Abstract
Young people in the USA who inject drugs, particularly those at a risk of residence instability, experience the highest incidence of hepatitis C (HCV) infections. This study examined associations between geographic mobility patterns and sociodemographic, behavioral, and social network characteristics of 164 young (ages 18–30) persons who inject drugs (PWID). We identified a potential bridge sub-population who reported residence in both urban and suburban areas in the past year (crossover transients) and higher-risk behaviors (receptive syringe sharing, multiple sex partners) compared to their residentially localized counterparts. Because they link suburban and urban networks, crossover transients may facilitate transmission of HIV and HCV between higher and lower prevalence areas. Interventions should address risk associated with residential instability, particularly among PWID who travel between urban and suburban areas.
Keywords: Persons who inject drugs, Injection drug use, Hepatitis C, Transience, Mobility, Suburban
Introduction
In the USA, alarming increases in injection drug use (IDU) [1, 2] and hepatitis C virus (HCV) infection incidence [3–7] are occurring among young persons who inject drugs (PWID) from non-urban communities [4, 8–14]. IDU is a well-established risk factor for HIV and the primary mode of HCV transmission in developed countries [15]. Until recently, young PWID from non-urban areas, a population predominantly composed of non-Hispanic (NH) whites, generally exhibited low to modest incidence and prevalence of HIV and HCV infection [16–18], despite engaging in high levels of risky injection practices [4, 5, 9, 10, 12, 19]. This disjunction between risk behavior and HIV/HCV infection status was likely due to social and environmental factors that minimized the likelihood of having an infected partner [16]. Recent trends suggest that these protections are eroding, particularly for hepatitis C, with high incidence and outbreaks of HCV infection are now common among non-urban PWID, with the highest rates observed among those aged 20–29 years [3–5]. High levels of risky injection and sexual behaviors [20, 21] and sexually transmitted infections (STIs) [22] are also common among PWID, who report more sex partners, greater likelihood of trading sex for money or drugs, and less consistent condom use than their non-drug-using counterparts [23]. Understanding the individual, social, and geographic factors that promote high-risk partner contact is imperative for developing targeted HIV/HCV interventions for the emerging population of young PWID.
Geographic mobility has important implications for HIV/HCV risk and prevention among PWID. Previous studies have examined various aspects of mobility, including homelessness, residential transience (i.e., having multiple residences over a short period), and travel distances (e.g., to drug markets) on HIV/HCV risk. Studies have consistently linked residential instability with high-risk behaviors (e.g., syringe sharing and exchanging sex for drugs or money) and HIV infection [24–28]. Residential instability may disrupt control of one’s physical environment, fosters use of high-risk strategies to accomplish goals or acquire resources (e.g., injecting in public spaces, syringe sharing, and exchanging sex for drugs or money), and alters social networks (e.g., size, and member characteristics) over time. These factors are all plausible mechanisms for the link between residential instability, high-risk behaviors, and HIV/HCV infection. Among the emerging population of young, predominantly non-urban PWID, little is known about residential transience and directional movement between urban and suburban areas on HIV/HCV risk. Residential transience could conceivably augment HIV/HCV transmission by bridging populations (e.g., older infected and younger uninfected) and regions of high and low prevalence (e.g., urban and suburban). Thus, understanding patterns of residential transience and associated risk is important to develop effective interventions targeted to this population.
Our study seeks to characterize mobility patterns, including residential transience and directional movement, and their association with sociodemographic, behavioral, and injection network characteristics and behaviors of young PWID from both urban and surrounding suburban areas of Chicago, Illinois, USA.
Methods
Subjects and Recruitment
We conducted a cross-sectional personal (egocentric) network study of 164 young PWID and their injection, social support, and sexual networks. Detailed descriptions of sample recruitment, study design, and data collection methods are previously described [29, 30]. Briefly, we recruited participants via flyers posted at four standalone field sites in Chicago located near major heroin and cocaine markets that attract both urban and suburban drug users, as well as at other venues that provide services to PWID in these areas. All individuals responding to posted flyers were directed to on-site research staff for eligibility screening during regular hours of operation between September 2012 and June 2013. Most participants were registered members of a large Chicago syringe services program (SSP) that operated out of the four field sites and a mobile unit. To be eligible for the study, individuals had to be between the ages of 18 and 30 and have injected drugs at least once in the past 30 days.
Trained research staff conducted personal interviews with participants to collect sociodemographic information, self-reported HIV and HCV status, drug and sexual behaviors for the past 6 months, and geographic data on the location and characteristics of places where they resided, purchased drugs, injected drugs, and met sex partners in the past year. Participants also provided similar data on each of their core injection network members, defined as up to 10 individuals they injected most often with in the past 6 months. All participants provided written informed consent. The study was approved by the Institutional Review Board of the University of Illinois at Chicago.
Measures
Sociodemographic Measures
These included gender, age, race/ethnicity, education, employment, place of birth, and homelessness, defined as living on the street or in a shelter at any time during the past 6 months.
Drug use, Sexual Behaviors, and HIV/HCV Infection Status
Participants reported on drug use type (e.g., heroin, crack), mode of administration (e.g., smoking, snorting, injection), and length of drug use (e.g., years injecting drugs). Participants also reported on injection-related risk practices, including frequency of injections per day, injecting with others, sharing syringes or injection paraphernalia (e.g., cotton, cooker, rinse water), syringe-mediated sharing (backloading), and source of syringes (e.g., SSP, street). Participants also provided data on their sexual behaviors, including number, gender, and type (e.g., steady or casual) of sexual partners, condom use with steady and casual sex partners, and exchanging sex for drugs or money. Participants self-reported HIV and HCV infection status, many of whom reported getting tested in the prior year as part of an on-site free service provided to all PWID, regardless of participation in the study.
Geographic Measures
Participants provided the locations (cross-streets, town, state) of all places where they resided, purchased drugs, injected drugs, and met sex partners in the past year. Informed by a substantive body of research among Chicago PWID in the past 30 years [16, 29, 31–35] along with published data on heroin and cocaine police arrest locations for Illinois [36], we used conservative street boundaries to define the three primary drug market areas located within Chicago city limits. We named these the West, South, and Southeast drug markets to correspond to the region of the city where each was located. We assessed movement distance for subjects with multiple residences in the past year, defined as the average distance between residences. For the primary outcome, we constructed three measures of geographic mobility. First, a binary measure of residential transience, defined as having more than one residence in the past year, regardless of location. Second, a three-category indicator variable termed residential movement that assessed whether PWID remained within the same geographic boundaries (urban or suburban Chicago), regardless of number of residences, or moved between geographic areas in the past year. For residential movement, participants were grouped into (i) urban, i.e., having ≥1 residence exclusively within the Chicago city limits (referent category); (ii) suburban, i.e., having ≥1 residence exclusively in suburban areas surrounding Chicago; or (iii) crossover, i.e., having ≥1 residence in urban and ≥1 residence in suburban areas in the past year. Third, among participants who reported transience (n = 96), we assigned a variable termed bridging transience that compared localized transience (i.e., having multiple residences in the same type of area) to crossover transience (i.e., category iii in residential movement).
Injection Network Measures
Participants also provided data by proxy on their core injection network (i.e., those they injected most often with in the past 6 months), including sociodemographic characteristics, risk behaviors, residential transience, residential movement, bridging transience, homelessness, and perceived HIV and HCV infection status. In addition, participants reported on their relationship with each network member, including engagement in injection-related (e.g., syringe sharing) and sexual behaviors. Summary network measures (e.g., size, proportion reporting syringe sharing) were calculated by summarizing across all injection partnerships reported by the participant.
Statistical Analysis
We compared sociodemographic characteristics, injection practices, and network features by residential movement using Pearson chi-square tests for categorical variables and Kruskal-Wallis tests for continuous variables. We used multivariable logistic regression to identify factors associated with residential transience and bridging transience and multinomial logistic regression to examine factors associated with residential movement. For all models, we entered all variables that were p < 0.2 in univariable analysis as well as those deemed conceptually important in multivariable models and removed non-significant variables one by one in an iterative process to build a parsimonious final model. We included gender, age, and race/ethnicity in all multivariable models regardless of statistical significance to compare to prior studies and to control for residual confounding. We conducted inferential statistical analyses using STATA/SE version 14 for Windows (STATA Corp, College Station, TX).
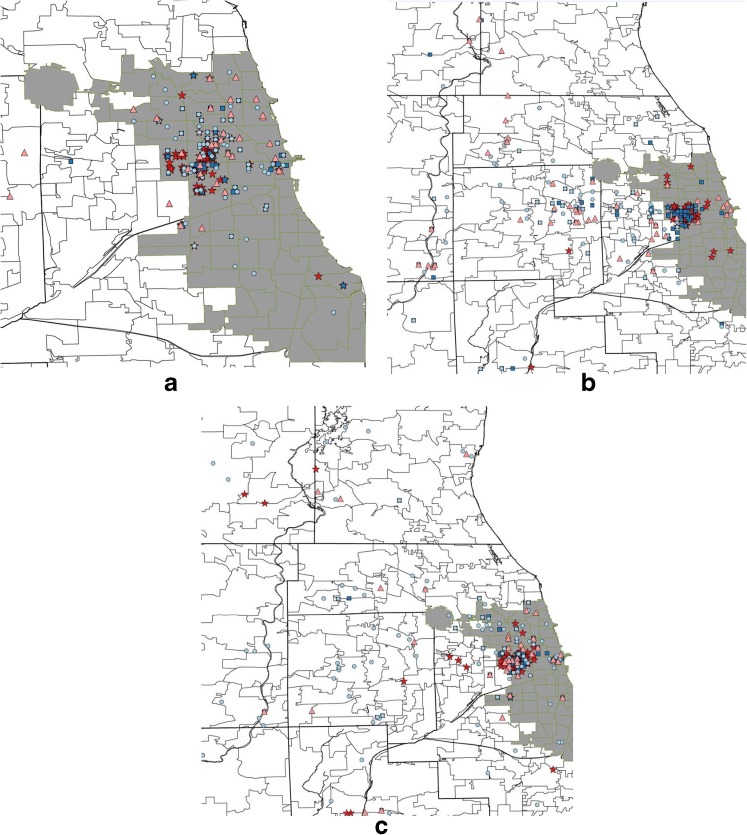
We also performed geographic analyses using Python (Python Software Foundation, Beaverton, OR) and R version 3.2 using the spatstat package. We geocoded geographic locations for analysis using the Google Geocoding API with GeoPy (Google, Mountainview, CA) and categorized them based on whether they were within the Chicago city limits (urban) or in the surrounding areas (suburban), and according to whether or not they were within the boundaries of the largest drug market area (West Side). We created maps to visualize relationships among residence, drug market areas, injection, and sex partner meeting locations (Fig. 1). We used logistic regression models with generalized estimating equations (GEE) to compare injection and sex partner meeting locations (urban/suburban and within the West Side drug market) by residential movement status.
Fig. 1.
a–c Places where young a urban (n = 59), b suburban (n = 60), and c crossover (n = 44) PWID resided, purchased drugs, injected drugs, and met sexual partners in the past year. Urban was defined as having ≥1 residence(s) exclusively within the Chicago city limits; suburban was defined as having ≥1 residence(s) exclusively in the suburban areas surrounding Chicago; and crossover was defined as having ≥1 residence(s) in urban and ≥1 residence(s) in suburban areas in the past year. Maps include multiple locations per person
Results
Participant characteristics by residential movement status are shown in Table 1. Among those who reported residence for the past year (n = 163, 99.4%), 37.0% were suburban (i.e., having ≥1 residence exclusively in the suburban areas surrounding Chicago), 27.0% were urban (i.e., having ≥1 residence exclusively within the Chicago city limits), and 36.0% were crossover (i.e., having ≥1 residence in Chicago and ≥1 residence in Chicago suburbs). Compared to urban and suburban PWID, crossover participants were significantly more likely to report, in the past 6 months, homelessness (81.4%), smoking crack (70.5%), backloading (42.9%), receptive syringe sharing (45.2%), and having 2 or more sex partners (54.6%) (p < 0.05 for all comparisons). Crossover participants were also significantly more likely than their localized counterparts to report sharing syringes with their network (p < 0.01). Compared to others, urban participants were significantly (p < 0.05) more likely to be older and to report an urban place of birth and least likely to report injecting with others, receptive syringe sharing, and syringe sharing with their network (Table 1).
Table 1.
Participant characteristics by residential movement status in the past year
| Suburban n = 60 (37.0%) | Crossover n = 44 (27.0%) | Urban N = 59 (36.0%) | p valuea | |
|---|---|---|---|---|
| Age | ||||
| 18–24 | 25 (41.7) | 16 (36.4) | 12 (20.3) | 0.037 |
| 25–30 | 35 (58.3) | 28 (63.6) | 47 (79.7) | |
| Gender | ||||
| Male | 37 (61.7) | 28 (63.6) | 40 (69.0) | 0.494 |
| Female | 23 (38.3) | 15 (34.1) | 18 (31.0) | |
| Transgender | 0 (0.0) | 1 (2.3) | 0 (0.0) | |
| Race/ethnicity | ||||
| NH White | 45 (75.0) | 33 (75.0) | 39 (66.1) | 0.587 |
| Hispanic | 9 (15.0) | 7 (15.9) | 9 (15.3) | |
| NH Black/other | 6 (10.0) | 4 (9.1) | 11 (18.6) | |
| Educational attainment | ||||
| Less than high school | 20 (33.3) | 9 (20.5) | 15 (25.4) | 0.278 |
| High school diploma | 20 (33.3) | 20 (45.5) | 30 (50.9) | |
| Any college | 20 (33.3) | 15 (34.1) | 14 (23.7) | |
| Place of birthc | ||||
| Urban Chicago | 4 (9.8) | 5 (19.2) | 22 (59.5) | <0.001 |
| Suburban Chicago | 31 (75.6) | 19 (73.1) | 10 (27.0) | |
| Out of state | 6 (14.6) | 2 (7.7) | 5 (13.5) | |
| Regular job | 29 (48.3) | 21 (47.7) | 22 (37.3) | 0.411 |
| Homeless in past 6 months | 19 (32.2) | 35 (81.4) | 30 (50.9) | <0.001 |
| Self-reported HCV positive | 5 (8.3) | 7 (15.9) | 3 (5.1) | 0.164 |
| Years injecting, median (IQR) | 5.5 (3–8) | 5.5 (3–8.5) | 6 (3–9) | 0.423 |
| Drug and sexual risk behaviors, past 6 months | ||||
| Smoked crack | 21 (35.0) | 31 (70.5) | 26 (44.1) | 0.001 |
| Poly-injection drug use | 18 (30.0) | 19 (44.2) | 17 (29.3) | 0.224 |
| Backloading | 13 (21.7) | 18 (42.9) | 11 (19.0) | 0.016 |
| Receptive syringe sharing | 18 (30.0) | 19 (45.2) | 11 (19.0) | 0.018 |
| Injected daily | 46 (76.7) | 33 (75.0) | 51 (86.4) | 0.272 |
| Injections per day | ||||
| 1 | 3 (5.0) | 1 (2.3) | 2 (3.4) | 0.443 |
| 2–4 | 45 (75.0) | 27 (61.4) | 41 (69.5) | |
| ≥5 | 12 (20.0) | 16 (36.4) | 16 (27.1) | |
| Frequency of injection with others | ||||
| Never | 6 (10.0) | 5 (11.4) | 10 (17.0) | 0.047 |
| Sometimes | 26 (43.3) | 30 (68.2) | 31 (52.5) | |
| Often | 28 (46.7) | 9 (20.5) | 18 (30.5) | |
| Exchanged sex for drugs or money | 10 (16.7) | 11 (25.0) | 9 (15.3) | 0.410 |
| Total sex partners | ||||
| 0 | 8 (13.3) | 5 (11.4) | 10 (17.0) | 0.033 |
| 1 | 27 (45.0) | 15 (34.1) | 35 (59.3) | |
| ≥2 | 25 (41.7) | 24 (54.6) | 14 (23.7) | |
| Geographic variables | ||||
| Total residences in the past year, median (IQR) | 1 (1.0–2.0) | 3 (2.5–4.5) | 1 (1.0–3.0) | <0.001 |
| Purchased drugs ≥1 on West Side | 54 (94.7) | 39 (95.1) | 49 (87.5) | 0.899 |
| Purchased drugs in multiple drug market areas | 14 (24.6) | 10 (24.4) | 13 (23.2) | 0.984 |
| Inject in multiple locations | 19 (33.3) | 15 (35.7) | 11 (20.0) | 0.167 |
| Inject drugs ≥1 in West Side drug market area | 32 (56.1) | 37 (88.1) | 36 (65.5) | 0.003 |
| Proportion of total injection episodes in West Side drug market area | 39.0% | 62.8% | 50.9% | <0.01 |
| Average stay in daysc, Median (IQR); n | 310.3 (184–776.5); 25 | 160.3 (100.2–301.5); 39 | 232.2 (153.4–341.5); 28 | 0.004 |
| Average movement distance in milesb, median (IQR); n | 11.1 (3.1–46.8); 25 | 24.2 (13.1–64.3); 38 | 5.4 (2.7–568.0); 28 | 0.079 |
| Change in distance from drug marketb, median (IQR) | 0 (−3.5, +2.5) | 0.24 (−3.1, +5.7) | −0.12 (−2.2, +0.32) | 0.718 |
| Injection network variables | ||||
| Core injection network size, median (IQR) (n) | 3 (2–5) [56] | 3.5 (1–7.5) [40] | 3 (2–5) [51] | 0.599 |
| Mean age of network members, median (IQR) (n) | 27.3 (25.1–30.1) [56] | 29.4 (24.3–33.0) [40] | 30.7 (27–35.8) [51] | 0.003 |
| Proportion of network members homeless, median (IQR) (n) | 0 (0–0) [56] | 0 (0–13%) [40] | 0 (0–33%) [51] | 0.079 |
| Proportion of network members NH white race, median (IQR) (n) | 100% (69–100%) [56] | 68% (45–100%) [40] | 80% (50–100%) [51] | 0.105 |
| Any network member is crossover | 11 (19.6) | 15 (37.5) | 19 (37.3) | 0.077 |
| Any network member is suburban | 50 (89.3) | 19 (47.5) | 13 (25.5) | <0.001 |
| Any network member is homeless | 7 (12.5) | 14 (35.0) | 17 (33.3) | 0.015 |
| Any member is HCV positive | 12 (21.4) | 7 (17.5) | 10 (19.6) | 0.892 |
| Shared syringes with an injection network member | 18 (32.1) | 21 (52.5) | 11 (21.6) | 0.008 |
One individual was missing residence data; analysis based on n = 163
aPearson chi-square or Wilcoxon rank-sum test
bPlace of birth was only available for representative sample (n = 104; 63%)
cAmong those with multiple residences
Predictors of Residential Transience, Residential Movement, and Bridging Transience
In multivariable logistic regression, controlling for age, race/ethnicity, gender, and education, residential transience was associated with homelessness in the past 6 months, (adjusted odds ratio (aOR) = 9.67; 95% CI 3.81–24.5), self-reported HCV positivity (aOR = 5.41; 95% CI 1.18–24.7), receptive syringe sharing (aOR = 1.74; 95% CI 1.07–2.84), exchanging sex for drugs or money (aOR = 9.30; 95% CI 1.65–52.4), and having any network members that met the definition for crossover in the past year (aOR = 3.69; 95% CI 1.15–11.8) (Table 2).
Table 2.
Results of univariable and multivariable logistic regression: factors associated with residential transience, n = 147
| Univariable OR (95% CI) | p value | Multivariable OR (95% CI) | p value | |
|---|---|---|---|---|
| Age 25–30 vs. 18–24 | 0.72 (0.37–1.42) | 0.346 | 0.95 (0.35–2.62) | 0.925 |
| Male gender | 0.91 (0.47–1.77) | 0.785 | 3.17 (1.08–9.32) | 0.036 |
| NH white race | 1.23 (0.62–2.44) | 0.556 | 0.89 (0.32–2.45) | 0.820 |
| Education (ref = less than HS) | ||||
| High school diploma | 1.53 (0.71–3.30) | 0.274 | 1.76 (0.45–6.86) | 0.418 |
| Any college | 2.96 (1.25–7.01) | 0.014 | 4.91 (1.25–19.3) | 0.023 |
| Suburban borna | 0.99 (0.45–2.17) | 0.975 | – | – |
| Regular job | 1.11 (0.59–2.08) | 0.748 | – | – |
| Homeless in past 6 months | 6.42 (3.19–12.9) | <0.001 | 9.67 (3.81–24.5) | <0.001 |
| Self-reported HCV positive | 5.09 (1.10–23.5) | 0.037 | 5.41 (1.18–24.7) | 0.029 |
| Years injecting | 0.99 (0.91–1.07) | 0.779 | – | – |
| Drug and sexual risk behaviors, past 6 months | ||||
| Smoked crack | 2.30 (1.21–4.39) | 0.011 | – | – |
| Poly-injection drug use | 1.99 (0.99–4.01) | 0.053 | – | – |
| Backloading | 2.76 (1.24–6.14) | 0.013 | – | – |
| Receptive syringe sharing | 3.25 (1.50–7.01) | 0.003 | 1.74 (1.07–2.84) | 0.025 |
| Injected daily | 0.59 (0.26–1.35) | 0.211 | – | – |
| Injections per day (ref = 1) | ||||
| 2–4 | 1.15 (0.22–6.00) | 0.865 | – | – |
| ≥5 | 2.75 (0.48–15.6) | 0.254 | – | – |
| Frequency of injection with others (ref = never) | ||||
| Sometimes | 2.37 (0.89–6.33) | 0.086 | – | – |
| Often | 3.16 (1.11–8.98) | 0.030 | – | – |
| Exchanged sex for drugs or money | 4.61 (1.66–12.8) | 0.003 | 9.30 (1.65–52.4) | 0.011 |
| ≥2 Sex partners past 6 months | 3.06 (1.53–6.11) | 0.002 | – | – |
| Geographic variables | ||||
| Drug markets in multiple places | 1.63 (0.75–3.57) | 0.218 | – | – |
| Inject in multiple locations | 1.88 (0.88–3.98) | 0.101 | – | – |
| Inject ≥1 in West side drug market area | 1.06 (0.53–2.12) | 0.870 | – | – |
| Injection network variables | ||||
| Core injection network size | 1.14 (1.01–1.28) | 0.039 | – | – |
| Any network member is suburban | 0.94 (0.82–1.09) | 0.434 | – | – |
| Percent network NH white | 0.45 (0.15–1.31) | 0.141 | – | – |
| Mean age of network members | 0.99 (0.94–1.05) | 0.761 | – | – |
| Any network member is homeless | 4.31 (1.66–11.2) | 0.003 | – | – |
| Any network member is crossover | 5.02 (2.04–12.3) | <0.001 | 3.69 (1.15–11.8) | 0.028 |
| Any network member reports syringe sharing | 2.93 (1.34–6.41) | 0.007 | – | – |
| Any network member is HCV positive | 2.23 (0.88–5.65) | 0.090 | – | – |
aPlace of birth was only available for representative sample (n = 104; 63%)
Table 3 reports on factors associated with residential movement in multinomial regression and shows that, compared to urban participants, crossover participants were more likely to report receptive syringe sharing (aOR = 2.03; 95% CI 1.02–4.01), having 2 or more sex partners (aOR = 5.11; 95% CI 1.52–17.3), injecting at locations within the West Side drug market area (aOR = 6.05; 95% CI 1.24–29.5), and having at least one suburban network member (aOR = 1.95; 95% CI 1.12–3.38). Individuals with exclusively suburban versus exclusively urban residence were significantly younger, less likely to report homelessness in the past past 6 months, and more likely to have suburban network members(p < 0.05 for all comparisons).
Table 3.
Multinomial logistic regression: factors associated with residential movement (including core drug-using network variables), N = 147
| Suburban vs. urban aOR (95% CI) | p value | Crossover transient vs. urban aOR (95% CI) | p value | |
|---|---|---|---|---|
| Age 25–30 vs. 18–24 | 0.27 (0.09–0.82) | 0.021 | 0.42 (0.13–1.37) | 0.151 |
| Male gender | 0.86 (0.29–2.57) | 0.786 | 1.43 (0.44–4.64) | 0.553 |
| NH white race | 2.06 (0.68–6.21) | 0.200 | 1.22 (0.34–4.31) | 0.763 |
| Homeless in past 6 months | 0.26 (0.08–0.82) | 0.022 | 2.74 (0.79–9.47) | 0.111 |
| Receptive syringe sharing past 6 months | 1.52 (0.84–2.75) | 0.171 | 2.03 (1.02–4.01) | 0.043 |
| ≥2 sex partners past 6 months | 2.45 (0.82–7.31) | 0.109 | 5.11 (1.52–17.3) | 0.009 |
| Inject drugs ≥ 1 in West Side drug market area | 0.63 (0.22–1.80) | 0.387 | 6.05 (1.24–29.5) | 0.026 |
| Any network member is suburban | 2.53 (1.51–4.24) | <0.001 | 1.95 (1.12–3.38) | 0.018 |
Estimates are adjusted for other variables presented. Models exclude one transgender individual. Seventeen observations were excluded due to missing data
aOR adjusted odds ratio
Among those reporting residential transience (n = 96), we examined the potential association between factors listed on Table 2 and bridging transience. In univariable analysis, crossover transients were more likely than localized transients to report having been born in the suburbs (OR = 3.12; 95% CI: 1.00–9.70) and, in the past year, homelessness (OR = 2.74; 95% CI: 1.05–7.15), smoking crack (OR = 2.91; 95% CI: 1.24–6.84), and injecting within the West Side drug market area (6.40; 95% CI: 2.15–19.1). However, in a multivariable model that controlled for age, race/ethnicity, and gender (all p > 0.05), only two strong predictors of bridging transience remained statistically significant: smoking crack (aOR = 3.62; 95% CI 1.16–11.3) and injecting within the West Side drug market area (aOR = 8.78; 95% CI 2.23–34.6).
Geographic Analyses
Overall, the median number of residences in the past year was significantly (p < 0.05) higher among crossover (3) compared to suburban (1) and urban (1) PWID (Table 1). Crossover participants also reported shorter stays (median days) at each residence (160.3 days) compared to those who exclusively resided in suburban (310.3 days) or urban (232.2 days) areas. As expected, among PWID reporting transience, crossover PWID reported slightly larger median residential movement distances (24.2 miles) compared to suburban (11.1 miles) and urban (5.5 miles) PWID (p < 0.1). Locations where participants resided, purchased drugs, injected drugs, and met sex partners over the past year by residential movement status are shown in Fig. 1a–c for Chicago (shaded) and surrounding suburban areas. Most young PWID purchased drugs within the urban West Side drug market area. As expected, most urban PWID (94.6%) reported injecting in urban areas at least once in the past year (Fig. 1a); however, a significant proportion of crossover (88.1%) (Fig. 1c) and a substantial number of suburban PWID (43.2%) (Fig. 1b) also reported injecting in urban areas. Specifically, crossover PWID were significantly (p < 0.01) more likely (88.1%) compared to urban (65.5%) and suburban PWID (56.1%) to inject in the large outdoor West Side drug market area at least once in the past year. Additionally, the proportion of total injection episodes performed in this urban drug market was also significantly (p < 0.01) higher among crossover (62.8%) compared to urban (50.9%) and suburban (39.0%) only residents. Crossover (45.3%) and urban (53.4%) PWID were also more likely than suburban PWID (11%) to meet sex partners in urban Chicago (p < 0.01); crossover PWID were more likely (21%) to meet sex partners within the West Side drug market boundaries compared to urban (12%) and suburban (2%) PWID (p = 0.06). Exchange sex was associated with meeting sex partners in Chicago (p < 0.01) and specifically with meeting sex partners within the West Side drug market boundaries (p < 0.01).
Discussion
Our study reports novel patterns of residential instability and associated HIV/HCV risk among predominantly non-urban young PWID in the USA. We identified a subgroup of young PWID (crossover transients) with higher-risk behaviors than their more residentially localized (urban or suburban) counterparts.
Our study results are consistent with prior studies in this region showing that young PWID are predominantly NH-whites from the suburban areas surrounding the city of Chicago (59–82%) [16, 18, 37]. Most young PWID who either reported living exclusively in the suburban (76%) or in both urban and suburban (73%) areas in the past year were born in the suburbs, compared to only 27% of those who reported urban only residence during that period. In Illinois, as with many states, HIV and HCV prevalence in urban [38] areas is dramatically higher than in surrounding suburbs [39].
Homelessness was consistently associated with all high-risk mobility patterns we found in our study. Prior studies have shown homelessness [40–43] and unstable housing [44, 45], including transience [24, 25], to be associated with HIV and HCV risk among PWID. Our study further shows a link between homelessness and high-risk mobility patterns. First, we show that residential transience (Table 2) and residential movement (Table 3) were both strongly associated with homelessness in the past 6 months in multivariable analysis. Moreover, for our bridging transience outcome, crossover transients were also significantly (p < 0.05) more likely to be homeless than localized urban or suburban transients (bridging transience, OR = 2.74; 95% CI 1.05–7.15); although this was only significant in univariable analysis.
Future research and interventions focused on homeless PWID populations should consider the role of mobility, particularly among crossover transients who may serve as potential “bridges” between areas. Bridge populations play a key role in the spread of infectious disease by linking subgroups that would otherwise not have been linked, thereby facilitating disease transmission [46, 47]. Because they link suburban and urban injection and sexual networks, crossover transients may facilitate transmission of HIV/HCV from higher (urban) to lower (suburban) HIV/HCV prevalence settings. In addition, compared to young PWID who reported transience within a localized area in our study, those reporting crossover transience were more likely to smoke crack and inject at locations within the West Side drug market area, suggesting that they are a distinct population with potentially elevated risk due to behavioral and geographic factors. The linkage between HIV/HCV high and low prevalence areas and the elevated risk practices of crossover transients may help explain why increases in HIV and HCV have been observed among suburban PWID populations where prevalence previously was low. Identifying bridging populations is important, therefore, because they represent a potentially significant intervention target.
Among all PWID, centralized urban outdoor drug market areas were key settings for activities beyond drug purchase, including injecting and meeting injection and sexual network partners. Proximity to and activities within urban areas may be key to understanding the evolution of HCV risk among young suburban PWID. Targeted harm reduction intervention in these areas might have a significant impact on HCV transmission risk over time. Our study showed that young PWID, particularly crossover transients, often have interactions (residences and sexual relations) both within drug market areas, which are higher risk, and outside of these areas, which is typically lower risk, and thus act as bridges in spreading the disease to new regions (Fig. 1a). Our geographic analyses provide support for potential bridging-related HIV/HCV transmission between areas of high and low HIV/HCV prevalence. Both urban and crossover PWID performed most injections in Chicago, though only approximately half of their reported sex partner meeting locations were in Chicago. While most suburban PWID reported meeting sex partners outside of Chicago, a substantial proportion of injection episodes (43%) among suburban PWID occurred in Chicago, and of these, the majority (83%) were within the West Side drug market area.
Injection and sexual risk behaviors (e.g., syringe sharing, exchange sex, multiple sex partners) were prevalent in our sample, particularly among crossover transient PWID, and thus mobility between urban and suburban areas could facilitate the spread of infections by linking networks of varying HIV/HCV prevalence. Additional research is warranted to understand the mechanisms by which mobility, and crossover transience in particular, may directly impact injection and sexual risk behavior and HIV/HCV transmission.
Residential instability likely leads to vulnerability by increasing the likelihood of injecting in riskier situations (e.g., injecting in public places or in drug markets) or with different and potentially higher-risk PWID networks. Prospective studies are needed to elucidate the temporal associations between housing instability, changes in network characteristics, and HIV/HCV risk among young, predominantly non-urban PWID. Future research should also focus on the evolution of interaction with drug market areas over the course of a PWID’s injecting career. For example, among those who travel long distances to these areas to purchase drugs, what characteristics or behaviors predict who might be more likely to inject and eventually take up residence in these areas, and how does this relate to HIV/HCV risk over time? A better understanding of this progression could lead to early identification of risk-prone individuals for targeted intervention.
Our study results have other important implications for interventions, especially with HCV infection. In 2014, the Department of Health & Human Services (DHHS) set national goals for reducing viral hepatitis by 2020 [48], including (i) increase the proportion of persons who are aware of their HCV infection from 45 to 66% and (ii) reduce by 25% the number of new HCV infections. In 2016, the National Academy of Sciences (NAS) examined the feasibly of HCV elimination in the USA, defined as cessation of viral transmission in this country [49]. In its phase one report, NAS determined that people at greatest risk for HCV are young PWID [49]. The recent availability of all oral direct-acting antivirals (DAAs) with high reported cure rates (e.g., >90% [50, 51]) that can prevent liver disease progression and HCV transmission [52, 53], combined with prevention and harm reduction strategies, make HCV elimination an attainable goal. However, barriers [50, 54–60], including the high cost of DAAs, poor linkage to care, low adherence to medication regimens, stigma, and possible reinfection, all warrant policy development and strategic planning to understand the factors that would most efficiently lead to HCV elimination among PWID. To address this need, we developed mathematical and low-dimensional agent-based models (ABMs) in our prior work to simulate HCV spread and treatment scale up among PWID in metropolitan Chicago [61, 62]. The current study provides important insights into strategies that might address key subpopulations that will inform the development of our future high-dimensional ABMs that would provide much more realistic predictions of how to design HCV elimination strategies.
Our study has several limitations. Small sample size limited our power for subgroup analyses and for testing of interactions and resulted in wide confidence intervals for some estimates. Second, the cross-sectional study design did not allow us to determine temporality of associations between individual and network characteristics and transience. Third, the study was limited to a single geographic region (i.e., metropolitan Chicago), and the majority of participants were recruited from a large urban SSP that attracts both urban and suburban PWID, which limits the generalizability of the findings to other populations of PWID. Fourth, data on participants were self-reported and collected through face-to-face interviews and, therefore, are subject to socially desirable responding and recall errors. Fifth, proxy data from participants on their network members may be inversely related to the strength of the relationship with the network member. However, the median injection network size for the sample was three and highly reliable measures of network density and composition are expected for up to five network members [63]. Moreover, sociodemographic and risk behaviors were only collected for core drug-using network members and were not available for those with a casual drug-using network. We also used a fairly broad definition of transience (i.e., more than one residence in the past year), which may have resulted in diluted effects. Refining the definition and measurement of residential instability as a construct may help to pinpoint associations with specific risk behaviors and identify mechanisms of impact, as well as facilitating comparison across studies.
Our study reports on an under-studied population of young PWID primarily emerging from suburban areas. We found that high-risk behaviors are associated with residential instability among young suburban PWID that points to a need for further empirical research and theoretical modeling to understand the underlying mechanisms by which transience shapes HCV and HIV transmission among young PWID. The association of crossover transience with high-risk behavior has important implications for interventions targeting this population, particularly given the potential for disease transmission associated with bridging between areas of high and low HIV/HCV prevalence.
Compliance with Ethical Standards
The study was approved by the Institutional Review Board of the University of Illinois at Chicago.
Funding Source
This study was funded by a pilot grant award from the Chicago Developmental Center for AIDS Research (Grant#5P30AI082151–04). Other funding to support work on this manuscript include an NIH grant (R01-AI078881) and internal funding from Loyola University Chicago. The funding sources were not directly involved in the collection, analysis, or interpretation of the data; in the writing of this report; or in the decision to submit the paper for publication.
Footnotes
Anna L. Hotton and Louis Shekhtman are equal contributors
References
- 1.Chatterjee S, Tempalski B, Pouget ER, Cooper HL, Cleland CM, Friedman SR. Changes in the prevalence of injection drug use among adolescents and young adults in large U.S. metropolitan areas. AIDS Behav. 2011;15(7):1570–1578. doi: 10.1007/s10461-011-9992-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tempalski B, Pouget ER, Cleland CM, et al. Trends in the population prevalence of people who inject drugs in US metropolitan areas 1992-2007. PLoS One. 2013;8(6):e64789. doi: 10.1371/journal.pone.0064789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Page K, Hahn JA, Evans J, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis. 2009;200(8):1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged </=30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. Morb Mortal Wkly Rep. 2015;64(17):453–458. [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention: Viral hepatitis surveillance. http://www.cdc.gov/hepatitis/statistics/2013surveillance/pdfs/2013hepsurveillancerpt.pdf (2013). Accessed July 20, 2016.
- 6.Centers for Disease Control and Prevention Hepatitis C virus infection among adolescents and young adults: Massachusetts, 2002-2009. Morb Mortal Wkly Rep. 2011;60(17):537–541. [PubMed] [Google Scholar]
- 7.Leuchner L, Lindstrom H, Burstein GR, et al. Use of enhanced surveillance for hepatitis C virus infection to detect a cluster among young injection-drug users—New York, November 2004-April 2007 (reprinted from Morb Mortal Wkly Rep., vol 57, pg 517-521, 2008) J Am Med Assoc. 2008;300(1):34–36. doi: 10.1001/jama.300.1.34. [DOI] [PubMed] [Google Scholar]
- 8.Cicero TJ, Kuehn BM. Driven by prescription drug abuse, heroin use increases among suburban and rural whites. J Am Med Assoc. 2014;312(2):118–119. doi: 10.1001/jama.2014.7404. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease C, Prevention Notes from the field: hepatitis C virus infections among young adults--rural Wisconsin, 2010. Morb Mortal Wkly Rep. 2012;61(19):358. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Use of enhanced surveillance for hepatitis C virus infection to detect a cluster among young injection-drug users, New York, November 2004-April 2007. Morb Mortal Wkly Rep. 2008;57(19):517–521. [PubMed] [Google Scholar]
- 11.Havens JR, Lofwall MR, Frost SD, Oser CB, Leukefeld CG, Crosby RA. Individual and network factors associated with prevalent hepatitis C infection among rural Appalachian injection drug users. Am J Public Health. 2013;103(1):e44–e52. doi: 10.2105/AJPH.2012.300874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006-2012. Clin Infect Dis. 2014;59(10):1411–1419. doi: 10.1093/cid/ciu643. [DOI] [PubMed] [Google Scholar]
- 13.Akyar E, Seneca KH, Akyar S, Schofield N, Schwartz MP, Nahass RG. Linkage to care for suburban heroin users with hepatitis C virus infection, New Jersey, USA. Emerg Infect Dis. 2016;22(5):907–909. doi: 10.3201/eid2205.151980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heimer R, Barbour R, Palacios WR, Nichols LG, Grau LE. Associations between injection risk and community disadvantage among suburban injection drug users in southwestern Connecticut. USA AIDS Behav. 2014;18(3):452–463. doi: 10.1007/s10461-013-0572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13(17):2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boodram B, Golub ET, Ouellet LJ. Socio-behavioral and geographic correlates of prevalent hepatitis C virus infection among young injection drug users in metropolitan Baltimore and Chicago. Drug Alcohol Depend. 2010;111(1–2):136–145. doi: 10.1016/j.drugalcdep.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Garfein RS, Swartzendruber A, Ouellet LJ, et al. Methods to recruit and retain a cohort of young-adult injection drug users for the Third Collaborative Injection Drug Users Study/Drug Users Intervention Trial (CIDUS III/DUIT) Drug Alcohol Depend. 2007;91(Suppl 1):S4–17. doi: 10.1016/j.drugalcdep.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Thorpe LE, Bailey SL, Huo D, Monterroso ER, Ouellet LJ. Injection-related risk behaviors in young urban and suburban injection drug users in Chicago (1997-1999) J Acquir Immune Defic Syndr. 2001;27(1):71–78. doi: 10.1097/00126334-200105010-00012. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report 2012;17(No. 4). http://www.cdc.gov/hiv/topics/surveillance/resources/reports/%23supplemental. Published December 2012. Accessed August 1, 2017.
- 20.Rondinelli AJ, Ouellet LJ, Strathdee SA, et al. Young adult injection drug users in the United States continue to practice HIV risk behaviors. Drug Alcohol Depend. 2009;104(1–2):167–174. doi: 10.1016/j.drugalcdep.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Spiller MW, Broz D, Wejnert C, Nerlander L, Paz-Bailey G. HIV infection and HIV-associated behaviors among persons who inject drugs—20 cities, United States, 2012. Morb Mortal Wkly Rep. 2015;64(10):270–275. [PMC free article] [PubMed] [Google Scholar]
- 22.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 23.Khan MR, Berger A, Hemberg J, O’Neill A, Dyer TP, Smyrk K. Non-injection and injection drug use and STI/HIV risk in the United States: the degree to which sexual risk behaviors versus sex with an STI-infected partner account for infection transmission among drug users. AIDS Behav. 2013;17(3):1185–1194. doi: 10.1007/s10461-012-0276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy E, Robert M, Vaillancourt E, Boivin JF, Vandermeerschen J, Martin I. Residential trajectory and HIV high-risk behaviors among Montreal street youth—a reciprocal relationship. J Urban Health. 2011;88(4):767–778. doi: 10.1007/s11524-011-9574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.German D, Davey MA, Latkin CA. Residential transience and HIV risk behaviors among injection drug users. AIDS Behav. 2007;11(6 Suppl):21–30. doi: 10.1007/s10461-007-9238-3. [DOI] [PubMed] [Google Scholar]
- 26.Aidala A, Cross JE, Stall R, Harre D, Sumartojo E. Housing status and HIV risk behaviors: implications for prevention and policy. AIDS Behav. 2005;9(3):251–265. doi: 10.1007/s10461-005-9000-7. [DOI] [PubMed] [Google Scholar]
- 27.Patrick DM, Strathdee SA, Archibald CP, et al. Determinants of HIV seroconversion in injection drug users during a period of rising prevalence in Vancouver. Int J STD AIDS. 1997;8(7):437–445. doi: 10.1258/0956462971920497. [DOI] [PubMed] [Google Scholar]
- 28.Smereck GA, Hockman EM. Prevalence of HIV infection and HIV risk behaviors associated with living place: on-the-street homeless drug users as a special target population for public health intervention. Am J Drug Alcohol Abuse. 1998;24(2):299–319. doi: 10.3109/00952999809001714. [DOI] [PubMed] [Google Scholar]
- 29.Boodram B, Mackesy-Amiti ME, Latkin C. The role of social networks and geography on risky injection behaviors of young persons who inject drugs. Drug Alcohol Depend. 2015;154:229–235. doi: 10.1016/j.drugalcdep.2015.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotton A, Boodram B. Gender, transience, network partnerships and risky sexual practices among young persons who inject drugs. AIDS Behav. 2017;21(4):982–993. doi: 10.1007/s10461-016-1555-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiebel WW, Jimenez A, Johnson W, et al. Risk behavior and HIV seroincidence among out-of-treatment injection drug users: a four-year prospective study. J Acquir Immune Defic Syndr. 1996;12(3):282–289. doi: 10.1097/00042560-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Thorpe LE, Ouellet LJ, Levy JR, Williams IT, Monterroso ER. Hepatitis C virus infection: prevalence, risk factors, and prevention opportunities among young injection drug users in Chicago, 1997-1999. J Infect Dis. 2000;182(6):1588–1594. doi: 10.1086/317607. [DOI] [PubMed] [Google Scholar]
- 33.Thorpe LE, Bailey SL, Huo D, Monterroso ER, Ouellet LJ. Injection-related risk behaviors in young urban and suburban injection drug users in Chicago, 1997-1999. J Acquir Immune Defic Syndr. 2001;27(1):71–78. doi: 10.1097/00126334-200105010-00012. [DOI] [PubMed] [Google Scholar]
- 34.Ouellet L, Huo D, Bailey SL. HIV risk practices among needle exchange users and nonusers in Chicago. J Acquir Immune Defic Syndr. 2004;37(1):1187–1196. doi: 10.1097/01.qai.0000120802.43677.ea. [DOI] [PubMed] [Google Scholar]
- 35.Huo D, Bailey SL, Hershow RC, Ouellet L. Drug use and HIV risk practices of secondary and primary needle exchange users. AIDS Educ Prev. 2005;17(2):170–184. doi: 10.1521/aeap.17.3.170.62900. [DOI] [PubMed] [Google Scholar]
- 36.Project Know. Arrests across America: the current drug landscape. http://www.projectknow.com/discover/drug-arrest-across-america/#chi. Accessed July 30, 2016.
- 37.Mackesy-Amiti ME, Donenberg GR, Ouellet LJ. Prevalence of psychiatric disorders among young injection drug users. Drug Alcohol Depend. 2012;124(1–2):70–78. doi: 10.1016/j.drugalcdep.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chicago Department of Public Health. HIV/STI Surveillance Report 2016. Chicago, IL: City of Chicago, December 2016.https://www.cityofchicago.org/content/dam/city/depts/cdph/HIV_STI/World_AIDS_Day_Report_012717.pdf. Accessed August 1, 2017.
- 39.Cook County Department of Public Health. HIV/AIDS Surveillance Report, 2006–2008. Oak Park, IL; 2010.www.cookcountypublichealth.org/files/pdf/HIVAIDS2006to2008Final.pdf. Accessed August 1, 2017.
- 40.Ennett ST, Bailey SL, Federman EB. Social network characteristics associated with risky behaviors among runaway and homeless youth. J Health Soc Behav. 1999;40(1):63–78. doi: 10.2307/2676379. [DOI] [PubMed] [Google Scholar]
- 41.Latkin CA, Mandell W, Knowlton AR, Vlahov D, Hawkins W. Personal network correlates and predictors of homelessness for injection drug users in Baltimore, Maryland. J Soc Distress Homel. 1998;7(4):263–278. doi: 10.1023/A:1022943311679. [DOI] [Google Scholar]
- 42.Metraux S, Metzger DS, Culhane DP. Homelessness and HIV risk behaviors among injection drug users. J Urban Health. 2004;81(4):618–629. doi: 10.1093/jurban/jth145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stein JA, Nyamathi A. Correlates of hepatitis C virus infection in homeless men: a latent variable approach. Drug Alcohol Depend. 2004;75(1):89–95. doi: 10.1016/j.drugalcdep.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Corneil TA, Kuyper LM, Shoveller J, et al. Unstable housing, associated risk behaviour, and increased risk for HIV infection among injection drug users. Health Place. 2006;12(1):79–85. doi: 10.1016/j.healthplace.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Weir BW, Bard RS, O’Brien K, Casciato CJ, Stark MJ. Uncovering patterns of HIV risk through multiple housing measures. AIDS Behav. 2007;11(6 Suppl):31–44. doi: 10.1007/s10461-007-9284-x. [DOI] [PubMed] [Google Scholar]
- 46.Aral SO. Behavioral aspects of sexually transmitted diseases: core groups and bridge populations. Sex Transm Dis. 2000;27(6):327–328. doi: 10.1097/00007435-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Youm Y, Mackesy-Amiti ME, Williams CT, Ouellet LJ. Identifying hidden sexual bridging communities in Chicago. J Urban Health. 2009;86(Suppl 1):107–120. doi: 10.1007/s11524-009-9371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.U.S. Department of Health and Human Services: Action plan for the prevention, care, & treatment of viral hepatitis. http://www.cdc.gov/hepatitis/HHS-ActionPlan.htm (2014). Accessed December 15, 2016.
- 49.Buckley GJ, Strom BL. Eliminating the public health problem of hepatitis B and C in the United States: phase one report. Washington, D.C.: National Academy of Sciences; 2016. [PubMed] [Google Scholar]
- 50.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 51.Sadler MD, Lee SS. Revolution in hepatitis C antiviral therapy. Br Med Bull.. 2015;113(1):31–44. [DOI] [PubMed]
- 52.Dahari H, Cotler SJ, Feld JJ. Cure prevents more than transmission of HCV. Hepatology. 2016;64(3):1003–4. [DOI] [PMC free article] [PubMed]
- 53.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. J Am Med Assoc. 2012;308(24):2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 54.Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of Sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med. 2015;163(3):215–223. doi: 10.7326/M15-0406. [DOI] [PubMed] [Google Scholar]
- 55.Grebely J, Genoway KA, Raffa JD, et al. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend. 2008;93(1–2):141–147. doi: 10.1016/j.drugalcdep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 56.Rhodes T, Treloar C. The social production of hepatitis C risk among injecting drug users: a qualitative synthesis. Addiction. 2008;103(10):1593–1603. doi: 10.1111/j.1360-0443.2008.02306.x. [DOI] [PubMed] [Google Scholar]
- 57.Zeremski M, Zibbell JE, Martinez AD, Kritz S, Smith BD, Talal AH. Hepatitis C virus control among persons who inject drugs requires overcoming barriers to care. World J Gastroenterol. 2013;19(44):7846–7851. doi: 10.3748/wjg.v19.i44.7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sethi N, Tapper EB, Vong A, Sethi S, Rourke M, Afdhal NH. Direct costs of first-generation protease inhibitors for the treatment of genotype 1 chronic hepatitis C viral infection. J Viral Hepat. 2015;22(12):974–976. doi: 10.1111/jvh.12421. [DOI] [PubMed] [Google Scholar]
- 59.Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58(5):1598–609. [DOI] [PMC free article] [PubMed]
- 60.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33(3):126–133. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gutfraind A, Boodram B, Prachand N, Hailegiorgis A, Dahari H, Major ME. Agent-based model forecasts aging of the population of people who inject drugs in metropolitan Chicago and changing prevalence of hepatitis C infections. PLoS One. 2015;10(9):e0137993. doi: 10.1371/journal.pone.0137993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Echevarria D, Gutfraind A, Boodram B, et al. Mathematical modeling of hepatitis C prevalence reduction with antiviral treatment scale-up in persons who inject drugs in metropolitan Chicago. PLoS One. 2015;10(8):e0135901. doi: 10.1371/journal.pone.0135901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marsden PV. The reliability of network density and composition measures. Soc Networks. 1993;15(4):399–421. doi: 10.1016/0378-8733(93)90014-C. [DOI] [Google Scholar]