Abstract
Repeated cycles of alcohol (ethanol; EtOH) intoxication and withdrawal dysregulate excitatory glutamatergic systems in the brain, and induce neuroadaptations in the medial prefrontal cortex (mPFC) that contribute to cognitive dysfunction. The mPFC is comprised of subdivisions that are functionally distinct, with dorsal regions facilitating drug-cue associations, and ventral regions modulating new learning in the absence of drug. A key modulator of glutamatergic activity is the holoenzyme calcium/calmodulin-dependent protein kinase II (CaMKII) that phosphorylates ionotropic glutamate receptors. Here, we examined the hypothesis that abstinence from chronic intermittent EtOH (CIE) exposure dysregulates CaMKII activity in the mPFC to impair cognitive flexibility. We used an operant model of strategy set-shifting in male Long-Evans rats demonstrating reduced susceptibility to trial omissions during performance in a visual cue-guided task versus albino strains. Relative to naïve controls, rats experiencing approximately 10 days of abstinence from CIE vapor exposure demonstrated impaired performance during a procedural shift from visual cue to spatial location discrimination. Phosphorylation of CaMKII subtype α was upregulated in the dorsal, but not ventral mPFC of CIE-exposed rats, and was positively correlated with perseverative-like responding during the set-shift. The findings suggest that abstinence from CIE exposure induces an undercurrent of kinase activity (e.g., CaMKII) which may promote aberrant glutamatergic responses in select regions of the mPFC. Given the role of the mPFC in modulating executive control of behavior, we propose that increased CaMKIIα activity reflects a dysregulated “top-down” circuit that interferes with adaptive behavioral performance under changing environmental demands.
Keywords: ethanol, prelimbic, cognition
INTRODUCTION
Impairments in executive function have been identified as a cardinal feature of addiction (Garavan and Stout, 2005). Deficits in impulse control, attention, working memory, planning and mental flexibility likely underlie the difficulties experienced in reversing drug-taking behaviors. Indeed, detoxified alcoholics display persistent cognitive impairments throughout abstinence (Parsons, 1998; Stavro et al., 2013) that are associated with heightened relapse risk (Bowden-Jones et al., 2005; Parsons, 1998). The transition from regular use to dependence is prefaced by drug-cue associations that are thought to become more reflexive in nature, and less susceptible to cognitive feedback (Baler and Volkow, 2006; Robinson and Berridge, 2003). These concepts overlap with executive control loops monitoring “bottom-up” (i.e., stimulus-driven responses) and “top-down” (i.e., feedback regulation) processes that may underlie the transition from impulsive to compulsive use in alcohol dependent individuals (Koob and Volkow, 2016).
Executive function is mediated in part by a collection of regions in the prefrontal cortex. The medial division of the prefrontal cortex (mPFC) modulates control of emotional behavior and motivation, is densely connected with limbic brain structures that encode reward and aversion, and is highly reactive to alcohol (ethanol; EtOH) cues in relapsing individuals (Goldstein and Volkow, 2002; Grusser et al., 2004). The mPFC is further divided along a dorsal-ventral axis. In rodents, the dorsal region is comprised of the anterior cingulate and prelimbic cortex, whereas the ventral mPFC is comprised mostly of the infralimbic cortex. These subregions exhibit distinct connectivity with cortical and subcortical structures including the striatum, thalamus, amygdala, hypothalamus and brain stem (Heidbreder and Groenewegen, 2003). Relevant to the present study, projections from the dorsal mPFC innervate medial aspects of the dorsal striatum, extending ventrally into the nucleus accumbens core, with sparse diffusion into the shell and olfactory tubercles at more ventral regions of the prelimbic cortex. Alternatively, the ventral mPFC regions almost exclusively innervate the ventral striatum at the nucleus accumbens shell (see Figure 1). From here, a multitude of open and closed circuits arise through downstream associations with basal ganglia structures (e.g., the ventral pallidum) and thalamic relay nuclei that reciprocate connections with the cortex (Groenewegen et al., 1999). Generally, it is thought that distinctions in cortico-striato-thalamo-cortical circuitry, as well as key interactions with amygdalar nuclei set the stage for differential processing in dorsal versus ventral regions guiding “go” and “stop” functions in behavior, respectively (Gourley and Taylor, 2016; Peters et al., 2009).
Figure 1. Cortico-striatal connectivity involved in drug-cue associations and extinction learning.
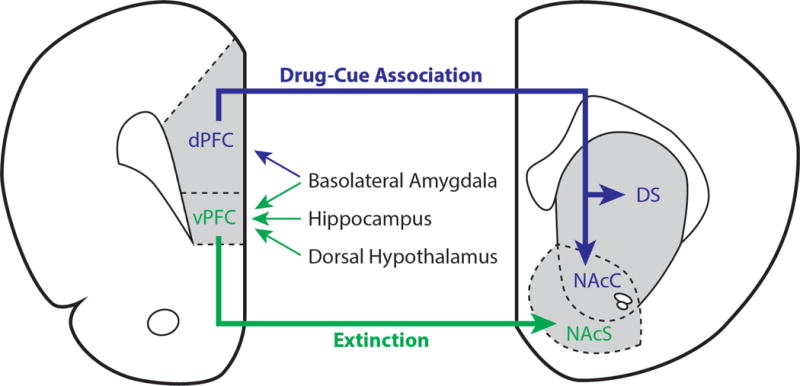
The medial prefrontal cortex (mPFC) can be subdivided into dorsal (dPFC) and ventral (vPFC) regions. The dPFC receives input from the basolateral amygdala, and projects to the dorsal striatum (DS) and nucleus accumbens core (NAcC) to facilitate drug-cue associations. The vPFC integrates input from the basolateral amygdala, hippocampus, and dorsal hypothalamus and primarily projects to the nucleus accumbens shell (NAcS) to facilitate extinction learning. For a more detailed discussion, please see the following reviews that address cortico-striatal connectivity and its importance in modulating drug-induced plasticity (Gass and Chandler, 2013; Gourley and Taylor, 2016; Peters et al., 2009).
Lesioning studies have provided a context for understanding the dissociable roles of dorsal versus ventral mPFC regions in learning and memory processes. For example, the learning of fear- (Giustino and Maren, 2015) and drug-conditioned cues (Gass and Chandler, 2013) is inhibited by lesions to the prelimbic cortex, whereas infralimbic lesions inhibit new learning in the absence of overt stimuli (e.g., extinction) (Holmes et al., 2012; Milad and Quirk, 2012). A similar parsing of function is evident in cognitive tasks employing cross-modal attentional designs that examine behavioral flexibility. More specifically, mPFC inactivation impairs the ability to complete new task requirements in place of old ones under both plus-maze (Ragozzino et al., 1999; Rich and Shapiro, 2007) and operant designs of strategy set-shifting (Darrah et al., 2008; Floresco et al., 2008). In addition to an increase in the number of trials required to successfully adapt to new task rules, inactivation of prelimbic versus infralimbic regions increases error responses in distinct manners (Ragozzino, 2007). The mechanisms that mediate proper responding in these assessments likely involve factors promoting synaptic plasticity, as recent work shows that the magnitude of early growth response genes correlate with higher attentional demands in the set-shift (DeSteno and Schmauss, 2008). In this regard, emerging studies support the role of excitatory signaling in mobilizing plasticity mechanisms in the mPFC, serving as a potential basis of distinction between prelimbic and infralimbic processing (Benn et al., 2016; Murphy et al., 2005).
EtOH modulates activity of the major excitatory transmitter in the brain, glutamate, via selective blockade of N-methyl-D-aspartate (NMDA) receptors. As a consequence of chronic intermittent EtOH (CIE) exposure, distinct neuroadaptations occur within the glutamate signaling system. Specifically, CIE exposure on neuronal cell cultures increases NMDA receptor expression containing NR1 and NR2 subunits that facilitate NMDA-mediated currents (Carpenter-Hyland et al., 2004). CIE exposure in rats also increases NMDA activity and long-term potentiation (LTP) in the prelimbic cortex via a similar pattern of NMDA receptor upregulation. Although expression is restored to baseline levels within a week, electrophysiological indices of LTP and the dendritic remodeling of synapses persist beyond the acute phases of abstinence (Kroener et al., 2012). Studies using similar methods of exposure report dendritic morphological changes persisting 3 weeks into abstinence, accompanied by changes in NR2B receptor phosphorylation, but not protein expression (Navarro and Mandyam, 2015). Conversely, NMDA activity and NR1 receptor expression are decreased in the infralimbic cortex following CIE exposure (Holmes et al., 2012). We recently observed that the emergence of impulsive action during protracted EtOH abstinence results from diminished stimulation of critical NR1 co-agonist sites in the infralimbic cortex (Irimia et al., 2017). Collectively, these findings indicate that abstinence from CIE exposure exerts dissociable responses in the mPFC and underscore the importance of identifying molecular signaling pathways that may underlie distinctions in the activation of mPFC glutamatergic circuits.
Here, we used an operant model of strategy set-shifting to evaluate cognitive flexibility in rats undergoing abstinence from CIE vapor inhalation. We first characterized the influence of rat strain in EtOH-naïve albino (Wistar) versus pigmented (Long-Evans) rats in our behavioral measures, as the latter strain is thought to possess superior visual acuity that might be expected to alter performance in visual cue-guided tasks (Kumar et al., 2015; Prusky et al., 2002). We then explored the possibility of dysregulated kinase signaling in the mPFC of CIE-exposed rats demonstrating inflexible behavior. In this regard, the holoenzyme calcium/calmodulin-dependent protein kinase II (CaMKII) is abundantly expressed in excitatory synapses, and is heavily implicated in synaptic plasticity and LTP via post-translational modifications of glutamate receptors (Lisman et al., 2012; Sanhueza and Lisman, 2013). Specifically, the autophosphorylation of threonine in the 286 position renders CaMKII subtype α constitutively active, targeting the phosphorylation of NMDA receptors that increase calcium channel conductance (Soderling and Derkach, 2000). Given regional distinctions in NMDA receptor activity following dependence-inducing regimens of EtOH, we hypothesized that CIE exposure confers increased phosphorylation of CaMKIIα in dorsal regions of the mPFC that may underlie inflexible performance during abstinence.
MATERIALS AND METHODS
Experiment 1: Examination of the influence of rat strain on strategy set-shifting
Subjects
Male albino Wistar and pigmented Long-Evans (n=12/strain) rats (Charles River, Wilmington, MA, USA) weighing approximately 300 g at the beginning of the experiments were group-housed in a humidity and temperature-controlled (22°C) vivarium on a 12h reverse light/dark cycle (lights off at 8 AM). Rats were food-restricted for 3d prior to the start of training procedures on strategy set-shifting, and were maintained at 85% of their free-feeding body weight throughout testing. All procedures were conducted in strict adherence to the NIH Guide for Care and Use of Laboratory Animals.
Apparatus
We utilized 12 standard operant conditioning chambers (Med Associates, St. Albany, VT) enclosed in sound-attenuating, ventilated environmental cubicles. Each chamber contained a centrally-positioned food magazine equipped with infrared photocell beams for detecting head entries into the basin. The magazine was flanked on either side by retractable levers with 3W cue lamps positioned overhead ~3 cm apart. A 1.1W house-light was located on the rear panel, opposite to the magazine and levers. Correct responses on the lever operandum were reinforced with the delivery of palatable 45 mg pellets (5TCY, Test Diet, Richmond, IN). Data collection and automated sequences were controlled by custom-made interfaces and software.
Strategy Set-shifting
We modified an approach for assessing strategy set-shifting in operant chambers based on previous work (Brady and Floresco, 2015; Darrah et al., 2008). On the day prior to training, rats received 3–5 g of food pellets in their home cages to reduce neophobia to the food reinforcer. On day 1 of training, rats were placed in dark chambers and exposed to an automated sequence resulting in food pellet delivery every 15 sec for a 10-min period. During this time, all chamber elements (i.e., levers and lights) remained inactive. Rats were left undisturbed for up to 1h to consume 40 dispensed pellets, and then returned to the vivarium. The following day, rats were placed in their respective chambers, and exposed to a single lever whereby responses on a fixed-ratio 1 (FR-1) schedule of reinforcement triggered the delivery of a single food pellet for a maximum of 40 rewards in 20 min. Unresponsive rats were given additional training procedures to facilitate responding. The next day, rats were exposed to a single lever on the alternate side using similar procedures. We alternated days of exposure to the lever operandum for additional training on a FR-3 schedule of reinforcement, requiring a total of 120 individual presses in a 20-min period. All rats completed training within 7d. Rats requiring additional training sessions during this period were counterbalanced for equivalent lever exposure.
Following training, rats were evaluated for performance on the initial discrimination (visual cue) task. Each testing session consisted of 120 trials separated into 12×10 trial blocks. The automated sequence began with the illumination of a house-light on a limited hold period (20 sec). During this time, rats were required to nose-poke into the food magazine to trigger a response interval sequence (20 sec), featuring the illumination of a single cue light and the extension of both levers. Lapses in performance resulted in the retraction of levers and extinguishing of cue- and house-lights for a timeout period (5 sec), followed by an inter-trial interval waiting period (5 sec) before proceeding to the next trial. The active lever was designated on the congruent side of the illuminated cue, whereby FR-3 responding triggered the retraction of levers, extinguishing of cue lights, and delivery of a single food pellet. The house-light remained on for a brief period (5 sec) while the animals collected their reward. Responding on the inactive lever (paired with the non-lit cue) triggered a similar response, but held no scheduled consequences and resulted in a timeout period. In either case, the end of the trial was signaled by the extinguishing of the house-light, followed by an inter-trial interval before proceeding to the next trial. The location of the active side was pseudo-randomized in a manner that produced equivalent left/right pairings in each 10-trial block. Data collection included: correct responses on the lever that was congruent with the visual cue, incorrect (error) responses on the lever that was opposite of the visual cue, and omissions of behavioral performance that failed to trigger the automated sequence (i.e., lever and cue light presentation). Rats were considered to have successfully completed the task when committing a total of 9 correct responses within a 10-trial block (binomial distribution p<0.01 for a 10-trial set). Rats were tested in successive trials for up to 7d until meeting criterion.
Following completion of the “set” task, we evaluated performance on a procedural shift to spatial response discrimination. Similar automated sequences followed as described above, with the exception that the illuminated cue no longer predicted the location of the active lever. Instead, the active lever was assigned a set position either left or right of the food magazine (counterbalanced across groups). The illumination of the cue light was pseudo-randomized in a manner that produced equivalent left/right cue exposures for each 10-trial block. Data collection included similar performance measures, using the spatial dimension to determine correct versus incorrect responses. Rats were tested in successive trials until meeting the same criterion.
Experiment 2: Examination of the influence of abstinence from CIE procedures on strategy set-shifting
EtOH vapor exposure
Based on the findings from Experiment 1, a new cohort of male Long-Evans rats (N=24) weighing approximately 250 g were trained on strategy set-shifting procedures in a similar manner. Following training, n=12 rats were group-housed in vapor inhalation chambers housed within a vivarium space under a similar light/dark schedule. Rats were exposed to 5 separate cycles of CIE exposure consisting of EtOH vapor inhalation (14h on/10h off) for 5d and intervening abstinence periods for 2d in their respective home cages outside of the vapor inhalation unit. The intervening abstinence periods allowed for rats to receive reminder food training sessions between each cycle of exposure. Blood alcohol levels (BALs) were determined once per week using an alcohol-enzyme assay (Analox Instrument, Stourbridge, England). The EtOH vapor model is a reliable method for inducing dependence in rodents by allowing the experimenter to control the dose, duration, and pattern of exposure within target BALs of ~200 mg/dL (Gilpin et al., 2008; O’Dell et al., 2004). Although direct measures of EtOH dependence were not examined in this study, it is well established that CIE exposure induces reliable indices of somatic and motivational withdrawal, coupled with increases in EtOH self-administration (Gilpin et al., 2008; O’Dell et al., 2004; Schulteis et al., 1995). Naïve rats (n=12) were housed in a separate vivarium space to ensure that they remained naïve to EtOH. Prior to the start of each cycle, all rats were food-deprived for 24h and given reminder food training sessions, alternating between levers as previously mentioned.
Strategy Set-Shifting
After the final cycle of EtOH exposure, rats were placed alongside naïve controls for 2d before administering the final training sessions. On day 5 of abstinence, rats were exposed to the visual cue task as previously mentioned, but with a lower criterion threshold of 8 correct responses in a 10-trial block. This substantially reduced the number of successive trials within range of previous work (Beas et al., 2016), while maintaining a reasonable threshold for indexing performance in a 10-trial set (binomial distribution p<0.05). This also allowed us to retest the visual cue association to distinguish between acquisition versus retention of task performance. Following successful completion of the task, rats were shifted to the spatial dimension and evaluated for performance using similar strategies.
Experiment 3: Examination of the influence of abstinence from CIE procedures on phosphorylated and total CaMKIIα levels in the mPFC
CIE Re-exposure and Tissue Collection
Following the assessment of cognitive flexibility in Experiment 2, rats were given an additional cycle of CIE vapor exposure for 5d. Rats were then withdrawn from EtOH vapor, and group-housed alongside naïve controls for an additional 10d that approximated the timeline of set-shift testing. Rats were euthanized and whole brains were extracted and dissected for dorsal and ventral mPFC regions using 12- and 18-gauge tissue extractors, respectively, on a ~2 mm thick coronal slice. Wet tissue collections were placed in individual vials, snap frozen in liquid nitrogen, and stored in a −80° freezer until molecular analyses described below.
Molecular Assays for CaMKIIα
Samples from each group (n=7) were analyzed for total and phosphorylated (threonine 286) CaMKIIα content. Tissue samples were thawed, homogenized, and total protein content was extracted in Pierce RIPA buffer (ThermoFisher Scientific, Waltham, MA) containing a protease/phosphatase inhibitor cocktail (78440, ThermoFisher Scientific). Protein concentrations were measured using a DC Assay (BioRad, Hercules, CA). Briefly, 20 μg of total protein extract per lane were resolved using 4–12% gradient bis tris gels and analyzed by western blot. Proteins were dry transferred to PVDF membranes which were blocked in tris-buffered saline (TBS) containing 5% (w/v) non-fat milk. Blots were probed using the following primary antibodies: rabbit anti-phospho-CaMKII (Threonine 286) (cat #12716, 1:1,000, Cell Signaling, Danvers, MA), mouse anti-CaMKIIα (05-532, 1:750, Millipore, Temecula, CA), and rabbit anti- glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (2118, 1:1000, Cell Signaling) for loading normalization. Blots were then incubated in biotinylated donkey anti-rabbit (1:1000) and DyLight 555-conjugated donkey anti-mouse secondaries for 1h at room temperature, followed by 30 min in Alexa Fluor 647-conjugated streptavidin (21374, 1:4000, ThermoFisher Scientific). Membranes were scanned on a ChemiDoc MP imager (BioRad) and bands were quantified using ImageLab software (BioRad) according to the protocols of the manufacturer. Pretreatment of sample with lambda phosphatase (sc-200312A, Santa Cruz Biotechnology, Santa Cruz, CA) dramatically reduced the phospho-CaMKIIα signal without affecting total CaMKIIα expression, demonstrating specificity of the phospho-CaMKIIα antibody.
Data Analysis
All data were analyzed with SPSS v.23 (IBM, Armonk, NY) and graphically displayed using Prism 7 (GraphPad, La Jolla, CA). Outlier values of the data were determined with Iglewicz and Hoaglin’s robust test (modified Z-score <3.5) as reported. Data for the cognitive flexibility experiments were analyzed separately for total number of trials, errors, and omissions until criterion was achieved for each task. In Experiment 1, performance measures were compared in Wistar versus Long-Evans rats using independent Student’s t-tests (two-tailed). In Experiment 2, performance measures were first analyzed using repeated measures analyses of variance (ANOVA), with EtOH history as the between-subject factor (CIE-exposed versus naïve) and test phase (acquisition versus retention) as the within-subject factor. Significant interactions were probed further with independent Student’s t-tests (two-tailed, Fisher’s protected least square differences) comparing CIE-exposed versus naïve rats. One-way ANOVAs examined whether the assigned lever position (left versus right) influenced performance during the set-shift. Given that the operant method utilized the same stimuli throughout testing, we evaluated the nature of errors committed during the set-shift. Specifically, we distinguished between errors that were previously reinforced (i.e., incorrect responses on the lever paired with the illuminated cue) and errors that were never reinforced (i.e., incorrect responses on the lever paired with the non-lit cue). “Previously-reinforced” errors reflect a form of perseveration that would have resulted in food reward delivery under the initial task, whereas “never-reinforced” responses index learning errors that would have been incorrect in either test procedure (Beas et al., 2016). Error analyses using independent Student’s t-tests (two-tailed) were performed only when significant group differences in total errors were detected. In Experiment 3, total protein and phosphorylated levels of CaMKIIα relative to the loading control GAPDH were first analyzed using independent Student’s t-tests (two-tailed) for each mPFC region. Significant effects were correlated further with trial performance measures during the set-shift (Spearman’s ρ), followed by correlations with error type when appropriate. In all cases, p<0.05 was considered significant.
RESULTS
Figure 2: Trial omissions in visual cue discrimination are lower in Long-Evans versus Wistar rats
Figure 2. Trial omissions in visual cue discrimination are lower in Long-Evans versus Wistar rats.
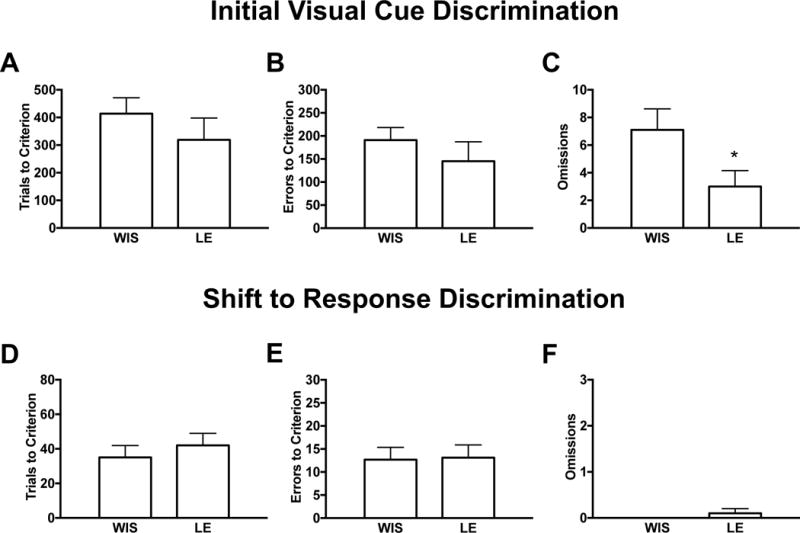
Behavioral performance on an operant model of strategy set-shifting was compared in ethanol-naïve male Wistar (WIS, n=10) and Long-Evans (LE, n=10) rats. The data are separated into two distinct operant procedures: the initial (visual cue) discrimination task in which the rats were required to respond on a lever located on the congruent side of the illuminated cue to receive a food reward, and the set-shift to a spatial discrimination task in which the rats were required to respond on a lever with a set position that was independent of visual cue illumination. Behavioral measurements (mean ± SEM) included total trials (A and D), errors (B and E) and omissions (C and F) until rats achieved a criterion threshold for each task. Generally, we observed no strain differences in cognitive performance across task procedures, except for reduced omissions (C) in LE versus WIS rats during the cue discrimination task. As high omission rates may complicate the assessment of cognitive flexibility, our subsequent studies evaluated the effects of ethanol abstinence in LE rats displaying reduced susceptibility to trial omissions. An asterisk (*) denotes significant group differences (p<0.05).
We characterized performance on strategy set-shifting in EtOH-naïve Wistar and Long-Evans (n=10/strain) rats (Figure 2). N=2/strain were excluded from the study for failure to achieve criterion within 7d of testing during the initial (visual cue) task. There were no group differences in the number of days needed to complete the initial task (Wistar: 3.9±0.4, Long-Evans: 3.0±0.7, p=n.s.), nor in the set-shift to the spatial dimension that was completed within the day of testing. Analyses revealed comparable performance in total trials (Figure 2A,D) and errors (Figure 2B,E) to criterion across task procedures, suggesting no overt strain differences in the discrimination of either visual cue or spatial dimensions. However, as compared to Wistars, Long-Evans displayed fewer incidences of omitted trials (t(18)= 2.16, p<0.05) during the cue discrimination task (Figure 2C), although no group differences emerged during the set-shift (Figure 2F). High omission rates may complicate the assessment of cognitive flexibility, given that the lack of behavioral performance is not readily interpretable. For this reason, subsequent studies investigating the effects of EtOH abstinence on cognitive flexibility were done in Long-Evans rats showing reduced susceptibility to trial omissions.
Figure 3: Abstinence from CIE exposure induces deficits in cognitive flexibility that are characterized by increased perseverative-like responding
Figure 3. Abstinence from chronic intermittent ethanol exposure induces deficits in cognitive flexibility that are characterized by increased perseverative-like responding.
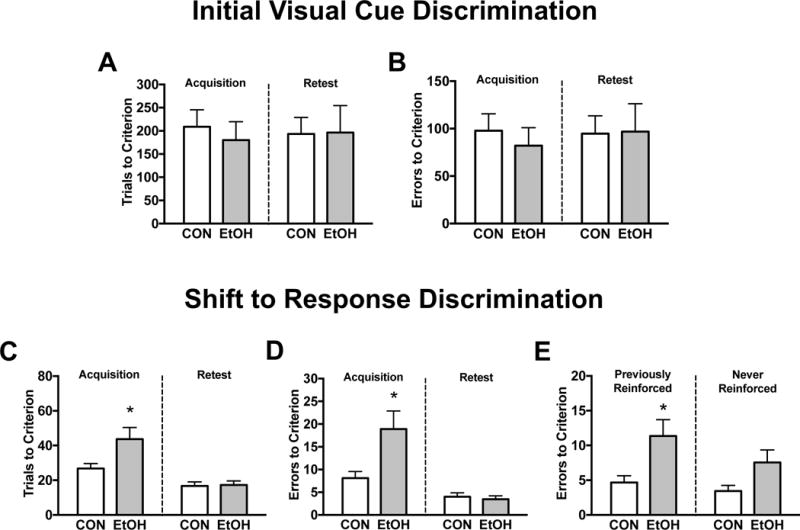
Behavioral performance on an operant model of strategy set-shifting was compared in male Long-Evans rats serving as ethanol-naïve controls (CON, n=9) or undergoing abstinence from chronic intermittent ethanol vapor exposure (EtOH, n=11). The data are separated into two distinct operant procedures: the initial (visual cue) discrimination task in which the rats were required to respond on a lever located on the congruent side of the illuminated cue to receive a food reward, and the set-shift to a spatial discrimination task in which the rats were required to respond on a lever with a set position that was independent of visual cue illumination. A retest of each operant procedure was administered to distinguish between acquisition and retention of task performance. Behavioral measurements (mean ± SEM) included total trials (A and C) and errors (B and D) until rats achieved a criterion threshold for each task. As compared to CON, EtOH rats undergoing abstinence did not display altered performance on the cue discrimination task (A and B) in either test phase. Alternatively, after approximately 10 days of abstinence, EtOH rats displayed higher trials (C) and errors (D) to criterion on the day of the set-shift, although no group differences emerged during the retest of this association. To gain further insight on impaired cognitive performance, we parsed the errors committed during the set-shift into previously- and never-reinforced (E) responses. As compared to CON, EtOH rats committed more errors that would have been correct under the initial task, although a marginal increase in indiscriminate learning errors was also evident. An asterisk (*) denotes significant group differences (p<0.05).
We characterized performance on strategy set-shifting in EtOH-naïve control (n=9) and CIE-exposed (n=11) Long-Evans rats undergoing abstinence (Figure 3). N=1/group were excluded from the study for failure to achieve retest criterion within 7d of testing during the initial (visual cue) task. In addition, n=1 control rat was eliminated due to an experimental error, and n=1 control was identified as an outlier based on an exceeding amount of trials and errors to criterion during the set-shift to the spatial dimension. Rats demonstrated an average BAL of 219.1±10.7 mg/dL, ranging between 176.8 and 268.1 mg/dL over the course of CIE exposure. There were no differences in the number of days needed to acquire (Control: 2.3±0.3, EtOH: 1.9±0.3 days, p=n.s.) or maintain (Control: 2.1±0.3, EtOH: 2.1±0.5 days, p=n.s.) performance on the initial task. Similarly, all rats completed set-shifting criteria within the day of testing. Analyses of performance on the initial task revealed comparable trials (Figure 3A) and errors (Figure 3B) to criterion across test phases (for trials: F(1,18)=0.0, p=n.s., for errors: F(1,18)=0.05, p=n.s.) and EtOH history (for trials: F(1,18)=0.14, p=n.s., for errors: F(1,18)=0.16, p=n.s.), with no evidence of an interaction of these variables (for trials: F(1,18)=0.09, p=n.s., for errors: F(1,18)=0.11, p=n.s.). During the set-shift, there was a significant interaction of test phase and EtOH history for both trials (F(1,18)=5.36, p<0.05) and errors (F(1,18)=6.83, p<0.05) to criterion. Post-hoc evaluations revealed that approximately 10.6±0.4 days into abstinence, EtOH rats displayed higher trials (Figure 3C) and errors (Figure 3D) to criterion during the acquisition, but not retention of the set-shift (p<0.05). The resulting differences were not influenced by a pre-existing side bias (for trials: F(1,18)=0.88, p=n.s., for errors: F(1,18)=0.16, p=n.s.). A parsing of the errors committed on the day of the set-shift (Figure 3E) revealed that EtOH rats displayed higher incorrect responses on the lever paired with the illuminated cue (t(18)=2.64, p<0.05), and marginal increases in errors that were never-reinforced (t(18)=2.06, p=0.06). Collectively, these data show that abstinence from CIE exposure discretely impairs flexibility performance during the set-shift. The factors that contribute to this deficiency likely involve retained aspects of behavior that were previously established, although the data are subthreshold for more generalized response impairments.
Figure 4: Abstinence from CIE exposure augments the phosphorylation of CaMKIIα in the dorsal mPFC, an effect that is associated with inflexible performance
Figure 4. Abstinence from chronic intermittent ethanol exposure augments the phosphorylation of CaMKII α in the dorsal medial prefrontal cortex, an effect that is associated with inflexible performance.
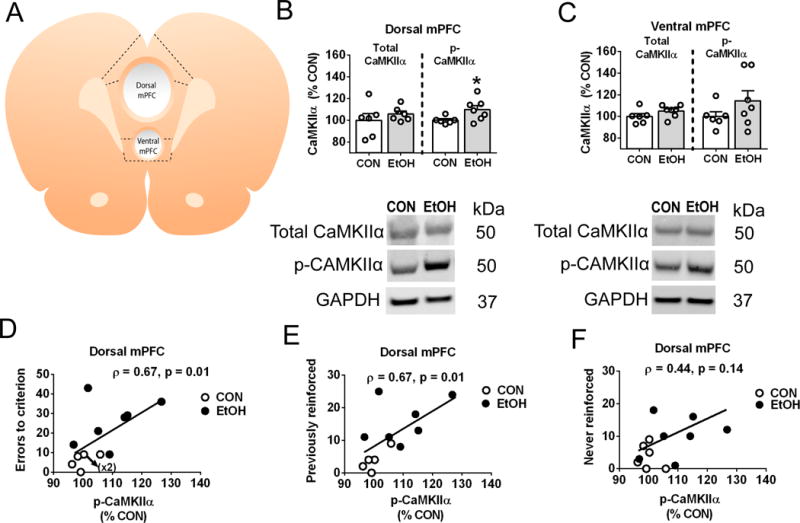
Total and phosphorylated levels of CaMKIIα (threonine 286) were estimated from microdissections of the medial prefrontal cortex (mPFC) in ethanol-naïve controls (CON, n=6) or rats that were re-exposed to ethanol vapor and abstinence procedures (EtOH, n=7) from the behavioral studies. The mPFC wet tissue slice (A) was dissected for regions comprising the dorsal (i.e., primarily prelimbic cortex) or ventral mPFC (i.e., primarily infralimbic cortex). Fluorescence values of immunoreactivity for total and phosphorylated CaMKIIα relative to the loading control glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were analyzed as a percentage change from CON levels (mean ± SEM) across brain regions (B and C). Representative images for each group illustrate the gel band and molecular weight of the proteins quantified in the analysis. Whereas total CaMKIIα levels remained comparable across groups and brain regions, EtOH rats undergoing 10 days of abstinence displayed an increase in phospho-CaMKIIα levels in the dorsal (B) but not ventral (C) mPFC as compared to CON. To gain insight on the role of upregulated phospho-CaMKIIα, we correlated these measures with respective performance during the set-shift (D, E, and F) in CON (open circles) and EtOH (closed circles) rats. Phospho-CaMKIIα in the dorsal mPFC correlated with inflexible performance, showing a positive association with increasing total errors (D), as well as indices of perseverative-like responding via previously-reinforced (E), but not never-reinforced (F) errors. An asterisk (*) denotes significant group differences (p<0.05).
The mPFC was analyzed for total and phosphorylated CaMKIIα levels in EtOH-naïve controls (n=6) and CIE-exposed (n=7) rats from the behavioral studies after a brief re-exposure to EtOH vapor and abstinence procedures (Figure 4). Wet tissue dissections isolated the dorsal and ventral mPFC, mostly containing the prelimbic and infralimbic cortex, respectively (Figure 4A). N=1 control was identified as an outlier based on exceeding fluorescence values of phospho-CaMKIIα across both brain regions. Analyses of immunoreactivity revealed comparable expression of total CaMKIIα across dorsal (Figure 4B) and ventral (Figure 4C) regions. However, as compared to controls, phospho-CaMKIIα levels in dorsal tissue dissections from EtOH rats were significantly elevated (t(11)= 2.3, p<0.05). Although a similar pattern of group differences emerged in the ventral mPFC, the findings were not statistically significant (t(11)= 1.3, p=n.s.). Based on evidence of regional distinctions, we examined whether phosphorylated levels of CaMKIIα in the dorsal mPFC correlated with respective performance observed during the set-shift. In this regard, phospho-CaMKIIα levels were positively correlated with increasing indices of impaired performance, as indicated by total trials (Spearman’s ρ=0.65, p=0.01, data not shown) and errors (Figure 4D, Spearman’s ρ=0.67, p<0.05) to criterion. A parsing of errors committed during the set-shift revealed a positive association with previously-reinforced responses (Figure 4E, Spearman’s ρ=0.67, p<0.05), whereas errors pertaining to never-reinforced responses lacked a significant association (Figure 4F, Spearman’s ρ=0.44, p=n.s.). The data suggest that increased autophosphorylation of CaMKIIα is associated with behavioral symptoms of inflexibility during a similar timeframe of EtOH abstinence.
DISCUSSION
Cognitive deficits are a hallmark symptom of substance use disorders that likely contribute to the loss of control in drug intake and relapse (Duka et al., 2011; Jentsch and Taylor, 1999; Koob and Volkow, 2016). As neuroadaptations in the mPFC may underlie clinical observations of alcohol-induced cognitive dysfunction, we utilized a rodent model of CIE vapor exposure to evaluate the long-term effects of dependence-like states on measures of behavioral flexibility. Our findings reveal that in the absence of EtOH exposure, CIE-exposed rats show impaired performance in a cognitive switching task, driven in part by increased perseverative-like responses during the set-shift. Inflexible performance during this period is associated with region-specific increases in CaMKIIα phosphorylation, a key biological factor known to facilitate glutamatergic signaling. The regional distinction is noteworthy, as levels of autonomous CaMKIIα in the dorsal mPFC correlated with indices of perseveration. Taken together as evidence of increased glutamatergic activity, our findings may reflect the persistence of a compulsivity circuit in the dorsal mPFC that diminishes the capacity for flexible performance.
Our behavioral findings offer insight into methodological considerations for operant models of cognitive flexibility. Long-Evans, and more broadly pigmented rats, are widely regarded for displaying enhanced visual acuity relative to albino strains (Kumar et al., 2015; Prusky et al., 2002). Impaired vision is commensurate with indices of poor performance in visuo-spatial and procedural learning tasks, independent of the animals’ learning ability (Prusky et al., 2000; Wong and Brown, 2006). Although the latter studies employed the use of complex visual stimuli, it is reasonable to suspect that impaired vision may defer critical learning processes inherent to the set-shift model. Here we show that while task performance was generally comparable across strain, albino Wistar rats omitted more trials during the visual cue task. High omission rates are notoriously difficult to reconcile in cognitive models, as the lack of behavioral performance is not readily interpretable and may lead to experimental attrition or other corrective strategies. In addition, the lack of strain differences during the spatial location task suggests that procedural shifts to visual cue dimensions may be highly susceptible to omission confounds. In support of this, we observed that rats from both strains required additional days of testing to complete the visual cue versus spatial location task. The findings are consistent with studies that do not restrict the number of successive trials before criterion is achieved (Beas et al., 2016; Cox et al., 2016; Harvey et al., 2013). Whereas increased trials imply an inherent difficulty in the discrimination of cue versus spatial dimensions, operant protocols that implement longer cue exposure prior to lever insertion are shown to facilitate the learning of the visual cue rule (Brady and Floresco, 2015; Floresco et al., 2008).
Our behavioral findings also support a growing consensus in the literature showing deficits in cognitive flexibility following abstinence from chronic exposure to various drugs of abuse, including alcohol (Kroener et al., 2012; Trantham-Davidson et al., 2014), nicotine (Parikh et al., 2016), and methamphetamine (Cox et al., 2016). Inflexible performance is manifest in the form of increased trials and errors on the day of the procedural shift to the spatial dimension task, although in the current study, group performance did not differ in measures that preceded or followed the set-shift. This indicates a discrete onset of the abstinence effect that is unveiled when stimulus-reward associations are changed, rather than in aspects involving operant training or maintenance of task performance. Moreover, we demonstrate that set-shifting impairments are characterized by increased errors committed on the congruent side of the cue light, in agreement with the strategy that was initially reinforced. We interpret the latter finding as evidence of perseveration that is consistent with a similar induction of error-prone behaviors following global mPFC inactivation (Ragozzino et al., 1999). However, we also noted marginal increases in errors committed on the incongruent side of the cue light, suggesting that in addition to perseveration, CIE-exposed rats may be exhibiting broader symptoms of impaired response inhibition. The interpretation is consistent with our previous work showing the emergence of impulsive- and compulsive-like traits during protracted EtOH abstinence using 5-choice operant procedures (Irimia et al., 2017; Irimia et al., 2015). Collectively, our findings point to the persistence of EtOH-induced cognitive dysfunction that transiently impairs attentional resources and adaptive function in the face of changing environmental demands. As EtOH abstinence may be associated with the rigorous retention of learned associations, increased perseveration has critical implications on compulsive behaviors that further index the loss of control in EtOH intake and relapse (Gass and Chandler, 2013).
Repeated cycles of EtOH intoxication and withdrawal progressively dysregulate brain amino acid systems, and are thought to induce a hyper-excitable state (Becker, 1998). Indeed, the emergence of cognitive dysfunction during abstinence is accompanied by increased indices of synaptic plasticity in the prelimbic cortex, albeit in the absence of overt changes in glutamate receptor expression or release (Kroener et al., 2012). Here, we provide initial evidence for the relation between upregulated glutamatergic function in the dorsal mPFC (as surveyed in the literature) and kinase signaling mechanisms known to potentiate glutamatergic transmission. High intracellular calcium concentrations promote the autophosphorylation of CaMKIIα, triggering autonomous activity that targets the phosphorylation of high-affinity serine sites on NMDA receptors shown to be critical for LTP (Chen and Roche, 2007; Sanhueza and Lisman, 2013). Coincident to the behavioral findings, we show that EtOH abstinence is accompanied by increased levels of autonomous CaMKIIα in the dorsal, but not ventral mPFC. Although the reported increase is modest in nature (~10% of control), the finding was devoid of changes in total protein, and falls within the expected range based on protocols using high frequency stimulation to induce LTP (Lisman et al., 2002). In addition, similar increases in phospho-CaMKIIα levels have been reported in the nucleus accumbens (Zhao et al., 2015) and central amygdala (Salling et al., 2016) of chronic EtOH-drinking mice experiencing acute abstinence. The lack of changes in the ventral mPFC might be expected, as deficits in infralimbic glutamatergic processing are shown to drive dysregulated extinction performance and impulsivity during EtOH abstinence (Gass et al., 2014b; Holmes et al., 2012; Irimia et al., 2017).
The correlational analyses allowed us to evaluate the relation between upregulated phospho-CaMKIIα in the dorsal mPFC and subsequent performance on the day of the set-shift. Indeed, a significant correlation of error performance emerged, particularly with those classified as previously-reinforced. We would infer from these data that worsening performance on the set-shift is linked with upregulated autonomous CaMKIIα, and is likely predicated on prior EtOH history. The lack of association with never-reinforced errors offers an intriguing divergence of behavioral repertoires that may influence cognitive dysfunction in distinct manners. In agreement with this, site-specific inactivation of dorsal mPFC regions results in increased perseverative-like responding under both 5-choice and set-shifting task procedures (Chudasama et al., 2003; Ragozzino, 2007). Conversely, inactivation of the infralimbic region impairs timing-dependent behaviors that are more akin to impulsivity and response inhibition (Chudasama et al., 2003; Dalley et al., 2004). Thus, while the factors contributing to inflexible performance may not be mutually exclusive in our assessments, we provide initial evidence that a critical component of this disorder (i.e., perseveration) likely involves increased CaMKIIα signaling, which may underlie the selective persistence of glutamatergic activity in the dorsal mPFC.
There are several important considerations. First, although we establish a rationale for the use of pigmented rats, it may be suggested that strain differences in chronic alcohol exposure differentially influences neuroadaptations in the mPFC. In this regard, strain comparisons in EtOH drinking studies report similar levels of intake and preference, with Long-Evans displaying all relevant areas of predictive validity such as intermittent-induced escalation, facilitation of intake by administration of pharmacological stressors, and reductions in this measure with gold-standard treatments (Simms et al., 2010; Simms et al., 2008). Moreover, CIE vapor exposure produces BALs within the expected range of intoxication (Morales et al., 2015; O’Dell et al., 2004), with no overt strain differences (Gilpin et al., 2008) or distinctions in withdrawal-related behaviors (Gass et al., 2014a; Morales et al., 2015; Ruwe et al., 1986). While emerging studies are unveiling the possibility of sex and strain differences in select behaviors (Priddy et al., 2017), it is reasonable to expect that vapor exposure induces similar neuroadaptive responses across strains. Second, it is possible that the source of glutamatergic dysregulation in the mPFC involves other neurotransmitter systems. In this regard, mesocortical dopamine projections from the midbrain innervate the mPFC and influence cognitive function in an inverted-U shaped manner, an effect related to the on-line stabilization of task-related representations and flexible updating of these processes (Cools and D’Esposito, 2011). Cortical dopamine receptors have been shown to modulate performance on set-shifting tasks (Floresco et al., 2006) and are also linked to psychiatric conditions involving attentional deficits (Cheng et al., 2017). Interestingly, CIE exposure is associated with the loss of inhibitory influence of dopamine receptors on mPFC pyramidal cell firing during abstinence (Trantham-Davidson et al., 2014). Taken together with evidence that activation of inhibitory dopamine receptors is under the influence of CaMKII signaling (Lauzon et al., 2012), it is possible that inflexible performance during EtOH abstinence also reflects an overactivation of cortical dopamine neurons, although the extent to which these mechanisms exhibit regional selectivity in the mPFC remains an important question. Similarly, we cannot rule out the possibility and/or contribution of alternative mechanisms that are dysregulated by CIE exposure, such as endogenous cannabinoids and other monoamines (Abernathy et al., 2010).
Clinical studies provide increasing evidence of a parsing of mPFC function among alcohol dependent individuals. The presentation of emotional stressors in alcoholic patients generally induces hypoactivation of ventral mPFC processing, coupled with observations of impaired impulse control (Seo et al., 2016). Conversely, tasks that challenge cognitive flexibility using proactive learning interference are resolved via the recruitment of dorsal mPFC regions in alcoholic patients versus healthy controls (De Rosa et al., 2004). Our findings begin to shed light on a potential mechanism in the mPFC that is critically aligned with learning and memory. Accordingly, increases in autonomous CaMKIIα following CIE exposure may confer a potentiated “top-down” circuit that persists beyond the transient rise in glutamatergic activity observed during early abstinence. Although it may seem peculiar that CIE exposure would facilitate a mechanism expected to improve cognition, the shifting between cue dimensions requires the coordination of multiple cognitive systems that are thought to inhibit previous response strategies in favor of new alternatives (Dalley et al., 2004; Floresco and Magyar, 2006). Thus, it is possible that overactive molecular mediators of LTP are maladaptive in the face of changing circumstances that require flexibility for behavioral adjustments. Future studies will endeavor to dissect the specific glutamatergic circuits and molecular framework under which autonomous CaMKIIα may exert differential influence in the mPFC during EtOH abstinence.
Acknowledgments
This is manuscript #29505 from The Scripps Research Institute. We are grateful for the support from our colleagues at the Pearson Center for Alcoholism and Addiction Research. We are grateful for the support provided by the National Institute on Alcohol Abuse and Alcoholism via the following mechanisms: R01-AA020404 (RM-F), R01-AA022249 (RM-F), R37-AA017447 (MR), R01-AA015566 (MR), P60-AA006420 (RM-F and MR), K99-AA025393 (LAN), F32-AA025257 (SAL), T32-AA007456 (MQS) and a Research Supplement to Promote Diversity (LAN). We sincerely appreciate the intellectual contributions from our laboratory colleagues Ann M. Gregus, Daniel B. McClatchy, David Stouffer, and John R. Yates III, as well as editorial assistance from Pedro Natividad, Jr. We humbly dedicate this work in loving memory of our friend and colleague, Dr. Loren (Larry) H. Parsons. He has been an invaluable influence on many students and colleagues, and his seminal contributions in the areas of neurochemistry and drug addiction will remain a driving force in better understanding alcohol dependence. We will always be grateful for his unparalleled support, mentorship and guidance as well as the inspiration to live by the qualities and ideals he promoted through his hard work, dedication, and loyalty. We will dearly miss him.
Footnotes
The authors state no competing financial interests, or interests otherwise that might be perceived to unduly influence the results and discussions generated in this report.
AUTHOR CONTRIBUTIONS
LHP and LAN designed the behavioral experiments. IP and RL developed the program for the operant chambers. LAN and IP executed the behavioral studies. LAN, MQS, and SAL collected brain tissue and together with MR, designed and executed the molecular studies. LAN, MQS and SAL analyzed all behavioral and molecular data. CI, MWB, and RM-F provided invaluable assistance in data analyses and/or the interpretation of the findings. LAN, MQS, and CI prepared the manuscript. All authors critically evaluated the content of the manuscript, and provided editorial changes where deemed appropriate.
References
- Abernathy K, Chandler LJ, Woodward JJ. Alcohol and the prefrontal cortex. Int Rev Neurobiol. 2010;91:289–320. doi: 10.1016/S0074-7742(10)91009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Beas BS, Setlow B, Bizon JL. Effects of acute administration of the GABA(B) receptor agonist baclofen on behavioral flexibility in rats. Psychopharmacology (Berl) 2016;233:2787–2797. doi: 10.1007/s00213-016-4321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Kindling in alcohol withdrawal. Alcohol Health Res World. 1998;22:25–33. [PMC free article] [PubMed] [Google Scholar]
- Benn A, Barker GR, Stuart SA, Roloff EV, Teschemacher AG, Warburton EC, Robinson ES. Optogenetic Stimulation of Prefrontal Glutamatergic Neurons Enhances Recognition Memory. J Neurosci. 2016;36:4930–4939. doi: 10.1523/JNEUROSCI.2933-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden-Jones H, McPhillips M, Rogers R, Hutton S, Joyce E. Risk-taking on tests sensitive to ventromedial prefrontal cortex dysfunction predicts early relapse in alcohol dependency: a pilot study. J Neuropsychiatry Clin Neurosci. 2005;17:417–420. doi: 10.1176/jnp.17.3.417. [DOI] [PubMed] [Google Scholar]
- Brady AM, Floresco SB. Operant procedures for assessing behavioral flexibility in rats. J Vis Exp. 2015:e52387. doi: 10.3791/52387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24:7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Liu A, Shi MY, Yan Z. Disrupted Glutamatergic Transmission in Prefrontal Cortex Contributes to Behavioral Abnormality in an Animal Model of ADHD. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Cope ZA, Parsegian A, Floresco SB, Aston-Jones G, See RE. Chronic methamphetamine self-administration alters cognitive flexibility in male rats. Psychopharmacology (Berl) 2016;233:2319–2327. doi: 10.1007/s00213-016-4283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav Pharmacol. 2008;19:225–234. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa E, Desmond JE, Anderson AK, Pfefferbaum A, Sullivan EV. The human basal forebrain integrates the old and the new. Neuron. 2004;41:825–837. doi: 10.1016/s0896-6273(04)00080-7. [DOI] [PubMed] [Google Scholar]
- DeSteno DA, Schmauss C. Induction of early growth response gene 2 expression in the forebrain of mice performing an attention-set-shifting task. Neuroscience. 2008;152:417–428. doi: 10.1016/j.neuroscience.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Crombag HS, Stephens DN. Experimental medicine in drug addiction: towards behavioral, cognitive and neurobiological biomarkers. J Psychopharmacol. 2011;25:1235–1255. doi: 10.1177/0269881110388324. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cogn Sci. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Gass JT, Chandler LJ. The Plasticity of Extinction: Contribution of the Prefrontal Cortex in Treating Addiction through Inhibitory Learning. Front Psychiatry. 2013;4:46. doi: 10.3389/fpsyt.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Glen WB, Jr, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, Yaxley R, Floresco SB, Chandler LJ. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology. 2014a;39:2570–2583. doi: 10.1038/npp.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Trantham-Davidson H, Kassab AS, Glen WB, Jr, Olive MF, Chandler LJ. Enhancement of extinction learning attenuates ethanol-seeking behavior and alters plasticity in the prefrontal cortex. J Neurosci. 2014b;34:7562–7574. doi: 10.1523/JNEUROSCI.5616-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci. 2008 Jul;Chapter 9(Unit 9.29) doi: 10.1002/0471142301.ns0929s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino TF, Maren S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Front Behav Neurosci. 2015;9:298. doi: 10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Taylor JR. Going and stopping: dichotomies in behavioral control by the prefrontal cortex. Nat Neurosci. 2016;19:656–664. doi: 10.1038/nn.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Galis-de Graaf Y, Smeets WJ. Integration and segregation of limbic cortico-striatal loops at the thalamic level: an experimental tracing study in rats. J Chem Neuroanat. 1999;16:167–185. doi: 10.1016/s0891-0618(99)00009-5. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Harvey RC, Jordan CJ, Tassin DH, Moody KR, Dwoskin LP, Kantak KM. Performance on a strategy set shifting task during adolescence in a genetic model of attention deficit/hyperactivity disorder: methylphenidate vs. atomoxetine treatments. Behav Brain Res. 2013;244:38–47. doi: 10.1016/j.bbr.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia C, Buczynski MW, Natividad LA, Laredo SA, Avalos N, Parsons LH. Dysregulated Glycine Signaling Contributes to Increased Impulsivity during Protracted Alcohol Abstinence. J Neurosci. 2017;37:1853–1861. doi: 10.1523/JNEUROSCI.2466-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia C, Wiskerke J, Natividad LA, Polis IY, de Vries TJ, Pattij T, Parsons LH. Increased impulsivity in rats as a result of repeated cycles of alcohol intoxication and abstinence. Addict Biol. 2015;20:263–274. doi: 10.1111/adb.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7:e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G, Talpos J, Steckler T. Strain-dependent effects on acquisition and reversal of visual and spatial tasks in a rat touchscreen battery of cognition. Physiol Behav. 2015;144:26–36. doi: 10.1016/j.physbeh.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Lauzon NM, Ahmad T, Laviolette SR. Dopamine D4 receptor transmission in the prefrontal cortex controls the salience of emotional memory via modulation of calcium calmodulin-dependent kinase II. Cereb Cortex. 2012;22:2486–2494. doi: 10.1093/cercor/bhr326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, McGinnis MM, McCool BA. Chronic ethanol exposure increases voluntary home cage intake in adult male, but not female, Long-Evans rats. Pharmacol Biochem Behav. 2015;139:67–76. doi: 10.1016/j.pbb.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ER, Dalley JW, Robbins TW. Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology (Berl) 2005;179:99–107. doi: 10.1007/s00213-004-2068-3. [DOI] [PubMed] [Google Scholar]
- Navarro AI, Mandyam CD. Protracted abstinence from chronic ethanol exposure alters the structure of neurons and expression of oligodendrocytes and myelin in the medial prefrontal cortex. Neuroscience. 2015;293:35–44. doi: 10.1016/j.neuroscience.2015.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Parikh V, Cole RD, Patel PJ, Poole RL, Gould TJ. Cognitive control deficits during mecamylamine-precipitated withdrawal in mice: Possible links to frontostriatal BDNF imbalance. Neurobiol Learn Mem. 2016;128:110–116. doi: 10.1016/j.nlm.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons OA. Neurocognitive deficits in alcoholics and social drinkers: a continuum? Alcohol Clin Exp Res. 1998;22:954–961. [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, Vendruscolo JC, Koob GF, Vendruscolo LF. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav. 2017;152:61–67. doi: 10.1016/j.pbb.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Harker KT, Douglas RM, Whishaw IQ. Variation in visual acuity within pigmented, and between pigmented and albino rat strains. Behav Brain Res. 2002;136:339–348. doi: 10.1016/s0166-4328(02)00126-2. [DOI] [PubMed] [Google Scholar]
- Prusky GT, West PW, Douglas RM. Reduced visual acuity impairs place but not cued learning in the Morris water task. Behav Brain Res. 2000;116:135–140. doi: 10.1016/s0166-4328(00)00267-9. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich EL, Shapiro ML. Prelimbic/infralimbic inactivation impairs memory for multiple task switches, but not flexible selection of familiar tasks. J Neurosci. 2007;27:4747–4755. doi: 10.1523/JNEUROSCI.0369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Ruwe WD, Bauce L, Flemons WW, Veale WL, Pittman QJ. Alcohol dependence and withdrawal in the rat. An effective means of induction and assessment. J Pharmacol Methods. 1986;15:225–234. doi: 10.1016/0160-5402(86)90052-5. [DOI] [PubMed] [Google Scholar]
- Salling MC, Faccidomo SP, Li C, Psilos K, Galunas C, Spanos M, Agoglia AE, Kash TL, Hodge CW. Moderate Alcohol Drinking and the Amygdala Proteome: Identification and Validation of Calcium/Calmodulin Dependent Kinase II and AMPA Receptor Activity as Novel Molecular Mechanisms of the Positive Reinforcing Effects of Alcohol. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza M, Lisman J. The CaMKII/NMDAR complex as a molecular memory. Mol Brain. 2013;6:10. doi: 10.1186/1756-6606-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Sinha R. Neural Correlates and Connectivity Underlying Stress-Related Impulse Control Difficulties in Alcoholism. Alcohol Clin Exp Res. 2016;40:1884–1894. doi: 10.1111/acer.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE. Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology. 2010;35:1453–1463. doi: 10.1038/npp.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling TR, Derkach VA. Postsynaptic protein phosphorylation and LTP. Trends Neurosci. 2000;23:75–80. doi: 10.1016/s0166-2236(99)01490-3. [DOI] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2013;18:203–213. doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW, Floresco SB, Chandler LJ. Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. J Neurosci. 2014;34:3706–3718. doi: 10.1523/JNEUROSCI.0623-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AA, Brown RE. Visual detection, pattern discrimination and visual acuity in 14 strains of mice. Genes Brain Behav. 2006;5:389–403. doi: 10.1111/j.1601-183X.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- Zhao B, Wang Y, Li Y, Qiao X, Yan P, Zhu Y, Lai J. Differential phosphorylation of NMDAR1-CaMKII-MAPKs in the rat nucleus accumbens following chronic ethanol exposure. Neurosci Lett. 2015;597:60–65. doi: 10.1016/j.neulet.2015.03.061. [DOI] [PubMed] [Google Scholar]


