Introduction and outline of the review
Particle beam irradiation is increasingly popular due to its physical characteristics. It has an inverted dose profile with low dose absorption on tissue entry and the point of maximum dose deposition at the Bragg-peak. Therefore, it decreases the dose to normal tissues and is expected to also decrease treatment-related side effects. Moreover, the deposited integral dose is lower compared to modern photon-based therapies (i.e., intensity modulated radiation therapy or volumetric modulated arc therapy) and thus holds the potential of reducing the risk of secondary neoplasms.
In recent years, proton beam therapy is being introduced for treatment of tumors of the central nervous system (CNS) in children and adults, e.g., ependymoma, medulloblastoma, meningioma, craniopharyngioma and glioma grade II–IV [10]. For chordoma and chondrosarcoma of the skull base and sacrum as well as for adenoid-cystic carcinomas, highly conformal proton therapy is considered the gold standard since even modern photon techniques fail to deliver the required radiation doses while keeping within the dose constraints of adjacent organs at risk [44], [48], [56]. Besides the “standard” indications, the potential benefit of proton beam irradiation is being investigated within clinical studies for several other tumor entities, such as esophageal cancer, non-small cell lung cancer, pancreatic cancer, and squamous cell carcinomas of the head and neck.
With ever more centers offering proton beam therapy in the near future and with growing patient numbers and follow-up time, concerns about the potential side effects of protons have risen during recent years. The effectiveness of different radiation modalities, i.e., photons, particles and carbon ions, regarding their potential to induce biological effects in the cells is weighted with the relative biological effectiveness (RBE). For photons, a reference RBE of 1.0 is generally used, whereas for carbon ions, most institutions use an RBE value of (approx.) 3.0. For protons, an RBE of 1.1 is used clinically, however, the uncertainty on this dose-weighting factor is thought to be one of the sources of normal tissue toxicity. The clinical evidence of RBE variations in patients is scarce since the complication rates for most treatment sites are low and follow-up times are currently too short to observe secondary malignant neoplasms in a sufficiently high number of patients as they typically arise more than 10–15 years following (radiation) treatment [15], [43], [51]. Thus far, (mainly pre-clinical) reports have postulated the RBE of protons compared to X-rays to be 1.1. However, it may well be that the RBE is higher than 1.1 and that it may vary depending on the position relative to the Bragg-peak, characterized by an increased linear energy transfer (LET). This uncertainty may lead to substantial dose increases to organs at risk, e.g., brainstem, temporal lobe, optic chiasm, in particular if they are in vicinity of the target volume.
This review summarizes recent abstracts from international meetings and international peer-reviewed publications on the potential variation of RBE and its possible side effects, and compares these with past publications on photon beam irradiation. Moreover, recent literature on how to deal with potential RBE variations and the resulting uncertainty during treatment planning, as well as solutions to correlate dose and LET distributions to subsequent (magnetic resonance) imaging changes, are presented. Finally, the current status on RBE measured in vitro and in vivo is reviewed with further discussion on how to bridge the existing gap between the laboratory and clinic.
Clinical reports on toxicity and RBE
In present years, increasing numbers of reports on treatment outcome and toxicity of patients treated with protons have been published. These encompass (pediatric and adult) chordoma and chondrosarcoma of the skull-base and axial skeleton, and ependymoma and posterior fossa malignancies [8], [18], [35], [44], [48], [56]. As the initial publications on the effect of the RBE on toxicity have focused on brain tumors, this review will also primarily focus on the toxicity reported for those tumors, and on the putative association with RBE.
The first studies discussed did not include a correlation with RBE. Murphy et al. [34] assessed a cohort of 236 patients with embryonal tumors who were treated with surgery, chemotherapy and proton beam therapy of the craniospinal axis and an additional boost to the primary tumor [(cumulative dose 55.8 Gy(RBE)]. In total, 8 patients developed brain necrosis (7 of the brainstem, 1 cerebellar), representing 3.7% of the entire cohort and 4.4% of those patients with an infratentorial tumor. A detailed analysis of the spectrum of brainstem injuries occurring in a cohort of 313 patients with tumors of the brain and skull base [dose > 50.4 Gy(RBE)] was published by Indelicato et al. [21] and reported a 2-year-incidence of brainstem toxicity ≥grade 2 of 3.8%. Risk factors for brainstem necrosis included a tumor location in the posterior fossa (actuarial rate 10.7%), age <5 years (12.5% versus 7.2% aged ≥5 years), ependymoma as primary tumor (crude rate 10.9%), but not chemotherapy. Notably, patients with ependymoma of the posterior fossa tend to be at higher risk since surgeons strive to achieve a (near-) complete tumor resection for better disease control. Higher risk is also due to the proximity of ependymoma to critical cranial nerves and vessels. Moreover, the authors established useful dosimetric constraints, including, (i) the maximum dose to the brainstem should not exceed 56.6 Gy(RBE) and, (ii) the mean dose to 50% of the brainstem should not be above 52.4 Gy(RBE). These parameters have since been incorporated in the Children’s Oncology Group (COG) proton therapy guidelines. The same first author has recently summarized the outcome of UK children referred for proton therapy to a North American facility [20]. Of the 166 patients in total, only 1 (0.6%) patient with a posterior fossa ependymoma developed a symptomatic brainstem necrosis with a dose of 55.1 Gy(RBE). In a retrospective review of clinical and radiological data in 60 pediatric patients with primary brain tumors treated with proton therapy [to the tumor (bed), in 21 patients combined with proton-based craniospinal irradiation to a mean total dose of 54 Gy(RBE); range 21 Gy-59.4 Gy(RBE)], Kralik et al. [24] reported an imaging-based radiation necrosis rate of 31% with a median onset time of 5.0 months (range, 3–11 months). They identified multiple (>3) chemotherapeutic agents and atypical teratoid rhabdoid tumor pathology as risk factors for developing radiation necrosis (p = 0.03, respectively). The median time to complete resolution was 5.3 months (range, 3–12 months), with complete resolution of enhancement seen in 50% of patients at 3 months, in 75% of patients at 6 months, and in 100% of patients at 12 months. Twenty-five percent of the patients with imaging changes (i.e., 8% of the whole patient group) of radiation necrosis required medical intervention for severe symptoms. The small locations of necrosis (largest focus of contrast enhancement measured 0.9 cm) did typically not occur immediately adjacent to the resection cavity, but instead in the periventricular white matter and corpus callosum as well as the pons and cerebellum.
The recent reports on toxicities following proton therapy may suggest that these unwanted side effects, even though still few in number, only occur when having applied proton beam therapy. In past series on 3D-conformal photon therapy, however, the rate of brainstem toxicity was also reported to be in the range of 2.5–18%, even though the definitions of the endpoint were heterogeneous [28], [45]. Contrary to the argument that increased precision using intensity-modulated radiation therapy (IMRT) or volumetric modulated arc therapy (VMAT) may overcome this issue of toxicity, Nanda et al. [35] recently reviewed 60 pediatric patients with infratentorial ependymomas (N = 16) and medulloblastomas (N = 44) who were treated with IMRT or VMAT following gross/near total resection (N = 43) or subtotal resection (N = 17). The median follow-up was 33.8 months, and 24.1 months for follow-up imaging studies, i.e., cerebral MRI with intravenous contrast agent. Fourteen patients (23.3%) experienced brainstem toxicity attributable to IMRT/VMAT, being grade 1 in 3 patients, grade 2 in 9 patients and grade 3–5 in 2 patients. One patient with grade 3 toxicity developed a radiation necrosis 118 days following radiotherapy requiring surgical intervention. The other patient died of sepsis and multi-organ failure after having developed severe brainstem toxicity 378 days after radiotherapy. No correlation between development of acute or late brainstem toxicity and clinical covariates was found. Merchant and colleagues [30] reported a brainstem necrosis rate of 2.5% at 7 years in a cohort of 153 ependymoma patients being treated to photon radiation doses of 54 Gy (below the age of 18 months) and 59.4 Gy (all other patients). Remarkably, none of these patients had received chemotherapy, but they all experienced peri-operative morbidity, which the authors concluded to be the cause of these side effects. Both studies had not considered constraints to the brainstem.
At present, there are 3 peer-reviewed publications that correlated CNS injury with LET or RBE in brain tumor patients [15], [18], [43]. Moreover, abstracts on this subject that were submitted to the 2017 ESTRO or ASTRO conferences are summarized in Table 1. The Boston group published the associations of CNS injury with LET and RBE in 111 medulloblastoma patients having undergone a craniospinal irradiation (passively scattered technique with involved field or whole posterior fossa boost) [16]. Patients with clinical symptoms of CNS injury (explicitly not only necrosis) were selected and the MRI findings for all patients were contoured on the original planning CT-scans. Ten patients had post-treatment changes on the MRI, of whom 4 patients developed CNS injury at a median of 9 months from the start of RT. Three of those patients developed brainstem injury 27.4 months from the start of RT, all at a median dose to the brainstem of >52.4 Gy(RBE). The LET distributions of these 3 patients did not differ from those of the remaining asymptomatic patients. Conversely, the RBE-weighted dose for the boost target and total craniospinal axis (according to the Carabe model [6]) was higher than that, assuming the constant RBE value of 1.1. The group from MD Anderson Cancer Center in Houston published 2 sequential papers, in which part of the data was re-used for a second, image-based analysis. First, Gunther et al. [18] assessed imaging changes (MRI at least 6 months after RT) in 72 ependymoma patients treated with postoperative RT (protons 37 patients, IMRT 35 patients). Twenty-two patients were found to have imaging changes (protons 16 and IMRT 6 patients, respectively), of whom 11 had changes in the brainstem. Grade 3 (evidence of hemorrhage) and 4 (encephalomalacia or focal necrosis) changes mainly occurred in the proton cohort. On multivariate logistic regression analysis (not specified for symptomatic patients or those with necrosis only), adverse factors for MRI detected changes were proton beam therapy (OR: 3.89, p = 0.024), early initiation of RT after surgery (31.0 versus 46.0 days; OR: 0.70, p = 0.068), and age below 3 years at diagnosis (OR: 0.38, p = 0.096). In the 16 proton patients, imaging changes were more frequently found in patients with higher mean doses [48.6 Gy(RBE) versus 36.8 Gy(RBE)] and higher median doses to the brainstem [56.0 Gy(RBE) versus 42.8 Gy(RBE)]. Second, the possibility of a variable RBE was further investigated in 34 ependymoma children (a subset of the aforementioned study) treated with proton beam therapy and followed up with T2-weighted MRI [43]. The T2-FLAIR hyperintensity (grade ≥1) was delineated, and the dose and LET distributions were calculated. Voxel-based changes on the post-treatment MR images were found to depend on the physical dose and the track-averaged LET. Furthermore, the authors developed a generalized linear model that describes the decrease in TD50% (tolerance dose at which a toxic effect is expected in 50% of the patients) for image changes as the proton LET increases. Validation of this model in independent cohorts as well as development of similar models for other body sites is still pending.
Table 1.
Reports on toxicity potentially attributed to the RBE effect.
| First Author [reference] | Tumor site; cohort size | Observations |
|---|---|---|
| Harrabi [19] | Meningioma, low grade glioma; 430 patients (276 protons, 154 photons) | Correlation of MRI findings (minimum follow-up 12 months) with LET and RBE distributions Cumulative incidence of necrosis after proton beam therapy: 3.3% No correlation with technique, number of beams, dose, concomitant chemotherapy Significant association with periventricular border and distal edge of SOPB |
| Zhang [60] | Nasopharyngeal carcinoma; 75 patients (61 protons, 14 IMRT) | Incidence temporal lobe necrosis (TLN) at 5.6 years: 7% for IMRT versus 14.8% in PBT; median interval 34 months (9–82 months). Asian race is the only clinical risk-factor for TLN. RBE estimated 1.12–1.25 |
| Merchant [29] | Craniopharyngioma; 97 proton patients (subset of NCT01419067) and 101 photon patients | Incidence of necrosis 2.68% (± SE 1.89%) for protons versus 1.98% (± SE 1.39%) for photons; permanent neurological deficits 4.15% (± SE 2.38%) versus 2.97% (± SE 1.70%) |
Correlation of imaging with side effects
Systematic toxicity studies with an adequate number of patients to reach a detectability threshold are needed to investigate potential correlations between RBE and clinical side effects. This is especially true when absolute numbers of reported toxicities are small. Also, due to the variable nature of RBE, with typically locally pronounced effects, it is important to be able to spatially resolve the biological effect in each patient. In this context, the analysis of follow-up imaging appears to be an appropriate approach for multiple reasons: (i) it allows for a spatially resolved analysis at the voxel level, (ii) direct correlation with treatment planning data on physical dose and LET, and iii. a quantitative analysis, due to the large number of voxels per patient. While quantitative MRI studies have been used earlier to verify proton beam ranges, e.g., by assessing MR changes of the vertebral spine [14], similar approaches can be applied to analyze the potential occurrence of fibrosis or necrosis after proton treatment. Apart from hyperintensity on T2 Flair images, as used in [43], T1 images with contrast agent may be relevant for detection of brain necrosis. To correlate the dose and LET (obtained from detailed Monte Carlo treatment plan simulations), MR images need to be co-registered with the treatment CT (voxel wise with MR grey values). An inherent difficulty in the analysis of MR images is the univocal differentiation between tumor recurrence and radiation induced injury. For improved differentiation, functional MRI, e.g., diffusion-weighted MRI, diffusion tensor imaging or magnetic resonance spectroscopy, or MRI combined with positron emission tomography (e.g., using 11C-methionine), has been proposed [17], [42]. Apart from MRI, quantitative changes may also be analyzed on follow-up CTs, e.g., comparing late-phase lung-density changes (indicative of asymptomatic fibrosis) for chest wall patients treated using protons and X-rays [50].
Factors influencing RBE
To acknowledge the elevated biological effectiveness of proton therapy, it is common practice to use a constant generic RBE of 1.1 (recommended by the ICRU Report 78) when planning treatments and analyzing outcomes, even though the RBE in preclinical studies varies across the spread-out Bragg-peak (SOBP). More precisely, the RBE for proton therapy is considered a complex function of radiation dose, LET, cell type, endpoint, etc. [40]. Hence, the assumption of a fixed RBE, together with the effects of physical uncertainties, may result in a biologically effective dose distribution experienced by the patient that significantly differs from that approved during treatment plan evaluation. This may contribute to unforeseen toxicities and/or failure to control the disease [32].
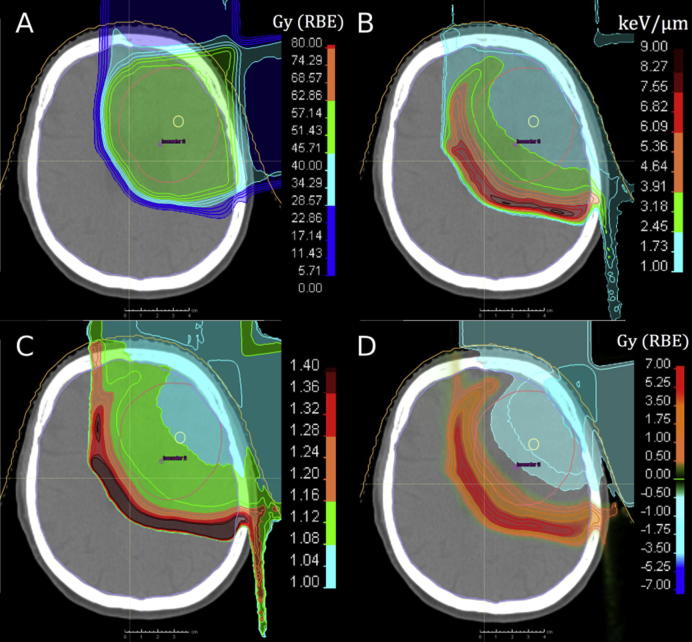
The assumption of a fixed RBE for protons is often justified with the uncertainties in the available RBE data, especially, the dependence on biological and clinical factors. On the other hand, the dependence on physical parameters as demonstrated in vitro is more accepted as a systematic effect. The analysis of large amounts of experimental data suggests that, averaged over all cell lines, the RBE for cell survival varies from 1.1 in the SOBP entrance, to 1.15 at its center, to 1.35 at the distal edge, and to 1.7 or even 4–6 in the distal fall-off at 2 Gy/fraction [11], [40]. This RBE increase is found to correlate with increasing dose-averaged LET. In other words, the proton RBE varies significantly with the LET along the beam path, especially near the end of the particle range. Accordingly, the predominant RBE effect would be expected in the normal tissue beyond the clinical target volume (CTV) as discussed by [24]. This is also shown in Fig. 1 for a clinical treatment plan of a primary brain tumor patient, where the physical proton dose used for patient treatment was weighted with variable in vitro RBE data for glioblastoma and normal tissue cells.
Fig. 1.
Proton treatment plan for a primary brain tumor patient with a prescribed dose of 60 Gy(RBE) to the CTV. (A) Absorbed physical dose Dphys; (B) LET distribution; (C) variable RBE distribution based on measured in vitro RBE data [7], which depend on dose and LET; (D) difference in RBE-weighted dose: Dphys × RBE − Dphys × 1.1. Courtesy of Jan Eulitz and Christian Hahn.
The RBE dependence on different cell lines or tissue types correlates to some extent with their fractionation sensitivity of α/β for photon irradiation – also due to RBE being defined as a ratio of photon and particle dose resulting in the same effect [26], [39], [40]. Low α/β value biosystems have the widest RBE ranges, showing a strong decrease of RBE with increases in dose per fraction. At clinical dose levels (≈2Gy), the RBE increases with LET often above 1.1, even within the SOBP [23]. On the other hand, tumors that are hardly fractionation-sensitive (α/β = 10–25 Gy) have the lowest RBEs, even below 1.1.
Incorporation of RBE in treatment planning and dose evaluation
Given the current knowledge on RBE in proton beam therapy, the question on how to proceed and deal with the resulting uncertainties in treatment planning and evaluation arises as a distinct future challenge. To enhance proton therapy and deliver safe patient treatments, a strategy should be advanced that builds upon different approaches.
-
(1)
Awareness for RBE uncertainty should be raised during plan evaluation.
-
(2)
Strategies to mitigate the RBE effect may be used during treatment planning, e.g., by modification of beam positioning.
-
(3)
Patient outcome data need to be analyzed to generate clinical RBE data.
-
(4)
Clinically relevant in vivo RBE studies have to be initiated.
While points (1) and (2) are discussed in this section, the last two points (3) and (4) are covered by the previous clinical and the subsequent biological section, respectively.
Regarding the first point, it appears important that all proton therapy practitioners are aware of the potential variability of RBE and where in the patient it could be expected. There is, on the other hand, the dilemma that assumptions currently made on the variability of the RBE already influence treatment planning decisions, e.g., by omitting certain beam angles. An automated application of RBE models within a treatment planning system seems, however, at the moment unfeasible due to the inconsistency of clinically relevant experimental data and the lack of adequate clinical validation of such variable RBE models. Accordingly, a focus is currently set on the second point, namely, mitigating the potential impact of proton RBE uncertainties in treatment planning decisions to ensure a safe treatment. Potential RBE mitigation strategies can be categorized into (a) beam angle selection and dose reduction in the distal part of the SOBP, (b) robust optimization, and (c) LET optimization, which are briefly presented.
(a) Beam angle selection and dose reduction in the distal part of the SOBP
In clinical practice, certain beam directions are avoided, e.g., proton beams stopping inside or just proximal to the normal tissue at risk [12], [15], [32]. These approaches are adopted due to concern for risk of injury around the distal edge, resulting from various uncertainties, such as in proton range, and in RBE, and by variations in patient anatomy and positioning. Treatment planning studies, e.g., for pediatric cancer patients, show that changing the field angles may lead to a reduction of LET and variable RBE-weighted dose in the brainstem [12]. Alternatively, the physical dose within the last millimeters of the proton SOBP may be reduced by a scaling factor based on established average in vitro sensitivities. However, the reduction of the prescribed doses by radiation oncologists realizing the potential of increased adverse effects, as reported in [32], requires acceptance of potentially compromised tumor control. This approach is counter-intuitive to health care practitioners and moreover not implemented in treatment plan optimizers. While the clinical practice of beam angle selection or dose reduction may be reasons for the still missing definitive clinical evidence to abandon the convention of a proton RBE of 1.1, they may also limit the true potential of proton therapy.
(b) Robust optimization
Robust optimization of the dose can yield treatment plans less sensitive to range variations, especially in the case of intensity-modulated proton therapy (IMPT). This is achieved by applying probabilistic and worst-case robust optimization instead of range-related uncertainty margins, which mitigate the clinical significance of range variations. In a similar way, robust optimization can be used to also moderate uncertainties associated with the RBE [52]. In IMPT, these uncertainties can occur anywhere within the target volume and are therefore more difficult to anticipate compared to passive scattering fields, where large RBE variations are primarily expected at the margins of the target volume. Accordingly, it appears advisable to also incorporate RBE uncertainties into proton plan robustness evaluation [36].
(c) LET optimization
Conventionally, the IMPT optimization criteria only include dose-based objectives assuming a constant RBE = 1.1. Different IMPT plans with similar physical dose distributions may yield greatly different LET distributions. Therefore, additional LET-based objectives can be implemented that aim at maximizing LET in target volumes while minimizing LET in critical structures and normal tissues [5], [38], [52]. The idea to optimize LET in addition to physical radiation dose is not novel and was suggested earlier for heavy ions [2], [3], to overcome radioresistance in hypoxic parts of the tumor. Practically speaking, the optimization process aims at shifting stopping protons from the margins of the treatment volume and in particular the critical structures towards more central regions of the CTV, e.g., by introducing proton track-end objectives [38]. LET optimization methods can be effective for treatments where serial organs at risk with a maximum dose constraint are located within or near the target, e.g., for intracranial tumors [52].
However, there may be a need for a tradeoff between the acceptability of physical dose and LET distributions, e.g., steep dose gradients are typically achieved by placing stopping protons at field edges [5]. The dosimetric effect of six different either dose- or LET-sparing treatment planning strategies applied in ependymoma patients using 2 or 3 passive scattering fields was compared in [15]. Strategies decreasing the LET values in the brainstem, e.g., by beam angle variation, were found to result in significantly higher brainstem volumes receiving high or full prescription dose.
Intra- and interfractional movement and anatomical changes are likely to reduce the high-LET volume applied to the patient compared to that predicted by the treatment plan. The resulting smearing-out of LET hotspots may also be a reason why, so far, only weak correlations have been established between (calculated) LET and variable RBE and observed clinical outcome data. However, proton beam facilities are increasingly implementing image-guided adaptive proton therapy, which aims at placing the proton beam spots at the same position in the patient on each treatment day. Ultimately, the increased precision may lead to more pronounced variable RBE effects. The potential impact of respiratory motion on variable RBE in proton therapy has been studied theoretically. For a patient with a liver tumor, intrafractional motion did not reduce the effects of variable RBE [49]. For breast cancer, breathing motion may have an impact on variable RBE, but may be negligible in most cases where the physical OAR doses are low [37]. However, more clinically relevant studies are necessary to investigate the effects of patient motion on RBE.
Biological considerations on relevance and assessment of a variable RBE
In the translational biological community, a constant RBE of 1.1–1.2 for protons and much higher and variable RBEs for heavier ions have been widely accepted, despite historical data showing very large variations of proton RBE in the in vitro and in vivo settings [41]. The translational potential of this historical data to the clinic was mainly limited by the use of primarily murine tumor cell lines and the in vivo endpoints of tumor growth delay and normal tissue complication - the latter being mainly early intestine reactions. Recently, our understanding of proton RBE has been updated based on experiments performed with human cell cultures. Depending on the position in the Bragg-peak, an increase of the RBE was shown in a LET-dependent manner being highest at the distal fall off of the proton Bragg-peak [4], [7], [40]. The clinical implications of these findings are thus far unclear, as others have found RBE values of 1.1 in a similar experimental setting [47]. However, 2-dimensional cell cultures may not be representative for the clinical treatment situation. Experiments with more sophisticated in vitro and in vivo models are therefore needed to evaluate the potential risks for the normal tumor-surrounding tissue and the potential benefits of an increased tumor cure. The possible improvement of preclinical radiobiology experiments to support clinical trials was recently reviewed for photon-based radiotherapy [9]. Most of the statements made are also true for proton radiobiology experiments, e.g., comparability of translational pre-clinical results across laboratories, which needs the development of thorough assays and cross-platform validation. Nonetheless, the restricted number of accessible proton beam lines for experimental purposes will still account for limited data sets in the nearby future. Therefore, it is of crucial relevance to design proton experiments for a translation to clinics. The relevance may be achieved by first, improving the experimental cell cultures system including translational endpoints and second, the use of a clinical exposure situation including sophisticated dosimetry and beam shaping systems.
Besides standard 2-dimensional mono-cultures, more physiological normal tissue equivalents for, e.g., skin have been developed and used to study irradiation effects [1], [46], [55]. These models contain generally more than one cell type in its organotypic differentiation state and allow for monitoring the cellular behavior over several days. The life span is limited since the stem cell compartment is missing. In the future, human pluripotent stem cell-derived 3-dimensional organoid culture system such as cerebral organoids may close this gap [25]. Currently, the technical challenges and high costs associated with growing those models impede routine use. For studying tumors in vitro, cell line derived 3-dimensional tumor spheroids are available which resemble the oxygen and nutrient gradients similar to small tumors [13], [57]. Closer to the clinic are patient-derived tumor organoids [22], [31]. In the past, a treatment individualization based on patient derived xenografts (PDX) was performed, however, the varying success rates, long latency times and slow growth rates of PDX hampered the high expectations of this approach for radiotherapy [59]. More reliably growing tumor models such as heterotopic and orthotopic cell line-derived xenografts seem to be more applicable for studying in vivo effects of proton irradiation. However, the need for immune deficient hosts to grow human tumor models conflicts with the increasingly recognized importance of the modulatory response of the immune system following irradiation [58]. Clinically, the biggest benefit of proton therapy is believed to be the protection of the normal tissue due to the volume effect: the inverted depth-dose profile of protons leads to less normal tissue exposed to low and intermediate doses. The proton beam used for such experiments should resemble a clinical composition of energies covering the target volume with a homogeneous dose. For 2- and 3-dimensional cell culture systems as well as organoids growing in regular cell culture plastic, this might be achieved with large field spread-out Bragg-peaks. However, the target volume in a rodent model will be much smaller when specific organs or subvolumes of organs are to be irradiated [53]. Partial body irradiation might be a first approach, but does not sufficiently simulate the clinical situation where attempts are made to expose the least amount of normal tissue. A particular challenge is the conformal exposure of experimental tumor/xenografts, e.g., 6 mm heterotopic subcutaneous tumors or <4 mm orthotopic brain tumors. In part, the challenge arises due to the limited accessibility to the proton facility, which is generally outside the animal housing unit. This requires an anesthetized and/or an immobilized animal in a sealed and transportable containment, which guarantees the stable hygiene status of the immunocompromised animal outside the facility. Furthermore, imaging devices for position verification at experimental beam lines is usually not available, while at clinical beam lines the installed imaging devices are not scaled for the volume resolution needed for small animals [33]. Dual-energy proton radiography might be a solution for inline positioning verification and treatment planning [33]. Dosimetry of small fields needed for experimental tumors, e.g., 5 mm in diameter, is the second part of the challenge. Independent of passive beam shaping or spot scanning proton application systems, the target volume in the rodent model will be in most cases smaller than the beam spot demanding for a collimation system. On the scale of a small animal, the dose gradient of a proton Bragg-beam is only moderate so that several millimeters of tissue will be irradiated with “the effective end” of a clinical proton beam. Additionally, the radial dose distribution of a low-energy 5 mm beam spot does not present a flat dose plateau but rather a Gaussian shape of the dose distribution. These particular features of a small proton field might call for a new concept for defining the applied dose to the animal, which might be orientated on the clinical standard for stereotactic body radiotherapy. However, recently an analytical treatment planning system was developed, which aimed at fulfilling the target accuracy constraints relevant for small-animal irradiation by improving the proton range calculations [54]. Differences between simulated and experimental proton stopping powers lead to proton range uncertainties and therewith to target accuracy deviations, which exceed the tolerable limit for mice of 0.1 mm, but might be suitable for irradiating rats or rabbits. Irradiation of spontaneous tumors of larger companion animals, e.g., dogs, may be more appropriate to test clinically relevant treatment planning and exposure techniques [27]. Lastly, all experimental proton results need to be evaluated in the context of a reference beam resulting in the same biological effect. In radiobiology, X-ray tubes (200–250 kVp) and photon sources (Co60, Cs137) are in use, which differ from the photon spectrum applied by clinical 6–18 MV photon linear accelerators. Therefore, parts of the discrepancies found in the literature on the RBE variabilities might be attributed to the reference beams. At least experiments aiming for clinical translation should consider using a clinical reference beam for RBE determination.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ctro.2018.01.006.
Appendix A. Supplementary data
References
- 1.Acheva A., Schettino G., Prise K.M. Pro-inflammatory signaling in a 3D organotypic skin model after Low LET irradiation-NF-kappaB, COX-2 activation, and impact on cell differentiation. Front Immunol. 2017;8:82. doi: 10.3389/fimmu.2017.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassler N., Jakel O., Sondergaard C.S., Petersen J.B. Dose- and LET-painting with particle therapy. Acta Oncol. 2010;49:1170–1176. doi: 10.3109/0284186X.2010.510640. [DOI] [PubMed] [Google Scholar]
- 3.Bassler N., Toftegaard J., Luhr A. LET-painting increases tumour control probability in hypoxic tumours. Acta Oncol. 2014;53:25–32. doi: 10.3109/0284186X.2013.832835. [DOI] [PubMed] [Google Scholar]
- 4.Britten R.A., Nazaryan V., Davis L.K. Variations in the RBE for cell killing along the depth-dose profile of a modulated proton therapy beam. Radiat Res. 2013;179:21–28. doi: 10.1667/RR2737.1. [DOI] [PubMed] [Google Scholar]
- 5.Cao W., Khabazian A., Yepes P.P. Linear energy transfer incorporated intensity modulated proton therapy optimization. Phys Med Biol. 2017 doi: 10.1088/1361-6560/aa9a2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carabe A., Moteabbed M., Depauw N., Schuemann J., Paganetti H. Range uncertainty in proton therapy due to variable biological effectiveness. Phys Med Biol. 2012;57:1159. doi: 10.1088/0031-9155/57/5/1159. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary P., Marshall T.I., Perozziello F.M. Relative biological effectiveness variation along monoenergetic and modulated Bragg peaks of a 62-MeV therapeutic proton beam: a preclinical assessment. Int J Radiat Oncol Biol Phys. 2014;90:27–35. doi: 10.1016/j.ijrobp.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y.L., Liebsch N., Kobayashi W. Definitive high-dose photon/proton radiotherapy for unresected mobile spine and sacral chordomas. Spine. 2013;38:E930–936. doi: 10.1097/BRS.0b013e318296e7d7. [DOI] [PubMed] [Google Scholar]
- 9.Coleman C.N., Higgins G.S., Brown J.M. Improving the predictive value of preclinical studies in support of radiotherapy clinical trials. Clin Cancer Res. 2016;22:3138–3147. doi: 10.1158/1078-0432.CCR-16-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Combs S.E. Does proton therapy have a future in CNS tumors? Curr Treat Options Neurol. 2017;19:12. doi: 10.1007/s11940-017-0447-4. [DOI] [PubMed] [Google Scholar]
- 11.Cuaron J.J., Chang C., Lovelock M. Exponential increase in relative biological effectiveness along distal edge of a proton bragg peak as measured by deoxyribonucleic acid double-strand breaks. Int J Radiat Oncol Biol Phys. 2016;95:62–69. doi: 10.1016/j.ijrobp.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fjaera L.F., Li Z., Ytre-Hauge K.S. Linear energy transfer distributions in the brainstem depending on tumour location in intensity-modulated proton therapy of paediatric cancer. Acta Oncol. 2017;56:763–768. doi: 10.1080/0284186X.2017.1314007. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich J., Ebner R., Kunz-Schughart L.A. Experimental anti-tumor therapy in 3-D: spheroids–old hat or new challenge? Int J Radiat Biol. 2007;83:849–871. doi: 10.1080/09553000701727531. [DOI] [PubMed] [Google Scholar]
- 14.Gensheimer M.F., Yock T.I., Liebsch N.J. In vivo proton beam range verification using spine MRI changes. Int J Radiat Oncol Biol Phys. 2010;78:268–275. doi: 10.1016/j.ijrobp.2009.11.060. [DOI] [PubMed] [Google Scholar]
- 15.Giantsoudi D., Adams J., MacDonald S.M., Paganetti H. Proton treatment techniques for posterior fossa tumors: consequences for linear energy transfer and dose-volume parameters for the brainstem and organs at risk. Int J Radiat Oncol Biol Phys. 2017;97:401–410. doi: 10.1016/j.ijrobp.2016.09.042. [DOI] [PubMed] [Google Scholar]
- 16.Giantsoudi D., Sethi R.V., Yeap B.Y. Incidence of CNS injury for a cohort of 111 patients treated with proton therapy for medulloblastoma: LET and RBE associations for areas of injury. Int J Radiat Oncol Biol Phys. 2016;95:287–296. doi: 10.1016/j.ijrobp.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Glaudemans A.W.J.M., Enting R.H., Heesters M.A.A.M., van Rheenen R.W.J., Dierckx R.A., Slart R.H.J.A. The value of 11C-methionine PET in the differential diagnosis between brain tumor recurrence and radionecrosis. In: Dierckx R., Otte A., de Vries E., van Waarde A., Leenders K., editors. PET and SPECT in neurology. Springer; Berlin, Heidelberg: 2014. [Google Scholar]
- 18.Gunther J.R., Sato M., Chintagumpala M. Imaging changes in pediatric intracranial ependymoma patients treated with proton beam radiation therapy compared to intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93:54–63. doi: 10.1016/j.ijrobp.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Harrabi S., Gudden C., Adeberg S. Radiation necrosis after proton beam therapy – when and where does it happen? ESTRO 36. 2017;123:S271. Wien. Elsevier. [Google Scholar]
- 20.Indelicato D.J., Bradley J.A., Sandler E.S. Clinical outcomes following proton therapy for children with central nervous system tumors referred overseas. Pediatr Blood Cancer. 2017;64 doi: 10.1002/pbc.26654. [DOI] [PubMed] [Google Scholar]
- 21.Indelicato D.J., Flampouri S., Rotondo R.L. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol. 2014;53:1298–1304. doi: 10.3109/0284186X.2014.957414. [DOI] [PubMed] [Google Scholar]
- 22.Jin M.Z., Han R.R., Qiu G.Z., Ju X.C., Lou G., Jin W.L. Organoids: an intermediate modeling platform in precision oncology. Cancer Lett. 2017;414:174–180. doi: 10.1016/j.canlet.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Jones B. Clinical radiobiology of proton therapy: modeling of RBE. Acta Oncol. 2017;56:1374–1378. doi: 10.1080/0284186X.2017.1343496. [DOI] [PubMed] [Google Scholar]
- 24.Kralik S.F., Ho C.Y., Finke W., Buchsbaum J.C., Haskins C.P., Shih C.-S. Radiation necrosis in pediatric patients with brain tumors treated with proton radiotherapy. Pediatrics. 2015;36:1572–1578. doi: 10.3174/ajnr.A4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lancaster M.A., Renner M., Martin C.A. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luhr A., von Neubeck C., Helmbrecht S., Baumann M., Enghardt W., Krause M. Modeling in vivo relative biological effectiveness in particle therapy for clinically relevant endpoints. Acta Oncol. 2017;56:1392–1398. doi: 10.1080/0284186X.2017.1356468. [DOI] [PubMed] [Google Scholar]
- 27.Mayer-Stankeova S., Fidel J., Wergin M.C. Proton spot scanning radiotherapy of spontaneous canine tumors. Vet Radiol Ultrasound. 2009;50:314–318. doi: 10.1111/j.1740-8261.2009.01542.x. [DOI] [PubMed] [Google Scholar]
- 28.Mayo C., Yorke E., Merchant T.E. Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys. 2010;76:S36–41. doi: 10.1016/j.ijrobp.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merchant TE, Hua CH, Sabin ND, et al. Necrosis, vasculopathy, and neurological complications after proton therapy for childhood craniopharyngioma: Results From a Prospective Trial and a Photon Cohort Comparison. In: Elsevier, ed. ASTRO. San Diego, 2017. p. S120.
- 30.Merchant T.E., Li C., Xiong X., Kun L.E., Boop F.A., Sanford R.A. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10:258–266. doi: 10.1016/S1470-2045(08)70342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merker S.R., Weitz J., Stange D.E. Gastrointestinal organoids: how they gut it out. Dev Biol. 2016;420:239–250. doi: 10.1016/j.ydbio.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Mohan R., Peeler C.R., Guan F., Bronk L., Cao W., Grosshans D.R. Radiobiological issues in proton therapy. Acta Oncol. 2017;56:1367–1373. doi: 10.1080/0284186X.2017.1348621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller J., Neubert C., von Neubeck C. Proton radiography for inline treatment planning and positioning verification of small animals. Acta Oncol. 2017;56:1399–1405. doi: 10.1080/0284186X.2017.1352102. [DOI] [PubMed] [Google Scholar]
- 34.Murphy E.S., Merchant T.E., Wu S. Necrosis after craniospinal irradiation: results from a prospective series of children with central nervous system embryonal tumors. Int J Radiat Oncol Biol Phys. 2012;83:e655–660. doi: 10.1016/j.ijrobp.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nanda R.H., Ganju R.G., Schreibmann E. Correlation of acute and late brainstem toxicities with dose-volume data for pediatric patients with posterior fossa malignancies. Int J Radiat Oncol Biol Phys. 2017;98:360–366. doi: 10.1016/j.ijrobp.2017.02.092. [DOI] [PubMed] [Google Scholar]
- 36.Oden J., Eriksson K., Toma-Dasu I. Incorporation of relative biological effectiveness uncertainties into proton plan robustness evaluation. Acta Oncol. 2017;56:769–778. doi: 10.1080/0284186X.2017.1290825. [DOI] [PubMed] [Google Scholar]
- 37.Oden J., Toma-Dasu I., Eriksson K., Flejmer A.M., Dasu A. The influence of breathing motion and a variable relative biological effectiveness in proton therapy of left-sided breast cancer. Acta Oncol. 2017;56:1428–1436. doi: 10.1080/0284186X.2017.1348625. [DOI] [PubMed] [Google Scholar]
- 38.Ödén J., Traneus E. Elsevier; ASTRO San Diego: 2017. Introducing proton track-end objectives as a tool to mitigate the elevated relative biological effectiveness in critical structures; p. E705. [DOI] [PubMed] [Google Scholar]
- 39.Paganetti H. Relating the proton relative biological effectiveness to tumor control and normal tissue complication probabilities assuming interpatient variability in alpha/beta. Acta Oncol. 2017;56:1379–1386. doi: 10.1080/0284186X.2017.1371325. [DOI] [PubMed] [Google Scholar]
- 40.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59:R419–472. doi: 10.1088/0031-9155/59/22/R419. [DOI] [PubMed] [Google Scholar]
- 41.Paganetti H., Niemierko A., Ancukiewicz M. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407–421. doi: 10.1016/s0360-3016(02)02754-2. [DOI] [PubMed] [Google Scholar]
- 42.Parvez K., Parvez A., Zadeh G. The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci. 2014;15:11832–11846. doi: 10.3390/ijms150711832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peeler C.R., Mirkovic D., Titt U. Clinical evidence of variable proton biological effectiveness in pediatric patients treated for ependymoma. Radiother Oncol. 2016;121:395–401. doi: 10.1016/j.radonc.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rombi B., Ares C., Hug E.B. Spot-scanning proton radiation therapy for pediatric chordoma and chondrosarcoma: clinical outcome of 26 patients treated at Paul Scherrer Institute. Int J Radiat Oncol Biol Phys. 2013;86:578–584. doi: 10.1016/j.ijrobp.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 45.Shaw E., Arusell R., Scheithauer B. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20:2267–2276. doi: 10.1200/JCO.2002.09.126. [DOI] [PubMed] [Google Scholar]
- 46.Simoniello P., Wiedemann J., Zink J. Exposure to carbon ions triggers proinflammatory signals and changes in homeostasis and epidermal tissue organization to a similar extent as photons. Front Oncol. 2015;5:294. doi: 10.3389/fonc.2015.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slonina D., Biesaga B., Swakon J. Relative biological effectiveness of the 60-MeV therapeutic proton beam at the Institute of Nuclear Physics (IFJ PAN) in Krakow, Poland. Radiat Environ Biophys. 2014;53:745–754. doi: 10.1007/s00411-014-0559-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stacchiotti S., Sommer J., Chordoma Global Consensus G Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol. 2015;16:e71–83. doi: 10.1016/S1470-2045(14)71190-8. [DOI] [PubMed] [Google Scholar]
- 49.Ulrich S., Wieser H.P., Cao W., Mohan R., Bangert M. Impact of respiratory motion on variable relative biological effectiveness in 4D-dose distributions of proton therapy. Acta Oncol. 2017;56:1420–1427. doi: 10.1080/0284186X.2017.1354131. [DOI] [PubMed] [Google Scholar]
- 50.Underwood T., Grassberger C., Bass R. Clinical evidence that end-of-range proton RBE exceeds 1.1: lung density changes following chest RT. Radiother Oncol. 2017;123:S123–S124. [Google Scholar]
- 51.Underwood T., Paganetti H. Variable proton relative biological effectiveness: how do we move forward? Int J Radiat Oncol Biol Phys. 2016;95:56–58. doi: 10.1016/j.ijrobp.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Unkelbach J., Botas P., Giantsoudi D., Gorissen B.L., Paganetti H. Reoptimization of intensity modulated proton therapy plans based on linear energy transfer. Int J Radiat Oncol Biol Phys. 2016;96:1097–1106. doi: 10.1016/j.ijrobp.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Luijk P., Pringle S., Deasy J.O. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci Transl Med. 2015;7:05ra147. doi: 10.1126/scitranslmed.aac4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vanstalle M., Constanzo J., Karakaya Y., Finck C., Rousseau M., Brasse D. Analytical dose modeling for preclinical proton irradiation of millimetric targets. Med Phys. 2017 doi: 10.1002/mp.12696. [DOI] [PubMed] [Google Scholar]
- 55.von Neubeck C., Geniza M.J., Kauer P.M., Robinson R.J., Chrisler W.B., Sowa M.B. The effect of low dose ionizing radiation on homeostasis and functional integrity in an organotypic human skin model. Mutat Res. 2015;775:10–18. doi: 10.1016/j.mrfmmm.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Weber D.C., Malyapa R., Albertini F. Long term outcomes of patients with skull-base low-grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiother Oncol. 2016;120:169–174. doi: 10.1016/j.radonc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 57.Weiswald L.B., Bellet D., Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia. 2015;17:1–15. doi: 10.1016/j.neo.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wennerberg E., Vanpouille-Box C., Bornstein S., Yamazaki T., Demaria S., Galluzzi L. Immune recognition of irradiated cancer cells. Immunol Rev. 2017;280:220–230. doi: 10.1111/imr.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willey C.D., Gilbert A.N., Anderson J.C., Gillespie G.Y. Patient-derived xenografts as a model system for radiation research. Semin Radiat Oncol. 2015;25:273–280. doi: 10.1016/j.semradonc.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y., Hui W., Adams J.A. Temporal lobe necrosis after proton for nasopharyngeal carcinoma: predictive factors and clinical RBE estimation. ASTRO 59. 2017;98:E386. San Diego. Elsevier. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.