Abstract
Platelet contraction provides a minimally invasive source for physiological information. In this paper we describe a device that directly measures the kinetics of platelet contraction. Whole blood is injected between acrylic plates and an adherent clot forms. The bottom plate is fixed, and the top plate is attached to a wire cantilever. Platelet contraction drives deflection of the wire cantilever which is captured by a camera. Force generated by the clot with time is derived using beam equations. Force derivations were verified using a microelectromechanical (MEMS) force sensor. Kinetics of clot contraction were defined, including maximum contraction force (FMAX), lift-off time (TLIFTOFF), and contraction rate (CR). Metrics were compared to optical aggregometry and thromboelastography. FMAX correlates with optical aggregometry maximal amplitude with a Spearman’s rho of 0.7904 and p = 0.0195 and thromboelastography maximal amplitude with a Spearman’s rho of 0.8857 and p = 0.0188. TLIFTOFF correlates with optical aggregometry lag time with a Spearman’s rho of 0.9048 and p = 0.002. This preliminary study demonstrates the repeatability of a useful platelet contraction device and its correlation with thromboelastography and optical aggregometry, the gold standard platelet function test.
Keywords: Platelet, contraction, medical device
Introduction
Platelet contraction is a biologic signal that potentially reflects hemostatic potential and systemic metabolic status. Thromboelastography (TEG) is commonly used to determine hemostatic potential in a bleeding patient. Studies show correlation of low maximum amplitude (MA) in TEG with increased risk of hemorrhage in trauma or surgery patients.1, 2 Platelet contraction must be a critical consideration in interpreting these results because it contributes up to 80% of the MA value.3 Platelet mitochondrial function is an established biomarker for systemic mitochondrial dysfunction.4–12 Platelet contraction, an energy dependent process, is a dynamic indicator of platelet mitochondrial function and a potential surrogate for systemic metabolic status.13 A device capable of measuring platelet contraction could potentially measure efficacy of anti-platelet drugs or hemostatic potential in critically ill patients. Medical devices like mechanical heart valves require antiplatelet therapy, and a platelet contraction assay could offer insight into optimizing anticoagulation. Although platelet contraction and its relationship to hemostatic potential and systemic metabolic status is a widely understood concept, a bulk platelet contraction assay is not in common clinical usage.
However, a number of investigative platelet contraction devices exist. One of the first devices described by Cohen et al attached thin strips of clots to a force sensor and measured platelet contraction forces under various conditions. This early concept allowed quantification of clot contraction forces due to platelet contraction and demonstrated its dependence on calcium. 14 Carr et al described a device with a strain gauge to measure contraction forces of a clot between two aluminum plates. This device did not require manual handling of clots and allowed greater throughput than Cohen’s. Assays run in this device demonstrated reproducible effects from anti-platelet drugs like aspirin, ticlodipine, and clopidogrel; establishing platelet contraction as a potential clinical measure of platelet function. 13 Recent devices focus on capturing contraction mechanics of individual platelets. Fegghi et al developed a device to measure platelet contraction force by recording deflection of an array of microposts. This study highlighted the importance of actin and myosin in platelet contraction using inhibitors to Rho and myosin light-chain kinase. This device demonstrates contraction dynamics of single platelets and allows study of platelets away from a bulk clot. 15, 16 Another similar device developed by Myers et al uses an array of fibrinogen microdots with variable stiffness to measure platelet contractile force. This device is unique in that it simulates physiologic shear stresses present in vivo. A major advantage of this device is the ability to fabricate and test microdot arrays with a wide range of variables such as stiffness.17 While these devices capture the platelet contraction signal, they have limitations including large sample volume, high level of skill required for assay completion, high-cost, or design complexity that hinders clinical translation.
In this study, we present preliminary results from a useful platelet contraction device. The device is unique because of its small sample volume, low-cost of disposable materials, and simple design. Device mechanics were validated using MEMS force sensors prior to human testing. Metrics describing contraction kinetics are defined and device repeatability over a healthy population is demonstrated. Assay results correlate with corresponding metrics in optical aggregometry and thromboelastography.
Materials and Methods
Device Design
Device components were designed using Solid Works (Dassault Systemes SolidWorks Corp., Waltham MA) and fabricated on a Haas computer numerical control machine (Haas Automation Inc., Oxnard CA). A schematic of the device is shown in Fig. 1. Image right depicts a cross section of the test chamber. Blood clots and adheres between concentric acrylic plates in a glass sample vial. The sample vial rests inside a heated aluminum block. The bottom plate is attached to the bottom of the sample vial. A nickel wire cantilever is attached to the top plate which displaces downward as platelets within the clot contract. The plates are 6 mm in diameter whereas the surrounding sample vial is 7 mm in inner diameter. A layer of silicone oil on top of the blood sample prevents blood evaporation. Image left depicts the device as it would appear during testing. After blood is added to the sample vial, the top plate is lowered to begin testing using an adjustable height stage to 1 mm above the bottom plate. The contracting clot volume between the plates is 27 μL, and total sample volume is 250 μL. Platelets in the blood clot between the two plates contract, causing downward displacement of the top plate. As the top plate displaces downwards, clot syneresis occurs and extruded plasma flows around and then above the top plate. A light emitting diode (LED) is aligned to project onto the cantilever. A 10x Nikon microscope objective (Nikon USA, Melville NY) attached to a Hitachi KP-F120CL digital camera (Hitachi Ltd., Marunouchi, Japan) captures displacement of the wire cantilever due to platelet contraction.
Figure 1.
Schematic of the device. (Left) Cross sectional view of sample vial. The sample vial is removable, composed of glass and rests inside a heated aluminum housing. The bottom plate is fixed to the bottom of the sample vial. The top plate is fixed to the nickel wire cantilever. Blood in the contraction volume between top and bottom acrylic plates forms an adherent clot causing downward movement of the top plate and deflection of the wire cantilever. Silicone oil protects blood from evaporation. (Right) Components of device including adjustable stage, wire chuck, LED light source, objective and aluminum housing.
Images are captured every 10 seconds using a LabVIEW (National Instruments, Austin TX) edge detection program. Wire deflection is measured in pixels. A micro-grid was used to calculate the size of a single pixel at the focal length of the Nikon lens and convert to micrometers. Force from platelet contraction is derived using the equation governing force generation for displacement of a cantilever:
| (1) |
Where F is force, δ is wire displacement, E is elastic modulus of nickel, I is moment of inertia of the wire cross section, and L is length of the wire.
Disposable device components include the sample vial, nickel wire cantilever and acrylic plates. These raw materials cost $0.75 per assay.
Validation of Cantilever Force Sensor
Force derivation from displacement of the cantilever was verified using a microelectromechanical (MEMS) force sensor (FemtoTools AG, Switzerland) in conjunction with a piezoelectric positioning stage (Physik Instrumente, Germany). Resolution of the piezoelectric positioning stage is ±0.1 nm with 0.02% positioning accuracy. Resolution of the MEMS force sensor is ±0.5 μN with a range of ±10,000 μN. Tests were conducted from 0 to 30 micrometers because this was the average deflection recorded during assays with whole blood. The experimental setup is shown in Fig. 2. The MEMS force sensor attaches to the piezoelectric positioning stage, allowing vertical translation in the z-axis. The tip of the MEMS force sensor contacts the top of the nickel wire at the end of the cantilever. Downward translation of the piezoelectric positioning stage causes an equal amount of deflection in the nickel wire, and the MEMS force sensor detects the resultant force in Newtons. Predicted wire deflection is derived using the recorded force and correlated to actual deflection of the piezoelectric positioning stage. This experimental setup modeled force exerted on the nickel wire by a contracting clot between the two plates.
Figure 2.
Experimental set up for validation of the cantilever force sensor. The MEMS force sensor is attached to the piezo stage. The tip of the MEMS force sensor contacts the distal end of the nickel wire cantilever. Downward displacement of the piezo causes wire deflection and the MEMS sensor measures resultant force.
Specimen Collection and Preparation
Human whole blood was collected utilizing universal precautions from healthy volunteers not taking any antiplatelet medications. Written informed consent was collected under approved protocol HSC-MS-10-0190 from the Internal Review Board at the University of Texas Health Science Center at Houston. Whole blood was collected by antecubital venipuncture into a polypropylene syringe and either run immediately in the platelet contraction device or stored in 3.2% sodium citrate for testing in optical aggregometry or TEG. Platelet rich plasma was prepared by centrifuging citrated whole blood at 200xg for 10 minutes. Platelet poor plasma was obtained by an additional centrifugation step at 2,000xg for 10 minutes.
Measuring Platelet Contraction
Platelet contraction assays were performed using native whole blood within 120 seconds after collection to avoid clotting of the sample. Native whole blood rather than citrated and re-calcified blood was used to exclude any effects of anticoagulation. Standard controls were run with whole blood at 37° C. 250 μL of blood was injected into the sample vial to insure filling of the test chamber and immersion of the top plate into the blood sample. Data recording commenced once the top plate is lowered into position and continued for 5,000 seconds. Assays were run in duplicate.
Optical Aggregometry
Optical aggregometry assays to correlate measures of platelet aggregation to platelet contraction were performed using a Chronolog Model 700 Whole Blood Optical Aggregometer (Chrono-log Corp., Havertown, PA). 500 μL of platelet rich plasma was pipetted into glass cuvettes and a stir bar insured adequate mixing of agonist. 5 μL of 50 mMol arachidonic acid was added to initiate aggregation. Maximum amplitude (MA), slope, lag time and area under the curve (AUC) was recorded. Assays were run in duplicate.
Thromboelastography
Thromboelastography (TEG) assays to correlate measures of clotting to platelet contraction were performed using a Haemoscope TEG 5000 Coagulation Analyzer (Haemoscope Corp., Niles, IL). 340 μL of citrated whole blood was mixed with 20 μL of 0.2 M calcium chloride in the TEG cup prior to assay initiation. Assays were run in duplicate. Maximum amplitude (MA), alpha angle, and r time were recorded.
Statistics
Average force curves were calculated from the mean of individual assays. Standard deviation was calculated at each time point and reported every 250 seconds. Data from platelet contraction assays was correlated to related metrics in optical aggregometry and TEG using Spearman’s rank correlation test.
Results
Validation of Cantilever Force Sensor
This experiment verified a nickel wire cantilever as a reliable indicator of clot contraction forces from platelets. It also verified the ability of the optical system to capture wire deflection. Fig. 3 shows correlation between piezoelectric positioning stage displacement on the x-axis and derived wire displacement on the y-axis. Derived wire displacement on the y-axis is calculated from the force recorded by the MEMS force sensor using the cantilever beam equation solved for δ. Derived wire displacement on the y-axis is calculated from the force recorded by the MEMS force sensor using equation (1). The experiment was run 5 times, each time with a new nickel wire cantilever.
Figure 3.
Verification of nickel wire cantilever force detection with MEMS microforce sensor. Derived wire displacement equaled actual with variation increasing 0.15 micrometers with each micrometer of cantilever deflection. Error increased linearly with increasing deflection because of minor variations in cantilever dimensions such as beam length. The experiment was run 5 times, with each point showing the mean and standard error of the mean.
Each nickel wire cantilever had differences in beam length of ±0.1 mm. This manufacturing variability led to variation in the derived wire displacement increasing at a rate of 0.15 micrometers per micrometer of cantilever deflection. While each experimental iteration was linear, the dispersion of these linear curves was responsible for the progressive increase in error seen in Fig. 3.
Linear fit of the mean curve was excellent with R2 = 0.999. Maximum standard deviation with 30 micrometers displacement was 4.6 micrometers, or 15.3%. These results demonstrate that the cantilever equation is valid within the range of expected deflection caused by a contracting clot. It also confirms that actual and predicted displacement of the nickel cantilever are equal, within the defined range of variation. This data set establishes the error range that is inherent to the device for a given amount of cantilever deflection. Induced deflection in the nickel wire was elastic and there was no noticed creep after cycling from 0 to 30 micrometers 4 times.
Validation of Platelet Contraction Biology
A series of initial assays confirmed the device signal was indeed from contracting platelets, shown in Figure 4. Phosphate-buffered saline (PBS) assays did not produce any contraction signal and defined the noise floor of the resting device at ±1 micrometer, or ± 125 μN. Citrated whole blood at control conditions without re-calcification showed no contraction signal with a similar noise floor as PBS. Platelet poor plasma (PPP) with platelet count of 20 platelets/μL run at control conditions formed an adherent clot between the plates but showed no contraction. Whole blood diluted 75% and 50% with PBS demonstrated a corresponding 75% and 50% decrease in signal magnitude. Citrated re-calcified blood demonstrated a similar signal as native blood, however with larger magnitude.
Figure 4.
Validation of platelet contraction biology. Native blood demonstrates decrease in signal magnitude corresponding with amount of dilution by PBS. Citrated blood, saline and 100% PPP all show minimal signal generation.
Description of Platelet Contraction Metrics
Whole blood is run in the device at control conditions to generate the contraction signal shown in Fig. 5. These metrics were calculated similarly for all following assays. With no clotting blood in the device, a baseline amount of expected noise in the deflection signal is expected. Lift-off time (TLIFTOFF) is when platelet contraction begins, and is defined as when the contraction force exceeds 500 μN. This value was chosen because assays uniformly demonstrate a period of steady state contraction after this force is reached. Contraction rate (CR) is the maximum average change in force with time. It is calculated starting at TLIFTOFF for 500 seconds. Ultimately the clot reaches a maximum contraction force (FMAX).
Figure 5.
Metrics derived from contraction curve. Lift-off time (TLIFTOFF) is when recorded force exceeds 500 μN; contraction rate (CR) is the average slope for 500 seconds starting at the TLIFTOFF; maximum contraction force (FMAX) is the maximum force recorded during the assay.
Reproducibility of Platelet Contraction Assay
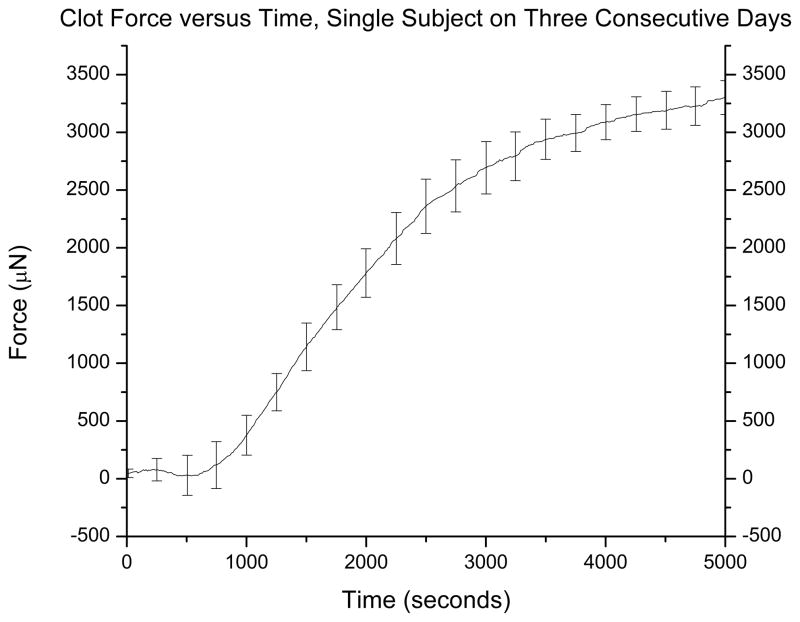
Whole blood from eight healthy adult donors was run in duplicate at control conditions to define person-to-person variability. Platelet count across all donors was 230,000 ± 50 platelets/μL. An average force curve is presented in Fig. 6A with standard deviation every 250 seconds. Assays were run on four identical devices; there was no significant variation between the four devices. For the eight donors, the platelet contraction curve was repeatable with standard deviation averaging ± 190 μN. Interassay variability of the device, measured as coefficient of variation of the FMAX, was 6.3%. To define variation among a single subject over time, whole blood from a single donor was run in duplicate on three consecutive days. The average force curve is presented in Fig. 6B. Standard deviation averaged ± 210 μN and coefficient of variation of FMAX was 6.7%. Published interassay variability of other platelet function devices such as TEG and Multiplate are 6.4% and 9.7%, respectively.18
Figure 6.
Clot force versus time for 8 healthy adults. Standard deviation for the curve averaged ±190 μN. Coefficient of variation (standard deviation divided by the mean) of FMAX was 6.3% (A). Clot force versus time for a single donor from three different days. Standard deviation average ±210 μN, coefficient of variation of FMAX was 6.7% (B).
Correlation with Optical Aggregometry
Platelet rich plasma from eight healthy adult donors was run in duplicate in the optical aggregometer and in parallel with whole blood assays in the platelet contraction device. Data is shown in Figure 7. Optical aggregometry maximum amplitude correlates with platelet contraction FMAX with a Spearman’s rho of 0.7904 and p = 0.0195. Optical aggregometry lag time correlates with platelet contraction TLIFTOFF with a Spearman’s rho of 0.9048 and p = 0.002.
Figure 7.
Linear regression analysis comparing the platelet contraction device to optical aggregometry. Platelet contraction TLIFTOFF and lag time (LT) in aggregometry correlate with a Spearman’s rho of 0.9048 and p = 0.002 (A). Platelet contraction FMAX and max amplitude (MA) in aggregometry correlate with a Spearman’s rho of 0.7904 and p = 0.0195 (B).
Correlation with Thromboelastography
Citrated re-calcified whole blood from six healthy donors was run in duplicate in TEG and in parallel with whole blood assays in the platelet contraction device. Data is shown in Figure 8. TEG maximum clot amplitude (MA) correlates with platelet contraction FMAX with a Spearman’s rho of 0.8857 and p = 0.0188.
Figure 8.
Linear regression analysis comparing TEG to platelet contraction. TEG MA and platelet contraction FMAX correlate with a Spearman’s rho of 0.8857 and p = 0.0188.
Discussion
In this paper we present a useful device that measures kinetics of platelet contraction. This work is significant because we believe that clot contraction is an underutilized and understudied biologic signal with high potential for correlation to clinical pathology and influence on clinical decision making. Specifically, it has the potential to measure efficacy of anticoagulation in patients with implanted artificial devices. For example, patients with left ventricular assist devices (LVADs) require anticoagulation and the present device may serve as an adjunct to titration of antiplatelet therapy.19 Furthermore, the present device is advantageous because of its simple design, small sample volume and low cost of disposable materials to run individual assays.
Of all the previously described platelet contraction devices in the literature, the present device is most similar to that presented by Carr et al.20 Both measure platelet contraction forces in a forming clot between two parallel plates. However, in the present device the plates are smaller (6 mm diameter versus 30 mm diameter), fabricated from acrylic rather than aluminum and fully submerged into the blood sample. The required blood volume is 250 μL as opposed to 2,000 μL. In the present device the actual contraction blood volume, or volume between the plates, is 27 μL. The present device used MEMS force sensor chips to initially validate the cantilever system, allowing use of cheap and disposable cartridge components. Additionally, the present device simulates in-vivo clot contraction by allowing free displacement of the top plate and increasing counter-forces provided by the cantilever beam as platelets contract.
When considering common tests of blood clotting, the present device is most similar to thromboelastography (TEG). For this reason correlative studies were performed between the two devices. Both devices measure extended function of platelets in a forming clot over a period of up to one hour. While TEG continually perturbs a forming clot with rotation to detect changes in shear, the present device allows clot forces to develop on their own. The present device measures platelet contraction directly with values reported in units of Newtons, while platelet contractile forces must be extrapolated in TEG using measures of clot elasticity.21 While TEG applies a constant torque to the blood sample via rotation of the sample cup, the present device provides dynamic and increasing force as clot contraction and cantilever beam deflection increases. Despite these differences, the two devices correlate with regards to final clot strength as shown in Figure 8. In future studies, TEG Platelet Mapping will be utilized to isolate the platelet contraction signal in TEG and allow for more direct comparisons between the two devices.
Optical aggregometry is less common than TEG and not routinely used clinically. However, it remains the historical gold standard for platelet function testing.22 Optical aggregometry is considerably different from the present device. It measures changes in light transmittance of a sample of platelet rich plasma as platelets aggregate.23 Metrics include aggregation lag time, maximal change in light transmittance, and maximum rate of change in transmittance. Despite differences in device design and the platelet mechanical signal recorded, both devices correlate when comparing respective metrics – lag time to TLIFTOFF, and FMAX to Max Amplitude.
The signal generated from the clot between the plates is indeed from contracting platelets. A number of simple initial experiments varying cellular components of whole blood were required to confirm this. Assays were performed with only PBS to confirm static buoyant forces do not act on the top plate. After one hour of testing, these assays demonstrated no force generation. Average recorded deflection in this case, ±1 micrometer, defined the noise floor of the device. In assays run with whole blood, the top insert is subjected to small changes in buoyant force as red blood cells (RBCs) settle in the sample vial before clot formation. RBC settling decreases the density of fluid surrounding the top insert and theoretically decreases its buoyant force. To investigate this, assays were performed with citrated whole blood without re-calcification to inhibit clot formation. These assays showed no force generation and no clot formation. Signal interference from changing buoyant forces was thus negated. Assays run with citrated and re-calcified platelet free plasma were performed to exclude the effects of platelet contraction and determine effects from clot formation. This assay developed a clot adherent to the plates but showed no force generation. Thus PPP will form a clot but will not contract. Assays run with whole blood diluted with PBS were run to verify the magnitude of force generation related to platelet count. These assays showed a relationship between decrease in signal magnitude with the amount of sample dilution, suggesting that clot contraction force is dependent on platelet count. Preservation of blood samples with citrate is logistically convenient, however assays run with citrated and re-calcified blood were noted to consistently have higher FMAX and earlier TLIFTOFF, as seen in the representative curve in Figure 4. This phenomena of signal difference due to anticoagulation is outside the scope of the current study and the focus of future work.
Platelets have cues in vivo to initiate clotting including soluble constituents in blood, exposed substrates in blood vessels, shear forces from blood flow and mechanical properties of their surroundings.24 All of these variables are accounted for in the device described here except for shear stress. The assay occurs in stasis without fluid flow. Calcium is a soluble agonist, acrylic is a foreign body acting as an exposed substrate and the wire cantilever provides a simplified source of stiffness and spring force felt by a contracting platelet. Previous studies have shown that platelets selectively contract against stiffer materials and this device replicates this in-vivo principle by providing increasing counter force through the cantilever beam.25
Conclusions
This paper presents a preliminary study of a useful device that measures platelet contraction. Non-disposable materials used for assays are low-cost, assay volume is on the micro scale, and the device design is simple relative to existing experimental platelet contraction devices. This study demonstrates the validity of the proposed platelet contraction device and warrants further investigation using platelet function as a clinical tool.
Several limitations exist in this study that must be addressed. First, experiments were run from a small population of healthy individuals to demonstrate repeatability of the device, however conclusions regarding variation over a larger population or effects from platelet inhibitors like aspirin cannot be made. Second, interaction of the forming blood clot with glass walls of the sample vial cannot be excluded, although the test volume between the acrylic plates is isolated from the glass vial surface. Third, device design required placement of an oil film over the blood sample to prevent drying which is not ideal when considering large scale testing.
Acknowledgments
Funding Source: Author Mitchell George is funded by an NIH T32 grant (No. 4T32GM008792-14). This study was funded in part by Coagulex, Inc.
Footnotes
Disclaimers: none
Disclosures: Authors Aroom, Cox and Gill report equity ownership in Coagulex, Inc., which develops blood coagulation assays.
References
- 1.Plotkin AJ, Wade CE, Jenkins DH, et al. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma. 2008;64(2 Suppl):S64–8. doi: 10.1097/TA.0b013e318160772d. [DOI] [PubMed] [Google Scholar]
- 2.Spiess BD, Tuman KJ, McCarthy RJ, DeLaria GA, Schillo R, Ivankovich AD. Thromboelastography as an indicator of post-cardiopulmonary bypass coagulopathies. J Clin Monit. 1987;3(1):25–30. doi: 10.1007/BF00770880. [DOI] [PubMed] [Google Scholar]
- 3.Harr JN, Moore EE, Chin TL, et al. Platelets are dominant contributors to hypercoagulability after injury. J Trauma Acute Care Surg. 2013;74(3):756–62. doi: 10.1097/TA.0b013e3182826d7e. discussion 762–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee I, Huttemann M. Energy crisis: the role of oxidative phosphorylation in acute inflammation and sepsis. Biochim Biophys Acta. 2014;1842(9):1579–86. doi: 10.1016/j.bbadis.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyrrell DJ, Bharadwaj MS, Jorgensen MJ, Register TC, Molina AJ. Blood cell respirometry is associated with skeletal and cardiac muscle bioenergetics: Implications for a minimally invasive biomarker of mitochondrial health. Redox Biol. 2016;10:65–77. doi: 10.1016/j.redox.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zharikov S, Shiva S. Platelet mitochondrial function: from regulation of thrombosis to biomarker of disease. Biochem Soc Trans. 2013;41(1):118–23. doi: 10.1042/BST20120327. [DOI] [PubMed] [Google Scholar]
- 7.Levy RJ. Mitochondrial dysfunction, bioenergetic impairment, and metabolic down-regulation in sepsis. Shock. 2007;28(1):24–8. doi: 10.1097/01.shk.0000235089.30550.2d. [DOI] [PubMed] [Google Scholar]
- 8.Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol. 2009;54(20):1891–8. doi: 10.1016/j.jacc.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montaigne D, Marechal X, Coisne A, et al. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation. 2014;130(7):554–64. doi: 10.1161/CIRCULATIONAHA.113.008476. [DOI] [PubMed] [Google Scholar]
- 10.Blake CI, Spitz E, Leehey M, Hoffer BJ, Boyson SJ. Platelet mitochondrial respiratory chain function in Parkinson’s disease. Mov Disord. 1997;12(1):3–8. doi: 10.1002/mds.870120103. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Shachar D, Bonne O, Chisin R, et al. Cerebral glucose utilization and platelet mitochondrial complex I activity in schizophrenia: A FDG-PET study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(4):807–13. doi: 10.1016/j.pnpbp.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol. 1989;26(6):719–23. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 13.Carr ME., Jr Development of platelet contractile force as a research and clinical measure of platelet function. Cell Biochem Biophys. 2003;38(1):55–78. doi: 10.1385/CBB:38:1:55. [DOI] [PubMed] [Google Scholar]
- 14.Cohen I, De Vries A. Platelet contractile regulation in an isometric system. Nature. 1973;246(5427):36–7. doi: 10.1038/246036a0. [DOI] [PubMed] [Google Scholar]
- 15.Feghhi S, Munday AD, Tooley WW, et al. Glycoprotein Ib-IX-V Complex Transmits Cytoskeletal Forces That Enhance Platelet Adhesion. Biophys J. 2016;111(3):601–8. doi: 10.1016/j.bpj.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feghhi S, Tooley WW, Sniadecki NJ. Nonmuscle Myosin IIA Regulates Platelet Contractile Forces Through Rho Kinase and Myosin Light-Chain Kinase. J Biomech Eng. 2016;138(10) doi: 10.1115/1.4034489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers DR, Qiu Y, Fay ME, et al. Single-platelet nanomechanics measured by high-throughput cytometry. Nat Mater. 2016 doi: 10.1038/nmat4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karon BS, Tolan NV, Koch CD, et al. Precision and reliability of 5 platelet function tests in healthy volunteers and donors on daily antiplatelet agent therapy. Clin Chem. 2014;60(12):1524–31. doi: 10.1373/clinchem.2014.226332. [DOI] [PubMed] [Google Scholar]
- 19.Baumann Kreuziger LM, Kim B, Wieselthaler GM. Antithrombotic therapy for left ventricular assist devices in adults: a systematic review. J Thromb Haemost. 2015;13(6):946–55. doi: 10.1111/jth.12948. [DOI] [PubMed] [Google Scholar]
- 20.Carr ME, Jr, Zekert SL. Measurement of platelet-mediated force development during plasma clot formation. Am J Med Sci. 1991;302(1):13–8. doi: 10.1097/00000441-199107000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Solomon C, Ranucci M, Hochleitner G, Schochl H, Schlimp CJ. Assessing the Methodology for Calculating Platelet Contribution to Clot Strength (Platelet Component) in Thromboelastometry and Thrombelastography. Anesth Analg. 2015;121(4):868–78. doi: 10.1213/ANE.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paniccia R, Priora R, Liotta AA, Abbate R. Platelet function tests: a comparative review. Vasc Health Risk Manag. 2015;11:133–48. doi: 10.2147/VHRM.S44469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–9. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- 24.Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood. 1996;88(5):1525–41. [PubMed] [Google Scholar]
- 25.Ehrlicher A, Hartwig JH. Cell mechanics: Contracting to stiffness. Nat Mater. 2011;10(1):12–3. doi: 10.1038/nmat2928. [DOI] [PubMed] [Google Scholar]