Abstract
OBJECTIVES
The effect of diabetes type and insulin dependence on short- and long-term outcomes after lower extremity revascularization for chronic limb-threatening ischemia (CLTI) warrants additional study and more targeted focus. We sought to address this paucity of information by evaluating outcomes in patients with insulin-dependent and noninsulin-dependent diabetes after any first-time revascularization.
METHODS
We reviewed all limbs undergoing a first-time infrainguinal bypass (BPG) or percutaneous transluminal angioplasty with or without stent (PTA/S) for CLTI at our institution from 2005–2014. Based on preoperative medication regimen, patients were categorized as having insulin-dependent diabetes (IDDM), noninsulin-dependent diabetes (NIDDM), or no diabetes (NDM). Outcomes included wound healing, major amputation, RAS events (revascularization, major amputation, or stenosis), major adverse limb events (MALE), and mortality. Outcomes were evaluated using Chi-square, Kaplan-Meier, and Cox regression analyses.
RESULTS
Of 2,869 infrainguinal revascularizations from 2005–2014, 1,294 limbs (646 BPG, 648 PTA/S) fit our criteria. Overall, our analysis included 703 IDDM, 262 NIDDM, and 329 NDM limbs. IDDM patients, compared to NIDDM and NDM, were younger (69 vs. 73 vs. 77 years; P<.001) and more often presented with tissue loss (89% vs. 77% vs. 67%; P<.001), coronary artery disease (57% vs. 48% vs. 43% P<.001), and end-stage renal disease (26% vs. 13% vs. 12%; P<.001). Perioperative complications, including mortality (3% vs. 2% vs. 5%; P=.07), did not differ between groups; however, complete wound healing at 6-month follow-up was significantly worse among IDDM patients (41% vs. 49% vs. 61%; P<.001). IDDM patients had significantly higher three-year major amputation rates (23% vs. 11% vs. 8%; P<.001). Multivariable analyses illustrated that, compared to NDM, IDDM was associated with significantly higher risk of both major amputation and RAS events following any first-time intervention (Hazard Ratio (HR) 2.0, 95% Confidence Interval [CI] 1.1–4.1 and 1.4 [1.1–1.8], respectively). Similar associations between IDDM and both major amputation and RAS events were found in patients undergoing a PTA/S-first intervention (4.1 [1.3–12.6] and 1.5 [1.1–2.2], respectively), while IDDM in BPG-first patients was only associated with incomplete wound healing (2.0 [1.4–4.5]). Lastly, when compared to NDM, NIDDM was associated with lower late mortality (0.7 [0.5–0.9]).
CONCLUSIONS
As compared to NDM, IDDM is associated with similar perioperative and long-term mortality but a higher risk of incomplete wound healing, major amputation, and future RAS events, especially after a PTA/S-first approach. NIDDM, on the other hand, is associated with lower long-term mortality and few adverse limb events. Overall, these data demonstrate both the importance in distinguishing between diabetes types, as well as potential long-term benefit of a bypass-first strategy in appropriately selected IDDM patients with CLTI.
INTRODUCTION
Despite advances in the management of diabetes, the profound effect of the estimated growth is still likely to yield a tremendous escalation in end-stage peripheral artery disease (PAD).1 Chronic limb-threatening ischemia (CLTI), broadly defined as the most advanced stages of PAD and demarcated by ischemic rest pain, non-healing ulcer, or gangrene, is significantly more likely in diabetic patients and is often a debilitating condition.2 Ultimately, the diagnosis of PAD in patients with diabetes is often delayed due to presence of neuropathy, as PAD-related symptoms go unnoticed until more severe CLTI symptoms develop.3 Given the prevalence and severity of such events, non-operative wound management and care may not be sufficient to avoid limb loss.
Although open surgical bypass (BPG) has been shown to have excellent results in patients with diabetes and PAD, contemporary management of CLTI has gradually favored the use of minimally invasive techniques that offer lower periprocedural morbidity and mortality, reduced costs, faster procedural times, and a shortened hospital stay.4 Several studies have compared the utility of both BPG and percutaneous transluminal angioplasty with or without stenting (PTA/S) in varying degrees of lower extremity limb ischemia, and in patients with and without diabetes; however, in the current endovascular era, few studies have evaluated the degree to which these subsets of patients fare in regard to procedure type.5–13 In this study, we sought to describe our institution’s long-term experience with BPG-first and PTA/S-first repair in insulin-dependent, noninsulin-dependent, and non-diabetic patients.
METHODS
Subjects and settings
We performed a retrospective review of all patients with CLTI undergoing a first-time lower extremity intervention at Beth Israel Deaconess Medical Center (BIDMC). Medical records of all BPG and PTA/S interventions from January 2005 to October 2014 were individually reviewed. Patients were categorized as having insulin-dependent diabetes (IDDM), noninsulin-dependent diabetes (NIDDM), or no diabetes (NDM). IDDM was defined as preoperative or at-home reliance on insulin administration to control diabetes at baseline. Patients with diabetes who were not prescribed insulin were categorized as having NIDDM. Importantly, for the purposes of this study, insulin dependence is not considered tantamount to type I diabetes, as it describes the patient-level pattern of insulin use at the time of revascularization. Patients who received previous interventions on the ipsilateral limb (whether at BIDMC or at an outside institution) or interventions solely at or proximal to the iliac arteries were excluded. Patients undergoing a concomitant procedure, including endarterectomy, profundaplasty, thrombectomy, atherectomy, or patch, were included and adjusted for in our multivariable analyses. Interval and modality for typical patient follow-up was every 3 to 4 months for 2 years and every 6 months afterwards, with arterial duplex ultrasound imaging and ankle-brachial indices with forefoot pulse volume recordings and/or toe pressures.
Our analysis included patients whose disease severity was distinctly classifiable as CLTI and who underwent either a first-time BPG or a first-time PTA/S. Indications for intervention included tissue loss (i.e., gangrene and ulcer) or rest pain. Patients presenting with more than one indication were assigned hierarchically, with gangrene constituting as the most severe indication, followed by ulcer, and, lastly, rest pain. Femoropopliteal lesion anatomy and severity were defined according to the modified Trans Atlantic Inter-society Consensus (TASC II) classification. As there was no updated TASC class for tibial lesions included in the modified TASC II classification, tibial lesion information was defined by TASC I.14,15
Measurements and outcome variables
Primary outcomes included perioperative complications, wound healing, major amputation, RAS events (a composite variable denoted by re-intervention, major amputation, or stenosis), major adverse limb events (MALE, a composite variable denoted by any major amputation or any major re-intervention, defined as creation of a new bypass graft, a jump graft revision, surgical thrombectomy with or without surgical patch angioplasty, and thrombectomy of an occluded graft or arterial segment using pharmacologic or mechanical thrombolysis), and mortality.16 Demographics, comorbidities, SVS WIfI information, restenosis, and re-intervention were also recorded.17 Perioperative complications included hematoma, acute myocardial infarction, and death. Cardiac enzymes and EKGs were not routinely obtained following revascularization. If patients developed chest pain, dyspnea, hemodynamic instability or other concerning signs/symptoms, an EKG was obtained with cardiac enzymes (if the patient had EKG changes or strong history of coronary disease). Criteria for restenosis was at least 75% stenosis by angiographic measurement, or a >3.5 fold increase in peak systolic velocity by duplex. Re-interventions included any ipsilateral surgical or endovascular revision and were most commonly performed for symptomatic graft restenosis or threatened asymptomatic grafts (peak systolic velocity ratio >3.5–4 or low graft velocities <30cm/second). Ordinarily, patients did not undergo re-interventions for an asymptomatic restenosis after PTA alone; however, attending physicians were more likely to re-intervene with PTA/S for an asymptomatic in-stent restenosis if the peak systolic velocity ratio was >3.5–4. Type of re-intervention strategy was surgeon-dependent and varied over time with the acquisition of endovascular skills: Generally, PTA/S-first strategies were done so at the clinical judgment of the attending physician at the time of the angiogram. Following BPG, patients were prescribed aspirin and a statin, and were not prescribed Plavix. Anticoagulation and cilostazol use was attending-dependent and varied with operative findings. Additionally, patients undergoing a PTA/S below the inguinal ligament received dual antiplatelet therapy for 30 days, followed by aspirin indefinitely. Routine statin use was introduced over time. Technical success following PTA/S was defined as less than 30% residual stenosis and no flow-limiting dissection, while technical success following BPG included a patent bypass graft at completion, which was defined as one without significant defect in the vein on angioscopy and continuous wave Doppler interrogation. Both preoperative vein mapping and angioscopy were used in all BPG cases, and all patients undergoing any revascularization received general anesthesia.18
Statistical Analyses
Contingent on the outcome of interest, analyses were performed on either a per-limb basis (i.e., wound healing, stenosis, re-intervention, amputation, RAS, MALE) or a per-patient basis (i.e., mortality), where, on per-patient outcomes, the initial limb was censored at the procedure date of the contralateral limb. Pearson chi-square and Fisher exact tests were used for categorical variable comparison. Continuous variables were compared using Student t-test or Mann-Whitney U test. Rates were compared across strata (IDDM, NIDDM, and NDM) using chi-square analysis. Treatment outcomes during the course of follow-up were analyzed using Kaplan-Meier methodology, and unadjusted time-to-failure curves were compared with the log-rank test. Covariates were selected using purposeful selection, incorporating backward selection after a univariate screen (P < .10) as well as including relevant patient factors previously identified.19 Multivariable Cox regression models were constructed to assess independent associations between diabetes type and time-dependent outcomes. Statistical significance was defined as P < .05. All statistical tests were done using STATA 13 (StataCorp, College Station, Tex). The Beth Israel Deaconess Medical Center Institutional Review Board approved this study and waived the need for patient consent.
RESULTS
Baseline Characteristics
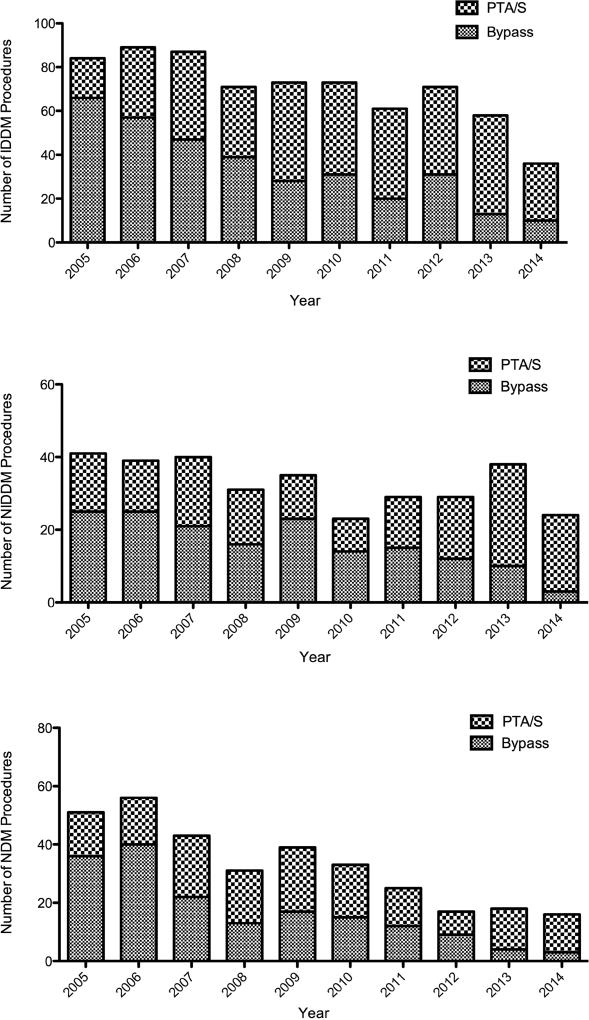
Of the 2,869 total lower extremity revascularizations performed between January 2005 and October 2014, 667 were performed on patients with non-CLTI symptoms, 475 were reinterventions, and 433 were performed on patients who had undergone interventions prior to 2005; ultimately, 1,294 limbs in 1,160 patients met our inclusion criteria (i.e., a first-time lower extremity intervention for CLTI with reliable insulin information): 646 undergoing a primary BPG and 648 undergoing a primary PTA/S. As Figure Ia illustrates, the number of IDDM limbs treated with a revascularization gradually decreased over the study period (from 84 procedures in 2005 to 58 in 2013), as did the number of IDDM limbs treated with a BPG-first approach (from 79% in 2005 to 22% in 2013). Additionally, as Figure Ib and Ic demonstrate, these decreasing trends remained relatively consistent across NIDDM and NDM limbs, with the former undergoing 61% BPG-first procedures in 2005 and 26% in 2013 and the latter falling from 71% BPG-first interventions to 22%.
Figure I.
Number of yearly first-time revascularization procedures performed on patients with chronic limb-threatening ischemia and a) insulin-dependent diabetes (IDDM), b) noninsulin-dependent diabetes (NIDDM), and c) no diabetes (NDM)
PTA/S, percutaneous angioplasty with or without stent; IDDM, insulin-dependent diabetes; NIDDM, noninsulin-dependent diabetes; NDM, non-diabetes
Overall, 703 IDDM, 262 NIDDM, and 329 NDM limbs were included in our analysis. IDDM patients, compared to NIDDM and NDM patients, respectively, were younger (69 vs. 73 vs. 77 years; P < .001) and more often presented with tissue loss (89% vs. 78% vs. 67%; P < .001), coronary artery disease (57% vs. 48% vs. 43% P < .001), and end-stage renal disease (26% vs. 13% vs. 12%; P < .001) (Table I). Conversely, NDM patients more commonly suffered from COPD (10% vs. 9% vs. 19%; P < .001) and more frequently smoked (57% vs. 58% vs. 69%; P = .001). Groups did not differ in male sex (62% vs. 57% vs. 56%; P = .13) or in rates of congestive heart failure (34% vs. 28% vs. 28%; P = .10). There was no difference in the proportion of patients undergoing a primary BPG by diabetes type (49% vs. 51% vs. 52%; P = .58). The prevalence of preoperative femoropopliteal TASC D lesions was lowest in IDDM patients (17% vs. 19% vs. 31%; P < .001), although this difference was not seen when directly comparing IDDM to NIDDM (P = .46). There was no difference in tibial TASC D lesions (32% vs. 29% vs. 32%; P = .69). Finally, WIfI clinical stage 4 was most prevalent among IDDM patients (52% vs. 43% vs. 31%; P < .02), potentially driven by the high WIfI wound component among these patients (1.6 vs. 1.4 vs. 1.2; P < .01).
Table I.
Demographics, co-morbidities, and pre-operative lesion characteristics between 1,294 patients with insulin-dependent diabetes, noninsulin-dependent diabetes, and no diabetes with chronic limb-threatening ischemia (CLTI)
| IDDM | NIDDM | NDM |
P -value (IDDM to NIDDM) |
P -value (IDDM to NDM) |
P -value (NIDDM to NDM) |
P-value | |
|---|---|---|---|---|---|---|---|
| (N=703) | (N=262) | (N=329) | |||||
| Demographics No. (%) | |||||||
| Age, mean (SD) | 68.9 (12.0) | 72.9 (11.0) | 76.6 (12.5) | <.001 | <.001 | <.001 | <.001 |
| Race | 0.03 | <.001 | 0.04 | <.001 | |||
| White | 518 (74) | 210 (81) | 286 (87) | ||||
| Non-white | 182 (26) | 50 (19) | 43 (13) | ||||
| Male sex | 436 (62) | 148 (57) | 185 (56) | .13 | .08 | .91 | .13 |
| Indication, No. (%) | |||||||
| Rest Pain | 78 (11) | 59 (23) | 108 (33) | <.001 | <.001 | <.01 | <.001 |
| Ulcer | 407 (58) | 124 (47) | 148 (45) | <.01 | <.001 | .57 | <.001 |
| Gangrene | 218 (31) | 79 (30) | 73 (22) | .80 | <.01 | .03 | .01 |
| Comorbidities, No. (%) | |||||||
| Coronary artery disease | 395 (57) | 123 (48) | 136 (43) | .01 | <.001 | .27 | <.001 |
| Hypertension | 622 (89) | 226 (87) | 242 (76) | .47 | <.001 | .001 | <.001 |
| Hyperlipidemia | 466 (67) | 145 (56) | 167 (53) | <.01 | <.001 | .43 | <.001 |
| Dialysis dependence | 185 (26) | 34 (13) | 38 (12) | <.001 | <.001 | .67 | <.001 |
| BMI, mean | 29.0 | 27.6 | 24.5 | <.01 | <.001 | <.001 | .04 |
| History of myocardial infarction | 215 (31) | 65 (25) | 54 (17) | .09 | <.001 | .02 | <.001 |
| Congestive heart failure | 234 (34) | 73 (28) | 88 (28) | .12 | .06 | .89 | .10 |
| COPD | 69 (10) | 23 (8.9) | 60 (19) | .66 | <.001 | .001 | <.001 |
| Smoking history | 401 (57) | 150 (58) | 220 (69) | .88 | <.001 | <.01 | .001 |
| WIfI clinical stage,16 No. (%) | |||||||
| Clinical stage 1 | 6 (1.2) | 2 (1.0) | 3 (1.2) | .85 | .98 | .85 | .98 |
| Clinical stage 2 | 99 (19) | 65 (33) | 116 (46) | <.001 | <.001 | <.01 | <.001 |
| Clinical stage 3 | 140 (27) | 48 (24) | 56 (22) | .37 | .13 | .66 | .28 |
| Clinical stage 4 | 268 (52) | 85 (43) | 77 (31) | .02 | <.001 | <.01 | <.001 |
| Fempop TASC classification, No. (%) | |||||||
| TASC A | 120 (19) | 50 (22) | 42 (15) | .49 | .11 | .05 | .13 |
| TASC B | 185 (30) | 78 (34) | 82 (29) | .30 | .83 | .28 | .50 |
| TASC C | 76 (12) | 30 (13) | 44 (16) | .80 | .17 | .38 | .38 |
| TASC D | 104 (17) | 44 (19) | 88 (31) | .46 | <.001 | .001 | <.001 |
| Tibial TASC classification, No. (%) | |||||||
| TASC A | 78 (13) | 30 (13) | 35 (13) | .83 | .97 | .87 | .98 |
| TASC B | 143 (23) | 46 (20) | 55 (20) | .36 | .29 | .95 | .46 |
| TASC C | 138 (23) | 50 (21) | 55 (20) | .91 | .43 | .58 | .72 |
| TASC D | 198 (32) | 66 (29) | 87 (32) | .40 | .89 | .53 | .70 |
IDDM, insulin-dependent diabetes; NIDDM, noninsulin-dependent diabetes; NDM, non-diabetes; COPD, chronic obstructive pulmonary disease; WIfI, wound, ischemia, and foot infection; TASC, Trans Atlantic Inter-society Consensus
Of the 646 BPG-first procedures, the femoral artery was the most common inflow artery (74% of all procedures), although significantly less so among IDDM patients (68% vs. 74% vs. 84%; P = .001). When directly comparing IDDM to NDM, the outflow artery among IDDM patients was less commonly the popliteal artery (29% vs. 40%; P = .01) and was more commonly the dorsalis pedis/pedal arteries (29% vs. 16%; P < .01) (Table II). Procedural details were not significantly different between IDDM and NIDDM patients nor between NIDDM and NDM patients. Single-segment great saphenous vein conduits were used in over three-quarters of procedures performed in each group (76% vs. 80% vs. 78%; P = .56), where non-reversed great saphenous vein was most the most common conduit (40% vs. 41% vs. 39%; P = .88). Composite vein conduit use (6% vs. 5% vs. 8%; P = .49) and synthetic conduit use (12% vs. 12% vs. 12%; P = .98) did not differ between diabetes type.
Table II.
Operative details of 646 insulin-dependent, noninsulin-dependent, and non-diabetic patients undergoing open surgical bypass for chronic limb-threatening ischemia (CLTI)
| IDDM | NIDDM | NDM |
P-value (IDDM to NIDDM) |
P-value (IDDM to NDM) |
P-value (NIDDM to NDM) |
P-value | |
|---|---|---|---|---|---|---|---|
| (N=342) | (N=133) | (N=171) | |||||
| Inflow artery, No. (%) | |||||||
| Femoral | 233 (68) | 99 (74) | 143 (84) | .18 | <.001 | .049 | .001 |
| Popliteal | 109 (32) | 34 (26) | 27 (16) | .18 | <.001 | .04 | <.001 |
| Tibial | 1 (0.6) | 0 (0) | 0 (0) | .16 | .38 | - | .25 |
| Outflow artery, No. (%) | |||||||
| Popliteal | 98 (29) | 45 (34) | 69 (40) | .27 | .01 | .29 | .04 |
| Tibial | 126 (37) | 41 (31) | 56 (33) | .16 | .27 | .73 | .29 |
| Peroneal | 21 (6.1) | 13 (10) | 18 (11) | .33 | .10 | .66 | .25 |
| Dorsalis pedis/pedal | 98 (29) | 33 (24) | 28 (16) | .26 | <.01 | .10 | <.01 |
| Conduit, No. (%) | |||||||
| In situ saphenous vein | 76 (22) | 29 (22) | 45 (26) | .92 | .30 | .36 | .53 |
| Reversed saphenous vein | 41 (12) | 21 (16) | 21 (12) | .27 | .92 | .38 | .52 |
| Non-reversed saphenous vein | 138 (40) | 55 (41) | 66 (39) | .84 | .70 | .63 | .88 |
| Arm vein | 36 (11) | 9 (6.8) | 13 (7.7) | .21 | .29 | .78 | .34 |
| Composite vein | 19 (5.6) | 6 (4.5) | 13 (7.7) | .65 | .37 | .27 | .49 |
| Synthetic | 42 (12) | 16 (12) | 20 (12) | .95 | .85 | .92 | .98 |
IDDM, insulin-dependent diabetes; NIDDM, noninsulin-dependent diabetes; NDM, non-diabetes
Finally, of the 648 PTA/S-first procedures, IDDM patients were less likely to undergo a superficial femoral artery angioplasty (57% vs. 67% vs. 75%; P < .001) and were more likely to undergo an anterior tibial angioplasty (31% vs. 11% vs. 16%; P < .001) (Table III). Further, there were no differences in multi-level interventions (42% vs. 42% vs. 49%; P = .34); however, femoropopliteal stenting was significantly less common among IDDM patients (26% vs. 31% vs. 42%; P = .001) – a significant difference that was most likely driven by the difference between IDDM patients and NDM patients (P < .001). NIDDM patients, when compared solely to NDM patients, were significantly less likely to undergo infrapopliteal stenting (3% vs. 9%; P = .045).
Table III.
Operative details of 648 insulin-dependent, noninsulin-dependent, and non-diabetic patients undergoing percutaneous transluminal angioplasty for chronic limb-threatening ischemia (CLTI)
| IDDM | NIDDM | NDM |
P-value (IDDM to NIDDM) |
P-value (IDDM to NDM) |
P-value (NIDDM to NDM) |
P -value | |
|---|---|---|---|---|---|---|---|
| (N=342) | (N=133) | (N=171) | |||||
| Proximal vessel, No. (%) | |||||||
| Femoral | 204 (57) | 86 (67) | 118 (75) | .04 | <.001 | .14 | <.001 |
| Popliteal | 126 (35) | 44 (34) | 69 (44) | .87 | .06 | .10 | .13 |
| Infrapopliteal vessel, No. (%) | |||||||
| Anterior tibial | 111 (31) | 14 (11) | 25 (16) | <.001 | <.001 | .22 | <.001 |
| Posterior tibial | 59 (16) | 13 (10) | 16 (10) | .08 | .06 | .99 | .07 |
| Peroneal | 73 (20) | 25 (19) | 24 (15) | .84 | .18 | .35 | .40 |
| Dorsalis pedis/pedal | 10 (3.0) | 0 (0.0) | 2 (1.3) | .06 | .29 | .20 | .11 |
| Multi-level (prox + infrapop) | 152 (42) | 54 (42) | 77 (49) | .96 | .16 | .25 | .34 |
| Stenting, No. (%) | |||||||
| Any | 109 (30) | 43 (33) | 71 (45) | .51 | .001 | .046 | <.01 |
| Femoropopliteal | 92 (26) | 40 (31) | 66 (42) | .23 | <.001 | .06 | .001 |
| Infrapopliteal | 26 (7.2) | 4 (3.1) | 14 (8.9) | .10 | .51 | .045 | .14 |
IDDM, insulin-dependent diabetes; NIDDM, noninsulin-dependent diabetes; NDM, non-diabetes
The median follow-up for IDDM, NIDDM, and NDM patients was 1.5 years (range <1– 10), 1.6 years (<1–10), and 1.3 years (<1–10), respectively.
Perioperative Outcomes
Following any lower extremity revascularization for CLTI, IDDM patients exhibited a significantly longer total hospital length of stay (LOS) (9.6 vs. 8.9 vs. 8.0 days; P < .01), most likely driven by the LOS difference between IDDM and NDM patients (P < .001) (Table IV). Further univariate analysis suggested that both perioperative mortality (3.0 vs. 1.5 vs. 4.9; P = .07) and perioperative complications (15% vs. 12% vs. 15%; P = .60) were similar between groups. Among BPG-first patients, perioperative surgical site infections did not differ (11% vs. 10% vs. 8%; P = .52). Regardless of procedure type, after adjusting for baseline characteristics, multivariable analysis found diabetes type to not be associated with perioperative death or complications.
Table IV.
Perioperative outcomes and complications between 1,294 insulin-dependent, noninsulin-dependent, and non-diabetic patients with chronic limb-threatening ischemia (CLTI)
| IDDM | NIDDM | NDM |
P -value (IDDM to NIDDM) |
P -value (IDDM to NDM) |
P-value (NIDDM to NDM) |
P-value | |
|---|---|---|---|---|---|---|---|
| (N=703) | (N=262) | (N=329) | |||||
| P erioperative Outcomes, No. (%) | |||||||
| Pre-operative LOS, mean days | 3.3 | 3.1 | 2.4 | .39 | <.001 | .05 | <.01 |
| Post-operative LOS, mean days | 6.3 | 5.8 | 5.6 | .23 | .05 | .62 | .11 |
| Total LOS, mean days | 9.6 | 8.9 | 8.0 | .22 | <.01 | .14 | <.01 |
| Hematoma | 40 (5.7) | 15 (5.7) | 20 (6.1) | .98 | .80 | .86 | .97 |
| Acute myocardial infarction | 13 (1.8) | 1 (0.4) | 4 (1.2) | .09 | .46 | .27 | .21 |
| Mortality | 21 (3.0) | 4 (1.5) | 16 (4.9) | .20 | .13 | .03 | .07 |
IDDM, insulin-dependent diabetes; NIDDM, noninsulin-dependent diabetes; NDM, non-diabetes; LOS, length of stay
Long-term Outcomes
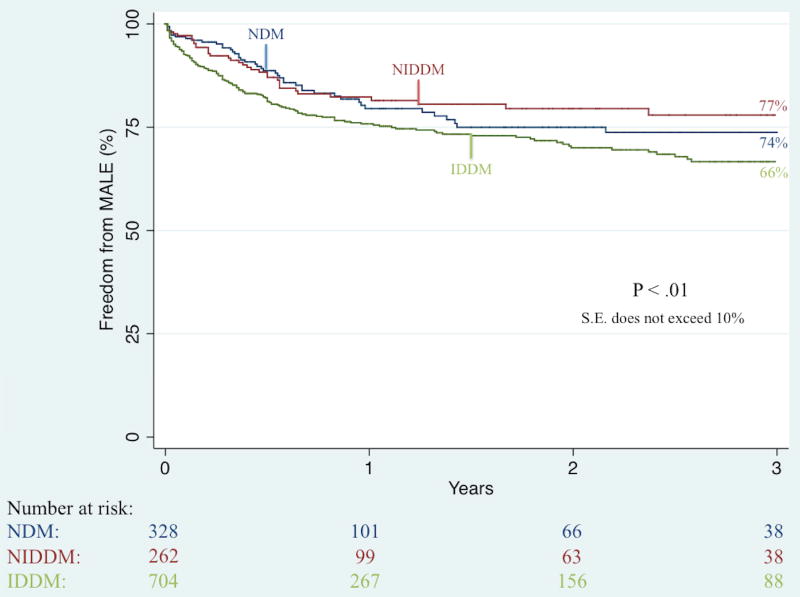
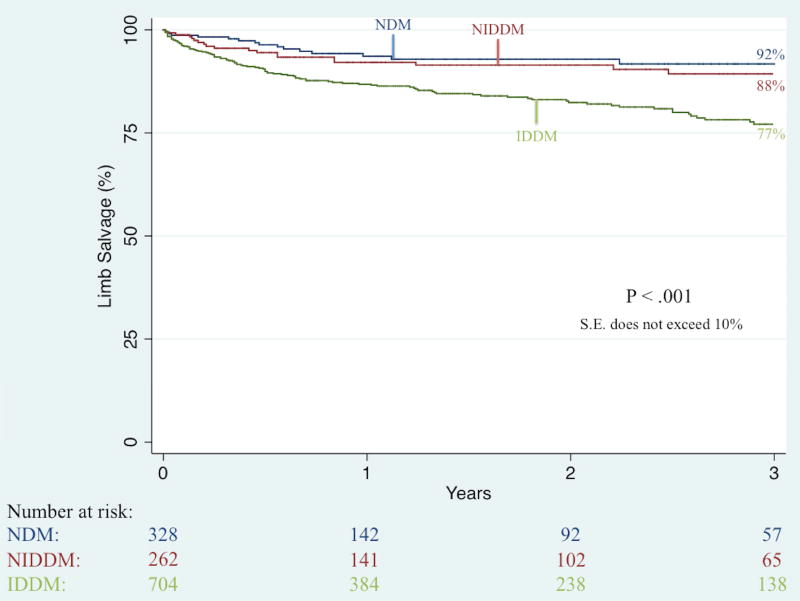
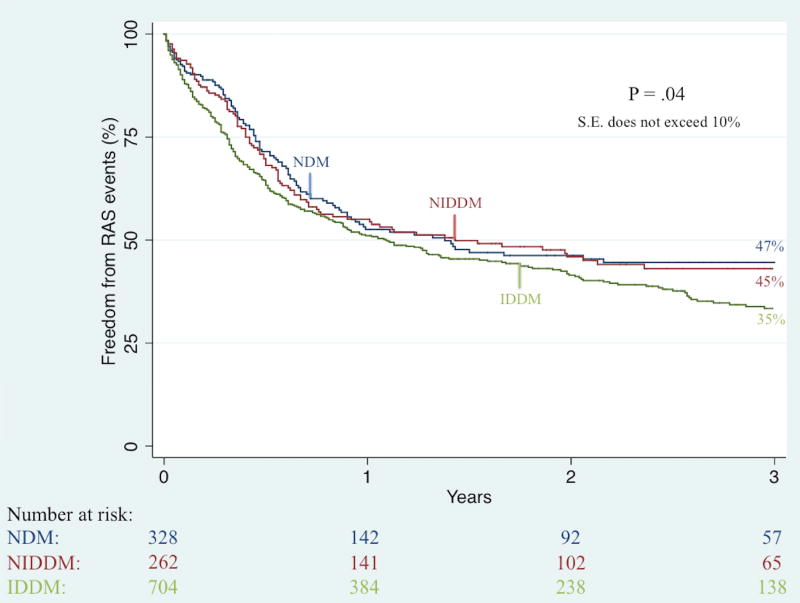
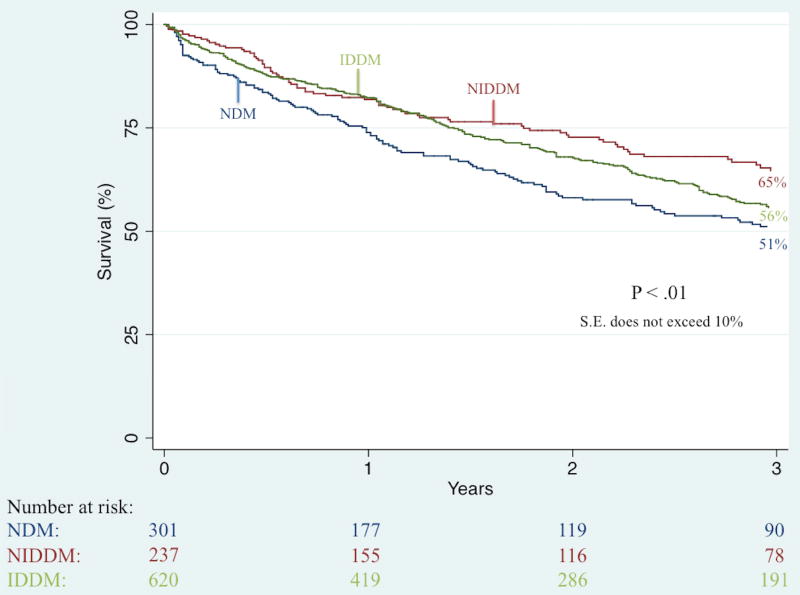
Unadjusted Kaplan-Meier analysis demonstrated that complete wound healing at 6-month follow-up was significantly worse among IDDM patients (41% vs. 49% vs. 61%; P < .001). Further unadjusted Kaplan-Meier analyses illustrated no significant difference in three-year rates of restenosis (50% vs. 46% vs. 38%; P = .36) and re-intervention (36% vs. 37% vs. 31%; P = .63) but did demonstrate significant differences in three-year rates of major amputation (23% vs. 12% vs. 8%; P < .001; Figure II), RAS events (65% vs. 55% vs. 53%; P = .04; Figure III), MALE (34% vs. 26% vs. 23%; P < .01; Figure IV), and death (44% vs. 35% vs. 49%; P < .01; Figure V).
Figure II.
Unadjusted effect of diabetes type on long-term limb salvage among patients undergoing any lower extremity revascularization for chronic limb-threatening ischemia (CLTI)
IDDM, insulin-dependent diabetes; NIDDM, noninsulin-dependent diabetes; NDM, non-diabetes; S.E., standard error
Figure III.
Unadjusted effect of diabetes type on long-term freedom from re-intervention, amputation, or stenosis (RAS) among patients undergoing any lower extremity revascularization for chronic limb-threatening ischemia (CLTI)
IDDM, insulin-dependent diabetes; NIDDM, noninsulin-dependent diabetes; NDM, non-diabetes; S.E., standard error
Figure IV.
Unadjusted effect of diabetes type on long-term freedom from any major adverse limb event (MALE) among patients undergoing any lower extremity revascularization for chronic limb-threatening ischemia (CLTI)
IDDM, insulin-dependent diabetes; NIDDM, noninsulin-dependent diabetes; NDM, non-diabetes; S.E., standard error
Figure V.
Unadjusted effect of diabetes type on long-term survival among patients undergoing any lower extremity revascularization for chronic limb-threatening ischemia (CLTI)
IDDM, insulin-dependent diabetes; NIDDM, noninsulin-dependent diabetes; NDM, non-diabetes; S.E., standard error
IDDM, insulin-dependent diabetes; NIDDM, noninsulin-dependent diabetes; NDM, non-diabetes; COPD, chronic obstructive pulmonary disease; WIfI, wound, ischemia, and foot infection; TASC, Trans Atlantic Inter-society Consensus
After adjustment, among all procedure types, diabetes type was not shown to independently affect restenosis or re-intervention. Conversely, among all revascularization strategies, with NDM as the reference group, IDDM was shown to independently heighten a patient’s risk of incomplete wound healing (Hazard Ratio (HR) 1.6, 95% Confidence Interval [CI], 1.2–3.4), major amputation (2.0 [1.1–4.1]), RAS events (1.4 [1.1–1.8]) and MALE (2.2 [1.3–3.6]) (Table V). Among BPG-first interventions, IDDM was shown to only independently heighten the risk of incomplete wound healing (2.0 [1.4–4.5]). Finally, among PTA/S-first interventions, IDDM was shown to independently heighten the risk of incomplete wound healing (1.4 [1.1–2.6]), major amputation (4.1 [1.3–12.6]), and RAS events (1.5 [1.1–2.2]). NIDDM, as compared to NDM, was shown to be significantly associated with incomplete wound healing among all procedure types (1.4 [1.1–2.2]) and BPG-first patients (1.9 [1.3–4.1]), but no other limb-related primary outcomes; however, interestingly, NIDDM, as compared to NDM, was associated with a significantly lower risk of mortality among patients undergoing any revascularization type (0.7 [0.5–0.9]), a BPG-first intervention (0.7 [0.5–0.9]), and a PTA/S-first intervention (0.7 [0.4–0.9]).
Table V.
Multivariable analyses of diabetes type on long-term major amputation, RAS events, and mortality
| Any intervention (N=1,294) |
Bypass-first (N=646) |
PTA/S-first (N=648) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Outcomes | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| |||||||||
| Mortality | |||||||||
| NDM (ref) | - | - | - | - | - | - | - | - | - |
| NIDDM | 0.7 | 0.5–0.9 | <.01 | 0.7 | 0.5–0.9 | .01 | 0.7 | 0.4–0.9 | .01 |
| IDDM | 0.9 | 0.8–1.3 | .91 | 0.9 | 0.8–1.2 | .90 | 0.9 | 0.7–1.4 | .88 |
|
| |||||||||
| Major amputation | |||||||||
| NDM (ref) | - | - | - | - | - | - | - | - | - |
| NIDDM | 1.5 | 0.6–3.3 | .28 | 1.3 | 0.4–4.0 | .68 | 1.5 | 0.4–5.5 | .52 |
| IDDM | 2.0 | 1.1–4.1 | .03 | 2.1 | 0.8–5.7 | .14 | 4.1 | 1.3–12.6 | .02 |
|
| |||||||||
| RAS | |||||||||
| NDM (ref) | - | - | - | - | - | - | - | - | - |
| NIDDM | 0.9 | 0.7–1.3 | .61 | 1.1 | 0.6–2.0 | .76 | 0.9 | 0.6–1.3 | .39 |
| IDDM | 1.4 | 1.1–1.8 | .04 | 1.4 | 0.8–2.3 | .26 | 1.5 | 1.1–2.2 | .02 |
|
| |||||||||
| MALE | |||||||||
| NDM (ref) | - | - | - | - | - | - | - | - | - |
| NIDDM | 1.2 | 0.6–2.2 | .60 | 1.1 | 0.5–2.8 | .77 | 1.0 | 0.3–2.6 | .85 |
| IDDM | 2.2 | 1.3–3.6 | <.01 | 1.6 | 0.8–3.5 | .21 | 1.7 | 0.8–3.9 | .19 |
|
| |||||||||
| Incomplete healing | |||||||||
| NDM (ref) | - | - | - | - | - | - | - | - | - |
| NIDDM | 1.4 | 1.1–2.2 | .02 | 1.9 | 1.3–4.1 | <.01 | 1.1 | 0.6–1.5 | .56 |
| IDDM | 1.6 | 1.2–3.4 | <.001 | 2.0 | 1.4–4.5 | .01 | 1.4 | 1.1–2.6 | .03 |
NDM, no diabetes; NIDDM, non-insulin-dependent diabetes; IDDM, insulin-dependent diabetes; RAS, re-intervention, amputation, or stenosis; HR, hazard ratio; CI, confidence interval; RAS, re-intervention, amputation, or stenosis; MALE, major adverse limb event. Additionally adjusted for age, gender, indication for intervention, symptom status ambulatory status, living status, race, renal disease, coronary artery disease, hypertension, hyperlipidemia, history of myocardial infarction, congestive heart failure, TASC classification, smoking history, COPD, WIfI mean score, year of procedure, and procedure type (for any intervention group only)
An important and final note is that, when combining IDDM and NIDDM patients across all revascularization strategies (i.e., comparing 965 diabetic patients vs. 329 non-diabetic patients), multivariable analysis demonstrated that any diabetes was significantly associated with higher risk of incomplete wound healing (1.5, 1.1–1.9]), major amputation (2.0 [1.0–4.0]), and MALE (1.7 [1.1–2.8]), but there was no difference in mortality (0.8 [0.7–1.1]; P = .11).
DISCUSSION
Our data illustrate that, in patients undergoing a first-time lower extremity revascularization for CLTI, those suffering from IDDM present at an earlier age and with more severe disease. Regardless of revascularization strategy, there are no differences in perioperative complications, restenosis, or re-intervention; however, IDDM was associated with longer pre-operative and total hospital lengths of stay, as well as a heightened risk of incomplete wound healing, major amputation, RAS events, and major adverse limb events. Conversely, NIDDM patients – seemingly the least diseased-burden of the three groups – were shown to have lower long-term mortality (compared to NDM), even after adjusting for the discrepancy in comorbidity burden. More specifically, as compared to NDM patients, IDDM patients undergoing a PTA/S-first strategy were shown to have a heightened risk of incomplete wound healing, RAS events, and major amputation. Conversely, IDDM patients undergoing a BPG-first intervention were shown to only be associated with poorer wound healing, suggesting that the oft-referenced adverse outcomes in IDDM patients may be most mitigated following a BPG-first strategy.
Prior studies have illustrated that the impact of diabetes on perioperative outcomes remains controversial, with several studies demonstrating higher risk of perioperative morbidity and mortality among patients with diabetes, whereas others report no added risk in this patient population.20–23 In 2004, Virkkunen et al. studied 5,709 lower extremity bypasses performed for CLTI and found that patients with diabetes, although not differing in perioperative mortality, demonstrated a higher risk of wound infection (Odds ratio (OR), 1.3), cardiac complications (OR, 1.5), and major amputation (OR, 1.7).20 Conversely, Akbari et al. demonstrated reduced perioperative mortality in patients with diabetes (as compared to those without; 0.9% vs. 4.2%), and no difference in five-year survival or limb salvage.23 Further, Hamdan et al. – reporting perioperative and long-term outcomes among 4,052 lower extremity procedures – also found diabetes to be associated with lower perioperative mortality (OR, 0.6) and to decrease five-year survival, although these were unadjusted rates and no multivariable analysis was performed.24 Importantly, however, these studies did not distinguish between diabetes type, which, as our data illustrate, may play individual and important roles in long-term risk.
Fortunately, several recent studies have elaborated on the potential importance of diabetes type following lower extremity revascularization. In 2007, Hertzer et al. – stratifying by diabetes type – examined a single surgeon’s experience with over 600 lower extremity bypasses for PAD and found no difference in perioperative mortality and significantly higher rates of one-year and five-year mortality among NIDDM (1.4 [1.1–1.8]) and IDDM (1.5 [1.2–1.8]) patients.25 This study also indicated that IDDM is a significant predictor of both short-term and long-term amputation (OR, 2.6 and OR, 1.8, respectively). Additionally, in 2012, Wallaert et al. analyzed the effect of diabetes type on 1,977 infrainguinal bypass patients with CLTI, demonstrating that diabetes type does not significantly affect perioperative mortality rates and that both NIDDM and IDDM increase perioperative risk of any major adverse event, a composite variable defined as myocardial infarction, dysrhythmia, congestive heart failure, renal insufficiency, wound infection, and major amputation (OR, 1.4 and OR 1.5, respectively). Unfortunately, both studies focus only on patients undergoing bypass, providing little information regarding a prevalent subset of patients who undergo PTA/S procedures.
Lastly, in 2007, Dick et al. performed a prospective cohort study of 426 limbs suffering from both diabetes and CLTI undergoing conservative treatment, endovascular treatment, or surgical treatment.26 This study demonstrated that one-year clinical success – defined as survival without major amputation or future target extremity revascularization – was significantly better in non-diabetic patients (HR, 0.48), and that, in both diabetic and non-diabetic patients, this success was not influenced by mode of initial revascularization. Further, diabetes was not shown to be significantly associated with higher one-year mortality (P = .064). Ultimately, diabetic patients within this cohort were shown to improve to the same degree as in non-diabetic patients, but only through multiple revascularization procedures and by means of close follow-up and timely repetition of target extremity revascularization.
Overall, our study both differs from and corroborates previous literature. Curiously, NIDDM patients within our study were shown to have lower long-term mortality, which is a novel finding compared to prior works. Generally, we believe that this outcome may be less reflective of the health of NIDDM patients and more reflective of the severity of disease among and between both IDDM and NDM patients, as NIDDM patients were less likely to have tissue loss, coronary artery disease, and congestive heart failure (as compared to IDDM), and decreased proportions of COPD, smoking history, and femoropopliteal TASC D lesions (as compared to NDM). Although a surprising finding, the lower mortality among NIDDM patients may further reveal better – or simpler – long-term medical management, or the potential additional increases in cardiac risk within the IDDM and NDM patients that is not presently captured within this analysis. Importantly, when combining IDDM and NIDDM groups, our study substantiates the insignificant differences in long-term mortality that several previous studies have demonstrated, further highlighting the importance of evaluating the distinction between insulin-dependent and noninsulin-dependent diabetes within CLTI patients.22,23,25,26
There are important limitations to this study. First, it was a retrospective, single-center review where patients were allocated to treatment based on surgeon preference, which changed over time. As our data represent the experience of one group of surgeons at a single institution, the potential for selection and information bias exists and our results are subject to the influence of specific referral patterns, surgeon experience, and patient selection preferences. Second, these data only include revascularization attempts and do not reflect outcomes for those patients treated with primary amputation or medical management as a contrast. Fortunately, several previously published studies have illustrated both the poor outcomes following medical management and the importance of revascularization in diabetic patients with CLTI.21,22,26–29 Third, information regarding onset of diabetes and diabetes symptoms were difficult to accurately capture within this study, which may be important to consider in regards to certain differences illustrated between diabetes types – perhaps most important noticed in patient age. Lastly, since supplementary measures of diabetes disease severity were not readily accessible for this study, including patient hemoglobin A1c, baseline insulin reliance and administration was used as a replacement for disease severity, which could increase potential for confounding factors. Ultimately, however, our data include one of the largest reported analyses of the effect of diabetes type on the initial revascularization for CLTI.
CONCLUSION
To conclude, our data suggest that insulin-dependence in patients undergoing any first-time revascularization for CLTI may have a disease severity-dependent limb effect on a variety of long-term outcomes. Noninsulin dependence is not associated with these long-term events and, as compared to non-diabetic patients, is actually associated with lower long-term mortality. Overall, these data demonstrate the importance in distinguishing between diabetes type, as insulin-dependent, noninsulin-dependent, and non-diabetic patients all present with differing degrees of disease and comorbid conditions that harbor varying degrees of limb-based and patient-based risk. Finally, although insulin-dependence is associated with the greatest risk of adverse outcomes, these data suggest that these adversities may be most mitigated in those IDDM patients that are appropriately selected and anatomically suitable for a bypass.
JVS-D-17-00409R1, Outcomes Following First-time Lower Extremity Revascularization for Chronic Limb-threatening Ischemia between Patients with and without Diabetes
Type of Research: Retrospective review of a prospectively maintained single center database
Take Home Message: In 1294 limbs undergoing a first-time infrainguinal revascularization for chronic limb-threatening ischemia (CLTI) insulin-dependent diabetes was associated with poorer wound healing, more major amputations and more frequent reinterventions and restenosis than non-insulin dependent diabetes or no diabetes at all.
Recommendation: This study suggests that increased attention should be paid to insulin dependency in diabetics with CLTI as it is associated with poorer outcomes following first time revascularization compared to non-insulin dependent diabetics or non-diabetics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 45rd annual symposium of the Society for Clinical Vascular Surgery, Orlando, Florida, March 21, 2017
Submitted in partial fulfillment of the requirements for the degree of Master of Science at Boston University, 2017
References
- 1.Fonseca VA, Kirkman MS, Darsow T, Ratner RE. The American Diabetes Association Diabetes Research Perspective. Diabetes. 2012;61(6):1338–45. doi: 10.2337/db12-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katwal AB, Dokun AO. Peripheral Arterial Disease in Diabetes: Is There a Role for Genetics? Curr Diab Rep. 2011;11(3):218–25. doi: 10.1007/s11892-011-0188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownrigg JR, Schaper NC, Hinchliffe RJ. Diagnosis and assessment of peripheral arterial disease in the diabetic foot. Diabet Med. 2015;32(6):738–47. doi: 10.1111/dme.12749. [DOI] [PubMed] [Google Scholar]
- 4.Papavassiliou VG, Walker SR, Bolia A, Fishwick G, London N. Techniques for the endovascular management of complications following lower limb percutaneous transluminal angioplasty. Eur J Vasc Endovasc Surg. 2003;25(2):125–30. doi: 10.1053/ejvs.2002.1822. [DOI] [PubMed] [Google Scholar]
- 5.Salas CA, Adam DJ, Papavassiliou VG, London NJ. Percutaneous transluminal angioplasty for critical limb ischaemia in octogenarians and nonagenarians. Eur J Vasc Endovasc Surg. 2004;28(2):142–5. doi: 10.1016/j.ejvs.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Saketkhoo RR, Razavi MK, Padidar A, Kee ST, Sze DY, Dake MD. Percutaneous bypass: subintimal recanalization of peripheral occlusive disease with IVUS guided luminal reentry. Tech Vasc Interv Radiol. 2004;7(1):23–7. doi: 10.1053/j.tvir.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Desgranges P, Boufi M, Lapeyre M, Tarquini G, van Laere O, Losy F, et al. Subintimal angioplasty: feasible and durable. Eur J Vasc Endovasc Surg. 2004;28(2):138–41. doi: 10.1016/j.ejvs.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Clair DG, Dayal R, Faries PL, Bernheim J, Nowygrod R, Lantis JC, 2nd, et al. Tibial angioplasty as an alternative strategy in patients with limb-threatening ischemia. Ann Vasc Surg. 2005;19(1):63–8. doi: 10.1007/s10016-004-0136-0. [DOI] [PubMed] [Google Scholar]
- 9.Atar E, Siegel Y, Avrahami R, Bartal G, Bachar GN, Belenky A. Balloon angioplasty of popliteal and crural arteries in elderly with critical chronic limb ischemia. Eur J Radiol. 2005;53(2):287–92. doi: 10.1016/j.ejrad.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Surowiec SM, Davies MG, Eberly SW, Rhodes JM, Illig KA, Shortell CK, et al. Percutaneous angioplasty and stenting of the superficial femoral artery. J Vasc Surg. 2005;41(2):269–78. doi: 10.1016/j.jvs.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Trocciola SM, Chaer R, Dayal R, Lin SC, Kumar N, Rhee J, et al. Comparison of results in endovascular interventions for infrainguinal lesions: claudication versus critical limb ischemia. Am Surg. 2005;71(6):474–9. doi: 10.1177/000313480507100605. discussion 9–80. [DOI] [PubMed] [Google Scholar]
- 12.Tefera G, Hoch J, Turnipseed WD. Limb-salvage angioplasty in vascular surgery practice. J Vasc Surg. 2005;41(6):988–93. doi: 10.1016/j.jvs.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Treiman GS. Subintimal angioplasty for infrainguinal occlusive disease. Surg Clin North Am. 2004;84(5):1365–80. viii. doi: 10.1016/j.suc.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC) J Vasc Surg. 2000;31(1 Pt 2):S1–s296. [PubMed] [Google Scholar]
- 15.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 16.Conte MS. Understanding objective performance goals for critical limb ischemia trials. Semin Vasc Surg. 2010;23(3):129–37. doi: 10.1053/j.semvascsurg.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Mills JL, Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI) J Vasc Surg. 2014;59(1):220–34. e1–2. doi: 10.1016/j.jvs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Pierce ET, Pomposelli FB, Jr, Stanley GD, Lewis KP, Cass JL, LoGerfo FW, et al. Anesthesia type does not influence early graft patency or limb salvage rates of lower extremity arterial bypass. J Vasc Surg. 1997;25(2):226–32. doi: 10.1016/s0741-5214(97)70345-8. discussion 32–3. [DOI] [PubMed] [Google Scholar]
- 19.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virkkunen J, Heikkinen M, Lepantalo M, Metsanoja R, Salenius JP. Diabetes as an independent risk factor for early postoperative complications in critical limb ischemia. J Vasc Surg. 2004;40(4):761–7. doi: 10.1016/j.jvs.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 21.Luther M, Lepantalo M. Femorotibial reconstructions for chronic critical leg ischaemia: influence on outcome by diabetes, gender and age. Eur J Vasc Endovasc Surg. 1997;13(6):569–77. doi: 10.1016/s1078-5884(97)80066-4. [DOI] [PubMed] [Google Scholar]
- 22.Awad S, Karkos CD, Serrachino-Inglott F, Cooper NJ, Butterfield JS, Ashleigh R, et al. The impact of diabetes on current revascularisation practice and clinical outcome in patients with critical lower limb ischaemia. Eur J Vasc Endovasc Surg. 2006;32(1):51–9. doi: 10.1016/j.ejvs.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Akbari CM, Pomposelli FB, Jr, Gibbons GW, Campbell DR, Pulling MC, Mydlarz D, et al. Lower extremity revascularization in diabetes: late observations. Arch Surg. 2000;135(4):452–6. doi: 10.1001/archsurg.135.4.452. [DOI] [PubMed] [Google Scholar]
- 24.Hamdan AD, Saltzberg SS, Sheahan M, Froelich J, Akbari CM, Campbell DR, et al. Lack of association of diabetes with increased postoperative mortality and cardiac morbidity: results of 6565 major vascular operations. Arch Surg. 2002;137(4):417–21. doi: 10.1001/archsurg.137.4.417. [DOI] [PubMed] [Google Scholar]
- 25.Hertzer NR, Bena JF, Karafa MT. A personal experience with the influence of diabetes and other factors on the outcome of infrainguinal bypass grafts for occlusive disease. J Vasc Surg. 2007;46(2):271–9. doi: 10.1016/j.jvs.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 26.Dick F, Diehm N, Galimanis A, Husmann M, Schmidli J, Baumgartner I. Surgical or endovascular revascularization in patients with critical limb ischemia: influence of diabetes mellitus on clinical outcome. J Vasc Surg. 2007;45(4):751–61. doi: 10.1016/j.jvs.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Faglia E, Clerici G, Losa S, Tavano D, Caminiti M, Miramonti M, et al. Limb revascularization feasibility in diabetic patients with critical limb ischemia: results from a cohort of 344 consecutive unselected diabetic patients evaluated in 2009. Diabetes Res Clin Pract. 2012;95(3):364–71. doi: 10.1016/j.diabres.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 28.LoGerfo FW, Gibbons GW, Pomposelli FB, Jr, Campbell DR, Miller A, Freeman DV, et al. Trends in the care of the diabetic foot. Expanded role of arterial reconstruction. Arch Surg. 1992;127(5):617–20. doi: 10.1001/archsurg.1992.01420050145019. discussion 20–1. [DOI] [PubMed] [Google Scholar]
- 29.Muhs BE, Gagne P, Sheehan P. Peripheral arterial disease: clinical assessment and indications for revascularization in the patient with diabetes. Curr Diab Rep. 2005;5(1):24–9. doi: 10.1007/s11892-005-0063-7. [DOI] [PubMed] [Google Scholar]