Abstract
Background
Visuospatial working memory (vsWM), which is impaired in schizophrenia, requires information transfer across multiple nodes in the cerebral cortex, including visual, posterior parietal, and dorsolateral prefrontal regions. Information is conveyed across these regions via the excitatory projections of glutamatergic pyramidal neurons located in layer 3, whose activity is modulated by local inhibitory GABAergic neurons. Key properties of these neurons differ across these cortical regions. Consequently, in schizophrenia, alterations in the expression of gene products regulating these properties could disrupt vsWM function in different ways, depending upon the region(s) affected.
Methods
Here, we quantified the expression of markers of glutamate and GABA neurotransmission selectively in layer 3 of four cortical regions in the vsWM network from 20 matched pairs of schizophrenia and unaffected comparison subjects.
Results
In comparison subjects, levels of glutamate transcripts tended to increase, whereas GABA transcript levels tended to decrease, from caudal-to-rostral across cortical regions of the vsWM network. Composite measures across all transcripts revealed a significant effect of region, with the glutamate measure lowest in primary visual cortex and highest in the dorsolateral prefrontal cortex, whereas the GABA measure showed the opposite pattern. In schizophrenia subjects, the expression levels of many of these transcripts were altered. However, this disease effect differed across regions such that the caudal-to-rostral increase in the glutamate measure was blunted and the caudal-to-rostral decline in the GABA measure was enhanced in the illness.
Conclusions
Differential alterations in layer 3 glutamate and GABA neurotransmission across cortical regions may contribute to vsWM deficits in schizophrenia.
Keywords: GABA, Glutamate, Prefrontal Cortex, Schizophrenia, Visual Cortex, Working Memory
Introduction
Long-term functional outcomes in schizophrenia are largely determined by the severity of cognitive impairments (1, 2). These impairments include disturbances in visuospatial working memory (vsWM) (3), the ability to transiently maintain and manipulate visuospatial information to guide thought and behavior (4). Deficits in vsWM not only characterize individuals with schizophrenia, but might also predict transition to psychosis in prodromal individuals (5).
In primates, vsWM is mediated by a distributed cortical network that includes nodes in the primary (V1) and association (V2) visual cortices of the occipital lobe which convey visual information to nodes in the posterior parietal (PPC) and dorsolateral prefrontal (DLPFC) cortices (6–8). This feedforward information is principally carried by excitatory projections from glutamatergic layer 3 pyramidal neurons in each region (9). Within each region, the activity of layer 3 pyramidal neurons during vsWM tasks is shaped by local inhibitory, γ-aminobutyric acid (GABA) neurons (10, 11).
Key elements regulating glutamatergic and GABAergic neurotransmission differ across regions in the vsWM network. For example, patterns of gene expression in cortical gray matter exhibit a caudal-to-rostral gradient, with V1 and DLPFC at opposite ends of this gradient (12–15). These patterns include lower levels of the glutamatergic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunit, GRIA2, in V1 relative to the DLPFC (16), and higher levels of the GABAA receptor α1 subunit in V1 than in the DLPFC (17). Similarly, the properties of layer 3 pyramidal and GABA neurons also exhibit regional differences. For example, layer 3 pyramidal neurons in V1 have lower dendritic spine densities, smaller soma sizes, and are more excitable compared to those in the DLPFC (18, 19). Together, these findings suggest that markers of glutamate and GABA neurotransmission in layer 3 are likely to differ across the cortical regions that mediate vsWM function.
Thus, in schizophrenia, alterations in these markers could disrupt vsWM function in different ways, depending upon the regions affected. Markers of glutamate neurotransmission have been examined in schizophrenia, but findings are inconsistent across studies and cortical regions (20). In contrast, consistent disease-related alterations in markers of cortical GABA neurotransmission have been reported (17, 21, 22), although most studies focused on the DLPFC (23). The few studies that examined GABA markers in multiple brain regions within the same subjects reported similar findings across cortical areas (17, 22, 24); however, nodes within the cortical vsWM network have not been examined systematically.
Consequently, we sought to answer three questions regarding markers of glutamate and GABA neurotransmission in layer 3 across regions of the human cortical vsWM network. First, do gene products regulating key elements of glutamate and GABA transmission normally exhibit regional differences in expression in layer 3? Second, is the expression of these gene products altered in schizophrenia, and if so, are those alterations region-specific or conserved across regions? Third, how do any disease effects on expression affect the normal regional patterns of glutamate and GABA transcript levels in the vsWM network?
To address these questions, we quantified the expression of key markers of glutamate and GABA neurotransmission in layer 3 from four regions of the vsWM network from 20 matched pairs of schizophrenia and unaffected comparison subjects. We selected the following, functionally-analogous markers of glutamate and GABA neurotransmission: 1) the enzymes that synthesize most cortical glutamate and GABA, glutaminase (GLS1) and the 67 kDa isoform of glutamic acid decarboxylase (GAD67), respectively; 2) the vesicular glutamate (vGLUT1) and GABA (vGAT) transporters that package their respective neurotransmitters into presynaptic vesicles; 3) the excitatory amino acid (EAAT2) and GABA membrane (GAT1) transporters involved in glutamate and GABA neurotransmitter reuptake, respectively; and 4) the obligatory N-methyl-D-aspartate (NMDA) receptor subunit (GRIN1), the calcium-impermeable AMPA receptor subunit (GRIA2), and the obligatory ionotropic GABA (GABAA) receptor subunit γ2 (GABRG2). Our experimental design that controlled for batch effects within subject pairs and across regions within subjects necessarily limited the number of transcripts that could be studied (See Methods and Materials, and Supplemental Methods), excluding the possibility of examining other glutamate or GABA receptor transcripts.
Methods and Materials
Human subjects
Human brain specimens (N=40) were obtained during routine autopsies conducted at the Allegheny County Medical Examiner’s Office (Pittsburgh, PA, USA) following consent obtained from the next-of-kin. Consensus DSM-IV diagnoses were made by an independent committee of experienced research clinicians using structured interviews with family members and review of prior medical records (25). The absence of a psychiatric diagnosis was confirmed in unaffected comparison subjects using the same approach.
To control for experimental variance and reduce between-group biological variance, each subject with schizophrenia was matched with one unaffected comparison subject for sex and as closely as possible for age. Subject groups did not differ in mean age, pH, RNA Integrity Number (RIN) (Agilent Bioanalyzer, Santa Clara, CA), postmortem interval (PMI) or tissue storage time at −80°C (Table 1; Supplemental Table S1). All procedures were approved by the University of Pittsburgh’s Committee for the Oversight of Research and Clinical Training Involving the Dead and Institutional Review Board for Biomedical Research.
Table 1.
Summary of demographic and postmortem characteristics of human subjects.
| Parameter | Unaffected Comparison Subjects |
Subjects with Schizophrenia |
Statistics |
|---|---|---|---|
| N | 20 | 20 | N/A |
| Sex (Male/Female) | 14/6 | 14/6 | N/A |
| Race (White/Black) | 16/4 | 13/7 | =1.1; p=0.29 |
| Age (Years) | 47.2 ± 9.9 | 45.6 ± 9.5 | t38=−0.5; p=0.62 |
| PMI* (Hours) | 15.4 ± 5.8 | 14.4 ± 6.2 | t38=−0.5; p=0.59 |
| Brain pH | 6.7 ± 0.2 | 6.5 ± 0.3 | t38=−1.6; p=0.11 |
| RIN** | 8.3 ± 0.5 | 8.2 ± 0.6 | t38=−0.6; p=0.54 |
| Storage time at −80°C (Months) | 134.7 ± 39.3 | 137.5 ± 49.3 | t38=−0.2; p=0.84 |
Values shown are mean ± SD; N/A = Not Applicable
Postmortem Interval
RNA Integrity Number
Laser microdissection procedure
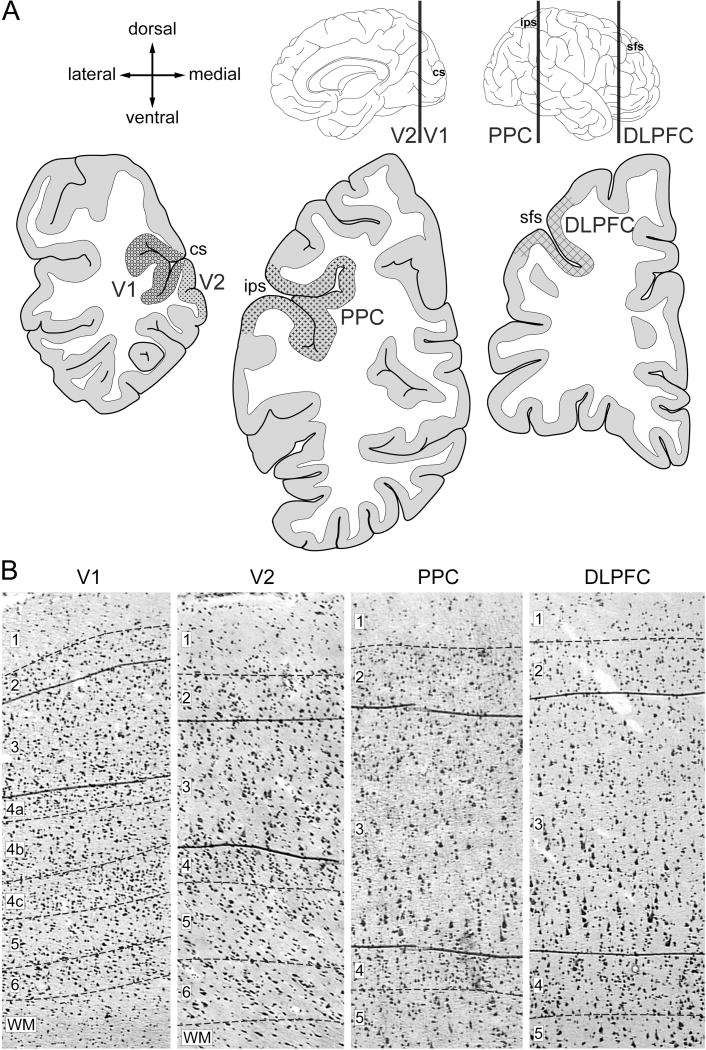
The right hemisphere of each brain was blocked coronally, immediately frozen and stored at −80°C as previously described (25). Four regions [V1 (Brodmann Area 17), V2 (Brodmann Area 18), PPC (Brodmann Area 7), DLPFC (Brodmann Area 46)] were sampled based on their anatomic location and cytoarchitectonic features (Figure 1). Cryostat sections (12 µm) were cut, thaw-mounted onto glass polyethylene naphthalate membrane slides (LEICA Microsystems, Bannockburn, IL) which were coded to blind subject number and diagnosis, dried and stored at −80°C as previously described (26). On the day of microdissection, tissue sections were stained for Nissl substance with thionin and layer 3 was identified based on its characteristic cytoarchitecture (Figure 1B) in portions of each section that were cut perpendicular to the pial surface. Strips (~10 million µm2) containing layer 3 from each region were dissected (Supplemental Figure S1) using a LEICA microdissection system (LMD 6500; 5× objective).
Figure 1. Sampling of cortical regions and layer 3.
(A) Schematic drawings of the medial and lateral surfaces of the right hemisphere of the human brain (top). Vertical lines indicate the approximate locations of the coronal sections (bottom). Locations of cortical regions of the vsWM network selected for sampling are shaded. Arrows labeled dorsal, medial, ventral, and lateral refer to the coronal sections (B) Representative Nissl-stained sections illustrating the laminar borders (dashed lines) and the borders as drawn of layer 3 for laser microdissection in each region. Numbers indicate cortical layers. V1, primary visual cortex; V2, association visual cortex; PPC, posterior parietal cortex; DLPFC, dorsolateral prefrontal cortex; sfs, superior frontal sulcus; ips, intraparietal sulcus; cs, calcarine sulcus.
qPCR analyses
Samples from all four regions of both subjects within each pair were processed together throughout the study. For each sample, RNA was extracted and purified using the RNAeasy Plus Micro Kit (QIAGEN, Inc, Valencia, CA). Total RNA was converted to cDNA using the qScript™ cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD). Forward and reverse primers were designed for each target mRNA to generate PCR amplicons of 85–120 base pairs (Supplemental Table S2). The specificity and efficiency of qPCR amplification for each target mRNA was demonstrated by high amplification efficiency (>96%) across a wide range of cDNA dilutions, and the presence of singular products in dissociation curve analysis.
Transcript expression levels were quantified by qPCR using Power SYBR green dye and ViiA™ 7 Real-Time PCR system (Life Technologies, Carlsbad, CA). For each subject pair, cDNA samples for all cortical regions were processed together on the same 384-well qPCR plate with four replicates per primer set. To avoid batch effects, all transcripts from all regions of both subjects in a pair were run on the same plate, limiting the number of transcripts that could be studied. Splice variants exist for each targeted transcript, but the primer sets amplified all variants for all transcripts except EAAT2 and GRIA2, where the synaptically-enriched splice variants were amplified (Supplemental Table S2).
Using an established method (27), the comparative cycle threshold (CT) approach was used to normalize transcript levels to the geometric mean of the internal reference genes (Beta-Actin and Cyclophilin-A), which were previously reported to have stable expression levels across multiple cortical regions and diagnoses (17, 25, 28–30) and, were not significantly different across region or diagnosis in this cohort (Supplemental Methods). Because the difference in cycle threshold (dCT) between the cycle thresholds of each target transcript and the mean of the reference genes represents the log2-transformed expression ratio of each target transcript to the reference genes mean, the relative expression ratio of each target transcript was determined as 2-dCT (17, 25).
Statistical analyses
To compare transcript levels across regions in unaffected comparison subjects, a mixed-model treating observations from the four regions for each subject as repeated measurements was performed for each transcript. The model included transcript as the dependent variable, region as a fixed effect, and age, sex, brain pH, RIN, PMI and tissue storage time as covariates. F-tests were used to assess the overall region effect, followed by post-hoc pairwise comparisons between regions, using Tukey’s method to control the overall type-I error.
To determine if the expression of any transcripts was altered in schizophrenia, a mixed-model was performed across all subjects for each individual transcript, where fixed effects included diagnosis, region and diagnosis-by-region interaction, while controlling for the covariates listed above. The diagnosis and diagnosis-by-region interaction effects were tested using F-tests. The potential confounding effect of antipsychotic medications, nicotine or other substances of abuse, suicide and other factors frequently comorbid with schizophrenia were also examined (Supplemental Table S3).
Composite scores of the glutamate and GABA measures were computed by summing the normalized (Z-score) expression levels for all glutamate and GABA transcripts, respectively (Supplemental Methods). For each composite measure, a mixed-model was performed across all subjects, where fixed effects included diagnosis, region and diagnosis-by-region interaction, while controlling for the covariates listed above. The diagnosis and diagnosis-by-region interaction effects were tested using F-tests.
All analyses were conducted on log-transformed data to stabilize the variance. Adjustments for multiple comparisons were made using the Benjamini-Hochberg method to control for the false discovery rate (FDR). For the main analyses, the uncorrected p-value is reported in the results. Corrected p-values for the comorbidity and main analyses are reported in Supplemental Tables S3 and S4. Models used SAS version 9.3 to implement PROC MIXED (SAS Institute, Cary, NC) using the restricted maximum likelihood method such that the default (“containment”) method was used to compute the denominator degrees of freedom.
Results
Glutamate and GABA transcript levels in the vsWM network of comparison subjects
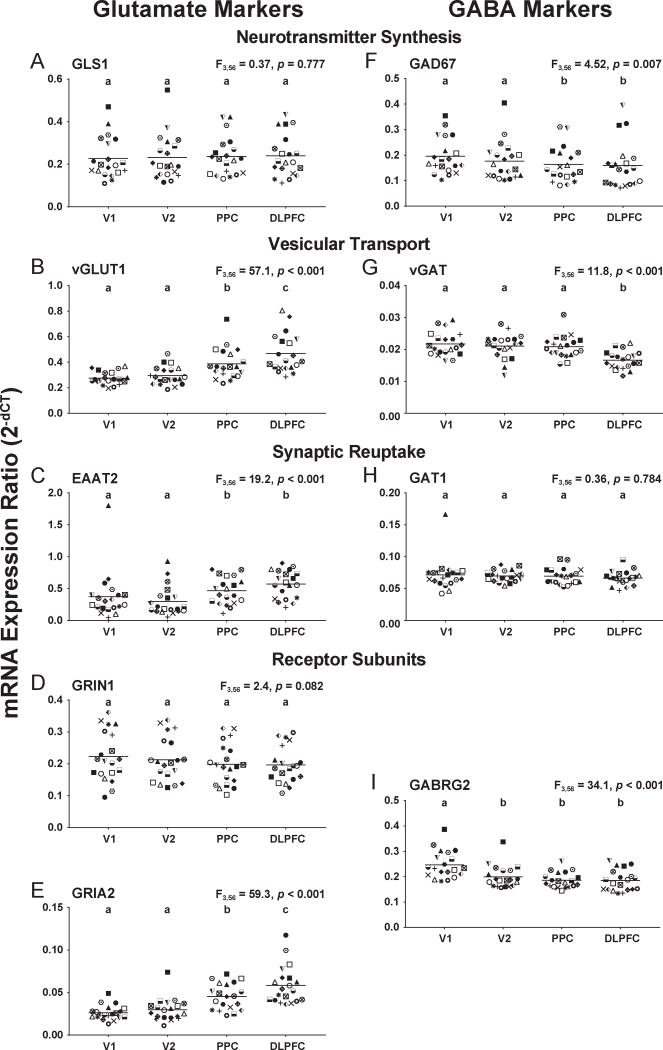
Three glutamate transcripts (Figure 2B,C,E, Supplemental Table S4), vGLUT1 (F3,56=57.1, p<0.0001), EAAT2 (F3,56=19.2, p<0.0001), and GRIA2 (F3,56=59.3, p<0.0001), showed similar patterns of increasing levels of expression from caudal-to-rostral regions (Figure 1A) of the vsWM network. Post-hoc analyses confirmed lower expression in V1 and V2 compared to PPC and DLPFC for each of these transcripts. For vGLUT1 and GRIA2, expression levels were also significantly lower in PPC than DLPFC. In contrast, GLS1 and GRIN1 mRNA levels did not differ across the cortical regions studied (Figure 2A,D).
Figure 2. Glutamate and GABA transcript levels in layer 3 across cortical regions of the vsWM network in unaffected comparison subjects.
For each panel (A-I), target transcript name is at the top center and ANCOVA results for the effect of region at the top right. Individual subjects are shown by the same symbol in all graphs. Horizontal bars represent group means. Regions within each graph that do not share the same letter are significantly different (p<0.05). V1, primary visual cortex; V2, association visual cortex; PPC, posterior parietal cortex; DLPFC, dorsolateral prefrontal cortex.
Three GABA transcripts (Figure 2F,G,I, Supplemental Table S4), GAD67 (F3,56=4.52, p<0.007), vGAT (F3,56=11.8, p<0.0001), and GABRG2 (F3,56=34.1, p<0.0001), shared a similar pattern of decreasing levels of expression from caudal-to-rostral regions of the vsWM network. Post-hoc analyses confirmed higher expression in V1 than DLPFC for each transcript. In contrast, GAT1 mRNA levels did not differ across the cortical regions studied (Figure 2H).
The consistency of expression differences between V1 and DLPFC was supported by findings within individual subjects. For glutamate transcripts, vGLUT1 and GRIA2 levels were lower in V1 than DLPFC in all 20 subjects, and EAAT2 was lower in V1 than DLPFC in 17 of 20 subjects (Supplemental Figure S2B,C,E). For GABA transcripts, GABRG2 levels were higher in V1 than DLPFC in all 20 subjects, and vGAT and GAD67 were higher in V1 than DLPFC in 17 and 16 of 20 subjects, respectively (Supplemental Figure S2F,G,I).
Effect of schizophrenia on expression of glutamate and GABA transcripts in the vsWM network
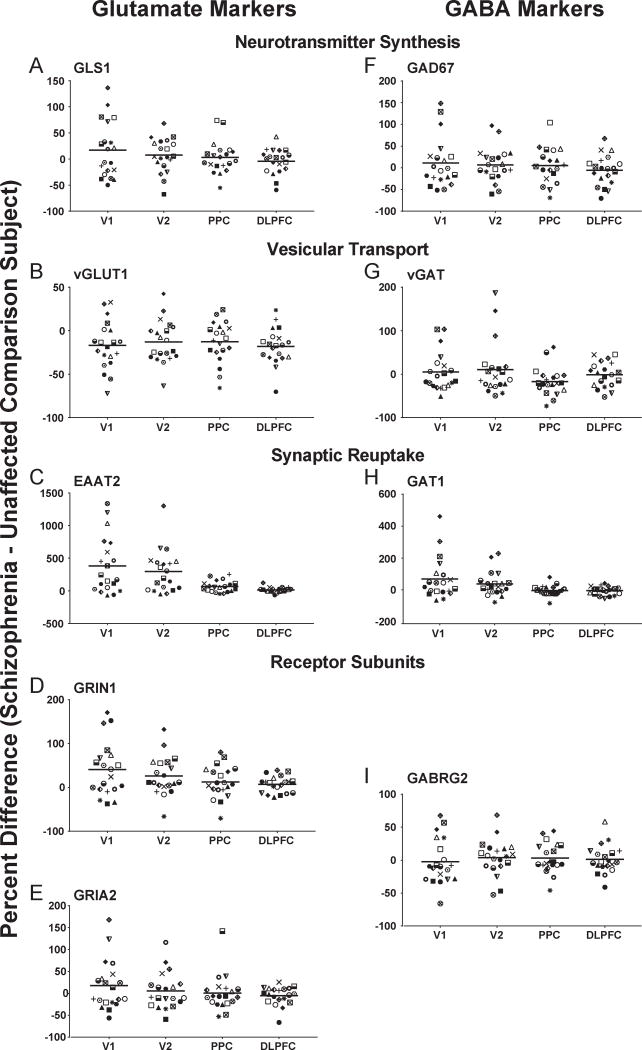
Next, we studied whether schizophrenia was associated with alterations in layer 3 glutamate and GABA transcript levels and whether any such alterations were conserved across regions (Figure 3). For glutamate transcripts, vGLUT1 mRNA expression significantly differed by diagnosis (F1,114=9.3, p<0.003) and region (F3,114=56.1, p<0.0001), but the interaction term was not significant. Post hoc analyses showed that mean transcript levels for vGLUT1 (Figure 3B) were significantly lower in schizophrenia in all regions studied (V1: −18%, p<0.005, corrected p=0.03; V2: −14%, p<0.03, corrected p=0.12; PPC: −14%, p<0.03, corrected p=0.12; and DLPFC: −22%, p=0.005, corrected p=0.04). Expression of EAAT2 mRNA showed significant effects of diagnosis (F1,114=5.6, p<0.02) and region (F3,114=4.5, p<0.005), as well as a significant region-by-diagnosis interaction (F3,114=16.0; p<0.0001). Mean EAAT2 mRNA levels (Figure 3C) were higher in V1 (+286%, p<0.0005, corrected p=0.003) and V2 (+258%, p<0.0001, corrected p=0.004) in schizophrenia (also see Supplemental Figure S3), and in the PPC (+61%) and DLPFC (+7%), although the disease effect in the latter two regions did not achieve statistical significance. For GLS1, GRIN1, and GRIA2 mRNAs (Figure 3A,D,E) neither the effect of diagnosis nor the region-by-diagnosis interaction were significant; however, the effects of region on mRNA levels of GRIA2 (F3,114=93.3, p<0.0001, corrected p<0.001) and GRIN1 (F3,114=9.9, p<0.0001, corrected p<0.001), but not GLS1, were significant. GRIN1 transcript expression (Figure 3D) was higher in V1 (+28%, p=0.02, corrected p=0.11) and in V2 (+18%, p=0.10, corrected p=0.33) in schizophrenia, although the latter finding in V2 did not achieve statistical significance.
Figure 3. Effect of schizophrenia on glutamate and GABA transcript levels across cortical regions of the vsWM network.
For each panel (A-I), target transcript name is at the top center. Individual subjects are shown by the same symbol in all graphs. Horizontal bars represent mean percent difference (schizophrenia – unaffected comparison subject). V1, primary visual cortex; V2, association visual cortex; PPC, posterior parietal cortex; DLPFC, dorsolateral prefrontal cortex.
For each GABA transcript, region had a significant effect on mRNA levels in layer 3 (all F1,114>8.0; all p<0.0001), but there was no effect of diagnosis for any transcript (all F1,114<2.7; all p>0.10). Region-by-diagnosis interaction was significant for vGAT (F3,114=2.8; p<0.05, corrected p=0.08) and GAT1 (F3,114=5.9; p<0.0001, corrected p=0.003), but not for GAD67 or GABRG2. Post-hoc analyses revealed that vGAT levels (Figure 3G) were significantly lower in the PPC (−19%, p<0.005, corrected p=0.03) in schizophrenia, but were not different in V1, V2 or DLPFC. GAT1 mRNA levels (Figure 3F) were higher in V1 (+57%, p=0.07, corrected p=0.29) and GAD67 mRNA levels were lower in DLPFC (−17%, p=0.10, corrected p=0.33) in schizophrenia, but these differences did not achieve statistical significance.
None of the schizophrenia-associated comorbid factors examined (death by suicide, or the use of antipsychotics, antidepressants, anticonvulsants/benzodiazepines, or nicotine at the time of death) had a significant effect on the levels of any transcript in any region.
Composite measures of glutamate and GABA transcripts across nodes of the vsWM network: Effects of schizophrenia
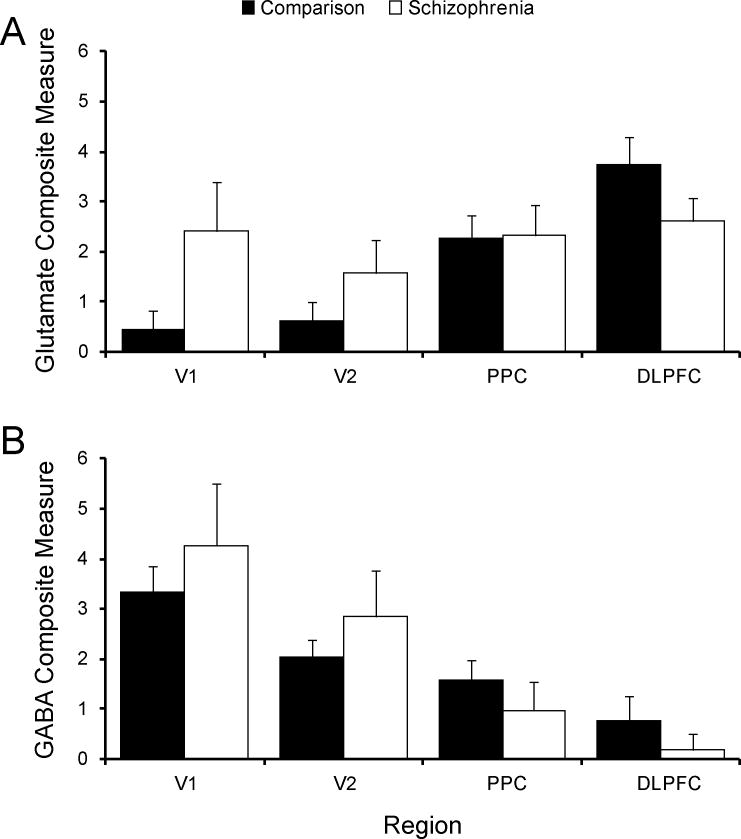
As described in the Methods and Materials, we computed composite glutamate and GABA measures from the normalized expression levels of the relevant transcripts. As expected, in unaffected comparison subjects the composite glutamate (F3,114=14.5, p<0.0001) and GABA (F3,114=5.4, p<0.002) measures confirmed significant regional differences in expression (Figure 4). Like most individual transcripts, the glutamate composite measure showed increasing expression and the GABA composite measure showing decreasing expression from the caudal-to-rostral regions of the vsWM network. However, in the schizophrenia subjects this caudal-to-rostral gradient was lost for the glutamate measure (F3,114=1.3, p=0.28), but was more highly significant for the GABA measure (F3,114=15.8, p<0.0001). For example, the difference between V1 and DLPFC in the composite glutamate measure was much greater in unaffected comparison subjects than in schizophrenia subjects, whereas for the GABA measure this difference was greater in schizophrenia than in unaffected comparison subjects (Figure 4). The diagnosis-by-region interaction was significant for glutamate (F3,114=5.1, p=0.002), but not for GABA (F3,114=1.7, p=0.18), and the 3-way interaction of diagnosis, region, and neurotransmitter composite type (glutamate or GABA) was not significant (F3,265=0.6, p=0.61). An additional analysis excluding EAAT2 from the glutamate composite measure showed a similar diagnosis-by-region interaction with reduced statistical significance (F3,114=2.7, p=0.05).
Figure 4. Effect of schizophrenia on composite measures of glutamate and GABA transcripts in the vsWM network.
In unaffected comparison subjects, the composite A) glutamate (F3,114=14.5, p<0.001) and B) GABA (F3,114=5.4, p=0.002) measures showed significant, and opposite, caudal-to-rostral gradients. Note that in the schizophrenia subjects, this gradient was lost for the glutamate measure (F3,114=1.3, p=0.28), but was more highly significant for the GABA measure (F3,114=15.8, p<0.001). Accordingly, the region-by-diagnosis interaction was significant for the glutamate (F3,114=5.1, p=0.002), but not for the GABA (F3,114=1.7, p=0.18), composite measure. V1, primary visual cortex; V2, association visual cortex; PPC, posterior parietal cortex; DLPFC, dorsolateral prefrontal cortex.
Discussion
In this study, we found that most glutamate and GABA transcripts examined exhibited opposite caudal-to-rostral gradients of expression across nodes of the cortical vsWM network, with glutamate transcript levels increasing and GABA transcript levels decreasing from caudal (V1) to rostral (DLPFC) regions. In schizophrenia subjects, the expression levels of many transcripts were altered, but the disease effect was not conserved across cortical regions. This differential effect of schizophrenia across regions altered the normal caudal-to-rostral gradients of expression in the vsWM network such that the glutamate marker gradient was blunted and the GABA marker gradient was enhanced in the illness.
Altered caudal-to-rostral gradients of glutamate and GABA neurotransmission markers in schizophrenia
Overall, schizophrenia was associated with region-specific alterations in the expression of glutamate and GABA transcripts in layer 3 of the cortical vsWM network such that both glutamate and GABA transcripts were upregulated in V1 and V2, downregulated in the DLPFC, and modestly downregulated or unchanged in the PPC (Figure 4). In combination, the region-specific changes in expression of glutamate and GABA transcripts in schizophrenia resulted in disease-related shifts in the normal caudal-to-rostral patterns of gene expression such that the glutamate marker gradient was blunted and the GABA marker gradient was enhanced in the illness. Thus, although speculative, in schizophrenia deficits in both glutamate and GABA neurotransmission in the DLPFC might impair the ability of layer 3 microcircuitry to increase the power of gamma oscillations required for vsWM function (31), whereas elevated levels of both glutamate and GABA neurotransmission in layer 3 of V1 might contribute to the elevated levels of visual gamma power reported in psychosis (32). Such disease-related shifts in regional patterns of gene expression might also provide a molecular basis for the observation that the normal caudal-to-rostral gradient in the natural frequency of cortical oscillations is disrupted in schizophrenia (33).
The shifts in the caudal-to-rostral gradients of glutamate and GABA markers reflect both conserved and region-specific effects of the illness on the expression of individual transcripts. For example, vGLUT1 mRNA expression was lower in schizophrenia in each of the four cortical regions examined, suggesting a deficit in the amount of glutamate available for synaptic release (34) across the cortical vsWM network which might contribute to the reduced activation of this network when individuals with schizophrenia are challenged with demanding working memory tasks (35). However, depending upon task conditions and possibly stage of illness, increased activation of the vsWM network has also been reported (36). It is important to note that alterations in vGLUT1 mRNA are likely to affect vGLUT1 protein in the axon terminals of both the local collaterals and long-range projections of layer 3 pyramidal neurons.
In contrast to the conserved regional alterations in vGLUT1 mRNA, mean GAD67 mRNA levels in layer 3 were not altered in V1 but were 17% lower levels in the DLPFC from subjects with schizophrenia. Although this difference did not reach statistical significance, the magnitude of the difference is quite similar to multiple prior studies which reported that mean GAD67 mRNA levels in total DLPFC gray matter were 12–36% lower in schizophrenia (23). Indeed, the largest single study (n=62 subject pairs) reported a 14% decrease in mean GAD67 mRNA levels in DLPFC gray matter (30). The absence of a difference of GAD67mRNA levels in V1 appears to contrast with a prior study (17). However, it is important to note that the latter study specifically selected schizophrenia subjects (n=9 pairs) with the largest deficits in GAD67 mRNA expression in the DLPFC and included all 6 layers of V1, whereas the present study examined only layer 3 in V1 from subject pairs selected based on highest quality of RNA independent of any prior knowledge of DLPFC GAD67 levels. In addition, our finding of no alterations in vGAT mRNA levels in the DLPFC is consistent with the prior findings of another group (29), and perhaps not materially different from our prior report of a 7% decrease (p=0.046) in schizophrenia in total DLPFC gray matter (37). Together, these comparisons suggest that disease-related transcript alterations might be selective for specific layers or cell types in certain cortical regions, an interpretation consistent with prior reports of schizophrenia-associated transcript alterations selective for pyramidal neurons (26) or subpopulations of GABAergic neurons (28).
Several factors need to be considered in interpreting the regional patterns of glutamate and GABA transcript levels. First, V1 has a much greater cell packing density relative to other cortical regions (38). In addition, in most regions of the primate neocortex, the ratio of interneurons to principal cells is 1:4, whereas in V1 the ratio is 1:3 (38). Thus, although we collected the same volume of layer 3 tissue from each region of every subject, V1 samples likely contained more neurons and a greater proportion of GABA neurons. Consistent with these findings in the unaffected comparison subjects, GABA transcript levels were higher in V1 than in other regions; however, glutamate transcript levels were lower (Figure 4). Furthermore, levels of glutamate and GABA transcripts were lower and higher, respectively, in V2 than in PPC and DLPFC, even though cell-packing densities in layer 3 appear to be similar across these three regions (38). Thus, our findings do not appear to be confounded by regional differences in cytoarchitecture.
Second, findings from transcriptome studies in monkeys and humans demonstrated that multiple gene groups show a caudal-to-rostral increase or decrease in expression levels (12, 14, 15, 39). Consistent with our results, these studies of tissue homogenates containing all cortical layers found the highest levels of vGLUT1 and GRIA2 mRNAs in the frontal lobe and the highest levels of GAD67 mRNA in the occipital lobe (12, 13). Other markers of glutamate neurotransmission (e.g., Ca2+/calmodulin-dependent kinase (CAMK2B) and certain AMPA and NMDA receptor subunits) had the highest levels of expression in the frontal cortex, whereas certain markers of GABA neurotransmission had the highest expression levels in the occipital lobe (13). These findings support the concept that caudal-to-rostral gradients of expression across cortical regions are present for multiple gene groups and are in the opposite direction for key gene products regulating glutamate and GABA neurotransmission. Importantly, the glutamate and GABA composite measures capture only the transcripts assessed in the present study and thus do not serve as measures of the entire glutamate or GABA neurotransmitter systems.
Third, like all postmortem studies, we cannot definitively exclude the potential influence of other factors comorbid with schizophrenia. Statistical analyses suggest that the factors investigated did not significantly affect transcript expression levels in this cohort (Supplemental Table S3). Furthermore, the mean body mass index (BMI) at time of death in the schizophrenia subjects was 29, which is at the top of the overweight range, suggesting that malnutrition is unlikely to account for altered gene expression in schizophrenia.
Functional Implications
Visuospatial information is conveyed in a hierarchical fashion from caudal (V1) to rostral (DLPFC) cortices (9, 40). Given their larger dendritic arbors and greater densities of dendritic spines (18, 19, 41), layer 3 pyramidal neurons in the DLPFC can receive more excitatory inputs than those in V1, which may support a more extensive level of information integration in higher order association areas of the vsWM network. Consistent with this idea, computational models support regional differences in the strength of local recurrent excitation, with greater strength in association than primary sensory cortices (42–44). Accordingly, our findings in unaffected comparison subjects suggest that a pattern of low glutamate/high GABA transcript expression may contribute to relatively lower local neural network activity in layer 3 of V1 compared to the high glutamate/low GABA transcript expression pattern in layer 3 of the DLPFC. Functionally, for example, high glutamate/low GABA transcripts in DLPFC might reflect an increase in sustained recurrent excitation that allows for the integration of information and enhanced signal-to-noise ratio required for working memory (43, 45), whereas low glutamate/high GABA transcript ratio in V1 might reflect a greater need for rapidly detecting and faithfully tracking dynamic stimuli in early sensory processing (43, 46). However, this interpretation is limited by the difficulty of determining the functional consequences of alterations in transcript levels. For example, because GRIA2, GRIN1 and GABRAG2 are expressed on pyramidal cells and GABAergic interneurons (47), the cellular source of the expression difference would have a substantial effect on its physiological consequences. Cell type-specific and protein level studies are needed to address these questions.
Comparisons between subject groups suggest that the set point for excitatory/inhibitory balance in layer 3 circuitry is lower in the DLPFC and higher in V1 in schizophrenia. These regional differences might help account for findings that excitatory/inhibitory balance seems to be maintained by either comparable increases or decreases in both excitation and inhibition in different cortical networks in schizophrenia (31, 48). However, in vivo studies of cortical network activity and of synaptic levels of glutamate and GABA across cortical regions are needed to determine if excitatory/inhibitory balance in schizophrenia is differentially altered as a function of cortical region or stage of the illness (49).
Finally, the precise balance of activity between glutamatergic pyramidal neurons and GABAergic interneurons in layer 3 is thought to be critical for optimal vsWM function (50). Our findings suggest that this balance might be altered in a region-specific manner in schizophrenia. Thus, disruptions in each region of the network could provide multiple, and potentially compounding, paths to vsWM dysfunction in the illness.
Supplementary Material
Acknowledgments
We would like to thank Dominique Arion, Mary Brady, Dibyadeep Datta and Kelly Rogers for their technical assistance and expertise. This study was supported by NIH grant MH103204 (DAL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: DAL currently receives investigator initiated research funding from Pfizer, and in 2017 served as a consultant to Merck. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70(10):1107–1112. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- 3.Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16(1):27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 5.Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA, et al. Association of Neurocognition With Transition to Psychosis: Baseline Functioning in the Second Phase of the North American Prodrome Longitudinal Study. JAMA Psychiatry. 2016;73(12):1239–1248. doi: 10.1001/jamapsychiatry.2016.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnsten AF. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci. 2015;18(10):1376–1385. doi: 10.1038/nn.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linden DE. The working memory networks of the human brain. Neuroscientist. 2007;13(3):257–267. doi: 10.1177/1073858406298480. [DOI] [PubMed] [Google Scholar]
- 8.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 9.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1(1):1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 10.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 11.Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci U S A. 2004;101(5):1368–1373. doi: 10.1073/pnas.0305337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernard A, Lubbers LS, Tanis KQ, Luo R, Podtelezhnikov AA, Finney EM, et al. Transcriptional architecture of the primate neocortex. Neuron. 2012;73(6):1083–1099. doi: 10.1016/j.neuron.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489(7416):391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, et al. Spatio-temporal transcriptome of the human brain. Nature. 2011;478(7370):483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pletikos M, Sousa AM, Sedmak G, Meyer KA, Zhu Y, Cheng F, et al. Temporal specification and bilaterality of human neocortical topographic gene expression. Neuron. 2014;81(2):321–332. doi: 10.1016/j.neuron.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beneyto M, Meador-Woodruff JH. Expression of transcripts encoding AMPA receptor subunits and associated postsynaptic proteins in the macaque brain. J Comp Neurol. 2004;468(4):530–554. doi: 10.1002/cne.10981. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165(4):479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amatrudo JM, Weaver CM, Crimins JL, Hof PR, Rosene DL, Luebke JI. Influence of highly distinctive structural properties on the excitability of pyramidal neurons in monkey visual and prefrontal cortices. J Neurosci. 2012;32(40):13644–13660. doi: 10.1523/JNEUROSCI.2581-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elston GN, Rosa MG. The occipitoparietal pathway of the macaque monkey: comparison of pyramidal cell morphology in layer III of functionally related cortical visual areas. Cereb Cortex. 1997;7(5):432–452. doi: 10.1093/cercor/7.5.432. [DOI] [PubMed] [Google Scholar]
- 20.Hu W, MacDonald ML, Elswick DE, Sweet RA. The glutamate hypothesis of schizophrenia: evidence from human brain tissue studies. Ann N Y Acad Sci. 2015;1338:38–57. doi: 10.1111/nyas.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE., Jr Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52(4):258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 22.Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43(11):970–977. doi: 10.1016/j.jpsychires.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12(4):335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katsel P, Davis KL, Gorman JM, Haroutunian V. Variations in differential gene expression patterns across multiple brain regions in schizophrenia. Schizophr Res. 2005;77(2–3):241–252. doi: 10.1016/j.schres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Volk DW, Eggan SM, Lewis DA. Alterations in metabotropic glutamate receptor 1alpha and regulator of G protein signaling 4 in the prefrontal cortex in schizophrenia. Am J Psychiatry. 2010;167(12):1489–1498. doi: 10.1176/appi.ajp.2010.10030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arion D, Corradi JP, Tang S, Datta D, Boothe F, He A, et al. Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Mol Psychiatry. 2015;20(11):1397–1405. doi: 10.1038/mp.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7) doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung DW, Volk DW, Arion D, Zhang Y, Sampson AR, Lewis DA. Dysregulated ErbB4 Splicing in Schizophrenia: Selective Effects on Parvalbumin Expression. Am J Psychiatry. 2016;173(1):60–68. doi: 10.1176/appi.ajp.2015.15020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fung SJ, Webster MJ, Weickert CS. Expression of VGluT1 and VGAT mRNAs in human dorsolateral prefrontal cortex during development and in schizophrenia. Brain Res. 2011;1388:22–31. doi: 10.1016/j.brainres.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Kimoto S, Bazmi HH, Lewis DA. Lower expression of glutamic acid decarboxylase 67 in the prefrontal cortex in schizophrenia: contribution of altered regulation by Zif268. Am J Psychiatry. 2014;171(9):969–978. doi: 10.1176/appi.ajp.2014.14010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77(12):1031–1040. doi: 10.1016/j.biopsych.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brealy JA, Shaw A, Richardson H, Singh KD, Muthukumaraswamy SD, Keedwell PA. Increased visual gamma power in schizoaffective bipolar disorder. Psychol Med. 2015;45(4):783–794. doi: 10.1017/S0033291714001846. [DOI] [PubMed] [Google Scholar]
- 33.Ferrarelli F, Sarasso S, Guller Y, Riedner BA, Peterson MJ, Bellesi M, et al. Reduced natural oscillatory frequency of frontal thalamocortical circuits in schizophrenia. Arch Gen Psychiatry. 2012;69(8):766–774. doi: 10.1001/archgenpsychiatry.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erickson JD, De Gois S, Varoqui H, Schafer MK, Weihe E. Activity-dependent regulation of vesicular glutamate and GABA transporters: a means to scale quantal size. Neurochem Int. 2006;48(6–7):643–649. doi: 10.1016/j.neuint.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 35.Barch DM, Csernansky JG. Abnormal parietal cortex activation during working memory in schizophrenia: verbal phonological coding disturbances versus domaingeneral executive dysfunction. Am J Psychiatry. 2007;164(7):1090–1098. doi: 10.1176/ajp.2007.164.7.1090. [DOI] [PubMed] [Google Scholar]
- 36.Hunt MJ, Kopell NJ, Traub RD, Whittington MA. Aberrant Network Activity in Schizophrenia. Trends Neurosci. 2017;40(6):371–382. doi: 10.1016/j.tins.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoftman GD, Volk DW, Bazmi HH, Li S, Sampson AR, Lewis DA. Altered cortical expression of GABA-related genes in schizophrenia: illness progression vs developmental disturbance. Schizophr Bull. 2015;41(1):180–191. doi: 10.1093/schbul/sbt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hendry SH, Schwark HD, Jones EG, Yan J. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci. 1987;7(5):1503–1519. doi: 10.1523/JNEUROSCI.07-05-01503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bakken TE, Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, et al. A comprehensive transcriptional map of primate brain development. Nature. 2016;535(7612):367–375. doi: 10.1038/nature18637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hochstein S, Ahissar M. View from the top: hierarchies and reverse hierarchies in the visual system. Neuron. 2002;36(5):791–804. doi: 10.1016/s0896-6273(02)01091-7. [DOI] [PubMed] [Google Scholar]
- 41.Elston GN, Benavides-Piccione R, DeFelipe J. The pyramidal cell in cognition: a comparative study in human and monkey. J Neurosci. 2001;21(17):RC163. doi: 10.1523/JNEUROSCI.21-17-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaudhuri R, Knoblauch K, Gariel MA, Kennedy H, Wang XJ. A Large-Scale Circuit Mechanism for Hierarchical Dynamical Processing in the Primate Cortex. Neuron. 2015;88(2):419–431. doi: 10.1016/j.neuron.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray JD, Bernacchia A, Freedman DJ, Romo R, Wallis JD, Cai X, et al. A hierarchy of intrinsic timescales across primate cortex. Nat Neurosci. 2014;17(12):1661–1663. doi: 10.1038/nn.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang XJ. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 2001;24(8):455–463. doi: 10.1016/s0166-2236(00)01868-3. [DOI] [PubMed] [Google Scholar]
- 45.Wang XJ. Probabilistic decision making by slow reverberation in cortical circuits. Neuron. 2002;36(5):955–968. doi: 10.1016/s0896-6273(02)01092-9. [DOI] [PubMed] [Google Scholar]
- 46.Buracas GT, Zador AM, DeWeese MR, Albright TD. Efficient discrimination of temporal patterns by motion-sensitive neurons in primate visual cortex. Neuron. 1998;20(5):959–969. doi: 10.1016/s0896-6273(00)80477-8. [DOI] [PubMed] [Google Scholar]
- 47.Lake BB, Ai R, Kaeser GE, Salathia NS, Yung YC, Liu R, et al. Neuronal subtypes and diversity revealed by single-nucleus RNA sequencing of the human brain. Science. 2016;352(6293):1586–1590. doi: 10.1126/science.aaf1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang GJ, Murray JD, Wang XJ, Glahn DC, Pearlson GD, Repovs G, et al. Functional hierarchy underlies preferential connectivity disturbances in schizophrenia. Proc Natl Acad Sci U S A. 2016;113(2):E219–228. doi: 10.1073/pnas.1508436113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anticevic A, Hu X, Xiao Y, Hu J, Li F, Bi F, et al. Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci. 2015;35(1):267–286. doi: 10.1523/JNEUROSCI.2310-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci U S A. 1996;93(24):13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.