Abstract
The ventromedial prefrontal cortex (vmPFC) has been implicated in a variety of social, cognitive, and affective functions that are commonly disrupted in mental illness. In this review, we summarize data from a diverse array of human and animal studies demonstrating that vmPFC is a key node of cortical and subcortical networks that subserve at least three broad domains of psychological function linked to psychopathology. One track of research indicates that vmPFC is critical for the representation of reward and value-based decision-making, through interactions with ventral striatum and amygdala. A second track of research demonstrates that vmPFC is critical for the generation and regulation of negative emotion, through its interactions with amygdala, bed nucleus of stria terminalis, periaqueductal gray, hippocampus, and dorsal anterior cingulate cortex. A third track of research shows the importance of vmPFC in multiple aspects of social cognition, such as facial emotion recognition, theory of mind ability, and processing self-relevant information, through its interactions with posterior cingulate cortex, precuneus, dorsomedial prefrontal cortex, and amygdala. We then present meta-analytic data revealing distinct subregions within vmPFC that correspond to each of these three functions, as well as the associations between these subregions and specific psychiatric disorders (depression, posttraumatic stress disorder, addiction, social anxiety disorder, bipolar disorder, schizophrenia, and attention-deficit/ hyperactivity disorder). We conclude by describing several translational possibilities for clinical studies of vmPFC-based circuits, including neuropsychological assessment of transdiagnostic functions, anatomical targets for intervention, predictors of treatment response, markers of treatment efficacy, and subtyping within disorders.
Keywords: prefrontal cortex, emotion, decision-making, social cognition, psychopathology, neuroanatomy
INTRODUCTION
A key step toward developing a neuropathophysiologically-based system of diagnosis and treatment for mental illness is characterizing the brain circuitry that underlies the critical domains of social, cognitive, affective function that are disrupted in psychiatric disorders. The ventromedial prefrontal cortex (vmPFC) has been one of the principal brain regions of empirical study in this regard. Decades of research studies have demonstrated the importance of vmPFC in social and affective function, yet the precise role of this brain region in various forms of psychopathology remains unclear. The purpose of this review is to collate and summarize research findings on vmPFC function at the neural systems level, in order to highlight areas of convergence, as well as discrepancies, gaps, and opportunities for future research and clinical translation.
This review begins with a brief anatomical overview of vmPFC, followed by a description of three major tracks of research—one highlighting the role of vmPFC in value representation, another emphasizing the role of vmPFC in emotion regulation, and a third examining the role of vmPFC social cognition. In relation to each of these psychological domains, we delineate the network of brain regions with which vmPFC interacts. The narrative portion of this review is not intended to be comprehensive or exhaustive; citations were selected as exemplars to illustrate the breadth of functions ascribed to vmPFC and their relevance to mental illness. We next present novel meta-analytic data that reveals distinct subregions of vmPFC corresponding to each of the three psychological domains, as well as the association of each subregion with different psychiatric disorders. We conclude by discussing efforts to translate the growing understanding of vmPFC functional anatomy into more targeted and efficacious treatments for psychopathology.
ANATOMY OF vmPFC
In the primate brain, “vmPFC” generally refers to an interconnected network of regions in the lower medial and orbital prefrontal cortices (1–4). In rodents, the infralimbic (IL) cortex is typically considered to be related to human and monkey Brodmann area 25, a component of vmPFC (2, 4). It is important to note that the term “vmPFC” does not refer to a discrete brain structure with clearly defined and uniformly applied anatomical borders, such as a specific nucleus or gyrus. The use of the term may depend on the anatomical precision of the experimental approach. For example, in human lesion studies, naturally-occurring lesions may span multiple Brodmann areas within an individual and include overlapping but distinct areas between individuals; in these studies “vmPFC” is the most specific anatomical label that can accurately be applied. By contrast, human functional imaging studies typically yield activation loci within more circumscribed regions of vmPFC, and these activations can be reported with more specific anatomical terms such as “anterior medial orbitofrontal cortex” or “subgenual cingulate cortex”. Similarly, non-human primate studies that target a specific sulcus, gyrus, or Brodmann area within vmPFC (e.g., with a focal lesion or recording electrode) may use those more specific anatomical terms, rather than “vmPFC”. (For more detailed consideration of the morphological and cytoarchitectural features of vmPFC, see (1–4)).
Regardless of the precise anatomical boundaries, the identification of a homologous vmPFC region across rodents, monkeys, and humans has afforded researchers the opportunity to generate a huge corpus of data on the function of this brain region. As detailed in the following sections, results from these studies implicate vmPFC in a variety of psychological and behavioral functions relevant to mental illness.
VALUE AND DECISION-MAKING
One of the seminal clinical observations of human neurological patients with focal vmPFC damage is a severe defect in value-based decision-making, despite intact performance on conventional measures of intelligence (5, 6). This behavioral defect was first captured in the laboratory with a gambling task that requires subjects to learn about rewards and punishments under conditions of risk, ambiguity, and reversing contingencies (7). Subsequent studies of vmPFC lesion patients have documented value-based decision making deficits in a wide variety of paradigms, including risky gambles (8, 9), probabilistic reinforcement learning (10, 11), economic exchange (12, 13), and simple binary item preference (14). In parallel with these demonstrations of decision-making deficits among vmPFC lesion patients, scores of human functional imaging studies have linked vmPFC activity with the representation of value and reward processing, in a variety of decision-making contexts (15, 16). Moreover, animal studies have demonstrated a critical role for vmPFC in representing and updating the reward values of stimuli and outcomes. For example, electrophysiological recording studies in both monkeys and rats demonstrate that vmPFC encodes the reward properties of stimuli (17, 18), while targeted vmPFC lesions in monkeys disrupt reward-guided decision-making (19, 20).
From a network standpoint, the reward processing and decision-making functions of vmPFC are thought to depend, in part, on interactions with the ventral striatum and amygdala. Human functional imaging data show that vmPFC and ventral striatum exhibit strong functional connectivity at rest (21–23) and are often co-activated during reward processing tasks (22). Moreover, animal research suggests a causal effect of vmPFC activity on ventral striatum activity. Rodent studies have shown that vmPFC has direct glutamatergic projections to the ventral striatum (24–26) and that inactivation of vmPFC alters neuronal activity in ventral striatum (27). Lesioning or inactivating both vmPFC and ventral striatum/accumbens disrupts behavioral responding during reward learning and reaction time tasks, indicating that adaptive decision-making depends on concurrent activation of both regions (28–34). Consistent with these animal studies, a recent functional imaging study of human neurological patients with vmPFC damage found attenuated ventral striatum activity during the anticipation of reward (35). Studies have also demonstrated the importance of interactions between the vmPFC and amygdala for reward processing and decision-making. In rodents, stimulation of a projection from the central nucleus of the amygdala to vmPFC modulates reward-related behavior (36). In monkeys, surgical disconnection of the amygdala and vmPFC impairs the ability to flexibly alter choice behavior according to the reward value of stimuli (37, 38), while targeted amygdala lesions reduce the percentage of neurons coding reward value in vmPFC (39). An analogous fMRI study of two human patients with focal bilateral amygdala damage found abnormal activity related to expected reward value in vmPFC (40).
Together, the rodent, monkey, and human data described in this section converge to characterize vmPFC as a key node in the neural circuitry underlying reward processing and value-based decision-making.
EMOTION REGULATION
A second domain of function in which vmPFC is theorized to play a major role is the regulation of negative emotion. An elegant series of rodent studies using a fear conditioning and extinction paradigm provided the foundational support for this model of vmPFC function. Following the initial demonstration that vmPFC damage impairs recall of extinction learning, as evidenced by elevated conditioned fear responses during the extinction period (41, 42), a subsequent electrophysiological study showed that vmPFC neurons fire during extinction recall, and that stimulation of vmPFC neurons reduces conditioned fear responses during the extinction phase (43). These findings, coupled with studies showing that amygdala is critical for the expression of conditioned fear (44) and that vmPFC stimulation suppresses amygdala activity (45, 46, c.f., 47), suggest a mechanism by which vmPFC regulates the expression of fear responses through inhibition of the amygdala. Furthermore, anatomical tracing studies in rodents and non-human primates have identified direct projections from vmPFC to inhibitory interneurons within the amygdala, indicating a viable anatomical substrate for the observed functional relationship (48, 49). Human functional imaging studies have yielded additional support for this model, showing that activity in vmPFC and amygdala is inversely related during the extinction of conditioned fear (50, 51), as well as during the volitional suppression of negative emotion (52–54); that vmPFC activity is greater in response to the CS− relative to CS+ (55); and that vmPFC damage is associated with elevated amygdala activity to aversive visual stimuli (56).
A related line of research supporting a role for vmPFC in regulating negative emotion involves the generalization of conditioned fear (i.e., fear responses to stimuli that are perceptually similar to the conditioned stimulus). A set of rodent studies using molecular manipulations of synaptic transmission and optogenetic activation provided circuit-level evidence for this model of vmPFC function. After an initial study demonstrated that global impairment of synaptic transmission in mPFC results in overgeneralization of fear memories (57), a subsequent study showed that inactivation of mPFC inputs to a specific thalamic nucleus, which in turn projects to hippocampus and back to mPFC, similarly increases the generalization of fear memories (58). These findings, along with anatomical studies showing direct links between mPFC, thalamus, and hippocampus (59, 60), suggest a circuit through which vmPFC may regulate fear memory generalization. Human functional imaging studies have provided additional support for this model, showing greater activity in vmPFC and hippocampus as stimuli became less similar to the conditioned stimulus (61, 62). These fear generalization findings converge with functional imaging findings showing greater vmPFC activity as threatening stimuli become more distal or unlikely (63, 64).
Yet another line of research implicating the vmPFC in regulating negative emotion involves the alleviation of pain through placebo or expectancy manipulations. A recent meta-analysis demonstrates that placebo effects and expectations for reduced pain elicit reliable increases in vmPFC activity, along with decreases in regions associated with aversive or noxious stimuli such as the amygdala, insula, and dorsal anterior cingulate cortex (65). Furthermore, placebo treatment enhances connectivity between vmPFC and periaqueductal gray, which could reflect descending regulation of autonomic responses related to pain (66, 67). Together, the studies linking vmPFC activity to fear extinction, fear generalization, and placebo analgesia suggest a general role in inhibiting negative emotion and/or signaling safety from threat.
Despite the considerable body of evidence for vmPFC mediating the inhibition of negative emotion (described above), there is also evidence that vmPFC plays a role in the generation of negative emotion. It has long been established that humans with vmPFC damage do not exhibit increases in negative affect, such as fear, anxiety, or guilt, following the acquisition of their lesions (as would be predicted by the inhibition model). To the contrary, humans with vmPFC lesions exhibit blunted affect and diminished physiological reactivity to aversive stimuli (6, 68), and a reduced susceptibility to depression and post-traumatic stress disorder (69, 70). This effect may depend on damage to the subjacent white matter, as monkey studies indicate that vmPFC lesions involving white matter reduce threat-induced fear responses (71), whereas vmPFC lesions sparing white matter have no such effect (72) or may increase fear responses (73). The vmPFC’s role in generating emotional responses may be explained by its dense projections to the basolateral and central nuclei of the amygdala (in addition to the inhibitory intercalated neurons, as highlighted in the inhibition model) as well as to visceromotor structures like the hypothalamus and periaqueductal gray (3, 74). The effects of human vmPFC lesions on the generation of emotional responses could also relate to damage to Brodmann area 32, which is homologous to the rodent prelimbic region implicated in driving (rather than inhibiting) the expression of fear (75). Furthermore, it has been shown that in both monkeys and humans, vmPFC damage reduces activity in the bed nucleus of the stria terminalis (76, 77), a subcortical structure that is centrally involved in anxiety (78). Collectively, the data reviewed in this section demonstrate the importance of vmPFC in both generating and inhibiting negative emotion.
SOCIAL COGNITION AND SELF-RELEVANCE
In addition to the substantial collection of results linking vmPFC to value processing, decision-making, and emotion regulation (described in the previous two sections), the vmPFC has also been implicated in a number of social cognitive functions relevant for mental illness. For example, patients with vmPFC damage exhibit deficits in empathy (6, 79) and facial emotion recognition (80, 81). Moreover, recent eye-tracking data indicate that vmPFC damage, like amygdala damage, is associated with reduced visual attention to the eye region of faces, and that instruction to attend the eye region can improve emotion recognition deficits in both types of lesion patients (82–84), suggesting a mutual role for these brain areas in allocating visual attention to stimuli with social-affective salience. The vmPFC is also consistently activated in human fMRI studies of moral cognition, and damage to this region yields aberrations in moral judgment (85, 86). Meta-analyses of fMRI data also indicate a role for vmPFC in theory of mind ability (87, 88). Another domain of social cognitive function putatively subserved by vmPFC is processing of self-relevant information. Human functional imaging studies have reliably shown vmPFC activity during tasks that require self-focused thought, such as judging whether a personality trait pertains to oneself, imagining one’s own feelings in a hypothetical situation, or recalling an autobiographical memory (89, 90). For this “self-relevant” processing function, vmPFC interacts with a network of brain regions known as the “default mode network” (91), which includes dorsomedial PFC as well as posterior cingulate cortex and precuneus.
SUBREGIONS WITHIN vmPFC AND ASSOCIATIONS WITH PSYCHOPATHOLOGY
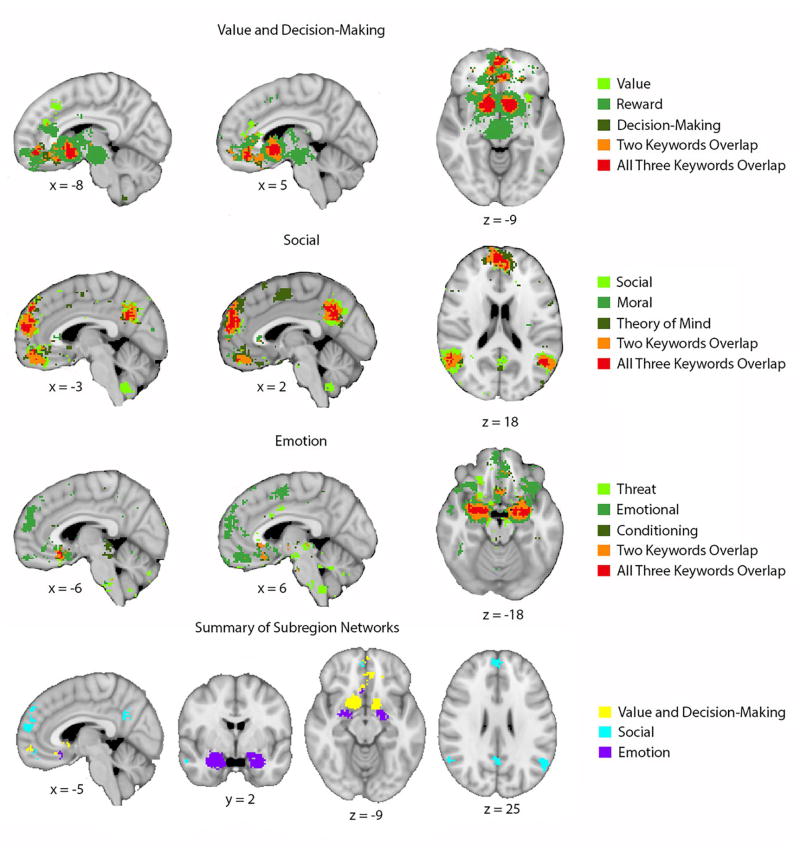
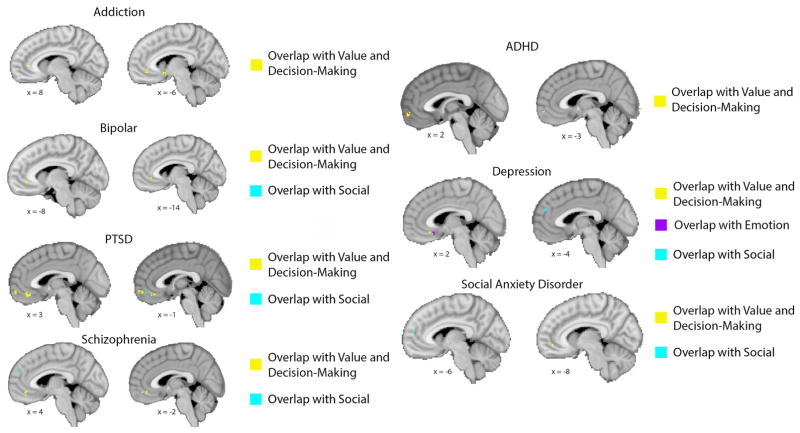
Given the variety of psychological functions ascribed to vmPFC, as well as the variety of brain regions with which vmPFC interacts, it is important to consider the possibility that there may be anatomically and/or functionally specialized subregions within vmPFC. To address this possibility empirically, we downloaded a series of meta-analyses of human fMRI data using Neurosynth (www.neurosynth.org; 92) to identify subregions within vmPFC that are associated with each domain of function (i.e., value and decision-making; emotion; social cognition), as well as other regions of the brain that are typically co-activated with each functionally-specialized vmPFC subregion (Figure 1). This analysis demonstrates distinct networks of activity associated with the three domains of function described above: for value and decision-making, a region of anterior/pregenual vmPFC and the ventral striatum; for emotion, a region of posterior/subgenual vmPFC and the amygdala; and for social cognition, a region of anterior/pregenual vmPFC and the dorsomedial PFC, precuneus, and temporoparietal cortex. These meta-analytic findings support a previously proposed subregion scheme that distinguishes a more anterior/perigenual region of vmPFC from a more posterior/subgenual region based on emotional valence, with the anterior region associated with positive valence (e.g., reward, value) and the posterior region associated with negative valence (e.g., threat, fear) (93, 94). Next, we sought to determine the association of the functionally specialized subregions of vmPFC with different forms of psychopathology. Using the vmPFC subregions derived from the meta-analyses described above (Figure 1), we analyzed the activation loci published in previous meta-analyses of fMRI findings related to specific disorders (e.g., depression, posttraumatic stress disorder, addiction, social anxiety disorder, bipolar disorder, schizophrenia, and attention-deficit/hyperactivity disorder) (Figure 2). This analysis yielded two general results. First, different forms of psychopathology are associated with abnormal activity in distinct as well as adjacent/overlapping regions of vmPFC (Supplementary Figure 1). Second, these disorder-related activation loci within vmPFC have distinct relationships with the functionally-specialized subregions described above. For example, depression-related activity is strongest in the posterior/subgenual subregion, but there are activation loci overlapping with each of the three functional subregions with vmPFC (corresponding to “value and decision-making”, “emotion”, and “social”, respectively). By contrast, addiction-related, bipolar-related, and ADHD-related activity all overlap exclusively with the “value and decision-making” subregion, whereas PTSD-related, schizophrenia-related, and SAD-related activity all overlap with both the “value and decision-making” and “social” subregions. This combination of meta-analyses thus yields novel data regarding the relationship between functional subregions of vmPFC and distinct forms of psychopathology.
Figure 1. Functionally specialized subregions of vmPFC.
Meta-analyses from Neurosynth yielded reverse inference statistical maps for each of the three domains (value and decision-making, emotion, and social). For each domain, we selected three keywords that we used as search terms to identify loci of domain-related activity (coded in different shades of green within each row). For the value and decision-making domain, we used search terms “value”, “reward”, and “decision-making”; for the emotion domain we used search terms “threat”, “emotional”, and “conditioning”; and for the social domain we used search terms “social”, “moral”, and “theory of mind”. The area of overlap within each domain (coded in red in the top three rows) was similar regardless of which specific domain-relevant search terms we used (i.e., using “fear” instead of “threat”, “empathy” instead of “moral”, or “reward anticipation” instead of “reward” yields nearly identical results). The bottom row shows the areas of overlap for each domain in different colors. This analysis demonstrates distinct subregions within vmPFC as well as distinct patterns of co-activation outside of vmPFC for each domain.
Figure 2. Association between vmPFC subregions and psychiatric disorders.
To define activation loci associated with each disorder, we used the results of previously published meta-analyses of fMRI findings related to each disorder to construct spheres representing the approximate volume of reported clusters at their respective peak coordinates (for details see Supplementary Methods, Supplementary Table 1, and Supplementary Figure 1). Meta-analyses were obtained by searching the PubMed database. The search terms included the combination of terms for each disorder (e.g., “depression”, “PTSD”, “addiction”, “social anxiety disorder”, etc.), “fMRI”, and “meta-analysis”. The search was restricted to meta-analyses of event-related fMRI studies of adults that were listed in the database on or after January 1st, 2006. Overlap between the disorder-related loci and functionally specialized vmPFC subregions (as defined in Figure 1) is shown in yellow (for value and decision-making), purple (for emotion), and aqua (for social), corresponding to the color scheme in the bottom row of Figure 1. Two sagittal slices were selected for each disorder to illustrate all regions of overlap.
CLINICAL IMPLICATIONS
As described in the preceding sections, vmPFC has been implicated in a number of social and affective psychological functions relevant for mental illness. In this section we describe several ways that assessments of vmPFC function could potentially impact clinical practice.
Neuropsychological assessment of transdiagnostic domains of function
Developing methods for the objective assessment of neural circuits responsible for particular domains of psychological or behavioral dysfunction that may be shared across traditional diagnoses is a critical step toward a neuropathophysiologically-based system of psychiatric diagnosis and treatment (95). As detailed above, the vmPFC is a key neural substrate for several relevant domains of psychological function. In Table 1, we provide examples of how neuroimaging measures of vmPFC activity have been linked to domains of psychological or behavioral dysfunction that are relevant for mental health, across multiple disorders. For each of these functional domains, there exist laboratory techniques for biological and behavioral assessment, including measures of task performance, peripheral physiology, and neural activity. The adaptation and application of these paradigms for studies of clinical utility is likely to accelerate in the near future.
Table 1.
Transdiagnostic links between vmPFC activity and psychological functions relevant for mental illness
| Domain of psychological function | Disorders in which functional domain is linked to abnormal vmPFC activity | Example references |
|---|---|---|
| Value representation/ Value-based decision-making | Major depression | (124, 125) |
| Substance use disorder | (126, 127) | |
| Autism spectrum disorder | (128, 129) | |
| Attention-deficit/hyperactivity disorder | (130) | |
| Obsessive-compulsive disorder | (131, 132) | |
|
| ||
| Fear extinction | Posttraumatic stress disorder | (133) |
| Obsessive-compulsive disorder | (134) | |
| Schizophrenia | (135) | |
|
| ||
| Emotion regulation | Major depression | (53) |
| Schizophrenia | (136) | |
|
| ||
| Fear generalization | Generalized anxiety disorder | (137) |
| Posttraumatic stress disorder | (138) | |
|
| ||
| Rumination/ Self-reflection | Major depression | (120) |
| Autism spectrum disorder | (139) | |
|
| ||
| Moral sensitivity | Obsessive-compulsive disorder | (140) |
| Psychopathy | (141) | |
|
| ||
| Uncertainty/ Unpredictability | Obsessive-compulsive disorder | (142) |
| Posttraumatic stress disorder | (143) | |
|
| ||
| Theory of mind/ Mental state inference | Autism spectrum disorder | (144) |
| Psychopathy | (145) | |
| Schizophrenia | (146, 147) | |
Anatomical target for intervention
Given the pivotal role that vmPFC appears to play in multiple facets of mental health, it has become an attractive target for therapies that can be localized to particular regions of the brain. Historically, surgical ablations for refractory mental illness have targeted subregions of vmPFC or its subjacent white matter (e.g., subcaudate tractotomy (96), orbitomedial leucotomy (97)). More recently, deep brain stimulation (DBS) of the subgenual region has been tested for treatment-resistant depression. While preliminary open-label studies of subgenual DBS with relatively small samples have shown efficacy in reducing depression symptom severity (98), further refinement of the technique and larger controlled trials will be necessary to more firmly establish this approach. Less invasive approaches, such as transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (TDCS), may be able to modulate activity in more superficial regions of vmPFC. These approaches have been employed to examine vmPFC-mediated psychological functions (99–101), but there are not yet data regarding their efficacy in treating mental illness.
Predictor of treatment response
Apart from comprising a direct target for intervention, the vmPFC has shown great promise as a predictor of response to a variety of treatments. For example, the efficacy of TMS applied to dorsolateral PFC for depression is related to the functional connectivity between the stimulation site and the subgenual vmPFC (102), while the efficacy of TMS applied to dorsomedial PFC for depression is related to greater pre-treatment resting-state functional connectivity between vmPFC and dorsomedial PFC (103), as well as to greater pre-treatment resting-state functional connectivity between vmPFC and striatum, ventral tegmental area, and dorsal PFC (104). A recent study found that subgenual vmPFC volume predicts response to electroconvulsive therapy (ECT) (105), while responders to DBS of the white matter underlying subgenual vmPFC exhibit stronger connections between vmPFC, anterior cingulate cortex, and ventral striatum, relative to non-responders (106). In studies of antidepressant medication for depression, pre-treatment resting-state functional connectivity between vmPFC and anterior cingulate cortex correlates with treatment response (107). In studies of cognitive-behavioral therapy (CBT) for depression, treatment response has been positively associated with pre-treatment activity in an anterior region of vmPFC during an emotional picture viewing task (108), but negatively associated with pre-treatment activity in a posterior region vmPFC during emotional word viewing task (109). (Interestingly, this anterior-posterior difference in treatment response prediction is consistent with the anterior-posterior vmPFC subregion scheme described above.) Together, these findings suggest that the efficacies of distinct treatment modalities (e.g., TMS, ECT, antidepressant medication, CBT) can be predicted by different neuroimaging markers of vmPFC function prior to treatment initiation. Building on this promising collection of results, prospective studies will be necessary to determine if neuroimaging assessments of vmPFC function can be used clinically to assign patients to the most effective available treatment option.
Marker of treatment efficacy
Consistent with the pre-treatment response prediction findings described above, a number of studies have demonstrated changes in vmPFC function from pre-treatment to post-treatment. For example, a meta-analysis of antidepressant effects shows increased activity in anterior vmPFC related to positive emotions (110). Similar task-related increases in anterior vmPFC activity have been observed in studies of psychotherapy for depression (108, 111). Decreases in activity in posterior vmPFC activity have been observed in studies of antidepressant medication (112, 113), TMS (114), and DBS (115). Again, note that the increases in activity in anterior vmPFC regions (for antidepressants and psychotherapy) and decreases in activity in posterior vmPFC regions (for antidepressants, psychotherapy, and DBS) are consistent with the anterior-posterior vmPFC subregion scheme described above.
Subtyping
One of the most challenging aspects of psychiatric patient care is the substantial heterogeneity present within a single categorical diagnosis. For example, the standard diagnostic criteria for major depression can be met by hundreds of possible symptom combinations. The elucidation of subtypes within a psychiatric diagnosis thus holds tremendous promise for more precise and effective patient care. While subtypes within various psychiatric disorders have been proposed based on psychological, behavioral, and/or etiological characteristics (116–118), it is possible that subtypes based on neurobiology could more closely correspond to differences in the proximal mechanisms of dysfunction, and thereby yield diagnostic groups with more homogenous treatment needs. Although efforts to define disorder subtypes based on clustering analyses of neuroimaging data are in the early stages, a promising recent study identified four subtypes of depression based on resting-state functional connectivity data (119). Despite differences in connectivity in a variety of cortical and subcortical regions, all four subtypes share abnormal connectivity of the vmPFC, among other regions. Given the widespread role that vmPFC plays in psychological functions relevant for mental illness, it is likely that future studies of this type will also reveal vmPFC function as a key factor in parsing the shared versus unique neural substrates of disorder subtypes.
CONCLUSION
Collectively, the findings reviewed in this article substantiate two general points. First, whereas recent review articles highlighting vmPFC dysfunction in mental illness have focused almost exclusively on either one psychological function and/or one category of mental illness (e.g., 120, 121–123), the data reviewed here establish the role of vmPFC in multiple functions, multiple brain networks, and multiple disorders. Second, there is a burgeoning collection of clinical studies that portend the translation of these neuroimaging measures of vmPFC-based circuits into clinically useful techniques for improving the diagnosis and treatment of mental illness.
Supplementary Material
Supplementary Figure S1. Association between vmPFC subregions and psychiatric disorders. To define activation loci associated with each disorder, we used the results of previously published meta-analyses of fMRI findings related to each disorder to construct spheres representing the approximate volume of reported clusters at their respective peak coordinates (for details see Supplementary Methods and Supplementary Table S1). We plotted these disorder-related activation loci (in red) along with the domain-related loci (in separate colors; corresponding to the bottom row of Figure 1). Overlap between the disorder-related loci and domain-related loci is shown in green (for value and decision-making), pink (for emotion), and orange (for social). Using this approach, we found relatively large regions of overlap in some cases (e.g., >30 voxels for the value and decision-making subregion and activation loci for addiction, depression, and schizophrenia, respectively), but fairly small regions of overlap in other cases (e.g., <5 voxels for the social subregion and activation loci for bipolar, depression, and social anxiety disorder, respectively).
Supplementary Table S1. Meta-analyses yielding disorder-related fMRI activation loci for Figure 2 and Supplementary Figure S1.
Acknowledgments
This work was supported by the National Institute of Mental Health (MH101162).
Footnotes
DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mackey S, Petrides M. Architecture and morphology of the human ventromedial prefrontal cortex. Eur J Neurosci. 2014;40:2777–2796. doi: 10.1111/ejn.12654. [DOI] [PubMed] [Google Scholar]
- 2.Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 3.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 4.Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- 6.Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol. 2000;18:355–381. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- 7.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 8.Camille N, Coricelli G, Sallet J, Pradat-Diehl P, Duhamel JR, Sirigu A. The involvement of the orbitofrontal cortex in the experience of regret. Science. 2004;304:1167–1170. doi: 10.1126/science.1094550. [DOI] [PubMed] [Google Scholar]
- 9.Pujara MS, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex damage alters relative risk tolerance for prospective gains and losses. Neuropsychologia. 2015;79:70–75. doi: 10.1016/j.neuropsychologia.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler EZ, Fellows LK. The human ventromedial frontal lobe is critical for learning from negative feedback. Brain. 2008;131:1323–1331. doi: 10.1093/brain/awn041. [DOI] [PubMed] [Google Scholar]
- 12.Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the Ultimatum Game. J Neurosci. 2007;27:951–956. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krajbich I, Adolphs R, Tranel D, Denburg NL, Camerer CF. Economic games quantify diminished sense of guilt in patients with damage to the prefrontal cortex. J Neurosci. 2009;29:2188–2192. doi: 10.1523/JNEUROSCI.5086-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henri-Bhargava A, Simioni A, Fellows LK. Ventromedial frontal lobe damage disrupts the accuracy, but not the speed, of value-based preference judgments. Neuropsychologia. 2012;50:1536–1542. doi: 10.1016/j.neuropsychologia.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience and biobehavioral reviews. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy DJ, Glimcher PW. The root of all value: a neural common currency for choice. Curr Opin Neurobiol. 2012;22:1027–1038. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 18.Lopatina N, McDannald MA, Styer CV, Peterson JF, Sadacca BF, Cheer JF, et al. Medial Orbitofrontal Neurons Preferentially Signal Cues Predicting Changes in Reward during Unblocking. J Neurosci. 2016;36:8416–8424. doi: 10.1523/JNEUROSCI.1101-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noonan MP, Walton ME, Behrens TE, Sallet J, Buckley MJ, Rushworth MF. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc Natl Acad Sci U S A. 2010;107:20547–20552. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 22.Cauda F, Cavanna AE, D’Agata F, Sacco K, Duca S, Geminiani GC. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J Cogn Neurosci. 2011;23:2864–2877. doi: 10.1162/jocn.2011.21624. [DOI] [PubMed] [Google Scholar]
- 23.Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 25.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends in neurosciences. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 27.Ghazizadeh A, Ambroggi F, Odean N, Fields HL. Prefrontal cortex mediates extinction of responding by two distinct neural mechanisms in accumbens shell. J Neurosci. 2012;32:726–737. doi: 10.1523/JNEUROSCI.3891-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christakou A, Robbins TW, Everitt BJ. Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci. 2004;24:773–780. doi: 10.1523/JNEUROSCI.0949-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.St Onge JR, Stopper CM, Zahm DS, Floresco SB. Separate prefrontal-subcortical circuits mediate different components of risk-based decision making. J Neurosci. 2012;32:2886–2899. doi: 10.1523/JNEUROSCI.5625-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, et al. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 2012;32:4982–4991. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richard JM, Berridge KC. Prefrontal cortex modulates desire and dread generated by nucleus accumbens glutamate disruption. Biol Psychiatry. 2013;73:360–370. doi: 10.1016/j.biopsych.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith KS, Graybiel AM. A dual operator view of habitual behavior reflecting cortical and striatal dynamics. Neuron. 2013;79:361–374. doi: 10.1016/j.neuron.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feja M, Koch M. Frontostriatal systems comprising connections between ventral medial prefrontal cortex and nucleus accumbens subregions differentially regulate motor impulse control in rats. Psychopharmacology (Berl) 2015;232:1291–1302. doi: 10.1007/s00213-014-3763-3. [DOI] [PubMed] [Google Scholar]
- 35.Pujara MS, Philippi CL, Motzkin JC, Baskaya MK, Koenigs M. Ventromedial Prefrontal Cortex Damage Is Associated with Decreased Ventral Striatum Volume and Response to Reward. J Neurosci. 2016;36:5047–5054. doi: 10.1523/JNEUROSCI.4236-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo DO, Funderburk SC, Bhatti DL, Motard LE, Newbold D, Girven KS, et al. A GABAergic Projection from the Centromedial Nuclei of the Amygdala to Ventromedial Prefrontal Cortex Modulates Reward Behavior. J Neurosci. 2016;36:10831–10842. doi: 10.1523/JNEUROSCI.1164-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiuzat EC, Rhodes SE, Murray EA. The role of orbitofrontal-amygdala interactions in updating action-outcome valuations in macaques. J Neurosci. 2017 doi: 10.1523/JNEUROSCI.1839-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudebeck PH, Mitz AR, Chacko RV, Murray EA. Effects of amygdala lesions on reward-value coding in orbital and medial prefrontal cortex. Neuron. 2013;80:1519–1531. doi: 10.1016/j.neuron.2013.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hampton AN, Adolphs R, Tyszka MJ, O’Doherty JP. Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron. 2007;55:545–555. doi: 10.1016/j.neuron.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 41.Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 42.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 44.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/a:1025048802629. [DOI] [PubMed] [Google Scholar]
- 45.Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 49.Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 50.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 51.Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Avila-Parcet A, et al. Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol Psychiatry. 2016;21:500–508. doi: 10.1038/mp.2015.88. [DOI] [PubMed] [Google Scholar]
- 56.Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry. 2015;77:276–284. doi: 10.1016/j.biopsych.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu W, Morishita W, Buckmaster PS, Pang ZP, Malenka RC, Sudhof TC. Distinct neuronal coding schemes in memory revealed by selective erasure of fast synchronous synaptic transmission. Neuron. 2012;73:990–1001. doi: 10.1016/j.neuron.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu W, Sudhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339:1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 60.Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull. 2007;71:601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica-Parodi LR. Neural reactivity tracks fear generalization gradients. Biol Psychol. 2013;92:2–8. doi: 10.1016/j.biopsycho.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 62.Lissek S, Bradford DE, Alvarez RP, Burton P, Espensen-Sturges T, Reynolds RC, et al. Neural substrates of classically conditioned fear-generalization in humans: a parametric fMRI study. Soc Cogn Affect Neurosci. 2014;9:1134–1142. doi: 10.1093/scan/nst096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mobbs D, Yu R, Rowe JB, Eich H, FeldmanHall O, Dalgleish T. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc Natl Acad Sci U S A. 2010;107:20582–20586. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Atlas LY, Wager TD. A meta-analysis of brain mechanisms of placebo analgesia: consistent findings and unanswered questions. Handb Exp Pharmacol. 2014;225:37–69. doi: 10.1007/978-3-662-44519-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 67.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 68.Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- 69.Koenigs M, Huey ED, Raymont V, Cheon B, Solomon J, Wassermann EM, et al. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci. 2008;11:232–237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J Neurosci. 2008;28:12341–12348. doi: 10.1523/JNEUROSCI.2324-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat Neurosci. 2013;16:1140–1145. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shiba Y, Kim C, Santangelo AM, Roberts AC. Lesions of either anterior orbitofrontal cortex or ventrolateral prefrontal cortex in marmoset monkeys heighten innate fear and attenuate active coping behaviors to predator threat. Front Syst Neurosci. 2014;8:250. doi: 10.3389/fnsys.2014.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci. 2010;30:7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Motzkin JC, Philippi CL, Oler JA, Kalin NH, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex damage alters resting blood flow to the bed nucleus of stria terminalis. Cortex. 2015;64:281–288. doi: 10.1016/j.cortex.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adhikari A. Distributed circuits underlying anxiety. Frontiers in behavioral neuroscience. 2014;8:112. doi: 10.3389/fnbeh.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–627. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- 80.Heberlein AS, Padon AA, Gillihan SJ, Farah MJ, Fellows LK. Ventromedial frontal lobe plays a critical role in facial emotion recognition. J Cogn Neurosci. 2008;20:721–733. doi: 10.1162/jocn.2008.20049. [DOI] [PubMed] [Google Scholar]
- 81.Tsuchida A, Fellows LK. Are you upset? Distinct roles for orbitofrontal and lateral prefrontal cortex in detecting and distinguishing facial expressions of emotion. Cereb Cortex. 2012;22:2904–2912. doi: 10.1093/cercor/bhr370. [DOI] [PubMed] [Google Scholar]
- 82.Wolf RC, Philippi CL, Motzkin JC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex mediates visual attention during facial emotion recognition. Brain. 2014 doi: 10.1093/brain/awu063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolf RC, Pujara M, Baskaya MK, Koenigs M. Emotion recognition deficits associated with ventromedial prefrontal cortex lesions are improved by gaze manipulation. Cortex. 2016;82:255–262. doi: 10.1016/j.cortex.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- 85.Young L, Koenigs M. Investigating emotion in moral cognition: a review of evidence from functional neuroimaging and neuropsychology. Br Med Bull. 2007;84:69–79. doi: 10.1093/bmb/ldm031. [DOI] [PubMed] [Google Scholar]
- 86.Fumagalli M, Priori A. Functional and clinical neuroanatomy of morality. Brain. 2012;135:2006–2021. doi: 10.1093/brain/awr334. [DOI] [PubMed] [Google Scholar]
- 87.Molenberghs P, Johnson H, Henry JD, Mattingley JB. Understanding the minds of others: A neuroimaging meta-analysis. Neuroscience and biobehavioral reviews. 2016;65:276–291. doi: 10.1016/j.neubiorev.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 88.Schurz M, Radua J, Aichhorn M, Richlan F, Perner J. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neuroscience and biobehavioral reviews. 2014;42:9–34. doi: 10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 89.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 90.Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol Psychiatry. 2012;17:132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC) toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 96.Bridges PK, Bartlett JR, Hale AS, Poynton AM, Malizia AL, Hodgkiss AD. Psychosurgery: stereotactic subcaudate tractomy. An indispensable treatment. Br J Psychiatry. 1994;165:599–611. doi: 10.1192/bjp.165.5.599. discussion 612–593. [DOI] [PubMed] [Google Scholar]
- 97.Sachdev PS, Sachdev J. Long-term outcome of neurosurgery for the treatment of resistant depression. J Neuropsychiatry Clin Neurosci. 2005;17:478–485. doi: 10.1176/jnp.17.4.478. [DOI] [PubMed] [Google Scholar]
- 98.Merkl A, Schneider GH, Schonecker T, Aust S, Kuhl KP, Kupsch A, et al. Antidepressant effects after short-term and chronic stimulation of the subgenual cingulate gyrus in treatment-resistant depression. Exp Neurol. 2013;249:160–168. doi: 10.1016/j.expneurol.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 99.Lev-Ran S, Shamay-Tsoory SG, Zangen A, Levkovitz Y. Transcranial magnetic stimulation of the ventromedial prefrontal cortex impairs theory of mind learning. Eur Psychiatry. 2012;27:285–289. doi: 10.1016/j.eurpsy.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 100.Zheng H, Huang D, Chen S, Wang S, Guo W, Luo J, et al. Modulating the Activity of Ventromedial Prefrontal Cortex by Anodal tDCS Enhances the Trustee’s Repayment through Altruism. Front Psychol. 2016;7:1437. doi: 10.3389/fpsyg.2016.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koenigs M, Ukueberuwa D, Campion P, Grafman J, Wassermann E. Bilateral frontal transcranial direct current stimulation: Failure to replicate classic findings in healthy subjects. Clin Neurophysiol. 2009;120:80–84. doi: 10.1016/j.clinph.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Salomons TV, Dunlop K, Kennedy SH, Flint A, Geraci J, Giacobbe P, et al. Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:488–498. doi: 10.1038/npp.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Downar J, Geraci J, Salomons TV, Dunlop K, Wheeler S, McAndrews MP, et al. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry. 2014;76:176–185. doi: 10.1016/j.biopsych.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 105.Redlich R, Opel N, Grotegerd D, Dohm K, Zaremba D, Burger C, et al. Prediction of Individual Response to Electroconvulsive Therapy via Machine Learning on Structural Magnetic Resonance Imaging Data. JAMA psychiatry. 2016;73:557–564. doi: 10.1001/jamapsychiatry.2016.0316. [DOI] [PubMed] [Google Scholar]
- 106.Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76:963–969. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kozel FA, Rao U, Lu H, Nakonezny PA, Grannemann B, McGregor T, et al. Functional connectivity of brain structures correlates with treatment outcome in major depressive disorder. Front Psychiatry. 2011;2:7. doi: 10.3389/fpsyt.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: changes with cognitive behavioral therapy and predictors of treatment response. J Psychiatr Res. 2011;45:577–587. doi: 10.1016/j.jpsychires.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- 110.Ma Y. Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Mol Psychiatry. 2015;20:311–319. doi: 10.1038/mp.2014.24. [DOI] [PubMed] [Google Scholar]
- 111.Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The effects of psychotherapy on neural responses to rewards in major depression. Biol Psychiatry. 2009;66:886–897. doi: 10.1016/j.biopsych.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kennedy SH, Konarski JZ, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am J Psychiatry. 2007;164:778–788. doi: 10.1176/ajp.2007.164.5.778. [DOI] [PubMed] [Google Scholar]
- 113.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 114.Kito S, Hasegawa T, Koga Y. Neuroanatomical correlates of therapeutic efficacy of low-frequency right prefrontal transcranial magnetic stimulation in treatment-resistant depression. Psychiatry and clinical neurosciences. 2011;65:175–182. doi: 10.1111/j.1440-1819.2010.02183.x. [DOI] [PubMed] [Google Scholar]
- 115.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 116.Dalenberg CJ, Glaser D, Alhassoon OM. Statistical support for subtypes in posttraumatic stress disorder: the how and why of subtype analysis. Depress Anxiety. 2012;29:671–678. doi: 10.1002/da.21926. [DOI] [PubMed] [Google Scholar]
- 117.Harald B, Gordon P. Meta-review of depressive subtyping models. J Affect Disord. 2012;139:126–140. doi: 10.1016/j.jad.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 118.Jablensky A. Subtyping schizophrenia: implications for genetic research. Mol Psychiatry. 2006;11:815–836. doi: 10.1038/sj.mp.4001857. [DOI] [PubMed] [Google Scholar]
- 119.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol Psychiatry. 2015;78:224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilcox CE, Pommy JM, Adinoff B. Neural Circuitry of Impaired Emotion Regulation in Substance Use Disorders. Am J Psychiatry. 2016;173:344–361. doi: 10.1176/appi.ajp.2015.15060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dejean C, Courtin J, Rozeske RR, Bonnet MC, Dousset V, Michelet T, et al. Neuronal Circuits for Fear Expression and Recovery: Recent Advances and Potential Therapeutic Strategies. Biol Psychiatry. 2015;78:298–306. doi: 10.1016/j.biopsych.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 123.Sonuga-Barke EJ, Fairchild G. Neuroeconomics of attention-deficit/hyperactivity disorder: differential influences of medial, dorsal, and ventral prefrontal brain networks on suboptimal decision making? Biol Psychiatry. 2012;72:126–133. doi: 10.1016/j.biopsych.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 124.Gong L, Yin Y, He C, Ye Q, Bai F, Yuan Y, et al. Disrupted reward circuits is associated with cognitive deficits and depression severity in major depressive disorder. J Psychiatr Res. 2017;84:9–17. doi: 10.1016/j.jpsychires.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 125.Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26:480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 127.Camchong J, MacDonald AW, 3rd, Nelson B, Bell C, Mueller BA, Specker S, et al. Frontal hyperconnectivity related to discounting and reversal learning in cocaine subjects. Biol Psychiatry. 2011;69:1117–1123. doi: 10.1016/j.biopsych.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Soc Cogn Affect Neurosci. 2012;7:160–172. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kohls G, Schulte-Ruther M, Nehrkorn B, Muller K, Fink GR, Kamp-Becker I, et al. Reward system dysfunction in autism spectrum disorders. Soc Cogn Affect Neurosci. 2013;8:565–572. doi: 10.1093/scan/nss033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wilbertz G, van Elst LT, Delgado MR, Maier S, Feige B, Philipsen A, et al. Orbitofrontal reward sensitivity and impulsivity in adult attention deficit hyperactivity disorder. Neuroimage. 2012;60:353–361. doi: 10.1016/j.neuroimage.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 131.Remijnse PL, Nielen MM, van Balkom AJ, Cath DC, van Oppen P, Uylings HB, et al. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:1225–1236. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- 132.Lagemann T, Rentzsch J, Montag C, Gallinat J, Jockers-Scherubl M, Winter C, et al. Early orbitofrontal hyperactivation in obsessive-compulsive disorder. Psychiatry Res. 2012;202:257–263. doi: 10.1016/j.pscychresns.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 133.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ, et al. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA psychiatry. 2013;70:608–618. doi: 10.1001/jamapsychiatry.2013.914. quiz 554. [DOI] [PubMed] [Google Scholar]
- 135.Holt DJ, Coombs G, Zeidan MA, Goff DC, Milad MR. Failure of neural responses to safety cues in schizophrenia. Arch Gen Psychiatry. 2012;69:893–903. doi: 10.1001/archgenpsychiatry.2011.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fan FM, Tan SP, Yang FD, Tan YL, Zhao YL, Chen N, et al. Ventral medial prefrontal functional connectivity and emotion regulation in chronic schizophrenia: a pilot study. Neurosci Bull. 2013;29:59–74. doi: 10.1007/s12264-013-1300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica-Parodi LR. Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depress Anxiety. 2013;30:242–250. doi: 10.1002/da.22016. [DOI] [PubMed] [Google Scholar]
- 138.Morey RA, Dunsmoor JE, Haswell CC, Brown VM, Vora A, Weiner J, et al. Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Transl Psychiatry. 2015;5:e700. doi: 10.1038/tp.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lombardo MV, Barnes JL, Wheelwright SJ, Baron-Cohen S. Self-referential cognition and empathy in autism. PloS one. 2007;2:e883. doi: 10.1371/journal.pone.0000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Harrison BJ, Pujol J, Soriano-Mas C, Hernadez-Ribas R, Lopez-Solà M, Ortiz H, et al. Neural Correlates of Moral Sensitivity in Obsessive-Compulsive Disorder. Archives of General Psychiatry. 2012;69:741–749. doi: 10.1001/archgenpsychiatry.2011.2165. [DOI] [PubMed] [Google Scholar]
- 141.Harenski CL, Harenski KA, Shane MS, Kiehl KA. Aberrant neural processing of moral violations in criminal psychopaths. J Abnorm Psychol. 2010;119:863–874. doi: 10.1037/a0020979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stern ER, Welsh RC, Gonzalez R, Fitzgerald KD, Abelson JL, Taylor SF. Subjective uncertainty and limbic hyperactivation in obsessive-compulsive disorder. Hum Brain Mapp. 2013;34:1956–1970. doi: 10.1002/hbm.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Grupe DW, Wielgosz J, Davidson RJ, Nitschke JB. Neurobiological correlates of distinct post-traumatic stress disorder symptom profiles during threat anticipation in combat veterans. Psychol Med. 2016;46:1885–1895. doi: 10.1017/S0033291716000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schulte-Ruther M, Greimel E, Markowitsch HJ, Kamp-Becker I, Remschmidt H, Fink GR, et al. Dysfunctions in brain networks supporting empathy: an fMRI study in adults with autism spectrum disorders. Soc Neurosci. 2011;6:1–21. doi: 10.1080/17470911003708032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Decety J, Skelly L, Yoder KJ, Kiehl KA. Neural processing of dynamic emotional facial expressions in psychopaths. Soc Neurosci. 2014;9:36–49. doi: 10.1080/17470919.2013.866905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hooker CI, Bruce L, Lincoln SH, Fisher M, Vinogradov S. Theory of mind skills are related to gray matter volume in the ventromedial prefrontal cortex in schizophrenia. Biol Psychiatry. 2011;70:1169–1178. doi: 10.1016/j.biopsych.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dodell-Feder D, Tully LM, Lincoln SH, Hooker CI. The neural basis of theory of mind and its relationship to social functioning and social anhedonia in individuals with schizophrenia. Neuroimage Clin. 2014;4:154–163. doi: 10.1016/j.nicl.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Association between vmPFC subregions and psychiatric disorders. To define activation loci associated with each disorder, we used the results of previously published meta-analyses of fMRI findings related to each disorder to construct spheres representing the approximate volume of reported clusters at their respective peak coordinates (for details see Supplementary Methods and Supplementary Table S1). We plotted these disorder-related activation loci (in red) along with the domain-related loci (in separate colors; corresponding to the bottom row of Figure 1). Overlap between the disorder-related loci and domain-related loci is shown in green (for value and decision-making), pink (for emotion), and orange (for social). Using this approach, we found relatively large regions of overlap in some cases (e.g., >30 voxels for the value and decision-making subregion and activation loci for addiction, depression, and schizophrenia, respectively), but fairly small regions of overlap in other cases (e.g., <5 voxels for the social subregion and activation loci for bipolar, depression, and social anxiety disorder, respectively).
Supplementary Table S1. Meta-analyses yielding disorder-related fMRI activation loci for Figure 2 and Supplementary Figure S1.