current distribution of CTVHR dose and volume in the EMBRACE study is shown (each point represents one patient). A number of 6 dose and volume groups are defined according to cut-points of 85 Gy and 95 Gy for the adaptive CTVHR D90 and of 30 cm3 for the CTVHR volume. For each dose-volume group the expected actuarial local control at 3 years is indicated, according to dose-effect data from the retroEMBRACE study [33].
Vaginal dose de-escalation
Given the dose-effect relationship for vaginal morbidity, it is hypothesised that limiting the dose to the ICRU recto-vaginal point to less than 65 Gy and the EBRT dose to 45 Gy will reduce the incidence of G2 or higher vaginal stenosis from 21% to 14% [37]. Multi-centre in silico-studies have shown that reduced source loading in the ring/ovoids and increased loading in the tandem (and needles if utilised) can be applied without compromising CTVHR and GTVres dose [38]. In typical standard loading patterns [64] and most clinical practices [38], the relative vaginal loading is usually around 50%. There is potential to decrease the vaginal loading to 33% which should reduce the ICRU recto-vaginal dose significantly [38].
There are also currently large differences between institutions regarding the definition of the lower field border of EBRT [40]. An increased awareness of the lower EBRT target border through a well-defined target concept for EBRT, and a specific vaginal dose reporting system referring to the Posterior-Inferior Border of the Symphysis (PIBS) [39], [40], should reduce the EBRT dose to the lower and mid vagina.
IMRT and IGRT
In EMBRACE I, the utilisation of IMRT and 3D CRT was 27% and 73%, respectively. PTV margins of 8–10 mm to the elective lymph node target are currently applied in many institutions [65]. Margins of this magnitude may be required for set-up uncertainties with patient positioning based on skin marks. With daily in-room imaging, now available in many institutions, bony image fusion, and couch correction, a reduction in PTV margin from 10 mm to 5 mm can be implemented without compromising target coverage [66].
In EMBRACE I patients, the mean volume irradiated to 43 Gy (V43 Gy) was 2500 cm3 for patients with pelvic irradiation and 3200 cm3 for patients with pelvic + para-aortic irradiation [67]. With the implementation of a consistent target contouring protocol, IMRT and daily IGRT and a 5 mm PTV margin, the V43Gy can be significantly reduced to ∼1500 cm3 for pelvic volumes and to ∼2200 cm3 for pelvic + para-aortic volumes [67], [68].
Nodal target selection
RetroEMBRACE and EMBRACE I outcome indicates that para-aortic failure is the major challenge for nodal control. Para-aortic nodal control can be addressed through pre-therapeutic laparoscopic para-aortic lymph node dissection or by increasing the use of para-aortic irradiation in selected patients with nodal involvement at diagnosis who are at higher risk of para-aortic and distant relapse. The number of nodes (≥3), location (common iliac), PET avidity [69], [70], [71], and to a lesser extent size of nodes [72], may be predictors of para-aortic and systemic failure. Systematic prophylactic para-aortic irradiation in node-positive patients has been shown to produce high levels of nodal control in mono-institutional reports [73].
Overall treatment time
In EMBRACE I 21% of patients were treated with OTT>50 days. To improve OTT, efficient organisation of the whole multimodal treatment is required. This includes delivering EBRT in a maximum of 25 fractions and use of simultaneous integrated boosts (SIB) in patients with lymph node involvement, minimising treatment interruptions as much as possible and by planning the timing of BT carefully.
Chemotherapy
In EMBRACE I, 70% of the patients received ≥5 cycles of chemotherapy, but there was a large variation between centres ranging from 15% to 85%. For optimal outcome particularly in high risk patients [57], the EMBRACE II protocol therefore emphasises administration of adequate doses of chemotherapy according to evidence from the EMBRACE I study and in accordance with international guidelines.
Study design, interventions, aims and hypotheses
The EMBRACE II study is an interventional prospective study with some areas for observational research. The study includes multiple interventions and multiple endpoints. Interventions address local, nodal and systemic treatment as well as exposure of OARs. Endpoints include local and nodal (pelvic) control within the specific EBRT and brachytherapy targets, physician-assessed morbidity and patient-reported outcome (PRO) related to OAR in the pelvis and the para-aortic region, quality of life (QoL) indicators, as well as systemic control, overall survival, disease free survival, and cancer specific survival. The study is designed to benchmark the clinical outcome (see hypotheses below) of the overall approach of advanced radiochemotherapy and brachytherapy. The accrual target was therefore calculated to achieve an appropriate confidence interval for benchmarking 3-year disease and morbidity outcome. The study aims to recruit 1000 patients from at least 30 institutions in 4 years and to monitor them for at least 5 years. With 1000 patients, actuarial outcome can be determined with a confidence interval of 1–3% for all disease and morbidity endpoints in the overall cohort. Subgroup analysis according to stage and risk groups can be determined with a confidence interval of 2–4%, except for stage III and IV subgroups where confidence intervals of 6% and 15% are predicted due to smaller patient numbers. Larger confidence intervals may also apply to other sub-questions with smaller patient cohorts. Furthermore, the EMBRACE II study will reach sufficient patient numbers to validate the patient- and treatment-related risk factors found in retroEMBRACE and EMBRACE I, including dose and volume effects.
Outcome will be analysed with actuarial statistics. Morbidity and PRO will also be evaluated by prevalence and a specific methodology developed within EMBRACE I for evaluating late, persistent, substantial, treatment-related symptoms (LAPERS).
Interventions are based on the evidence and rationale previously described and imply a change of practice for EBRT, brachytherapy and chemotherapy for many centres:
-
•
Increased use of IC/IS brachytherapy
-
•
Reduction of brachytherapy vaginal source loading
-
•
Protocol for target and OAR contouring for EBRT and BT
-
•
Adaptation of EBRT nodal elective CTV according to risk of nodal and systemic recurrence
-
•
Use of IMRT/VMAT and daily IGRT for EBRT delivery (45 Gy in 25 fractions to elective target volume)
-
•
Use of simultaneous integrated boost for pathological lymph nodes
-
•
Systematic application of concomitant chemotherapy
-
•
Reduction of overall treatment time
The general aims of the EMBRACE II study are:
-
•
To systematically apply IMRT/VMAT with daily IGRT as well as advanced IGABT in a prospective multi-centre setting
-
•
To systematically implement an adaptive dose volume prescription protocol for IGABT
-
•
To increase the tumour and target dose in patients with a large CTVHR and/or GTVres at brachytherapy and to decrease the tumour and target dose in patients with a limited size CTVHR and limited or no GTVres at brachytherapy
-
•
To decrease the EBRT and BT dose to the vagina
-
•
To decrease the dose to adjacent OARs, particularly in patients with limited size CTVHR
-
•
To implement systematic contouring, prescription and reporting for tumour and node -related EBRT CTVs, ITV, PTVs and OARs.
-
•
To administer EBRT in different targets which are adapted to the risk of nodal and systemic failure: to improve para-aortic and systemic control in high risk patients and maintain lymph node control in low risk and intermediate risk patients
-
•
To decrease overall EBRT volume through reduced PTV margins, appropriate target selection, systematic contouring and meticulous treatment planning for IMRT/VMAT
-
•
To administer concomitant chemotherapy to prescribed doses in as many patients as possible, particularly in high risk patients
-
•
To benchmark an outstanding level of local, nodal and systemic control as well as survival with application of advanced EBRT, brachytherapy and chemotherapy within a limited OTT
-
•
To benchmark a low incidence of intermediate and major morbidity as well as a high quality of life with application of advanced EBRT, brachytherapy and chemotherapy
Besides these general aims, there are several specific aims which refer to the prospective validation of dose -volume parameters from the previous EMBRACE analyses, to explore and evaluate dose-volume parameters for EBRT and to identify prognostic and predictive factors for disease recurrence and morbidity.
General and specific hypotheses are formulated for the feasibility of the various interventions (IGABT, IMRT, chemotherapy) and for major endpoints (disease, morbidity, quality of life). The major hypotheses are included in Table 1, while a comprehensive list of EMBRACE II hypotheses can be found in the EMBRACE II protocol (see Appendix A).
Table 1.
Hypotheses of the EMBRACE II study for disease outcome and morbidity (EMBRACE II protocol, Table 5.2 modified). The hypotheses given as actuarial estimates are based on the clinical outcome of retroEMBRACE and EMBRACE. Limitation: the numbers for EMBRACE represent the status of clinical evidence available in 8/2015. For the final definition of the assumed benchmark of EMBRACE II, the final mature EMBRACE I outcome (when available) has to be taken into account as baseline for both disease related outcome as well as morbidity. Confidence intervals for the EMBRACE II hypotheses are explained in the text.
| Based on retroEMBRACE 3y/5y | Based on EMBRACE I 3y | Hypothesis EMBRACE II 3y | |
|---|---|---|---|
| Local control | |||
| Overall | 91%/89% | 91% | 93% |
| ≤30 cm3 CTVHR | 96% | 96% | 96% |
| >30 cm3 CTVHR | 87% | 88% | 91% |
| Stage IB, IIA | 98%/98% | 95% | 98% |
| Stage IIB | 93%/91% | 90% | 94% |
| Stage III | 79%/75% | 88% | 89% |
| Stage IVA | 76%/76% | 87% | 89% |
| Nodal control (incl. para-aortic) | |||
| Overall | 88% | 84% | 90% |
| N0 and Stage I + II | 93% | 91% | 94% |
| N1 and Stage III + IVA | 83% | 79% | 87% |
| Pelvic nodal control | |||
| Overall | 94% | 89% | 95% |
| Pelvic control (local + nodal) | |||
| Overall | 87%/84% | 90% | |
| Systemic control (excluding para-aortic failures) | |||
| Overall | 83%/79% | 83% | 86% |
| N0 and Stage I + II | 90% | 89% | 91% |
| N1 and Stage III + IVA | 74% | 79% | 79% |
| Cancer specific survival | Consecutive ChT | ||
| Overall | 81%/74% | NA | 85%/78% |
| N0 and Stage I + II | 90%/87% | NA | 91%/88% |
| N1 and Stage III + IVA | 69%/57% | NA | 76%/64% |
| Overall survival | Consecutive ChT | ||
| Overall | 77%/67% | NA | 81%/71% |
| N0 and Stage I + II | 87%/82% | NA | 88%/83% |
| N1 and Stage III + IVA | 64%/49% | NA | 71%/56% |
| Morbidity | |||
| Bladder CTCAE ≥ G2 | 26% | 21% | |
| Bladder CTCAE ≥ G3 | 7% | 6% | |
| Rectum CTCAE ≥ G2 | 11% | 9% | |
| Rectum CTCAE ≥ G3 | 2% | 2% | |
| Bowel CTCAE ≥ G2 | 17% | 12% | |
| Bowel CTCAE ≥ G3 | 5% | 4% | |
| Vaginal CTCAE ≥ G2 | 27% (stenosis) 31% (all) | 20% (stenosis) 24% (all) | |
| Vaginal CTCAE ≥ G3 | 4% (all) | 3% (all) | |
Patient inclusion
All patients with biopsy proven squamous cell carcinoma, adenocarcinoma or adeno-squamous carcinoma of the cervix, FIGO stage IB-IVA (and nodal status according to TNM as N0 and N1) in whom definitive radio-chemotherapy with curative intent is planned are eligible for the study. Patients must be suitable for treatment with IMRT with IGRT and MRI-based IGABT and ≥5 cycles of cisplatin. Patients with para-aortic metastatic nodes (stage IVB) to the level of L2 are also eligible, but patients with further dissemination are not.
Treatment
All patients will receive EBRT with concomitant chemotherapy and brachytherapy. Summation of EBRT and brachytherapy doses will be performed by calculation of a biologically equieffective dose in 2 Gy per fraction (EQD2) using the linear-quadratic model with α/β = 10 Gy for tumour effects and α/β = 3 Gy for late normal tissue damage. The repair half time is assumed to be 1.5 h. The maximum OTT (including both EBRT and brachytherapy) is 50 days.
External beam radiotherapy
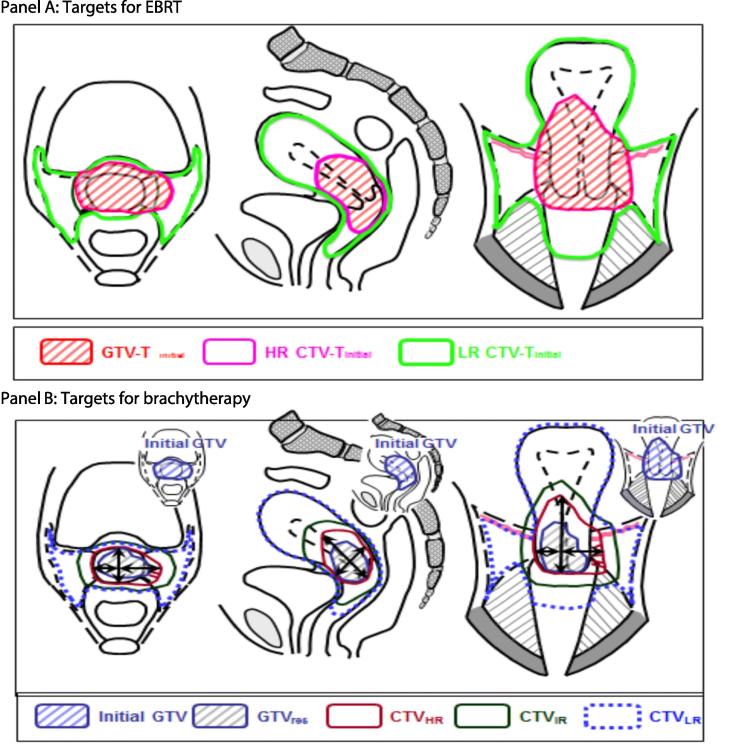
EBRT must be delivered as IMRT or VMAT (volumetric modulated arc therapy). The dose is 45 Gy in 25 fractions over 5 weeks. Daily image guidance is mandatory with couch correction according to bony structures. The definition of primary tumour targets is CT and MRI-based with an initial GTV, HR and LR CTV-T (Fig. 2). An individualised ITV-T is recommended based on patient anatomy and target motion on multiple pre-treatment EBRT MR and (PET) CT imaging. CT or MRI-based nodal Target (CTV-E) is defined according to risk of nodal spread - “Small Pelvis”, “Large Pelvis” or “Large Pelvis + Para-aortic” (Table 2 and Fig. 3). The overall CTV-E/ITV-T to PTV margin is 5 mm. Involved nodes are boosted using SIB to reach a total EBRT plus BT dose of 60 Gy EQD2 [69]. A coverage probability planning approach is recommended for nodal boosting, which delivers 90% of the prescribed dose to the edge of the nodal PTV to reflect the locational probability of the nodal GTV during EBRT [68], [74]. For PTV45 coverage, the aim is for 95% of the volume to receive 95% of the prescribed dose. This allows 5% of the PTV45 to be slightly underdosed to allow for normal tissue sparing. For the various OARs (contoured according to protocol), several DVH parameters, classified as soft planning aims and hard dose constraints, have been specified for treatment planning (Table 3).
Fig. 2.
Schematic diagram for EBRT and brachytherapy targets in cervical cancer, stage IIB bulky disease and good response after chemo-radiotherapy: coronal, transversal and sagittal view. For further details see figure 3.3 and 9.6 in the EMBRACE II protocol adapted from figure 5.10 from ICRU report 89 [9]. Panel A: EBRT targets: large GTV-Tinit, initial CTV-THR, and initial CTV-TLR. Panel B: Brachytherapy targets: limited GTV-Tres (residual GTV), adaptive CTV-THR, and CTV-TIR (GTV-Tinit plus margins around the CTV-THR). Maximum width, thickness and height of the adaptive CTV-THR are indicated.
Table 2.
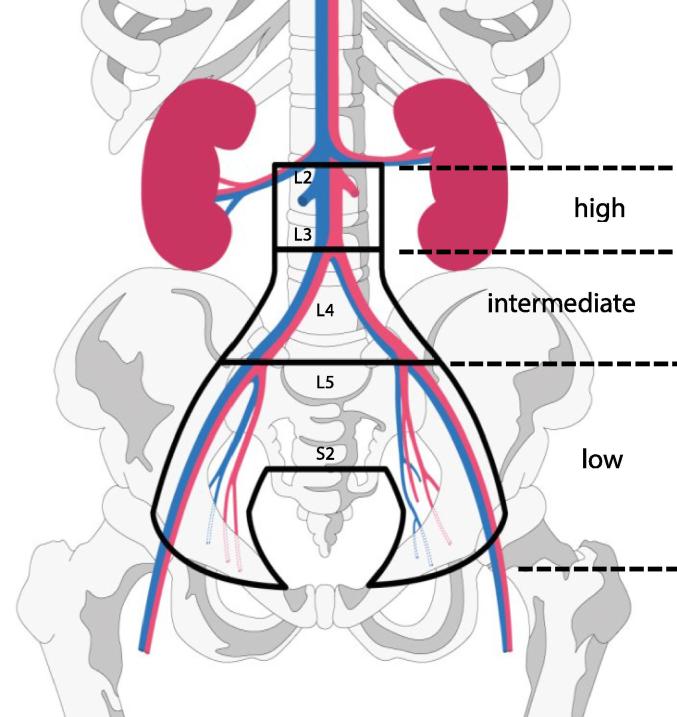
Risk groups for defining the elective clinical target volumes for lymph nodes and corresponding nodal targets defining the radiation field extensions.
| Risk Group LN | Definition | EBRT lymph node regions |
|---|---|---|
| Low Risk (LR LN) |
Tumour size ≤ 4 cm AND stage IA/IB1/IIA1 AND N0 AND squamous cell carcinoma AND no uterine invasion |
“Small Pelvis” internal iliac external iliac obturator presacral |
| Intermediate Risk (IR LN) |
Not low risk No high risk features |
“Large Pelvis” Nodes included in “Small Pelvis” and common iliac region (including the aortic bifurcation) In addition:
|
| High Risk (HR LN) |
Based on nodal pathology: ≥1 pathologic node at common iliac or above OR ≥3 pathologic nodes | “Large Pelvis + Para-aortic” Nodes included in “Large Pelvis” and para-aortic region with the upper border of CTV at the level of renal veins (usually incl. L2), and at least 3 cm cranial of the highest pathological node in case of para-aortic nodes] |
Fig. 3.
Schematic Diagram for lymph node elective CTVs based on risk of lymphatic spread, “Small Pelvis”, “Large Pelvis”, “Large Pelvis + para-aortic”. The risk groups are defined in .
Table 3.
Dose constraints for EBRT for N0 and N1 patients. This table is an update of table 9.4 of the EMBRACE II study protocol version 1.0.
| No lymph node involvement |
Involved lymph nodes |
|||
|---|---|---|---|---|
| Hard dose constraints | Soft dose constraints | Hard dose constraints | Soft dose constraints | |
| PTV45 | V42.75 Gy > 95% Dmax < 107% |
V42.75 Gy = 95% | V42.75 Gy > 95% | V42.75 Gy = 95% Dmax < 107% for helper structure: PTV45 – (PTV-N(#) + 1 cm) |
| ITV45 | Dmin > 95% | Dmin > 95% | ||
| CTV-HR + 10 mm | Dmax < 103% | Dmax < 103% for helper structure: CTV-HR + 10 mm – (PTV-N(#) + 1 cm) |
||
| PTV-N(#) | D98% > 90% of prescribed LN dose Dmax < 107% of prescribed LN dose |
D98% = 90% of prescribed LN dose | ||
| CTV-N(#) | D98% > 100% of prescribed LN dose | D50% > 102% of prescribed LN dose | ||
| Bowel | Dmax < 105% | V40Gy < 250 cm3* V30Gy < 500 cm3* |
Dmax < 105% in regions outside 10–15 mm from PTV-N |
When no para-aortic irradiation: V40Gy < 250 cm3* V30Gy < 500 cm3* For para-aortic irradiation: V40Gy < 300 cm3* V30Gy < 650 cm3* |
| Sigmoid | Dmax < 105% | Dmax < 105% in regions outside 10–15 mm from PTV-N |
||
| Bladder | Dmax < 105% | V40Gy < 60%* V30Gy < 80%* |
Dmax < 105% in regions outside 10–15 mm from PTV-N |
V40Gy < 60%* V30Gy < 80%* |
| Rectum | Dmax < 105% | V40Gy < 75%* V30Gy < 95%* |
Dmax < 105% in regions outside 10–15 mm from PTV-N |
V40Gy < 75%* V30Gy < 95%* |
| Spinal cord | Dmax < 48 Gy | Dmax < 48 Gy | ||
| Femoral heads | Dmax < 50 Gy | Dmax < 50 Gy | ||
| Kidney | Dmean < 15 Gy | Dmean < 10 Gy | Dmean < 15 Gy | Dmean < 10 Gy |
| Body | Dmax < 107% | Dmax < 107% in regions outside 10–15 mm from PTV-N |
||
| Vagina (if not involved) | DPIBS-2cm < 5 Gy | DPIBS-2cm < 5 Gy | ||
| Conformality | 1.10 (V43/Volume of PTV) 1.55 (V36Gy/Volume of PTV) |
1.10 (V43Gy/Volume of PTV) 1.55 (V36Gy/Volume of PTV) |
||
| Transposed ovaries | Dmean < 8 Gy | Dmean < 5 Gy | Dmean < 8 Gy | Dmean < 5 Gy |
| Duodenum | V55 < 15 cm3 | V55 < 15 cm3 | ||
Percentages of 45 Gy unless stated otherwise for nodes.
Dmax and Dmin for MC plans based on D99.9% and D0.1%.
Soft constraints which can be used in the treatment plan optimisation. Values are based on DVH parameters of EMBRACE II patients entered in the study before June 2017. The constraints are not supposed to be fulfilled in all patients, but by ∼70–80% of the patients.
Brachytherapy
The primary imaging modality is MRI with the applicator in situ which enables definition of the relevant volumes of interest directly on the images for treatment planning including applicator reconstruction (Fig. 2). Brachytherapy dose planning is based on a prescription protocol for targets and OARs (Table 4). Planning aims include dose and volume parameters for GTVres, CTVHR, CTVIR and OARs as well as the dose to the recto-vaginal point. The heterogeneous brachytherapy dose distribution is exploited to adapt the dose level to the burden of disease at diagnosis and at time of brachytherapy. If the planning aims cannot be achieved, limits for the final prescribed dose are defined for GTVres, CTVHR, CTVIR, point A, bladder, rectum, sigmoid bowel and vagina. Hard constraints must be fulfilled for 80–90% of the patients, and soft constraints should be fulfilled as often as possible. To achieve the target and OAR constraints, IC/IS brachytherapy must be used in at least 20% of patients in each centre (given a typical stage distribution of ∼20% IB, ∼50% IIB, ∼20% IIIB and ∼10% others). The vaginal dose should be de-escalated by reducing the loading of the vaginal sources (ring or ovoids) as much as possible without compromising CTVHR and CTVIR coverage. Primary and secondary dose planning aims for the vaginal dose are listed in Table 5.
Table 4.
Planning aims (soft constraints) and limits for prescribed dose (hard constraints) for treatment planning in EMBRACE II. The EQD2 is calculated using α/β = 10 for targets, α/β = 3 for OAR and a repair halftime of 1.5 h. The total EQD2 include 45 Gy/25 fractions delivered by EBRT.
| Target | D90 CTVHR EQD210 |
D98 CTVHR EQD210 |
D98 GTVres EQD210 |
D98 CTVIR EQD210 |
Point A EQD210 |
|---|---|---|---|---|---|
| Planning Aims | >90 Gy <95 Gy |
>75 Gy | >95 Gy | >60 Gy | >65 Gy |
| Limits for Prescribed Dose | >85 Gy | – | >90 Gy | – | – |
| OAR | Bladder D2cm3 EQD23 | Rectum D2cm3 EQD23 | Recto-vaginal point EQD23 | Sigmoid D2cm3 EQD23 | Bowel D2cm3 EQD23 |
| Planning Aims | <80 Gy | <65 Gy | <65 Gy | <70 Gy* | <70 Gy* |
| Limits for Prescribed Dose | <90 Gy | <75 Gy | <75 Gy | <75 Gy* | <75 Gy* |
For the sigmoid/bowel structures these dose constraints are valid in case of non-mobile bowel loops resulting in the situation that the most exposed volume is located at a similar part of the organ.
Table 5.
Parameters and constraints for vaginal dose control.
| Aim | Priority | |
|---|---|---|
| ICRU recto-vaginal point dose | <65 Gy EQD2 (EBRT + brachytherapy) | Primary |
| The ratio of vaginal TRAK and total TRAK | <30–40% | Secondary |
| Vaginal lateral dose points at 5 mm | <85 Gy EQD2 (EBRT + brachytherapy) | Secondary |
| Visual inspection of the 140% isodose | Intruding as little as possible into vaginal tissue, and preferentially located within the applicator | Secondary |
| PIBS – 2 cm | When vagina is not involved: DPIBS-2cm < 5 Gy | Secondary |
Chemotherapy
The standard chemotherapy regimen is weekly Cisplatin (40 mg/m2) unless chemotherapy is precluded by patient age, co-morbidity and morbidity. The aim is to deliver a minimum of 5 cycles in at least 80% of patients, particularly in advanced disease. Reduced chemotherapy dose per cycle is encouraged when the full dose cannot be given.
Follow-up
Follow-up with gynaecological examination will occur at 6, 9, 12, 18, 24, 30, 36, 48 and 60 months after treatment. Pelvic MRI ± para-aortic CT will be repeated at 12 months after treatment or in case of suspected recurrence. Tumour and nodal remission will be assessed as complete, uncertain complete, partial, stable or progressive disease at 3 months after treatment by gynaecological examination and pelvic MRI ± para-aortic CT. For uncertain remission, further imaging will be carried out at six months.
Morbidity, PRO and QoL will be scored at baseline and at each follow-up visit. Morbidity will be scored by the physician using the Common Terminology Criteria for Adverse Events (CTCAE v3.0/4.0). PRO and QoL will be assessed according to the scoring manual of the EORTC QoL study group.
Accreditation and quality assurance
Accreditation for each centre requires a positive evaluation by the study coordinators of: (1) a compliance questionnaire documenting clinical practice and infrastructure, (2) contouring and dose planning, and (3) registration and submission of patient cases. Centres that have previously contributed to EMBRACE I are required to undergo the quality assurance (QA) procedure for IMRT/IGRT. New centres must undergo QA for both brachytherapy and IMRT/IGRT. A unique feature of the study is a continuous web-based education programme developed for all study participants to highlight and reinforce key aspects of the protocol in addition to the annual workshops and EMBRACE meetings. This programme is hosted on the Cambridge Cancer Medicine Online platform (CCMO, ccmo.co.uk) and includes a contouring tool (the Addenbrooke’s Contouring Tool, ACT) with bespoke functionality for self-learning, workshops and accreditation.
Sub-studies
Vaginal morbidity sub-study
A study on vaginal morbidity was initiated within EMBRACE I and continues accrual within EMBRACE II. In a prospective longitudinal design, dosimetric and treatment parameters will be correlated with detailed information on vaginal morbidity (including 3-dimensional mapping of symptoms) and linked to patients’ reports on vaginal symptoms, sexual functioning and psychosocial impact. In addition, the impact of regular vaginal dilatation on the vaginal adhesions, length and width will be evaluated.
Translational tissue-based research
Molecular studies within selected EMBRACE I centres indicate that expression of hypoxia gene signature [75], stemness-related proteins, and epithelial mesenchymal translation markers are prognostic factors in cervix cancer. Furthermore, HPV subtype, integration site and activity of infection may be associated with metastatic potential and response to treatment. Immune response has been found to predict metastatic risk and response to therapy. The recently published results of The Cancer Genome Atlas study revealed distinct molecular subtypes with differences in methylation, copy number variation and upregulated resistance pathways [76], and results of the BioRAIDS study are awaited [77].
A multicentre pilot study using selected immunohistochemistry markers is currently being planned in a subset of EMBRACE I patients. One of the goals of this translational tissue-based initiative is to build a network for further translational collaboration within the EMBRACE group.
In EMBRACE II, informed consent for collecting additional biopsy and blood will be obtained and research will focus on validating biomarkers of distant metastasis, local and nodal recurrence in this unique prospective cohort of locally advanced cervical cancer patients treated with a state of the art uniform protocol. Contribution to this sub-study is optional and participating centres can choose different levels of participation. The basic level involves supplying paraffin-embedded tumour tissue while the higher level includes frozen tissue, blood and plasma. In a subset of patients, both tissue and imaging biomarkers will be available. In addition, translation research into late effects of OARs will be initiated.
Bio-imaging
The EMBRACE II study will also implement an imaging sub-study (Appendix B, IQ-EMBRACE protocol), which will evaluate the value of multiparametric quantitative MR imaging as biomarker for identifying patients at increased risk of local, nodal and systemic recurrence. The baseline MRI examination in these patients will include quantitative imaging for assessing quantitative T2, the apparent diffusion coefficient (ADC) from diffusion-weighted MRI and tracer kinetic model parameters from dynamic contrast-enhanced (DCE-) MRI. To ensure consistent quantification between participating centres, a QA procedure, consisting of imaging of calibration phantoms with the proposed sequences for the study, will be carried out in each centre prior to inclusion of the first patient.
Expected outcome and further prospects
The publication of the GEC-ESTRO recommendations more than one decade ago was a significant step forward for reaching international consensus on target definition and dose reporting in IGABT in cervix cancer. Through worldwide spread in clinical practice [5], [6], [7], [8], [9], the guidelines have proven their broad acceptance and applicability in clinical practice. IGABT holds furthermore promises for economic gain [78]. However, a unified approach to contouring and reporting does not imply unified treatment, and currently both EBRT and brachytherapy are administered using a wide variety of techniques and schedules. Emerging clinical results have demonstrated that the different prescription schedules and techniques have an impact on outcome. There is now convincing evidence to recommend use of advanced techniques such as IC/IS brachytherapy, IMRT and IGRT, and various targets for dose prescription. These recommendations will be prospectively tested in the EMBRACE II study and are included in the upcoming “ESGO-ESTRO-ESP Guidelines for the Management of Patients with Cervical Cancer”.
While excellent local and nodal control is expected with current state of the art treatment [22], 10–30% of patients will still develop distant metastases depending on local and nodal stage. Currently, results of adjuvant and neo-adjuvant chemotherapy are awaited from the OUTBACK (NCT01414608) and INTERLACE (NCT01566240) protocols, respectively. A real breakthrough for preventing distant metastatic disease will require development of novel targeted therapy, immunotherapy, vaccines or new applications of existing systemic agents. Translational research will allow identification of predictive factors to select those patients who will benefit from intensification of systemic therapy, and critical biological characteristics which can potentially be targeted through specific drugs. EMBRACE II is organising prospective collection of tissue to facilitate a translational research programme across centres. Furthermore, as EMBRACE II aims for optimal delivery of chemo-radiotherapy based on the most advanced techniques in order to achieve the best possible clinical results, it constitutes an ideal consortium for implementation of additional study protocols on new drugs or other systemic interventions in the future.
Advances in radiotherapy beyond EMBRACE II may include application of MR guided EBRT or particle therapy which are modalities currently growing in availability. With the MR linac further margin reduction may be possible with a promise for reduction of morbidity [79]. Proton and carbon ion therapy may also allow further reduction of normal tissue irradiation, particularly bowel and bladder [80]. Carbon ion radiotherapy may be beneficial for improving tumour and nodal control [81], [82]. While proton radiotherapy is mainly used for established indications at present, there is room to develop clinical protocols for new indications where a potential benefit may be expected, e.g. for morbidity endpoints [83], [84].
IGABT requires volumetric imaging, preferably MRI, and the availability of advanced IC/IS applicators. Lack of imaging scanners and personnel are barriers to implementation in large parts of the world and trans-rectal ultrasound (US) based IGABT may allow IGABT to become more widely available. A successful IGRT and IGABT programme requires multidisciplinary knowledge, experience and skills involving radiation oncology, medical physics, radiology, anaesthesiology, psychology and nursing. Teaching and training is crucial for high-quality IGABT [65] and EBRT [85], [86]. Many educational activities are already carried out through major national and international societies (e.g. ESTRO, GOG, ABS, AROI, IAEA). Examples include the ESTRO school (http://www.estro.org/school) [87], [88], workshops organised by Medical University of Vienna (supported by Elekta, Stockholm, Sweden) and Aarhus University Hospital (supported by Varian Medical Systems, Palo Alto, CA, USA) as well as the Dutch QA project [89]. These programmes need to be sustained and further disseminated to make advanced radiotherapy in cervix cancer available for as many patients as possible in the western world and worldwide. The EMBRACE II study group has developed a web-based training module for IMRT, IGRT and IGABT (http://ccmo.co.uk). The purpose of this module is to train departments to high performance in contouring and treatment planning before they embark on the accreditation process of EMBRACE II. Such teaching modules also have potential to be further exploited beyond the EMBRACE II participants.
Conclusion
The major current challenges in treatment of locally advanced cervical cancer are (1) local control in advanced tumours, (2) treatment-related morbidity and quality of life, (3) distant metastatic spread with the para-aortic region being one of the most common sites of distant failure, (4) selection of patients for additional systemic treatment. EMBRACE II will address these challenges through state of the art brachytherapy applicators, advanced EBRT, increased administration of para-aortic irradiation and systematic use of chemotherapy (≥5 cycles) in an international multicentre setting. The EMBRACE II study is expected to further improve disease control, morbidity and QoL compared to EMBRACE I and retroEMBRACE.
Conflict of interest
The EMBRACE I and II studies have been supported by Elekta and Varian Medical Systems.
Acknowledgements
We would like to thank the large group of researchers who have worked in the frame of the EMBRACE research group. This group of dedicated researchers have contributed with new knowledge for continuous developments in cervix cancer radiotherapy including the EMBRACE II protocol: Søren Bentzen, Kjersti Bruheim, Cyrus Chargari, Wolfgang Dörr, Mario Federico, Elena Fidarova, Taran Hellebust, Dina Najjari Jamal, Noha Jastaniyah, Katarina Majercakova, Renaud Mazeron, Sandy Mohamed, Karen Nkiwane, Primoz Petric, Anne Ramlov, Monica Serban, Stephanie Smet, Henrike Westerveld, and Kenji Yoshida. For development of functional imaging research protocols we would like to thank Jesper Kallehauge.
The GEC-ESTRO GYN working group has contributed during a long time period with the various aspects of image guided brachytherapy in gynaecological cancer such as imaging, contouring, applicator reconstruction, dose planning and applicator development. Apart from the authors of this paper, the following names should be specifically mentioned: Isabelle Barillot, Daniel Berger, Marisol de Brabandere, Edith Briot, Johannes Dimopolous, Isabelle Dumas, Beth Erickson, Taran Hellebust, Robert Hudej, Stefan Lang, Gerry Lowe, An Nulens, Erik Morre Pedersen, José Pérez-Calatayud, Primoz Petric, Peter Petrow, Jason Rownd, Jamema Swamidas, Natascha Wachter-Gerstner, Akila Viswanathan, André Wambersie, and Rachel Wills.
We would like to acknowledge the EMBRACE study office at Medical University of Vienna for coordination of the study, data management and communication with centers: Thomas Liederer, Ian Dilworth, Eva Weisz, and Tamara Rumpold.
The EMBRACE studies have been supported by Varian Medical Systems, Elekta, Danish Cancer Society, and the Christian Doppler Laboratory for Medical Radiation Research for Radiation Oncology (Medical University of Vienna, Austria). The GEC-ESTRO GYN working group has been supported by Elekta, Varian Medical Systems, and Bebig.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ctro.2018.01.001.
Appendix C. EMBRACE Collaborative Group
Department of Radiotherapy, Gustave-Roussy, Villejuif, France: Christine Haie-Meder, Isabelle Dumas, Cyrus Chargari.
Department of Oncology, Aarhus University Hospital, Denmark: Jacob Lindegaard, Kari Tanderup, Lars Fokdal.
Department of Radiation Oncology, Comprehensive Cancer Center, Medical University of Vienna/General, Hospital of Vienna, Vienna, Austria: Richard Pötter, Christian Kirisits, Alina Sturdza.
Department of Radiation Oncology, Tata Memorial Hospital, Mumbai, India: Umesh Mahantshetty, Jamema Swamidas, Shyam Kishore Shrivastava.
Cancer Centre, Mount Vernon Hospital, London, UK: Peter Hoskin, Gerry Lowe.
Department of Radiation Oncology, University Medical Centre Utrecht, The Netherlands: Ina M. Jürgenliemk-Schulz, Astrid De Leeuw.
Department of Radiotherapy, Institute of Oncology Ljubljana, Slovenia: Barbara Segedin, Robert Hudej.
Department of Oncology, The Norwegian Radium Hospital, Oslo University Hospital, Oslo, Norway: Kjersti Bruheim, Taran Paulsen Hellebust.
Department of Oncology, Cross Cancer Institute and University of Alberta, Edmonton, Canada: Fleur Huang, Geetha Menon.
Department of Radiotherapy and Oncology, Postgraduate Institute of Medical Education and Research, Chandigarh, India: Bhavana Rai, Arun S. Oinam.
Leeds Cancer Centre, St James's University Hospital, Leeds, UK: Rachel Cooper, Peter Bownes.
Department of Radiation Oncology, UZ Leuven, Belgium: Erik Van Limbergen.
Department of Radiotherapy, Arnhem, The Netherlands: Elzbieta Van Der Steen Banasik.
Clinic of Oncology and Women's Clinic, St. Olavs Hospital, Trondheim, Norway: Marit Sundset.
Department of Radiation Oncology Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands: Bradley Pieters.
Oncology Centre, Cambridge University Hospitals NHS Foundation Trust, Addenbrooke's Hospital, Cambridge, UK: Li Tee Tan.
Department of Radiation Oncology, Leiden University Medical Center, The Netherlands: Remi A. Nout.
Maastricht Radiation Oncology (MAASTRO) clinic, Maastricht, The Netherlands: Ludy C.H.W. Lutgens.
Department of Radiation Oncology, Hospital of Navarra, Pamplona, Spain: Elena Villafranca.
Department of Oncoradiology, University of Kaposvár, Healthsciences Center, Kaposvar, Hungary: Janaki Hadjiev.
Department of Radiation Oncology, British Columbia Cancer Agency, British Columbia, Canada: Francois Bachand.
Department of Radiation Oncology, Medical College of Wisconsin, Milwaukee, USA: Beth Erickson.
Department of Radiation Oncology, University of Iowa, Iowa, USA: Geraldine Jacobson.
Department of Obstetrics and Gynecology, Kuopio University Hospital, Finland: Maarit Anttila.
Appendices A and B. Supplementary data
Study protocol.
IQ-EMBRACE protocol.
References
- 1.Haie-Meder C., Pötter R., Van Limbergen E., Briot E., De Brabandere M., Dimopoulos J. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group☆ (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74:235–245. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Pötter R., Haie-Meder C., Van Limbergen E., Barillot I., De Brabandere M., Dimopoulos J. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Hellebust T.P., Kirisits C., Berger D., Pérez-Calatayud J., De Brabandere M., De Leeuw A. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group: considerations and pitfalls in commissioning and applicator reconstruction in 3D image-based treatment planning of cervix cancer brachytherapy. Radiother Oncol. 2010;96:153–160. doi: 10.1016/j.radonc.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Dimopoulos J.C.A., Petrow P., Tanderup K., Petric P., Berger D., Kirisits C. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (IV): Basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapy. Radiother Oncol. 2012;103:113–122. doi: 10.1016/j.radonc.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan L.T. Implementation of Image-guided Brachytherapy for Cervix Cancer in the UK: progress Update. Clin Oncol. 2011;23:681–684. doi: 10.1016/j.clon.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Grover S., Harkenrider M.M., Cho L.P., Erickson B., Small C., Small W. Image Guided Cervical Brachytherapy: 2014 Survey of the American Brachytherapy Society. Int J Radiat Oncol. 2016;94:598–604. doi: 10.1016/j.ijrobp.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Mahantshetty U., Swamidas J., Khanna N., Engineer R., Merchant N.H., Deshpande D.D. Reporting and Validation of Gynaecological Groupe Euopeen de Curietherapie European Society for Therapeutic Radiology and Oncology (ESTRO) Brachytherapy Recommendations for MR Image-Based Dose Volume Parameters and Clinical Outcome With High Dose-Rate Brachytherapy in Cervical Cancers. Int J Gynecol Cancer. 2011;21:1110–1116. doi: 10.1097/IGC.0b013e31821caa55. [DOI] [PubMed] [Google Scholar]
- 8.Tharavichitkul E., Chakrabandhu S., Wanwilairat S., Tippanya D., Nobnop W., Pukanhaphan N. Intermediate-term results of image-guided brachytherapy and high-technology external beam radiotherapy in cervical cancer: Chiang Mai University experience. Gynecol Oncol. 2013;130:81–85. doi: 10.1016/j.ygyno.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 9.International Commission on Radiation Units and Measurements. Prescribing, Recording, and Reporting Brachytherapy for Cancer of the Cervix (ICRU report 89). Bethesda: 2013.
- 10.EMBRACE www.embracestudy.dk.
- 11.retroEMBRACE www.retroEMBRACE.com.
- 12.Pötter R., Georg P., Dimopoulos J.C.A., Grimm M., Berger D., Nesvacil N. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol. 2011;100:116–123. doi: 10.1016/j.radonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindegaard J.C., Fokdal L.U., Nielsen S.K., Juul-Christensen J., Tanderup K. MRI-guided adaptive radiotherapy in locally advanced cervical cancer from a Nordic perspective. Acta Oncol. 2013;52:1510–1519. doi: 10.3109/0284186X.2013.818253. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro I., Janssen H., De Brabandere M., Nulens A., De Bal D., Vergote I. Long term experience with 3D image guided brachytherapy and clinical outcome in cervical cancer patients. Radiother Oncol, 2016;120:447–454. doi: 10.1016/j.radonc.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Nomden C.N., de Leeuw A.A.C., Roesink J.M., Tersteeg R.J.H.A., Moerland M.A., Witteveen P.O. Clinical outcome and dosimetric parameters of chemo-radiation including MRI guided adaptive brachytherapy with tandem-ovoid applicators for cervical cancer patients: a single institution experience. Radiother Oncol. 2013;107:69–74. doi: 10.1016/j.radonc.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Gill B.S., Kim H., Houser C.J., Kelley J.L., Sukumvanich P., Edwards R.P. MRI-guided high-dose-rate intracavitary brachytherapy for treatment of cervical cancer: the University of Pittsburgh experience. Int J Radiat Oncol Biol Phys. 2015;91:540–547. doi: 10.1016/j.ijrobp.2014.10.053. [DOI] [PubMed] [Google Scholar]
- 17.Rijkmans E.C., Nout R.A., Rutten I.H.H.M., Ketelaars M., Neelis K.J., Laman M.S. Improved survival of patients with cervical cancer treated with image-guided brachytherapy compared with conventional brachytherapy. Gynecol Oncol. 2014;135:231–238. doi: 10.1016/j.ygyno.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Charra-Brunaud C., Harter V., Delannes M., Haie-Meder C., Quetin P., Kerr C. Impact of 3D image-based PDR brachytherapy on outcome of patients treated for cervix carcinoma in France: results of the French STIC prospective study. Radiother Oncol. 2012;103:305–313. doi: 10.1016/j.radonc.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Tinkle C.L., Weinberg V., Chen L.-M., Littell R., Cunha J.A.M., Sethi R.A. Inverse Planned High-Dose-Rate Brachytherapy for Locoregionally Advanced Cervical Cancer: 4-Year Outcomes. Int J Radiat Oncol Biol Phys. 2015;92:1093–1100. doi: 10.1016/j.ijrobp.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Castelnau-Marchand P., Chargari C., Maroun P., Dumas I., Del Campo E.R., Cao K. Clinical outcomes of definitive chemoradiation followed by intracavitary pulsed-dose rate image-guided adaptive brachytherapy in locally advanced cervical cancer. Gynecol Oncol. 2015;139:288–294. doi: 10.1016/j.ygyno.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Mahantshetty U., Krishnatry R., Hande V., Jamema S., Ghadi Y., Engineer R. Magnetic resonance image guided adaptive brachytherapy in locally advanced cervical cancer: an experience from a tertiary cancer centre in a low-middle income country setting. Int J Radiat Oncol. 2017;99:608–617. doi: 10.1016/j.ijrobp.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Sturdza A., Pötter R., Fokdal L.U., Haie-Meder C., Tan L.T., Mazeron R. Image guided brachytherapy in locally advanced cervical cancer: improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol. 2016;120:428–433. doi: 10.1016/j.radonc.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Jensen N.B.K., Kirchheiner K., Fokdal L.U., Lindegaard J.C., Kirisits C., Mazeron R. Bowel morbidity in cervix cancer after RCHT+IGABT; physician and patient reported outcome - EMBRACE. Radiother Oncol. 2017;123:S26–7. doi: 10.1016/j.radonc.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Fokdal L.U., Kirchheiner K., Kibsgaard Jensen N., Lindegaard J.C., Kirisits K., Chagari C. Physician assessed and patient reported bladder morbidity after RCHT and IGABT for cervical cancer. Radiother Oncol. 2017;123:S23–4. doi: 10.1016/j.radonc.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Kang H.-C., Shin K.H., Park S.-Y., Kim J.-Y. 3D CT-based high-dose-rate brachytherapy for cervical cancer: clinical impact on late rectal bleeding and local control. Radiother Oncol. 2010;97:507–513. doi: 10.1016/j.radonc.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Smet S., Najjari-Jamal D., Bk Jensen N., Fokdal L., Lindegaard J.C., Kirisits C. Fatigue, insomnia, hot flashes (CTCAE) after definitive RCHT+IGABT for cervical cancer (EMBRACE) Radiother Oncol. 2017;123:S22–3. [Google Scholar]
- 27.Nesvacil N., Schmid M.P., Pötter R., Kronreif G., Kirisits C. Combining transrectal ultrasound and CT for image-guided adaptive brachytherapy of cervical cancer: Proof of concept. Brachytherapy. 2016;15(6):839–844. doi: 10.1016/j.brachy.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Kirchheiner K., Pötter R., Tanderup K., Lindegaard J.C., Haie-Meder C., Petrič P. Health-related quality of life in locally advanced cervical cancer patients after definitive chemoradiation therapy including image guided adaptive brachytherapy: an analysis from the EMBRACE study. Int J Radiat Oncol Biol Phys. 2016;94:1088–1098. doi: 10.1016/j.ijrobp.2015.12.363. [DOI] [PubMed] [Google Scholar]
- 29.Petrič P., Hudej R., Rogelj P., Blas M., Tanderup K., Fidarova E. Uncertainties of target volume delineation in MRI guided adaptive brachytherapy of cervix cancer: a multi-institutional study. Radiother Oncol. 2013;107:6–12. doi: 10.1016/j.radonc.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Hellebust T.P., Tanderup K., Lervåg C., Fidarova E., Berger D., Malinen E. Dosimetric impact of interobserver variability in MRI-based delineation for cervical cancer brachytherapy. Radiother Oncol. 2013;107:13–19. doi: 10.1016/j.radonc.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 31.Tanderup K., Lindegaard J.C., Kirisits C., Haie-Meder C., Kirchheiner K., de Leeuw A. Image Guided Adaptive Brachytherapy in cervix cancer: a new paradigm changing clinical practice and outcome. Radiother Oncol. 2016;120:365–369. doi: 10.1016/j.radonc.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Schmid M., Haie-Meder C., Mahanshetty U., Jürgenliemk-Schulz I.M., Segedin B., Hoskin P. Local failures after radiochemotherapy and MR-image-guided brachytherapy in cervical cancer patients. Radiother Oncol. 2017;123:S26. [Google Scholar]
- 33.Tanderup K., Fokdal L.U., Sturdza A., Haie-Meder C., Mazeron R., van Limbergen E. Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. Radiother Oncol. 2016;120:441–446. doi: 10.1016/j.radonc.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Fokdal L., Sturdza A., Mazeron R., Haie-Meder C., Tan L.T., Gillham C. Image guided adaptive brachytherapy with combined intracavitary and interstitial technique improves the therapeutic ratio in locally advanced cervical cancer: analysis from the retroEMBRACE study. Radiother Oncol. 2016;120:434–440. doi: 10.1016/j.radonc.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Fortin I., Tanderup K., Haie-Meder C., Lindegaard J.C., Mahantshetty U., Segedin B. Image guided brachytherapy in cervical cancer: a comparison between intracavitary and combined intracavitary/interstitial brachytherapy in regard to doses to HR CTV, OARs and late morbidity – early results from the embrace study in 999 patients. Brachytherapy. 2016;15:S21. [Google Scholar]
- 36.Kirchheiner K., Nout R.A., Tanderup K., Lindegaard J.C., Westerveld H., Haie-Meder C. Manifestation pattern of early-late vaginal morbidity after definitive radiation (chemo)therapy and image-guided adaptive brachytherapy for locally advanced cervical cancer: an analysis from the EMBRACE study. Int J Radiat Oncol. 2014;89:88–95. doi: 10.1016/j.ijrobp.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 37.Kirchheiner K., Nout R.A., Lindegaard J.C., Haie-Meder C., Mahantshetty U., Segedin B. Dose-effect relationship and risk factors for vaginal stenosis after definitive radio(chemo)therapy with image-guided brachytherapy for locally advanced cervical cancer in the EMBRACE study. Radiother Oncol. 2016;118:160–166. doi: 10.1016/j.radonc.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 38.Mohamed S., Lindegaard J.C., de Leeuw A.A.C., Jürgenliemk-Schulz I., Kirchheiner K., Kirisits C. Vaginal dose de-escalation in image guided adaptive brachytherapy for locally advanced cervical cancer. Radiother Oncol. 2016;120:480–485. doi: 10.1016/j.radonc.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 39.Westerveld H., Pötter R., Berger D., Dankulchai P., Dörr W., Sora M.-C. Vaginal dose point reporting in cervical cancer patients treated with combined 2D/3D external beam radiotherapy and 2D/3D brachytherapy. Radiother Oncol. 2013;107:99–105. doi: 10.1016/j.radonc.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Westerveld H., de Leeuw A., Kirchheiner K., Dankulchai P., Oosterveld B., Oinam A. Multicentre evaluation of a novel vaginal dose reporting method in 153 cervical cancer patients. Radiother Oncol. 2016;120:420–427. doi: 10.1016/j.radonc.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Mazeron R., Fokdal L.U., Kirchheiner K., Georg P., Jastaniyah N., Šegedin B. Dose-volume effect relationships for late rectal morbidity in patients treated with chemoradiation and MRI-guided adaptive brachytherapy for locally advanced cervical cancer: results from the prospective multicenter EMBRACE study. Radiother Oncol. 2016;120:412–419. doi: 10.1016/j.radonc.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Georg P., Pötter R., Georg D., Lang S., Dimopoulos J.C.A., Sturdza A.E. Dose Effect Relationship for Late Side Effects of the Rectum and Urinary Bladder in Magnetic Resonance Image-Guided Adaptive Cervix Cancer Brachytherapy. Int J Radiat Oncol. 2012;82:653–657. doi: 10.1016/j.ijrobp.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 43.Koom W.S., Sohn D.K., Kim J.-Y., Kim J.W., Shin K.H., Yoon S.M. Computed tomography-based high-dose-rate intracavitary brachytherapy for uterine cervical cancer: preliminary demonstration of correlation between dose-volume parameters and rectal mucosal changes observed by flexible sigmoidoscopy. Int J Radiat Oncol. 2007;68:1446–1454. doi: 10.1016/j.ijrobp.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Mazeron R., Maroun P., Castelnau-Marchand P., Dumas I., del Campo E.R., Cao K. Pulsed-dose rate image-guided adaptive brachytherapy in cervical cancer: dose-volume effect relationships for the rectum and bladder. Radiother Oncol. 2015;116:226–232. doi: 10.1016/j.radonc.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 45.Chopra S., Dora T., Engineer R., Mechanery S., Agarwal P., Kannan S. Late rectal toxicity after image-based high-dose-rate interstitial brachytherapy for postoperative recurrent and/or residual cervical cancers: EQD2 predictors for Grade ≥II toxicity. Brachytherapy. 2015;14:881–888. doi: 10.1016/j.brachy.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Ujaimi R., Milosevic M., Fyles A., Beiki-Ardakani A., Carlone M., Jiang H. Intermediate dose–volume parameters and the development of late rectal toxicity after MRI-guided brachytherapy for locally advanced cervix cancer. Brachytherapy. 2017;16:968–975. doi: 10.1016/j.brachy.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Stanic S., Mayadev J.S. Tolerance of the Small Bowel to Therapeutic Irradiation. Int J Gynecol Cancer. 2013;23:592–597. doi: 10.1097/IGC.0b013e318286aa68. [DOI] [PubMed] [Google Scholar]
- 48.Søndergaard J, Holmberg M, Jakobsen AR, Agerbæk M, Muren LP, Høyer M. A comparison of morbidity following conformal versus intensity-modulated radiotherapy for urinary bladder cancer. Acta Oncol (Madr) 2014;53:1321–8. [DOI] [PubMed]
- 49.Letschert J.G., Lebesque J.V., Aleman B.M., Bosset J.F., Horiot J.C., Bartelink H. The volume effect in radiation-related late small bowel complications: results of a clinical study of the EORTC Radiotherapy Cooperative Group in patients treated for rectal carcinoma. Radiother Oncol. 1994;32:116–123. doi: 10.1016/0167-8140(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 50.Naik A., Gurjar O.P., Gupta K.L., Singh K., Nag P., Bhandari V. Comparison of dosimetric parameters and acute toxicity of intensity-modulated and three-dimensional radiotherapy in patients with cervix carcinoma: a randomized prospective study. Cancer Radiothérapie J La Société Fr Radiothérapie Oncol. 2016;20:370–376. doi: 10.1016/j.canrad.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 51.Gandhi A.K., Sharma D.N., Rath G.K., Julka P.K., Subramani V., Sharma S. Early clinical outcomes and toxicity of intensity modulated versus conventional pelvic radiation therapy for locally advanced cervix carcinoma: a prospective randomized study. Int J Radiat Oncol Biol Phys. 2013;87:542–548. doi: 10.1016/j.ijrobp.2013.06.2059. [DOI] [PubMed] [Google Scholar]
- 52.Mundt A.J., Mell L.K., Roeske J.C. Preliminary analysis of chronic gastrointestinal toxicity in gynecology patients treated with intensity-modulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys. 2003;56:1354–1360. doi: 10.1016/s0360-3016(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 53.Chopra S., Engineer R., Mahantshetty U.M., Dora T., Kannan S., Phurailatpam R. Phase III RCT of Postoperative Adjuvant Conventional Radiation (3DCRT) Versus IGIMRT for Reducing Late Bowel Toxicity in Cervical Cancer (PARCER) ( NCT01279135/CTRI2012/120349): results of Interim Analyses. Int J Radiat Oncol Biol Phys. 2015;93:S4. [Google Scholar]
- 54.Klopp A.H., Yeung A.R., Deshmukh S., Gil K.M., Wenzel L., Westin S.N. A Phase III Randomized Trial Comparing Patient-Reported Toxicity and Quality of Life (QOL) During Pelvic Intensity Modulated Radiation Therapy as Compared to Conventional Radiation Therapy. Int J Radiat Oncol. 2016;96:S3. [Google Scholar]
- 55.Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: individual patient data meta-analysis. Cochrane Database Syst Rev 2010:CD008285. [DOI] [PMC free article] [PubMed]
- 56.Green JA, Kirwan JJ, Tierney J, Vale CL, Symonds PR, Fresco LL, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. In: Green JA, editor. Cochrane Database Syst. Rev., Chichester, UK: John Wiley & Sons, Ltd; 2005, p. CD002225. [DOI] [PMC free article] [PubMed]
- 57.Schmid M.P., Franckena M., Kirchheiner K., Sturdza A., Georg P., Dörr W. Distant metastasis in patients with cervical cancer after primary radiotherapy with or without chemotherapy and image guided adaptive brachytherapy. Gynecol Oncol. 2014;133:256–262. doi: 10.1016/j.ygyno.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Fortin I., Jürgenliemk-Schulz I., Mahantshetty U.M., Haie-Meder C., Hoskin P., Segedin B. Distant metastases in locally advanced cervical cancer pattern of relapse and prognostic factors: early results from the EMBRACE study. Int J Radiat Oncol. 2015;93:S8–9. [Google Scholar]
- 59.Nomden C., de Leeuw A.A.C., Tanderup K., Lindegaard J.C., Kirisits C., Haie-Meder C. Nodal failure after chemoradiation and magnetic resonance imaging guided adaptive BT in cervical cancer: a subanalysis within embrace. Int J Radiat Oncol Biol Phys. 2016;96:S12. [Google Scholar]
- 60.Beadle B.M., Jhingran A., Yom S.S., Ramirez P.T., Eifel P.J. Patterns of regional recurrence after definitive radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2010;76:1396–1403. doi: 10.1016/j.ijrobp.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Dimopoulos J.C.A., Kirisits C., Petric P., Georg P., Lang S., Berger D. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: clinical feasibility and preliminary results. Int J Radiat Oncol. 2006;66:83–90. doi: 10.1016/j.ijrobp.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 62.Kirisits C., Lang S., Dimopoulos J., Berger D., Georg D., Pötter R. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: design, application, treatment planning, and dosimetric results. Int J Radiat Oncol Biol Phys. 2006;65:624–630. doi: 10.1016/j.ijrobp.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 63.Fokdal L., Tanderup K., Hokland S.B., Røhl L., Pedersen E.M., Nielsen S.K. Clinical feasibility of combined intracavitary/interstitial brachytherapy in locally advanced cervical cancer employing MRI with a tandem/ring applicator in situ and virtual preplanning of the interstitial component. Radiother Oncol. 2013;107:63–68. doi: 10.1016/j.radonc.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Nkiwane K.S., Pötter R., Tanderup K., Federico M., Lindegaard J.C., Kirisits C. Single line source with and without vaginal loading and the impact on target coverage and organ at risk doses for cervix cancer Stages IB, II, and IIIB: treatment planning simulation in patients treated with MRI-guided adaptive brachytherapy in a multicen. Brachytherapy. 2013;12:317–323. doi: 10.1016/j.brachy.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 65.Kirisits C., Federico M., Nkiwane K., Fidarova E., Jürgenliemk-Schulz I., de Leeuw A. Quality assurance in MR image guided adaptive brachytherapy for cervical cancer: final results of the EMBRACE study dummy run. Radiother Oncol. 2015;117:548–554. doi: 10.1016/j.radonc.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Laursen L.V., Elstrøm U.V., Vestergaard A., Muren L.P., Petersen J.B., Lindegaard J.C. Residual rotational set-up errors after daily cone-beam CT image guided radiotherapy of locally advanced cervical cancer. Radiother Oncol. 2012;105:220–225. doi: 10.1016/j.radonc.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 67.Berger T., Assenholt M., Seppenwoolde Y., Mahantshetty U.M., Jürgenliemk-Schulz I.M., Hoskin P. Importance of technique, dose prescription, and contouring in cervix external beam radiation therapy: current and future practice in a large multicenter study (EMBRACE) Int J Radiat Oncol Biol Phys. 2016;96:E292. [Google Scholar]
- 68.Lindegaard J.C., Assenholt M.S., Ramlov A., Fokdal L.U., Alber M., Tanderup K. Early clinical outcome of coverage probability based treatment planning in locally advanced cervical cancer for simultaneous integrated boost of nodes. Acta Oncol. 2017;56:1479–1486. doi: 10.1080/0284186X.2017.1349335. [DOI] [PubMed] [Google Scholar]
- 69.Ramlov A., Kroon P.S., Jürgenliemk-Schulz I.M., De Leeuw A.A.C., Gormsen L.C., Fokdal L.U. Impact of radiation dose and standardized uptake value of (18)FDG PET on nodal control in locally advanced cervical cancer. Acta Oncol. 2015;54:1567–1573. doi: 10.3109/0284186X.2015.1061693. [DOI] [PubMed] [Google Scholar]
- 70.Kidd E.A., Siegel B.A., Dehdashti F., Grigsby P.W. Pelvic lymph node F-18 fluorodeoxyglucose uptake as a prognostic biomarker in newly diagnosed patients with locally advanced cervical cancer. Cancer. 2010;116:1469–1475. doi: 10.1002/cncr.24972. [DOI] [PubMed] [Google Scholar]
- 71.Kim D.H., Kim W.T., Bae J.S., Ki Y.K., Park D., Suh D.S. Maximum Standardized Uptake Value of Pelvic Lymph Nodes in [18F]-Fluorodeoxyglucose Positron Emission Tomography Is a Prognostic Factor for Para-Aortic Lymph Node Recurrence in Pelvic Node-Positive Cervical Cancer Treated With Definitive Chemoradiotherapy. Int J Gynecol Cancer. 2016;26:1274–1280. doi: 10.1097/IGC.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 72.Song S., Kim J.-Y., Kim Y.-J., Yoo H.J., Kim S.H., Kim S.-K. The size of the metastatic lymph node is an independent prognostic factor for the patients with cervical cancer treated by definitive radiotherapy. Radiother Oncol. 2013;108:168–173. doi: 10.1016/j.radonc.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 73.Vargo J.A., Kim H., Choi S., Sukumvanich P., Olawaiye A.B., Kelley J.L. Extended field intensity modulated radiation therapy with concomitant boost for lymph node-positive cervical cancer: analysis of regional control and recurrence patterns in the positron emission tomography/computed tomography era. Int J Radiat Oncol Biol Phys. 2014;90:1091–1098. doi: 10.1016/j.ijrobp.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 74.Ramlov A., Assenholt M.S., Jensen M.F., Grønborg C., Nout R., Alber M. Clinical implementation of coverage probability planning for nodal boosting in locally advanced cervical cancer. Radiother Oncol. 2017;123:158–163. doi: 10.1016/j.radonc.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 75.Halle C., Andersen E., Lando M., Aarnes E.-K., Hasvold G., Holden M. Hypoxia-Induced Gene Expression in Chemoradioresistant Cervical Cancer Revealed by Dynamic Contrast-Enhanced MRI. Cancer Res. 2012;72:5285–5295. doi: 10.1158/0008-5472.CAN-12-1085. [DOI] [PubMed] [Google Scholar]
- 76.Samuels S., Balint B., von der Leyen H., Hupé P., de Koning L., Kamoun C. Precision medicine in cancer: challenges and recommendations from an EU-funded cervical cancer biobanking study. Br J Cancer. 2016;115:1575–1583. doi: 10.1038/bjc.2016.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ngo C., Samuels S., Bagrintseva K., Slocker A., Hupé P., Kenter G. From prospective biobanking to precision medicine: BIO-RAIDs – an EU study protocol in cervical cancer. BMC Cancer. 2015;15:842. doi: 10.1186/s12885-015-1801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chakraborty S., Mahantshetty U., Chopra S., Lewis S., Hande V., Gudi S. Income generated by women treated with magnetic resonance imaging-based brachytherapy: a simulation study evaluating the macroeconomic benefits of implementing a high-end technology in a public sector healthcare setting. Brachytherapy. 2017 doi: 10.1016/j.brachy.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 79.Kerkmeijer L.G.W., Fuller C.D., Verkooijen H.M., Verheij M., Choudhury A., Harrington K.J. The MRI-linear accelerator consortium: evidence-based clinical introduction of an innovation in radiation oncology connecting researchers, methodology, data collection, quality assurance, and technical development. Front Oncol. 2016;6:215. doi: 10.3389/fonc.2016.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van de Schoot A.J.A.J., de Boer P., Crama K.F., Visser J., Stalpers L.J.A., Rasch C.R.N. Dosimetric advantages of proton therapy compared with photon therapy using an adaptive strategy in cervical cancer. Acta Oncol (Madr) 2016;55:892–899. doi: 10.3109/0284186X.2016.1139179. [DOI] [PubMed] [Google Scholar]
- 81.Wakatsuki M., Kato S., Ohno T., Karasawa K., Kiyohara H., Tamaki T. Clinical outcomes of carbon ion radiotherapy for locally advanced adenocarcinoma of the uterine cervix in phase 1/2 clinical trial (protocol 9704) Cancer. 2014;120:1663–1669. doi: 10.1002/cncr.28621. [DOI] [PubMed] [Google Scholar]
- 82.Wakatsuki M., Kato S., Ohno T., Karasawa K., Ando K., Kiyohara H. Dose–escalation study of carbon ion radiotherapy for locally advanced squamous cell carcinoma of the uterine cervix (9902) Gynecol Oncol. 2014;132:87–92. doi: 10.1016/j.ygyno.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 83.Langendijk J.A., Lambin P., De Ruysscher D., Widder J., Bos M., Verheij M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother Oncol. 2013;107:267–273. doi: 10.1016/j.radonc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 84.Widder J., van der Schaaf A., Lambin P., Marijnen C.A.M., Pignol J.-P., Rasch C.R. The quest for evidence for proton therapy: model-based approach and precision medicine. Int J Radiat Oncol. 2016;95:30–36. doi: 10.1016/j.ijrobp.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 85.Eminowicz G., McCormack M. Variability of clinical target volume delineation for definitive radiotherapy in cervix cancer. Radiother Oncol. 2015;117:542–547. doi: 10.1016/j.radonc.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 86.Eminowicz G., Rompokos V., Stacey C., McCormack M. The dosimetric impact of target volume delineation variation for cervical cancer radiotherapy. Radiother Oncol. 2016;120:493–499. doi: 10.1016/j.radonc.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 87.Turner S., Eriksen J.G., Trotter T., Verfaillie C., Benstead K., Giuliani M. Establishing a Global Radiation Oncology Collaboration in Education (GRaCE): objectives and priorities. Radiother Oncol. 2015;117:188–192. doi: 10.1016/j.radonc.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 88.Eriksen J.G., Leech M., Benstead K., Verfaillie C. Perspectives on medical education in radiation oncology and the role of the ESTRO School. Clin Transl Radiat Oncol. 2016;1:15–18. doi: 10.1016/j.ctro.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nout R., de Leeuw A.A.C., van Leuwen R., Mans A., Verhoef C., Jürgenliemk-Schulz I.M. Implementatie en “quality assurance” van “state-of-the-art” radiotherapie voor gevorderd cervixcarcinoom in Nederland: een kwaliteitsbevorderingsproject. Int J Radiat Oncol Biol Phys. 2017;14:117–121. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study protocol.
IQ-EMBRACE protocol.