Abstract
Background
Very poorly controlled (VPC) asthma in children is associated with ongoing acute exacerbations but factors associated with VPC are understudied.
Objective
To examine risk factors associated with VPC asthma in urban minority children.
Methods
This descriptive study examined asthma control levels (Well-Controlled (WC); Not Well-Controlled (NWC) and VPC) at baseline and 6 months in children participating in an ongoing randomized controlled trial of an Emergency Department (ED)/home environmental control intervention. Data collection occurred during the index ED visit and included allergen-specific IgE and salivary cotinine testing and caregiver interview of sociodemographic and child health characteristics. Follow-up data was collected at 6 months. Unadjusted analyses examined association of sociodemographic and health characteristics by level of asthma control. Multivariate analysis tested significant factors associated with VPC asthma at 6 months.
Results
At baseline most children were categorized with VPC asthma (WC: 0%, NWC: 47%, VPC: 53%) and rates of VPC minimally improved at 6 months (WC: 13%, NWC: 41%, VPC: 46%). Risk for VPC asthma was twice as likely in children with allergic rhinitis (OR 2.42), having ≥ 2 PCP asthma visits within the past 3 months (OR 2.77), or caregiver worry about medication side effects (OR 2.13) and three to four times more likely when asthma control was assessed during the fall or spring seasons (OR: Fall, 3.32; Spring, 4.14).
Conclusions
Improving asthma control in low-income, high risk children with VPC asthma requires treatment of co-morbidities, attention to caregiver medication beliefs and adept use of stepwise therapy.
Keywords: asthma control, very poorly controlled asthma, stepwise therapy, children
Introduction
Among the 8 million U.S. children with asthma, approximately 50% experience an acute asthma exacerbation each year(1) representing uncontrolled asthma.(2) Not only is uncontrolled asthma associated with increased emergency department (ED) visits(3) and unscheduled medical visits,(4) but it also places considerable burden on the child and family.(5) The primary goal of asthma management is to achieve well-controlled (WC) asthma. The National Asthma Education and Prevention Program (NAEPP) guidelines present recommendations to achieve WC asthma based on frequency of symptoms and short acting β2 agonist use, child activity limitation, number of ED visits and hospitalizations.(2) Despite these guidelines, approximately 50% of adult and pediatric patients with asthma remain not well-controlled or poorly controlled.(6)
The heterogeneity of childhood asthma manifests with varied symptom profiles including age at onset, atopy, co-morbidities and response to therapy creating specific asthma phenotypes.(7) Recognizing these phenotypes can inform the specifics of treatment decisions such as stepwise therapy (altering the dosage of medication and/or adding other medications) to achieve well-controlled asthma. A “very poorly controlled asthma (VPC) phenotype” has been described,(8) however the risk factors associated with this phenotype and the characteristics of children more susceptible to VPC asthma are not well understood.(9)
Risk factors that may be related to VPC or Not Well-controlled (NWC) asthma include exposure to indoor allergens, respiratory infections and second hand smoke (SHS),(10) co-morbid allergic rhinitis (AR)(11) or eczema,(12) improper medication delivery device technique, poor adherence to medication, parental misperception of their child’s level of asthma control(13) and parental beliefs about asthma medications. In particular, second hand smoke (SHS) is associated with a dose-related increase in cysteinyl leukotriene production that triggers contractile and inflammatory airway responses.(14) Nasal secretions occurring with allergic rhinitis expose the lower respiratory tract to allergic and/or infectious secretions that release systemic mediators leading to airway inflammation.(15, 16) Poor adherence to or improper delivery of controller medication can lead to insufficient medication delivered to the airways.(17, 18) Caregiver stressors i.e., poverty, violence exposure, poor housing and low caregiver quality of life are associated with poor asthma control.(4, 5, 19, 20)
However, specific sociodemographic and clinical risk factors for children with VPC asthma are often not recognized by clinicians treating children with asthma.(3) The goals of this descriptive study were (1) to examine change in asthma control levels (WC, NWC or VPC asthma) over 6 months and (2) to explore factors associated with VPC asthma in urban minority children with persistent asthma and frequent asthma ED visits.
Methods
Design and study setting
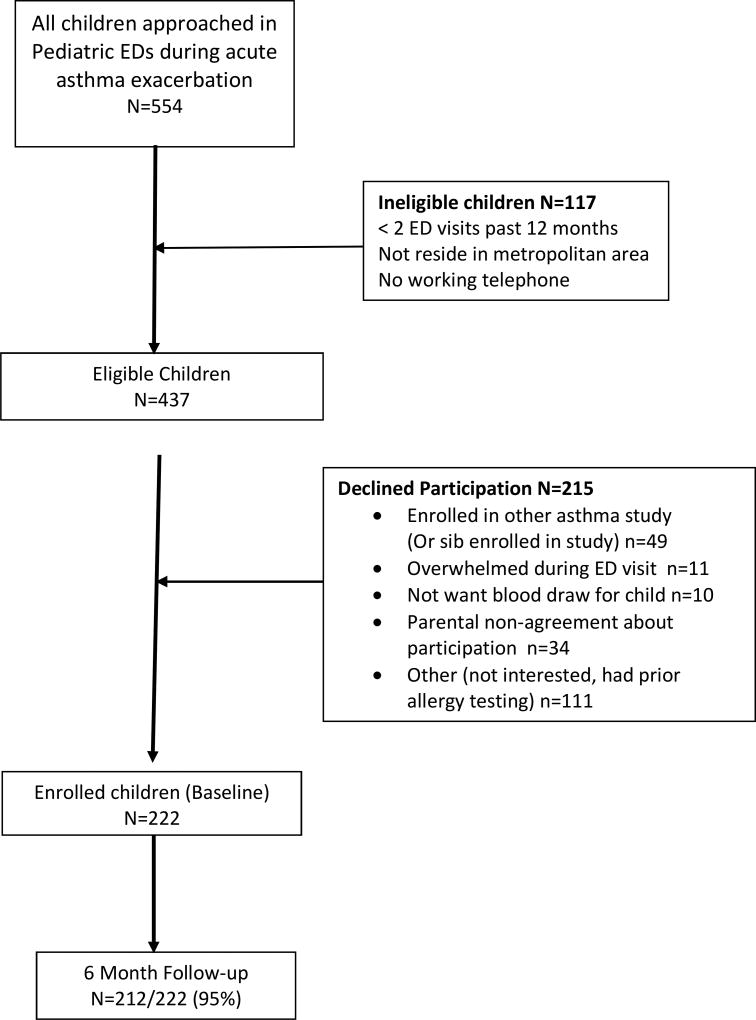
This descriptive study was a sub-analysis of data obtained from an ongoing randomized controlled trial testing the efficacy of an ED/home-based environmental control intervention in young inner-city children who had frequent ED visits for asthma.(21) Families of children aged 3–12 years were recruited and enrolled during an asthma ED visit from August 2013 through February 2016. Inclusion criteria were: physician diagnosed persistent and uncontrolled (NWC or VPC) asthma based on current national asthma guidelines,(2) having 2 or more ED asthma visits or ≥ 1 hospitalization over the past 12 months and residing in the Baltimore metropolitan area. Children were excluded if they had significant other non-asthma respiratory conditions, i.e., cystic fibrosis. The study is registered with Clinical Trials.gov (NCT01981564) and was approved by The Johns Hopkins Medical Institutional and the University of Maryland Institutional Review Boards. Written informed consent was obtained from each child’s primary caregiver/legal guardian and all children over age 8 years provided verbal assent to participate. Out of the 554 child/caregivers screened for study enrollment (Figure 1), 215 caregivers declined to participate and another 117 children were ineligible for enrollment, resulting in enrollment of 222 children into the study. Study attrition was minimal with 95% of participants retained at 6 months. During the index ED visit, all children received serum allergen-specific IgE serologic tests measured by fluorescent enzyme immunoassay (FEIA) to identify allergen sensitization to common environmental allergens and salivary cotinine measurement to screen for exposure to second hand smoke (SHS). Baseline and 6 month surveys were administered by a trained research assistant using the REDCap© web based application. Families received $30.00 for completing each survey.
Figure 1.
Recruitment and Retention Flow Diagram
Description of Interventions
Children assigned to the ED/home-based environmental control intervention received: (1) a medical follow-up visit in the ED and within 7 days of asthma index ED visit, (2) two home nurse visits for targeted environmental control education based on positive IgE results, (3) a brief motivational interview for caregivers of children with positive cotinine levels (> 1.0 ng/ml) to implement a total home smoking ban and (4) assistance scheduling follow-up asthma care with the child’s PCP. The control group children and their caregivers received three home nurse visits to provide asthma education regarding guideline-based medication use (rescue vs. controller medication), standard environmental trigger avoidance information, and assistance in scheduling a follow-up asthma visit with the child’s PCP.
Measures
Assessment of Asthma Severity and Asthma Control
Asthma severity was measured at baseline using the NAEPP guidelines.(2) Consistent with national guidelines, asthma control level was based on a caregiver report of several variables including (1) the number of symptom days over past 14 days and symptom nights over the past 30 nights, (2) short-acting β2-agonist (SABA) use over past two weeks for symptom control, (3) activity limitation over the past 7 days, (4) number of oral corticosteroid courses past year and (5) number of asthma ED visit or hospitalizations over the past 12 months.(2) These variables were included in an algorithm to categorize each child into one of three asthma control levels: WC, NWC or VPC. Asthma control was calculated at baseline and 6 months. Concurrently, caregivers rated their child’s asthma control over the past 4 weeks at baseline and 6 months as “controlled”, “not controlled” or “unsure” based on one item from The Asthma Control Test.(22)
Asthma Medication Use and Caregiver Medication Beliefs
All asthma medication names, doses and current use during the past two weeks for short acting β2 agonist (SABA), oral corticosteroid and controller medications were based on caregiver report at baseline and the 6 month follow-up. Pharmacy fill data was obtained from every pharmacy used based on caregiver’s report at six months. Oral corticosteroid fills were examined at the time of the ED index visit and up to 30 days after the visit. Amount of rescue and controller medication use was based on caregiver report of “The length of time a canister of rescue inhaler and controller inhaler will last” with the response categories of “< 1 month, 1–2 months, 3 or more months”. Caregiver concern over side effects of asthma medication was ascertained using a single item from the Pediatric Asthma Caregiver Quality of Life Questionnaire (PACQLQ) Scale (“How worried or concerned are you about your child’s asthma medications and side effects?”)(23) and children were categorized into two groups as “very, fairly, somewhat worried” versus “a little, hardly or not worried”.
Asthma Morbidity and Healthcare Utilization
The number of lifetime ICU asthma admissions, acute asthma ED visits and hospitalizations for asthma, primary care provider (PCP) visits for non-urgent asthma care over the past 3 months, receipt of any asthma specialty care over the past two years and type of health insurance were recorded based on caregiver report. Verification of the number of lifetime asthma ICU admissions and type of medical insurance was conducted by review of electronic medical record (EMR). High agreement was noted between caregiver report and EMR for number of ICU admissions (81% agreement) and type of health insurance (99% agreement).
Serologic allergen specific IgE test
Serum specific IgE testing, using serum collected during the index ED visit, was performed by a private commercial laboratory using the (ImmunoCap®) fluorescent enzyme immunoassay (FEIA) and measured specific IgE antibodies to ten common environmental allergens: mouse, cockroach, cat, dog, timothy grass, alternaria and aspergillus molds, oak tree, common ragweed and house dust mite. The degree of sensitization ranged from <0.35 to >100 kU/L of specific IgE to any of the allergens tested and results >0.35 kU/L were considered positive.
Cotinine Analysis for Second Hand Smoke (SHS) Exposure
Saliva samples were collected during the index ED asthma visit using a 3-cm cotton swab that was placed under the child’s tongue for 1 minute to absorb 1 ml of saliva (Salimetrics, State College, PA). Then the cotton roll was placed in a 2 ml vial and stored at −20° Centigrade prior to transport to the lab, then centrifuged and analyzed at the Johns Hopkins Institute for Clinical and Translational Research (ICTR) lab using Enzyme Immunoassay (EIA) analysis. The cotinine analysis serves as a biomarker of nicotine exposure level over the prior 24 hours. The lower limit of cotinine sensitivity was 0.05 ng/ml and average intra and inter-assay coefficients of variation were less than 5.8% and 7.9%, respectively. A cotinine cutoff level of 1.0 ng/ml was used to define positive SHS exposure based on prior assessment of SHS exposure among inner-city children with asthma.(24)
Statistical Analysis
Standard frequencies and means (SD) were used to describe sociodemographic and health characteristics of all children and caregivers at baseline and six months. Unadjusted analyses were conducted to examine bivariate relationships between select sociodemographic and health characteristics with level of asthma control (WC, NWC or VPC) using Chi-square tests. Odds ratios were calculated to highlight factors associated with VPC versus NWC/WC asthma control level. Multivariate regression models assessing the risk of VPC asthma were generated with covariates that were significant at p <0.10 in the unadjusted analyses. Pearson Chi-square was used to test for model goodness of fit. Odds ratios were based on GEE analyses with specification of binomial distribution. Analyses were conducted using SPSS Version 22 software.(25)
Results
Baseline Health and Sociodemographic Characteristics
Children were primarily male (65%), African American (93%) and Medicaid insured (94%) with a mean age of 6.4 (SD 2.7) years at baseline. (Table 1) Caregivers were the child’s biological mother (92%), single (74%), had a mean age of 31.3 (SD 7.5) years, were high school graduates or higher educated (80%), and poor based on a household income < $30,000 (61%). At baseline most children were categorized with moderate persistent (46%) or severe persistent (29%) asthma and the majority were categorized with VPC asthma (WC: 0%, NWC: 47%, and VPC: 53%). Asthma morbidity was high with a mean (SD) symptom days over the past 2 weeks at 5.9 (2.4) days, symptom nights over the past 4 weeks at 7.0 (2.6) nights. A prescription for oral corticosteroid medication was filled by almost half (49.5%, 105/212) of all children within 30 days after the index ED visit. Most children had the prescription filled within 3 days of their index ED visit (86%, 90/105).
Table 1.
Baseline patient characteristics
| Patient Characteristics (Baseline) | N=222 N (%) |
|
| |
| Child age | |
| 3–5 years | 104 (46.8) |
| 6–12 years | 118 (53.2) |
|
| |
| Gender: Female | 78 (35.1) |
|
| |
| Allergic Rhinitis Diagnosis (HX): Yes | 86 (38.7) |
|
| |
| Atopic: positive to one or more allergensa | 183 (82.4) |
|
| |
| Specialist care in past 2 years: Yes | 47 (21.2) |
|
| |
| ICU admission prior to baseline: Yes | 63 (28.4) |
|
| |
| Parent worried about medication side effects: Yes (very, fairly, or somewhat) | 84 (37.8) |
|
| |
| Asthma Severity at Baseline | N=221 |
| Mild Persistent | 56 (25.3) |
| Moderate Persistent | 101 (45.7) |
| Severe Persistent | 64 (29.0) |
|
| |
| Season of baseline visit: | |
|
| |
| Fall | 77 (34.7%) |
|
| |
| Winter | 57 (25.7%) |
|
| |
| Spring | 45 (20.2%) |
|
| |
| Summer | 43 (19.4%) |
Allergen sensitization results known in 185 patients
ED utilization was high with mean (SD) asthma ED visits in past 3 months at 1.3 (1.1) excluding the index enrollment ED visit. Over one-quarter of children (28%) had a prior lifetime ICU asthma admission and this was significantly higher in VPC children (WC: 13%, NWC: 38%, VPC: 59%; p=0.01). Atopy was prominent with 83% children testing positive to one or more allergens and 39% reporting allergic rhinitis. Based on the available de-identified data for children screened for study participation, no significant differences between enrolled and non-enrolled children were noted for child age, race/ethnicity or neighborhood zip codes.
Change in Level of Asthma Control from Baseline to 6 months
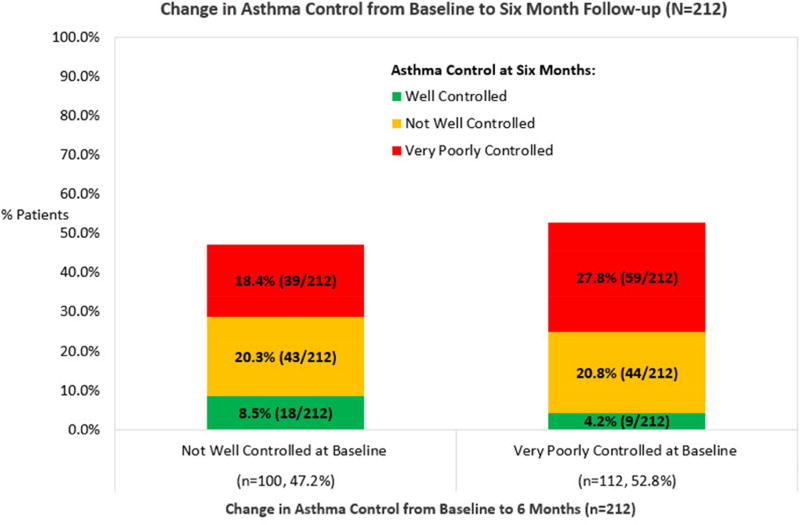
At 6 months asthma control slightly improved (WC: 12.7%, NWC: 41.1% and VPC: 46.2%). As shown in Figure 2, most NWC children remained with NWC asthma (20.3%) and almost one out of five children deteriorated to VPC (18.4%). Only 8.5% of NWC children improved to WC asthma. Further, most children with VPC asthma at baseline remained at VPC over the 6 months (28%) and very few children improved from VPC to WC (4.2%). Overall, very few children achieved WC asthma at 6 months.
Figure 2.
Change in Asthma Control from Baseline to Six Month Follow-up (N=212).
*At baseline, no child participant was categorized with Well-controlled asthma.
Factors Associated with Asthma Control at 6 months
Several factors were significantly associated with the level of asthma control at 6 months in the unadjusted analysis. (Table 2) Regarding medication use for asthma, almost 58% of VPC children reported any albuterol use within the past two weeks (WC: 0%, NWC: 42%, VPC: 58%, p<0.001). The proportion of caregivers reporting their child’s rescue medication inhaler lasting <1 month was significantly higher in the VPC group (WC: 3%, NWC: 30%, VPC: 67%; p<0.001). However, the length of time for a controller medication inhaler to last (< 2 months vs. 3 or more months) did not differ by level of asthma control. Caregiver medication and asthma control beliefs were different with caregivers of children with VPC asthma significantly more worried about asthma medication side effects than other groups (WC: 4%; NWC: 39%, VPC: 57%, p=.009). Interestingly, nearly 40% of caregivers of children with VPC asthma described their child’s asthma as well-controlled over the past 4 weeks. Most (73%) of these VPC children reported controller medication use over the past two weeks and over half reported albuterol use over the same time period. Correlation between caregiver rating of asthma control and NAEPP asthma control algorithm was low at 6 months (Spearman r=0.22). Reported allergic rhinitis was more common in the VPC children (WC: 8%; NWC: 37%; VPC: 55%, p=0.002). Finally, children with VPC asthma were more likely to be followed up and assessed for asthma control during the fall and spring seasons at 6 months (VPC: fall 59%, winter: 33%, spring: 59%, summer: 29%; p=0.008).
Table 2.
Factors Associated with Level of Asthma Control at 6 months (Unadjusted) (N=212)
| Characteristic | Well Controlled N=27 (12.7%) |
Not Well Controlled N=87 (41.1%) |
Very Poorly Controlled N=98 (46.2%) |
Total N=212 (100%) |
Statistic |
|---|---|---|---|---|---|
|
| |||||
| Asthma Medication Use | |||||
|
| |||||
| Use rescue medication past 2 weeks? | X2 =60.11, df=2, p<0.001 | ||||
| YES | 0 (0.0) | 58 (42.3) | 79 (57.7) | 137 (100) | |
| NO | 26 (35.6) | 29 (39.7) | 18 (24.7) | 73 (100) | |
|
| |||||
| Duration of time rescue inhaler lasts? | X2 =24.35, df=4, p< 0.001 | ||||
| < 1 month | 2(3.0) | 20 (30.3) | 44 (66.7) | 66 (100) | |
| 1–2 months | 10 (12.8) | 38 (48.7) | 30 (38.5) | 78 (100) | |
| 3 or more months | 15 (25.4) | 25 (42.4) | 19 (32.2) | 59 (100) | |
|
| |||||
| Length of time controller inhaler lasts?a | X2 =3.17, df=2, P=0.21 | ||||
| < 2 month | 14 (9.1) | 65 (42.2) | 75 (48.7) | 144 (100) | |
| 3 or more months | 4 (20.0) | 11 (44.0) | 9 (36.0) | 31 (100) | |
|
| |||||
| Caregiver Asthma Medication and Asthma Control Status Beliefs | |||||
|
| |||||
| Worried about side effects of medication | X2 = 9.47, df=2, p=0.009 | ||||
| Minimal worry | 24 (17.6) | 57 (41.9) | 55 (40.4) | 136 (100) | |
| Worriedb | 3 (4.2) | 28 (38.9) | 41 (56.9) | 72 (100) | |
|
| |||||
| Parental belief asthma was well-controlled past 4 weeks | X2 =8.90 df=2, p=0.01 | ||||
| YES | 23 (15.4) | 67 (45.0) | 59 (39.6) | 149 (100) | |
| NO | 4 (6.9) | 18 (31.0) | 36 (62.1) | 58 (100) | |
|
| |||||
| Allergen Sensitization, Co-morbid Allergic Rhinitis and Seasonality of Interviews | |||||
|
| |||||
| Mouse | X2 =0.91 df=2, p=0.63 | ||||
| Negative | 9 (10.8) | 34 (41.0) | 40 (48.2) | 83 (100) | |
| Positive | 15 (15.6) | 36 (37.5) | 45 (46.9) | 96 (100) | |
|
| |||||
| Cockroach | X2 =0.97, df=2, p=0.62 | ||||
| Negative | 12 (11.2) | 43 (40.2) | 52 (48.6) | 107 (100) | |
| Positive | 12 (16.0) | 30 (40.0) | 33 (44.0) | 75 (100) | |
|
| |||||
| Timothy Grass | X2 =0.32, df=2, p=0.85 | ||||
| Negative | 14 (13.6) | 43 (41.7) | 46 (44.7) | 103 (100) | |
| Positive | 10 (12,2) | 32 (39.0) | 40 (48.8) | 82 (100) | |
|
| |||||
| Oak Tree | X2 =0.60, df=2, p=0.74 | ||||
| Negative | 14 (13.9) | 42 (41.6) | 45 (44.6) | 101 (100) | |
| Positive | 9 (10.8) | 33 (39.8) | 41 (49.4) | 83 (100) | |
|
| |||||
| Ragweed | X2 =2.09, df=2, p=0.35 | ||||
| Negative | 18 (15.8) | 46 (40.4) | 50 (43.9) | 114 (100) | |
| Positive | 6 (8.7) | 28 (40.6) | 35 (50.7) | 69 (100) | |
|
| |||||
| Positive Allergen Tests | X2 =6.43, df=6, p=0.38 | ||||
| None | 6 (18.2) | 16 (48.5) | 11 (33.3) | 33 (100) | |
| 1–2 | 3 (10.3) | 8 (27.6) | 18 (62.1) | 29 (100) | |
| 3–4 | 3 (8.3) | 17 (47.2) | 16 (44.4) | 36 (100) | |
| >=5 | 12 (13.8) | 34 (39.1) | 41 (47.1) | 87 (100) | |
|
| |||||
| Allergic Rhinitis (history) | X2 =12.57, df=2, p=0.002 | ||||
| Yes | 11 (8.3) | 49 (37.1) | 72 (54.5) | 132 (100) | |
| No | 16 (20.3) | 38 (48.1) | 25 (31.6) | 79 (100) | |
|
| |||||
| Season at 6 month Interview | X2 =17.35 df=6, p=0.008 | ||||
| Fall | 4 (9.8) | 13 (31.7) | 24 (58.5) | 41 (100) | |
| Winter | 6 (15.0) | 21 (52.5) | 13 (32.5) | 40 (100) | |
| Spring | 7 (9.2) | 24 (31.6) | 45 (59.2) | 76 (100) | |
| Summer | 10 (18.2) | 29 (52.7) | 16 (29.1) | 55 (100) | |
|
| |||||
| Health Care Utilization Characteristics | |||||
|
| |||||
| Asthma Specialist | X2 =2.04, df=2, p=0.36 | ||||
| YES | 4 (7.5) | 25 (47.2) | 24 (45.3) | 53 (100) | |
| NO | 22 (14.0) | 61 (38.9) | 74 (47.1) | 157 (100) | |
|
| |||||
| ICU asthma admission lifetime (Verified EHR) | X2 =8.86, df=2, p=0.01 | ||||
| YES | 2 (13.2) | 22 (37.9) | 34 (58.6) | 58 (100) | |
| NO | 25 (17.0) | 62 (42.2) | 60 (40.8) | 147 (100) | |
|
| |||||
| Number of PCP visits for non-urgent asthma care past 3 months? | Kruskal-Wallis test Mean Rank p=0.003 | ||||
| Mean (SD) | 0.44 (0.58) | 0.85 (0.9) | 1.26 (1.3) | 0.99 (1.1) | |
|
| |||||
| Second Hand Smoke Exposure Characteristics | |||||
|
| |||||
| Home Smoking Ban Restrictions | X2 =.91, df=2, p=0.63 | ||||
| Partial/No restrict | 7 (15.9) | 19 (43.2) | 18 (40.9) | 44 (100) | |
| Total Ban | 19 (11.9) | 64 (40.0) | 77 (48.1) | 160 (100) | |
|
| |||||
| Cotinine Status at 6 months | X2 = 0.76, df=2, p=0.69 | ||||
| Negative | 11 (13.8) | 35 (43.8) | 34 (42.5) | 80 (100) | |
| Positive | 15 (12.8) | 45 (38.5) | 57 (48.7) | 117 (100) | |
|
| |||||
| Cotinine | F=0.41, df=2, p=0.67 | ||||
| Mean (SD) | 4.46 (7.5) | 3.50 (6.9) | 3.23 (5.0) | 3.50 (6.1) | |
Flovent, Advair, Pulmicort, Qvar
Minimal worry = a little, hardly worried, not worried; Worried = very, fairly or somewhat worried.
Non-urgent health care utilization also differed by asthma control level with mean number of PCP visits for non-urgent asthma care over past three months significantly higher in the VPC group (mean (SD) for PCP visits: WC: 0.44 (0.58), NWC: 0.85 (0.9); VPC: 1.26 (1.3); Kruskal-Wallis test p=0.003). In contrast, receipt of asthma specialty care, positive allergen sensitization and cotinine results, and indoor exposure to mice and/or cockroach did not differ by asthma control level.
Factors Associated with VPC Asthma
As seen in Table 3, risk for VPC versus NWC or WC asthma was more than two times as likely in children with a history of allergic rhinitis (OR: 2.59, 95% CI: 1.44, 4.65), three times more likely with report two or more PCP visits for non-urgent asthma care within the past 3 months (OR:3.25, 95%CI:1.6, 6.8), and almost twice as likely in children whose caregiver reported being worried about medication side effects (OR 1.95, 95%CI: 1.1–3.5). Children with VPC asthma were also three times more likely to be assessed for asthma control during the fall (OR 3.17, 95% CI: 1.4, 7.2) or spring (OR 3.54, 95% CI: 1.7, 7.4) seasons calculated at six months forward from baseline. Child age, gender, atopic status, mean number of positive allergen sensitization tests, prior ICU admission and duration of controller medication inhaler lasting did not significantly influence the odds of having VPC versus NWC/WC asthma. As seen in Table 4, in the final model that adjusted for all significant factors, increased odds of VPC asthma was associated with allergic rhinitis, having ≥ 2 PCP non-urgent asthma visits, caregiver worry about medication side effects and asthma control follow-up assessment in the fall and spring seasons. Notably, VPC children were three times more likely to have 2 or more PCP visits for non-urgent asthma and have allergic rhinitis and four times more likely to be followed-up and assessed in the spring season than NWC/WC children. The Pearson Chi-square value for the final model was 1.027, indicating goodness of fit.
Table 3.
Odds Ratios for Factors Associated with Very Poorly Controlled Asthma at 6 months, unadjusted.
| Asthma Control Status at Six Months | Outcome: Asthma VPC | ||
|---|---|---|---|
| NWC or WC N=114 (53.8%) |
VPC N=98 (46.2%) |
OR (95% CI), p-valuea | |
| Child age @ baseline: <6 years | 44.6 | 50.5% | 1.27 (0.74–2.17), p=0.397 |
| Child Gender: Female | 34.5% | 37.1% | 1.12 (0.64–1.96), p=0.695 |
| Atopic: + >=1 allergen test | 77.8% | 87.2% | 1.95 (0.88–4.29), p=0.098 |
| # + allergen tests, mean (SD) | 4.4 (3.4) | 4.6 (3.3) | 1.02 (0.94–1.11), p=0.649 |
| Allergic Rhinitis: YesҰ | 52.6% | 74.2% | 2.59 (1.44–4.65), p=0.001 |
| # PCP visits past 3 monthsҰ: | P=.004 | ||
| None | 45.6% | 33.7% | Reference |
| One | 40.4% | 32.7% | 1.10 (0.59–2.05), p=0.774 |
| Two or more | 14.0% | 33.7% | 3.25 (1.55–6.81), p=0.002 |
| ICS use (past 2 weeks) parent report: | P=.911 | ||
| None | 23.4% | 24.5% | Reference |
| Monotherapy | 39.6% | 41.5% | 1.00 (0.49–2.03), p=0.996 |
| Combination Therapy | 36.9% | 34.0% | 0.88 (0.43–1.83), p=0.736 |
| How long a controller medication inhaler lasts? | P=.449 | ||
| Less than 1 month | 55.8% | 63.1% | 1.78 (0.72–4.38), p=0.211 |
| 1–2 months | 27.4% | 26.2% | 1.50 (0.56–4.07), p=0.421 |
| >=3 months | 16.8% | 10.7% | Reference |
| Caregiver worried about medication side effects (Yes)Ұ: | 27.7% | 42.7% | 1.95 (1.10–3.47), p=0.024 |
| Season of enrollmentҰ: | P=.041 | ||
| Fall | 27.0% | 46.8% | 2.93 (1.30–6.60), p=0.009 |
| Winter | 35.1% | 14.9% | 0.72 (0.29–1.77), p=0.472 |
| Spring | 14.4% | 24.5% | 2.88 (1.14–7.23), p=0.025 |
| Summer | 23.4% | 13.8% | Reference |
| Season of six month follow-upҰ: | P=.001 | ||
| Fall | 17.7% | 26.8% | 3.17 (1.39–7.22), p=0.006 |
| Winter | 20.4% | 10.3% | 1.06 (0.41–2.72), p=0.904 |
| Spring | 27.4% | 46.4% | 3.54 (1.69–7.42), p=0.001 |
| Summer | 34.5% | 16.5% | Reference |
P<.05, distributional difference between VPC patients and those NWC or WC significant based on Chi-square test for categorical factors and independent t-test for continuous factors. OR=Odds ratio based on GEE with binomial distribution specified (continuous variables ~ odds of very poorly controlled disease per one unit increase)
Table 4.
Odds Ratios for Factors Associated with Very Poorly Controlled Asthma compared to Not Well-Controlled or Well-Controlled at 6 months, adjusted for all factors listed in table.
| OR for VPC Asthma | |
|---|---|
| OR (95% CI), p-valuea | |
| Allergic Rhinitis (Y vs. N) | 2.93 (1.39–6.24), p=0.005 |
| Atopic (Y vs. N) | 1.99 (0.91–4.36), p=0.086 |
| Routine PCP Visits: (>=2 vs. <2) | 3.24 (1.48–7.09), p=0.003 |
| Caregiver worried about medication side effects (Y vs. N) | 2.13 (1.07–4.24), p=0.032 |
| Season (p=.001): | |
| Fall | 3.17 (1.15–8.32), p=0.026 |
| Winter | 1.00 (0.33–3.07), p=0.996 |
| Spring | 4.73 (1.89–11.85), p=.001 |
| Summer | Reference |
Discussion
In a group of young urban minority children with persistent asthma and high ED utilization, almost half (46%) had persistent VPC asthma over 6 months. The lack of improvement in asthma control confirms the challenge in managing uncontrolled asthma in urban minority children without individualizing treatment for each child. Personalized management should include identifying biomarkers of allergen sensitization and environmental exposures, and assessing allergic co-morbidities, as performed in our study, to inform use of stepwise or novel therapies.(8) However, the personalized environmental control behavioral intervention utilized in this study was not associated with any significant improvement in asthma control or reduction in health care utilization at six months for the VPC children assigned to the intervention group when compared to the VPC control group. Implementation of stepwise or novel therapies may be inconsistently applied in the acute or primary care settings, as suggested in our data, since children with VPC asthma had a significantly higher number of non-urgent asthma PCP visits than NWC or WC children over 6 months. These missed opportunities by acute and PCP clinicians to adjust medications, provide education about environmental control and symptom recognition, indicates that increased healthcare utilization does not translate into well-controlled asthma(26) nor prevent future asthma exacerbations. Children receiving specialty asthma care are more likely to be prescribed appropriate controller medication and a higher level of therapy (e.g., combination therapy versus monotherapy).(21, 27) Yet, less than one-quarter of the VPC children had received specialty care. Achieving well-controlled asthma requires that clinicians become adept in stepwise therapy, medication adherence and medication device technique,(9) and commit sufficient time for patient counseling required to implement step-up therapy as delivered in asthma specialty care.(9, 27) Our findings indicate that maintaining step-up therapy during fall and spring seasons is especially critical for children with VPC asthma and premature step-down may lead to poor asthma control. Underlying atopy in our group of children with VPC asthma may have been undertreated or undermanaged leading to continued asthma symptoms.
Our study rate of allergic rhinitis (39%) is significantly lower than the rate of rhinitis symptoms noted in an earlier study of young children with persistent asthma (98%)(11) and was much lower than our rate of atopy measured by serum IgE. This may be due to caregiver recall bias or lack of recognition of the symptoms of allergy by the caregiver or clinician. This discordance is important to explore since asthma and allergic rhinitis have interrelated inflammatory processes in the upper and lower respiratory tract.(16,28,29) Increased nasal secretions associated with allergic rhinitis may result in release of inflammatory mediators locally and systemically as well as secretions that can be aspirated into the lower airway. Additionally, nasal turbinate edema can result in direct inhalation of irritants, allergens and cold air; all potentially causing bronchoconstriction.(15, 16) These factors may explain the high association between allergic rhinitis and VPC asthma noted in our study.
Caregiver worry about the side effects of anti-inflammatory medications is common and has been shown to be associated with poorly controlled asthma.21, 30–32) Steroid phobia is often more prevalent for inhaled steroids than oral corticosteroids. Yet, only half of caregivers filled a prescription for oral corticosteroids after the child’s index ED visit. Caregiver belief that a short course of oral corticosteroids results in less steroid exposure and side effects than the use of daily anti-inflammatory medications is unsubstantiated and may lead to poor adherence to inhaled anti-inflammatory medications.(5) However, actual comparison of a daily, low dose inhaled Fluticasone lung exposure for a full year (i.e., 44 mcg at 2 puffs twice a day for 1 year = 64.24 mg) provides significantly less corticosteroid exposure than a five-day course of 60 mg prednisone systemic exposure (Prednisone 60 mg daily × 5 days = 60 mg * 1000 mcg/mg * 5 = 300,000 micrograms = 300 mg). Moreover, systemic exposure to oral corticosteroid medication is substantial considering the estimate that only 10–15% of the inhaled dose of medication actually is delivered into the lungs even with the best inhaler technique including use of a spacer. This points out an area where further parent and patient education might be very useful in boosting adherence to daily controller medication.
Significant discordance was noted between caregiver perceptions of asthma control versus national guideline criteria for well-controlled asthma. Over three-quarters (84%) of caregivers with children categorized with NWC or VPC asthma reported their child’s asthma was “well-controlled”. Most of the VPC children who were perceived to have well controlled asthma reported recent use of controller and rescue medication. It is unclear if this misperception of well controlled asthma was due to (1) caregiver belief that medication use equated to well controlled asthma or (2) lack of caregiver or child symptom recognition. Yoos (1999) reported similar discordance between patient-perceived and physician assessed asthma control based on symptom recognition.(33) Misperception of asthma control is critical for clinicians to address with patients and caregivers, since underestimation of asthma symptoms often results in inadequate step-up therapy(13, 34) and may lead to acute exacerbations and ED visits.
There are potential limitations associated with this study. History of allergic rhinitis in the child was based on parental report and may be subject to recall bias or a poor estimation of symptoms. Actual dates of seasons were used to calculate season of enrollment, i.e. fall season defined as 9/21 to 12/21 and some misclassification of season may have occurred. However, in all but four participants, the season at the six month follow-up was two seasons ahead (e.g. 74 patients who enrolled in the fall season had their six month follow-up in the spring). Further, generalizability of the results is limited in that we purposely recruited children with more severe or uncontrolled asthma for our study in order to maximize our ability to detect a difference in asthma morbidity and healthcare utilization with our intervention. Thus, our sample of inner-city minority children may represent a higher percentage of very poorly controlled asthma patients than the general pediatric asthma population. Despite these limitations, a major strength this study is the characterization of factors associated with VPC asthma in a very high risk subgroup of children with frequent asthma ED visits.
In conclusion, in a group of young urban minority children with VPC asthma and high ED utilization, most remained poorly controlled after 6 months. Achieving well-controlled asthma in high risk children with VPC asthma requires treatment of co-morbidities, attention to caregiver medication and symptom recognition beliefs and adept use of stepwise therapy. Despite visits to primary care providers for asthma follow-up care, children remained uncontrolled and reported non-guideline based care. Allowing time during ED and primary care asthma visits to efficiently adjust medication, reinforce education about environmental control and symptom recognition may improve asthma control in high risk children.
Highlights Box.
What is already known about this topic?
Very poorly controlled (VPC) asthma is associated with a higher risk of future asthma exacerbations and increased healthcare utilization. However, specific sociodemographic and health characteristics have been understudied as risk factors of VPC asthma.
What does this article add to our knowledge?
Although children with VPC asthma may have increased acute and primary healthcare visits, this increased utilization does not translate into well-controlled asthma. Identifying and treating allergic co-morbidities, addressing caregiver medication beliefs and adept use of stepwise therapy are all indicated for treating children with VPC asthma.
How does this study impact current management guidelines?
Identifying allergen sensitization and environmental exposures informs treatment decisions including stepwise therapy. Stepwise therapy may be underutilized in the acute and primary care settings and should be implemented for children with atopic asthma. Timing of step-up and step-down therapy is complex in managing children with VPC asthma.
Acknowledgments
This work was supported by the National Institute of Nursing Research, NIH (NR013486). The study is registered with ClinicalTrials.gov with number NCT01981564. This publication was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR) which is funded in part by Grant Number UL1 TR 000424-06 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS or NIH. We appreciate Mary Gates, Amanda Manning, and Cheyenne McCray for the collection of the data and the families for their participation in the study.
Abbreviations
- WC
Well-controlled
- NWC
Not well-controlled
- VPC
Very poorly controlled
- PCP
Primary care provider
- AR
Allergic Rhinitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States. 1980–2007. Pediatrics. 2009;123(Suppl 3):S131–145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 2.USDHHS. The National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR3): Guidelines for the Diagnosis and Management of Asthma. 2007 NIH Publication No. 07-4051. [Google Scholar]
- 3.Haselkorn T, Fish JE, Zeiger RS, Szefler SJ, Miller DP, Chipps BE, et al. Consistently very poorly controlled asthma, as defined by the impairment domain of the Expert Panel Report 3 guidelines, increases risk for future severe asthma exacerbations in the Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol. 2009;124:895–902. doi: 10.1016/j.jaci.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 4.Guilbert TW, Garris C, Jhingran P, Bonafede M, Tomaszewski KJ, Bonus T, et al. Asthma that is not well controlled is associated with increased healthcare utilization and decreased quality of life. J Asthma. 2011;48:126–132. doi: 10.3109/02770903.2010.535879. [DOI] [PubMed] [Google Scholar]
- 5.Bellin MH, Land C, Newsome A, Kub J, Mudd SS, Bollinger ME, et al. Caregiver perception of asthma management of children in the context of poverty. J Asthma. 2017;54(2):162–172. doi: 10.1080/02770903.2016.1198375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chipps BE, Zeiger RS, Dorenbaum A, Borish L, Wenzel SE, Miller DP, et al. TENOR Study Group. Assessment of asthma control and asthma exacerbations in the epidemiology and natural history of asthma, outcomes and treatment regimens (TENOR) observation cohort. Curr Respir Care Rep. 2012;1(4):259–269. doi: 10.1007/s13665-012-0025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schatz M, Hsu JY, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2014;133:1549–1556. doi: 10.1016/j.jaci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Opina MT, Moore WC. Phenotype-driven therapeutics in severe asthma. Curr Allergy Asthma Rep. 2017;17:10–23. doi: 10.1007/s11882-017-0678-1. [DOI] [PubMed] [Google Scholar]
- 9.Chipps BE, Corren J, Israel E, Katial R, Lang DM, Panettieri RA, Jr, et al. Asthma Yardstick: Practical recommendations for a sustained step-up in asthma therapy for poorly controlled asthma. Ann Allergy Asthma Immunol. 2017;118:133–142. doi: 10.1016/j.anai.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Lang JE, Dozor AJ, Holbrook JT, Mougey E, Krishnan S, Sweeten S, et al. Biologic mechanisms of environmental tobacco smoke in children with poorly controlled asthma: results from a multicenter clinical trial. J Allergy Clin Immunol: In Practice. 2013;1(2):172–180. doi: 10.1016/j.jaip.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esteban CA, Klein RB, Kopel SJ, McQuaid EL, Fritz GK, Seifer R, et al. Underdiagnosed and undertreated allergic rhinitis in urban school-aged children with asthma. Ped Allergy, Immunol and Pulmonology. 2014;27(2):75–81. doi: 10.1089/ped.2014.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Magalhaes Simoes S, DaCunha SS, Cruz AA. A community study of factors related to poorly controlled asthma among Brazilian urban children. Plos One. 2012;7:e37050. doi: 10.1371/journal.pone.0037050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammer SC, Robroeks CM, van Rij C, Heynens J, Droog R, Jobsis Q, et al. Actual asthma control in a paediatric outpatient clinic population: Do patients perceive their actual level of control? Pediatr Allergy Immunol. 2008;19:626–633. doi: 10.1111/j.1399-3038.2007.00705.x. [DOI] [PubMed] [Google Scholar]
- 14.Fauler J, Frolich JC. Cigarette smoking stimulates cysteinyl leukotriene production in man. Eur J Clin Invest. 1997;27(1):43–47. doi: 10.1046/j.1365-2362.1997.650619.x. [DOI] [PubMed] [Google Scholar]
- 15.Corren J. Allergic rhinitis and asthma: how important is the link? J Allergy Clin Immunol. 1997;99:S781–S786. doi: 10.1016/s0091-6749(97)70127-1. [DOI] [PubMed] [Google Scholar]
- 16.Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003;111:1171–1183. doi: 10.1067/mai.2003.1592. [DOI] [PubMed] [Google Scholar]
- 17.Feldman JM, Perez EA, Canino G. The role of caregiver major depression in the relationship between anxiety disorders and asthma attacks in Island Puerto Rican youth and young adults. J Nerv Ment Dis. 2011;199:313–318. doi: 10.1097/NMD.0b013e3182174e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim JH, Wood BL, Miller BD. Maternal depression and parenting in relation to child internalizing symptoms and asthma disease activity. J Fam Psychol. 2008;22:264–273. doi: 10.1037/0893-3200.22.2.264. [DOI] [PubMed] [Google Scholar]
- 19.Booster GD, Oland AA, Bender BG. Psychosocial factors in severe pediatric asthma. Immunol Allergy Clin North Am. 2016;36(3):449–460. doi: 10.1016/j.iac.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Bellin MH, Osteen P, Kub J, Bollinger ME, Tsoukleris M, Chaikind L, et al. Stress and Quality of life in urban caregivers of children with poorly controlled asthma: a longitudinal analysis. J Pediatr Health Care. 2015;29(6):536–546. doi: 10.1016/j.pedhc.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butz A, Morphew T, Lewis-Land C, Kub J, Bellin M, Ogborn J, et al. Factors associated with poor controller medication use in children with high asthma emergency department use. Ann Allergy Asthma Immunol. 2017;118(4):419–426. doi: 10.1016/j.anai.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juniper EF, Graffydd-Jones K, Ward S, Svenson K. Asthma Control Questionnaire in children: validation, measurement properties, interpretation. Eur Resp J. 2010;36(6):1410–1416. doi: 10.1183/09031936.00117509. [DOI] [PubMed] [Google Scholar]
- 23.Juniper EF, Guyatt GH, Feeny DH, Griffith LE, Townsend M. Measuring quality of life in the parents of children with asthma. Quality of Life Research. 1996;5:27–34. doi: 10.1007/BF00435966. [DOI] [PubMed] [Google Scholar]
- 24.McCarville M, Sohn M, Oh E, Weiss K, Gupta R. Environmental tobacco smoke and asthma exacerbations and severity: difference between measured and reported exposure. Arch Dis Chil. 2013;98:510–514. doi: 10.1136/archdischild-2012-303109. [DOI] [PubMed] [Google Scholar]
- 25.IBM Corp. IBM SPSS Statistics for Windows Version 22.0. Amonk, NY: IBM Corp; Released 2013. [Google Scholar]
- 26.Pines JM, Asplin BR, Kaji AH, Lowe RA, Magid DJ, Raven M, et al. Frequent users of emergency department services: gaps in knowledge and a proposed research agenda. Acad Emerg Med. 2011;18:e64–e69. doi: 10.1111/j.1553-2712.2011.01086.x. [DOI] [PubMed] [Google Scholar]
- 27.Zeiger RS, Schatz M, Qiaowu L, Zhang F, Purdum AS, Chen W. Step-up care improves impairment in uncontrolled asthma: Am administrative data study. Am J Manag Care. 2010;16(12):897–906. [PubMed] [Google Scholar]
- 28.Bousquet J, Gaugris S, Sazonov-Kocevar V, Zhang Q, Yin D, Polos P, et al. Increased risk of asthma attacks and emergency room visits among asthma patients with allergic rhinitis: a subgroup analysis of the improving asthma control trial. Clin Exp Allergy. 2005;35:723–727. doi: 10.1111/j.1365-2222.2005.02251.x. [DOI] [PubMed] [Google Scholar]
- 29.Valovirta E, Pawankar R. Survey on the impact of comorbid allergic rhinitis in patients with asthma. BMC Pulmonary Medicine. 2006;6(Suppl 1):S3, 1–10. doi: 10.1186/1471-2466-6-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheares BJ, Du Y, Vasquez TL, Mellins RB, Evans D. Use of written treatment plans for asthma by specialist physicians. Pediatr Pulmonol. 2007;42:348–356. doi: 10.1002/ppul.20586. [DOI] [PubMed] [Google Scholar]
- 31.Klok T, Lubbers S, Kaptein AA, Brand PL. Every parent tells a story: why non-adherence may persist in children receiving guideline-based comprehensive asthma care. J. Asthma. 2014;51:106–112. doi: 10.3109/02770903.2013.841191. [DOI] [PubMed] [Google Scholar]
- 32.Yilmaz O, Eroglu N, Ozalp D, Yuksel H. Beliefs about medications in asthmatic children presenting to emergency department and their parents. J Asthma. 2012;49:282–287. doi: 10.3109/02770903.2011.654021. [DOI] [PubMed] [Google Scholar]
- 33.Yoos HL, McMullen A. Symptom perception and evaluation in childhood asthma. Nurs Res. 1999;48:2–8. doi: 10.1097/00006199-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Holgate ST, Price D, Valovirta E. Asthma out of control? A structured review of recent patient surveys. BMC Pulm Med. 2006;6(Suppl 1):S2. doi: 10.1186/1471-2466-6-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]