Abstract
Motor activity in healthy young humans displays intrinsic fluctuations that are scale-invariant over a wide range of time scales (from minutes to hours). Human postmortem and animal lesion studies showed that the intact function of the suprachiasmatic nucleus (SCN) is required to maintain such scale-invariant patterns. We therefore hypothesized that scale invariance is degraded in patients treated for suprasellar tumors that compress the SCN. To test the hypothesis, we investigated 68 patients with nonfunctioning pituitary macroadenoma and 22 patients with craniopharyngioma, as well as 72 age-matched healthy controls (age range 21.0–70.6 years). Spontaneous wrist locomotor activity was measured for 7 days with actigraphy, and detrended fluctuation analysis was applied to assess correlations over a range of time scales from minutes to 24 h. For all the subjects, complex scale-invariant correlations were only present for time scales smaller than 1.5 h, and became more random at time scales 1.5–10 h. Patients with suprasellar tumors showed a larger decrease in correlations at 1.5–10 h as compared to healthy controls. Within healthy subject, gender and age >33 year were associated with attenuated scale invariance. Conversely, activity patterns at time scales between 10 and 24 h were significantly more regular than all other time scales, and this was mostly associated with age.
In conclusion, scale invariance is degraded in healthy subjects at the ages of >33 year as characterized by attenuation of correlations at time scales 1.5–10 h. In addition, scale invariance was more degraded in patients with suprasellar tumors as compared to healthy subjects.
Keywords: Circadian rhythmicity, craniopharyngioma, detrended fluctuation analysis, nonfunctioning pituitary macroadenoma, scale invariance, suprachiasmatic nucleus
Introduction
Although our daily movements might seem completely voluntary, motor activity in both humans and rats is enslaved by an underlying intrinsic pattern that is similar across different time scales ranging from minutes to hours, independent of extrinsic scheduled events, environmental influences and activity levels (Hu et al., 2004; Hu et al., 2007; Hu et al., 2012). This phenomenon of self-similarity or fractality, in which temporal fluctuation patterns are repeated across different time scales, is known as scale invariance.
Researchers have sought intensively for anatomical structures responsible for this regulation. Lesions of the suprachiasmatic nucleus (SCN) in rodents abolish scale-invariant correlations of locomotor activity at scales larger than 4 h (Hu et al., 2007). This means that the hypothalamic clock orchestrating circadian (~24 h) rhythms in physiology and behavior (Welsh et al., 2010) also directs fluctuation patterns at time scales smaller than 24 h, either as a self-contained multi-oscillator system or by interacting with a network of other activity control nodes. Furthermore, compared to young people, older individuals show reductions in scale-invariant correlations for scales larger than 1.5 h, with an additional decrease in those with Alzheimer’s disease (Hu et al., 2009). Both aging and Alzheimer’s disease are associated with disturbances of sleep and daily activity, which are thought to be caused by associated neuroanatomical changes in the SCN (Swaab et al., 1985; Zhou et al., 1995; Liu et al., 2000; Wu et al., 2006; Harper et al., 2008). Therefore, these results further support the role of the SCN in scale invariance also in humans (Pittman-Polletta et al., 2013) such that scale invariance of motor activity may potentially be used as a noninvasive marker of SCN function in humans (Hu et al., 2013).
However, previous observations of alterations in scale invariance were based on a relatively small sample size, with analyses limited to data during daytime, time scales smaller than 8 h, and subjects under extreme conditions such as Alzheimer’s disease and very old age (e.g., no or few healthy subjects between 30–50 years old). To better understand the effects of aging and SCN dysfunction on scale invariance, we assessed scale invariance of motor activity over a broader range of time scales from minutes up to 24 h, in much larger groups of healthy individuals (n = 72) and patients with a history of compression of the SCN (n = 90) – i.e., patients treated for large benign suprasellar tumors such as nonfunctioning pituitary macroadenomas (NFMA) and craniopharyngiomas. These tumors compress surrounding tissue and present with pituitary insufficiency, visual field defects and headache (Jaffe, 2006; Stamm et al., 2011). Following transsphenoidal adenomectomy, patients chronically suffer from daytime somnolence, reduced sleep efficiency, fragmented sleep-wake patterns, and alterations of diurnal melatonin and temperature rhythmicity (Ullrich et al., 2005; Biermasz et al., 2011; Joustra et al., 2014a; Joustra et al., 2014b; Pickering et al., 2014; Pickering et al., 2017). These symptoms are strongly associated with suprasellar tumor extension (irrespective of hypopituitarism), implying damage to suprasellar structures, e.g., the optic tract or the ventral hypothalamus (most notably the adjacent SCN). The goal was to investigate the robustness of the scale invariance measure to detect healthy and possibly altered SCN functioning. We hypothesized that scale invariance is degraded in patients treated for suprasellar tumors and in a physiological condition of attenuated day–night rhythmicity, i.e., in older individuals.
Subjects and methods
Design
For this study, we analyzed wrist motor activity data collected from patients treated for NFMA or craniopharyngioma, and age-matched healthy individuals during normal daily activity at home. Using these data, detrended fluctuation analysis was performed to assess scale invariance. The Medical Ethics Committees of the Leiden University Medical Center and Copenhagen University Hospital approved the study protocols (P12.237 and H3.2011.057, respectively), and all subjects gave written informed consent.
Subjects
We examined wrist motor activity recordings of 72 healthy individuals (57 Dutch and 15 Danish; 34 women and 38 men; 20–70 years old) and 90 patients treated for NFMA or craniopharyngioma (75 Dutch and 15 Danish; 40 women and 35 men; 18–70 years old) that were collected from two previous studies (Joustra et al., 2014a; Joustra et al., 2014b; Pickering et al., 2014). Inclusion criteria for NFMA and craniopharyngioma were long-term remission (absence of, or stable residual adenoma) after transsphenoidal surgery for these adenomas at least one year ago, adequate and stable hormone replacement therapy for hypopituitarism (see below), and age between 18 and 70 years. Surgery was followed by radiotherapy in selected cases. We excluded patients with diagnosed sleep disorders (e.g., obstructive sleep apnea or periodic legs movement) or use of sleep-promoting- or psychotropic medication. The NFMA were staged according to the classification by Hardy–Wilson (Hardy, 1979). Visual field defects were diagnosed by standard Goldmann perimetry. An endocrinologist periodically evaluated the patients. Pituitary insufficiency (see Supplementary data for definitions) was treated with hydrocortisone, levothyroxine, recombinant human growth hormone (unless contraindicated or not preferred), testosterone in men and estrogen in combination with progestogens in premenopausal women. Dosages were monitored and adjusted as required. Stable replacement was assumed when medication was not adjusted for 4 months, complaints were absent, and basal hormone levels showed adequate replacement.
The age-matched healthy participants fulfilled the same inclusion and exclusion criteria, except for pituitary pathology.
All Dutch subjects were evaluated at the Leiden University Medical Center in the Netherlands, mostly during the spring (68.6%) and winter (21.2%) of 2013. All Danish subjects were evaluated at the Rigshospitalet in Copenhagen Denmark, mostly in the fall (40.0%), winter (46.7%) and spring (13.3%) of 2011–2012.
Actigraphy
Motor activity levels were assessed continuously for 7 consecutive days and nights using an Actiwatch AW7 (CamNtech, Cambridge, UK) in Dutch subjects and an Actiwatch Spectrum (Philips Respironics, Murrysville, PA, USA) in Danish subjects, worn on the nondominant wrist. Subjects maintained their habitual sleep-wake schedules. The accelerometer records the highest amplitude of wrist movement per second in counts (one count representing 0.04 g), and sums these counts in epochs of 1 minute. Actigraphic measurements from Denmark were initially recorded in epochs of 30 seconds for 14 days. For the current analysis, only the first 7 days of the Danish data were used, and 1-minute epochs were formed by summation of two subsequent 30-second epochs.
Using nonparametric calculations, we calculated the interdaily stability, which quantifies similarity of rest-activity rhythms between days, and the intradaily variability, which indicates fragmentation of the day–night rhythm within individual days (Van Someren et al., 1999). Furthermore, averaging the activity patterns of the 7 registration days created an average 24-h activity pattern. From this 24-h pattern, the average movement per epoch of 1 minute was calculated for the hours spent awake, as determined by the actiwatch sleep analysis sleep scoring algorithm. To avoid any influence of differences in actigraphy sensitivity, analyses that include activity levels were only performed in Dutch subjects.
We refer to our previous work for the results of standard actigraphic variables as well as subjective sleep quality, fatigue and daytime sleepiness in these patients (Joustra et al., 2014a; Joustra et al., 2014b; Pickering et al., 2014).
Assessment of scale invariance using detrended fluctuation analysis
Detrended fluctuation analysis was used to determine correlations of activity fluctuations at time scales from minutes to 24 h. It derives the amplitude of activity fluctuations, F(n), at different time scales n. To eliminate the potential effects of trends in the recordings, the second order polynomial function was used to detrend the data in the analysis. Scale invariance is characterized by a power-law form of the fluctuation function, F(n)≈nα, with the scaling exponent α indicating correlations in fluctuations: α = 0.5 represents no correlation in activity fluctuations, indicating complete randomness (or white noise) which is thought to reduce the system’s ability to orchestrate its subsystems appropriately in response to external stimuli; α > 0.5 indicates positive correlations in activity fluctuations (i.e., large values have more probability of being followed by large values and vice versa); and α = 1.5 indicates too much regularity (or Brownian/red noise), which restricts the functional responsiveness of the system, making it vulnerable to catastrophic events (Huikuri et al., 2000; Perkiomaki et al., 2001). Healthy physiological systems show a delicate balance between these two at α ~ 1.0 (pink noise) (Goldberger et al., 2002) indicating strong positive correlations with the highest complexity. Detailed description of the method is described in Gu et al. (Gu et al., 2015). The scaling exponent α was calculated using regression to obtain the best power-law fit of data: F(n)≈nα.
Statistical analysis
Statistical significance of mean differences between healthy participants and either of the patient groups were assessed using the two-tailed independent Student’s t-test or, in case the assumption of normality was not met (Shapiro–Wilk test), the Mann–Whitney U test. Categorical data were compared using the Chi-square test or, when the expected cell count was <5, the Fisher’s exact test. Paired t-test was used to calculate statistical significance of differences between α1, α2 and α3. Three types of generalized linear regression models were used to assess determinants of outcome variables. To test the hypothesis that aging and disease are associated with degraded scale invariance, model 1 assessed the influence of age, gender, mean activity levels and disease (NFMA and craniopharyngioma combined) and their interactions on α1 (scaling exponent at time scales of 0.25–1.5 h), α2 (1.5–10 h) or α3 (10–24 h). To test the hypothesis that degraded scale invariance is associated with objective and subjective parameters of altered circadian rhythmicity, model 2 assessed the influences of both α2 and Δα1–α2 (as well as age, gender and activity levels) on actigraphic sleep duration, sleep efficiency, intradaily variability or interdaily stability (Joustra et al., 2014a), and subjective sleep quality (Pittsburgh sleep quality index (Buysse et al., 1989)), fatigue (multidimensional fatigue inventory (Smets et al., 1995)), or daytime sleepiness (Epworth sleepiness scale (Johns, 1991)). Lastly, to test the hypothesis that suprasellar extension causing visual field defects is associated with degraded scale invariance, model 3 assessed the effect of hypopituitarism, previous radiotherapy and preoperative visual field defects (as well as age, gender and activity levels) and their interactions on each α. In each model, the least-significant co-variates were removed sequentially, until all covariates significantly contributed to the model. Then, Akaike’s information criterion, which measures the tradeoff between goodness of fit and complexity of the model, was used to determine whether the model could be further simplified. The regression coefficient B was used to express effect size. Differences were considered statistically significant at p < 0.05.
Results
Clinical characteristics
The 72 healthy subjects had a median age of 55.5 years (range 21.0–70.6 years) and 47.2% were women.
Patients with NFMA (n = 68, 48.5% women) in long-term remission after transsphenoidal surgery for suprasellar tumor extension had a median age of 59.4 years (range 26.0–70.1 years) (Table 1). Before surgery, suprasellar tumor extension was observed in 94% of cases, and visual field defects in 77.9%. At the time of the actigraphic evaluation, hypopituitarism was present in 82.4%, and all patients received proper and stable replacement therapy except for optional growth hormone replacement, which was left untreated in 15 of 49 growth hormone deficient patients. NFMA patients displayed more intradaily variability (p = 0.005) and less interdaily stability (p = 0.026) than the healthy group.
Table 1.
Clinical characteristics.
| Healthy participants (n = 72) | NFMA patients (n = 68) | Craniopharyngioma patients (n = 22) | |
|---|---|---|---|
| Age (years) | 55.5 (44.0–64.0) | 59.4 (51.5–63.5) | 51.7 (33.3–62.1) |
| Women | 34 (47.2%) | 33 (48.5%) | 7 (31.8%) |
| BMI (kg/m2) | 26.2 (23.4–29.8) | 27.2 (25.3–29.5) | 28.9 (25.8–32.6)* |
| Adjuvant radiotherapy | 20 (29.4%) | 7 (31.8%) | |
| Preoperative VFD | 53 (77.9%) | 14 (63.6%) | |
| Suprasellar extensiona | 64 (94%) | 22 (100%) | |
| Hypopituitarism | 56 (82.4%) | 22 (100%) | |
| ACTH deficiency | 31 (45.6%) | 18 (81.8%) | |
| TSH deficiency | 41 (60.3%) | 22 (100%) | |
| GH deficiency | 49 (72.1%) | 20 (90.9%) | |
| LH/FSH deficiency | 38 (55.9%) | 19 (86.4%) | |
| Vasopressin deficiency | 7 (10.3%) | 14 (63.6%) | |
| Intradaily variability | 0.35 (0.30–0.43) | 0.40 (0.34–0.50)** | 0.38 (0.34–0.51) |
| Interdaily stability | 0.84 (0.76–0.89) | 0.80 (0.68–0.84)* | 0.78 (0.67–0.87) |
| Activity during wake (counts)b | 254 (211–323) | 243 (194–280) | 236 (215–295) |
Data represent median (interquartile range) or number (percentage).
Hardy–Wilson classification (Hardy, 1979) minimal II-B.
Average activity per minute during hours spend awake in counts, each count representing 0.04 g of wrist acceleration per second.
NFMA: nonfunctioning pituitary macroadenoma. VFD: visual field defects.
p < 0.05 or
p < 0.01 compared to healthy subjects.
Furthermore, 22 patients in long-term remission after surgery for craniopharyngioma (31.8% women), with a median age of 51.7 years (range 18.2–70.2 years) were studied. The majority of the patients had visual field defects preoperatively (63.6%). At the time of the actigraphic evaluation, all had hypopituitarism, vasopressin deficiency was present in 63.6%, and all received proper and stable replacement therapy. Differences between craniopharyngioma patients (CP) and the healthy group in intradaily variability and interdaily stability did not reach statistical significance (p = 0.109 and p = 0.058, respectively). CP displayed a higher body mass index than healthy participants (p = 0.027). Dutch and Danish participants, both patients and healthy subjects, did not differ significantly in gender or age.
Scale invariance of activity fluctuations
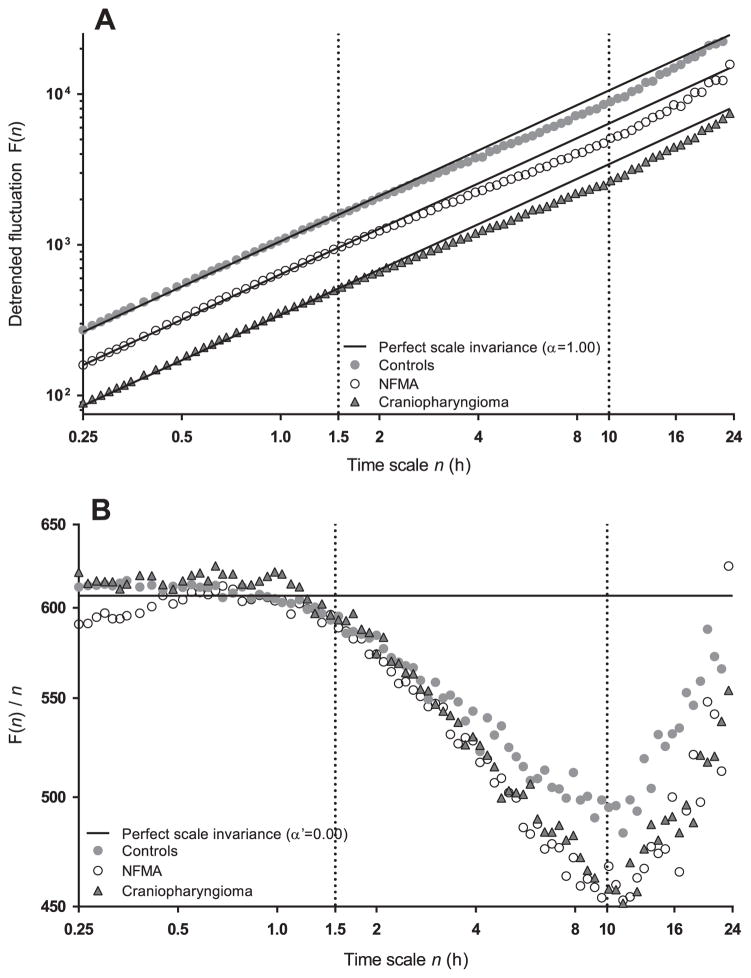
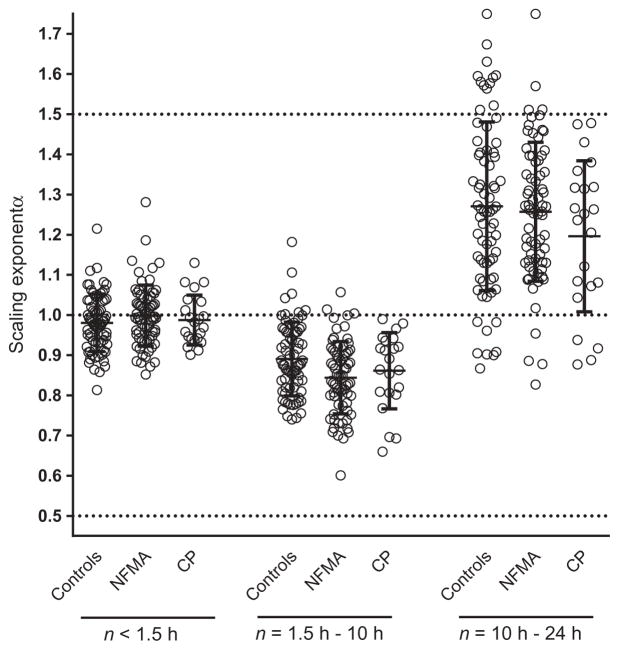
Figure 1A shows the averaged detrended fluctuation function F(n) across time scales 0.25–24 h for each group. As can be observed, scaling behavior differed in three regions: n1 ≈ 0.25–1.5 h, n2 ≈ 1.5–10 h and n3 ≈ 10–24 h. These breakpoints can be seen more clearly in Figure 1B, representing the change in F(n) per n [F(n)/n] with slope α′ (which is α–1). Slopes α1, α2 and α3 were calculated for the three regions n1, n2 and n3, respectively (Figure 2). Complex activity fluctuations with strong correlations (α1 ≈ 1) were observed across the lower time scales (α1 at n1 < 1.5 h) in healthy subjects (α1 [mean±SD]: 0.98 ± 0.07), NFMA (α1: 0.99 ± 0.07) and craniopharyngioma (α1: 0.99 ± 0.06). For scales from 1.5 to 10 h (n2), all groups showed a reduction in scale-invariant correlations, as the scaling exponent α2 decreased significantly (all p < 0.001) in healthy subjects (α2: 0.89 ± 0.09), NFMA (α2: 0.85 ± 0.09) and craniopharyngioma patients (α2: 0.86 ± 0.09). For the time scales from 10 to 24 h (n3), a dramatic inversion of scale-invariant correlations (all p < 0.001) toward red noise (also known as Brownian noise) was observed in healthy subjects (α3: 1.27 ± 0.21), NFMA (α3: 1.26 ± 0.17) and craniopharyngioma (α3: 1.20 ± 0.19).
Figure 1.
Average detrended fluctuation functions F(n) from locomotor activity data of 72 healthy controls, 68 NFMA patients and 22 craniopharyngioma patients. Data are shown on log-log plots and curves are vertically shifted in [A] for better visualization of differences between groups (vertical offset does not alter the slope). The scaling behavior can be separated in three time scale regions based on two breakpoints at ~1.5 h and ~10 h. The break points can be seen more clearly when F(n) is divided by time scale n in [B], indicating the change in F(n) per time unit n. The straight lines represent perfect scale-invariance α = 1.00 [A] or α′ = 0.00 [B].
Figure 2.
Scaling exponent α in 72 healthy controls, 68 NFMA patients and 22 craniopharyngioma patients (CP), obtained from detrended fluctuation analysis, separated in three time scale (n) regions of the 24-h analysis period. The dotted lines represent too much randomness or white noise at α = 0.5, too much regularity or red noise at α = 1.5, and the delicate balance between the two in healthy systems known as complex scale invariance or pink noise at α = 1.0. Bars represent mean ± SD.
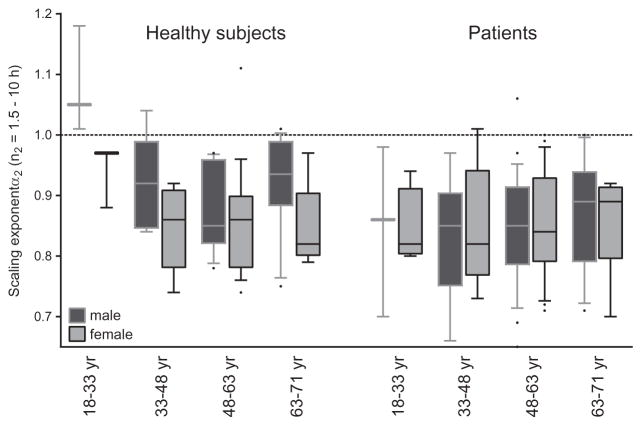
There were no significant effects of activity levels, age, gender or disease on α1 (model 1). For the second timescale region n2, α2 was lower (more random activity fluctuations) in patients as compared to controls (p = 0.014) (Table 2). Disease and gender showed two interaction effects on α2 (p = 0.010): women showed lower α2 than men only within healthy subjects, and the decrease in α2 in patients was significantly stronger in men (Figure 3). Within the healthy control group, one-way ANOVA showed a difference in α2 between the age groups (p = 0.003), i.e., α2 was around 1.0 (indicating the most complex fluctuations) in the youngest subgroup (18–33 years) and was significantly smaller in the other age subgroups (Tukey post hoc). The age groups were based on those used in the study of Hu et al. (Hu et al., 2009), and no effects of age groups were observed in patients (p = 0.595). We also observed a small interaction effect of age and activity levels on α2 (p = 0.021), as the positive association between activity and α2 was stronger in older subjects. The difference between α1 and α2 (Δ = α1–α2), as a direct test of a decrease in scale invariance, was solely influenced by disease (p = 0.006). For the largest time-scale region n3, a larger α3 correlated with age (p = 0.003, +0.193 per decade), and with higher activity level (p = 0.001, +0.472 for every increase of 100 activity counts per minute). In addition, the association between α3 and activity levels was influenced by age, i.e., it was positive in subjects <66 years old and negative at > 66 years old (Supplemental Figure S2). Although statistically significant, this interaction effect was considerably smaller than the individual effects of age and activity (p = 0.004, B = −0.069).
Table 2.
Associations with the scaling exponent α.
| B | 95% CI | p-Value | |
|---|---|---|---|
| α2 at time scales 1.5–10 h | |||
| Disease | −0.074 | −0.114–−0.034 | 0.014 |
| Disease* gender | −0.068a | −0.113–−0.024 | 0.010 |
| Age* activity levels | 0.003b,c | 0.001–0.006 | 0.021 |
| Only in patients | |||
| Radiotherapy* activity levels | 0.041b | 0.012–0.070 | 0.019 |
| Δ = α1–α2 | |||
| Disease | 0.058 | 0.016–0.099 | 0.006 |
| α3 at time scales 10–24 h | |||
| Age | 0.193c | 0.066–0.320 | 0.003 |
| Activity levels | 0.472b | 0.197–0.747 | 0.001 |
| Age* activity levels | −0.069b,c | −0.021–−0.116 | 0.004 |
Data represented factors included in the linear regression model that were best able to predict scaling exponent α at different time scales.
I.e., the disease effect is stronger in men than women.
B for higher activity level: increase of 100 counts per 1-minute epoch.
B for age: increase of 10 years.
interaction. Disease: NFMA or craniopharyngioma.
Figure 3.
Scaling exponent α2 (times scales 1.5–10 h) in 72 healthy subjects and 90 patients (NFMA and craniopharyngioma). Data are separated by gender and in four age groups. In healthy subjects, group size for each age group in men is 3, 8, 11 and 16, and for women 3, 7, 19 and 5. For patients, group size for each age group in men is 3, 9, 25 and 13, and for women 4, 6, 21 and 9. The dotted line represents complex scale invariance with α = 1.0. Boxes display the 25th, 50th and 75th percentile, bars the 10th and 90th percentile and dots the outliers.
Furthermore, lower α2 was associated with more fatigue (p = 0.017) and larger Δ = α1–α2 with better intradaily stability (p = 0.032) (model 2). Neither α2 nor Δ = α1–α2 were associated with subjective sleep quality, daytime sleepiness, sleep duration, sleep efficiency or intradaily variability.
Lastly, within patients, we observed a significant interaction between the effects of activity levels and radiotherapy on α2 (model 3), as the association between higher activity levels and higher α2 was only present in patients without radiotherapy (p = 0.014, B = −0.067, Supplemental Figure S1). No effects of patient characteristics (model 3) on α1, α1–α2 or α3 were observed.
Discussion
The results of this study demonstrate that scale invariance of activity patterns in humans degrades already during midlife. Larger attenuations of scale invariance were observed in patients treated for suprasellar tumors, as well as in women, with older age, and with lower activity levels.
Within the entire time scale window from minutes to 24 h, complex scale-invariant correlations were only observed for time scales smaller than 1.5 h, and became more random at time scales 1.5–10 h. Hu et al. observed a remarkably similar breakpoint at 1.5 h in older individuals (80.8 ± 8.6 years old) and in very old patients with Alzheimer’s disease (Hu et al., 2009). The attenuation of scale-invariant correlations for scales 1.5–10 h in those groups were larger than we observed in our patients and healthy subjects, which may be explained by more severe SCN dysfunction in old age and Alzheimer’s disease (Swaab et al., 1985; Zhou et al., 1995; Liu et al., 2000; Wu et al., 2006; Harper et al., 2008). The study reported no breakpoint in scale invariance in healthy young subjects (n = 13, 25.5 ± 6.1 years) (Hu et al., 2009), which was confirmed in our study as Figure 3 shows an α2 around 1.0 in the corresponding healthy young age group (18–33 years). In addition, our results show that α2 decreases quickly after the age of 33 year, especially in women.
Our observation of a second breakpoint at ~10 h has not been reported previously, either because large time scales were not assessed (Hu et al., 2009) or because the exponent α was only calculated for the entire observation window (Hu et al., 2007), although the two breakpoints were visible in their display of data (Hu et al., 2007). As we observed no effect of disease on activity correlations at time scales larger than 10 h, the increased regularity in this time scale region may depend less on SCN functioning and more on environmental and behavioral cues, (e.g., our environmental light-dark cycle, sleep-wake schedule and daily work schedule). Therefore, although complexity (alpha close to one) is a biomarker of healthy physiology, this might not be the case, or the intrinsic patterns can be masked, at larger time scales where imposed environmental rhythms play an important role. Aging was associated with further increased regularity at this larger time scale, which might imply reduced integration and orchestration of the SCN with other oscillators of motor activity control, or might reflect more dominating environmental or behavioral influences.
We observed an additional decrease of α2 in patients treated for tumors with suprasellar extension. Furthermore, Figure 3 shows that complex scale-invariant correlations during time scales 1.5–10 h are already decreased in young patients as compared to healthy controls. A history of supra-sellar tumor extension is therefore an additional risk factor for a decrease in the scale-invariant correlations at time scales that have previously been shown to be affected by the SCN. Within patients, we observed an association between higher activity levels and larger α2 (close to 1) only in those that without a history of radiotherapy, but there was no main effect of radiotherapy on α2. The explanation for this phenomenon remains unclear, but due to the observational nature of the data we cannot exclude confounding factors, e.g., by indications for radiotherapy.
Although Gu et al. observed strong effects of regular exercise on scale invariance in mice (Gu et al., 2015), we observed marginal associations of α2 with differences in activity level. As our study is observational, we cannot draw causal conclusions, and variations in activity might have been too small to show an effect. Future intervention studies in cohorts of young versus middle-aged healthy individuals should be performed to determine whether significantly improving activity levels results in a change in scale-invariant correlations.
The observed attenuation of scale-invariant correlations at time scales between 1.5–10 h in our healthy subjects indicated that even in healthy middle-aged individuals, the physiological balance between regularity and randomness is suboptimal. The complex physiological fluctuations that resemble pink noise are thought to reflect system plasticity and adaptability in response to unpredictable stimuli and stressors because the alterations have been associated with pathologic conditions. For instance, altered correlations in heart rate fluctuations are observed in patients with heart failure (Huikuri et al., 2000; Makikallio et al., 2001a; Makikallio et al., 2001b) and atrial fibrillation (Vikman et al., 1999), and more random gait is observed in Huntington’s disease (Hausdorff et al., 1997). The clinical significance of suboptimal scale invariance in activity patterns has not been established yet, although lower activity correlation at 1.5–10 h or its difference from the correlation at smaller time scales has been associated with increased age, with Alzheimer’s disease and with neurotransmitter content in the SCN in postmortem studies (Hu et al., 2009; Hu et al., 2013). In the current study, decreased α2 was associated with complaints of fatigue. Future studies are warranted to investigate the mechanism underlying the association.
Strengths of the current study were the large groups included in the study, allowing investigation of various associations. Limitations include the sensitivity of the actigraph, whose signal-to-noise ratio becomes more unfavorable during periods of low upper extremity movements, especially during the sleep/nap episodes. We did not consider the potential effects of light intensity on activity patterns. The sample size in our younger subgroups was small, requiring further studies to determine the exact time course of deterioration of α2 across age. Also, the extent of SCN dysfunctioning in our patients was not known as no direct measure of SCN functioning exists. However, patients had other indirect signs of SCN dysfunctioning, i.e., altered objective and subjective sleep-wake cycle (Biermasz et al., 2011; Joustra et al., 2014a; Pickering et al., 2014). In a subset of patients, rhythmicity of melatonin, temperature and sleep quality were measured and found to be disturbed, albeit in variable patterns (Joustra et al., 2014b).
In conclusion, the results of this study have demonstrated that healthy middle-aged subjects show robust scale invariance of activity patterns at time scales of up to 1.5 h, which attenuated at larger scales leading to more random activity fluctuations at time scales from 1.5 to 10 h and more regular activity patterns at time scales from 10 to 24 h. Activity patterns at time scales between 1.5 and 10 h were more affected in patients with suprasellar tumors, in line with previous reports associating these time scales with SCN function. We also observed effects of gender on activity patterns at time scales 1.5–10 h, as well as a rapid decline in activity correlations with age (starting as early as approximately 33 years old). Scale invariance is therefore already starting to become fragile in healthy middle-aged subjects.
Supplementary Material
Acknowledgments
Mr. Y. Robbers is acknowledged for his advice in the statistical analyses.
Funding
Dr. Scheer has received speaker fees from Bayer Healthcare, Sentara Healthcare and Philips. Dr. N.R. Biermasz was supported by the Netherlands Organization for Health Research and Development (Clinical Fellows 90700195, Veni 91613125), dr. C. Gu by the Netherlands Organization for Scientific Research grant no. 645.000.010 and by the National Natural Science Foundation of China grant no. 11505114, dr. F.A. Scheer in part by a National Institutes of Health grant R01 HL118601, dr. K. Hu in part by a National Institutes of Health grant R01AG048108-01A1 and Ulla Feldt-Rasmussen in part by the NovoNordic Foundation.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Supplemental data for this article can be accessed on the publisher’s website.
References
- Biermasz NR, Joustra SD, Donga E, Pereira AM, Van Duinen N, Van Dijk M, Van Der Klaauw AA, Corssmit EP, Lammers GJ, Van Kralingen KW, et al. Patients previously treated for nonfunctioning pituitary macroadenomas have disturbed sleep characteristics, circadian movement rhythm, and subjective sleep quality. J Clin Endocrinol Metab. 2011;96:1524–32. doi: 10.1210/jc.2010-2742. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Goldberger AL, Amaral LA, Hausdorff JM, Ivanov PC, Peng CK, Stanley HE. Fractal dynamics in physiology: Alterations with disease and aging. Proc Natl Acad Sci U S A. 2002;99(Suppl 1):2466–72. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Coomans CP, Hu K, Scheer FA, Stanley HE, Meijer JH. Lack of exercise leads to significant and reversible loss of scale invariance in both aged and young mice. Proc Natl Acad Sci U S A. 2015;112:2320–24. doi: 10.1073/pnas.1424706112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. The transsphenoidal surgical approach to the pituitary. Hosp Pract. 1979;14:81–89. doi: 10.1080/21548331.1979.11707562. [DOI] [PubMed] [Google Scholar]
- Harper DG, Stopa EG, Kuo-Leblanc V, McKee AC, Asayama K, Volicer L, Kowall N, Satlin A. Dorsomedial SCN neuronal subpopulations subserve different functions in human dementia. Brain. 2008;131:1609–17. doi: 10.1093/brain/awn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Mitchell SL, Firtion R, Peng CK, Cudkowicz ME, Wei JY, Goldberger AL. Altered fractal dynamics of gait: Reduced stride-interval correlations with aging and Huntington’s disease. J Appl Physiol (1985) 1997;82:262–69. doi: 10.1152/jappl.1997.82.1.262. [DOI] [PubMed] [Google Scholar]
- Hu K, Harper DG, Shea SA, Stopa EG, Scheer FA. Noninvasive fractal biomarker of clock neurotransmitter disturbance in humans with dementia. Sci Rep. 2013;3:2229. doi: 10.1038/srep02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Ivanov PC, Chen Z, Hilton MF, Stanley HE, Shea SA. Non-random fluctuations and multi-scale dynamics regulation of human activity. Physica A. 2004;337:307–18. doi: 10.1016/j.physa.2004.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Meijer JH, Shea SA, vander Leest HT, Pittman-Polletta B, Houben T, Van OF DT, Scheer FA. Fractal patterns of neural activity exist within the suprachiasmatic nucleus and require extrinsic network interactions. PLoS One. 2012;7:e48927. doi: 10.1371/journal.pone.0048927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Scheer FA, Ivanov PC, Buijs RM, Shea SA. The suprachiasmatic nucleus functions beyond circadian rhythm generation. Neuroscience. 2007;149:508–17. doi: 10.1016/j.neuroscience.2007.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Van Someren EJ, Shea SA, Scheer FA. Reduction of scale invariance of activity fluctuations with aging and Alzheimer’s disease: Involvement of the circadian pacemaker. Proc Natl Acad Sci U S A. 2009;106:2490–94. doi: 10.1073/pnas.0806087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huikuri HV, Makikallio TH, Peng CK, Goldberger AL, Hintze U, Moller M. Fractal correlation properties of R-R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation. 2000;101:47–53. doi: 10.1161/01.cir.101.1.47. [DOI] [PubMed] [Google Scholar]
- Jaffe CA. Clinically non-functioning pituitary adenoma. Pituitary. 2006;9:317–21. doi: 10.1007/s11102-006-0412-9. [DOI] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–45. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Joustra SD, Kruijssen E, Verstegen MJ, Pereira AM, Biermasz NR. Determinants of altered sleep-wake rhythmicity in patients treated for nonfunctioning pituitary macro-adenomas. J Clin Endocrinol Metab. 2014a;99(12):4497–505. doi: 10.1210/jc.2014-2602. [DOI] [PubMed] [Google Scholar]
- Joustra SD, Thijs RD, Van Den Berg R, Van Dijk M, Pereira AM, Lammers GJ, Van Someren EJ, Romijn JA, Biermasz NR. Alterations in diurnal rhythmicity in patients treated for nonfunctioning pituitary macroadenoma; a controlled study and literature review. Eur J Endocrinol. 2014b;171(2):217–28. doi: 10.1530/EJE-14-0172. [DOI] [PubMed] [Google Scholar]
- Liu RY, Zhou JN, Hoogendijk WJ, Van Heerikhuize J, Kamphorst W, Ua U, Ma H, Df S. Decreased vasopressin gene expression in the biological clock of Alzheimer disease patients with and without depression. J Neuropathol Exp Neurol. 2000;59:314–22. doi: 10.1093/jnen/59.4.314. [DOI] [PubMed] [Google Scholar]
- Makikallio TH, Huikuri HV, Hintze U, Videbaek J, Mitrani RD, Castellanos A, Myerburg RJ, Moller M. Fractal analysis and time- and frequency-domain measures of heart rate variability as predictors of mortality in patients with heart failure. Am J Cardiol. 2001a;87:178–82. doi: 10.1016/s0002-9149(00)01312-6. [DOI] [PubMed] [Google Scholar]
- Makikallio TH, Huikuri HV, Makikallio A, Sourander LB, Mitrani RD, Castellanos A, Myerburg RJ. Prediction of sudden cardiac death by fractal analysis of heart rate variability in elderly subjects. J Am Coll Cardiol. 2001b;37:1395–402. doi: 10.1016/s0735-1097(01)01171-8. [DOI] [PubMed] [Google Scholar]
- Perkiomaki JS, Zareba W, Daubert JP, Couderc JP, Corsello A, Kremer K. Fractal correlation properties of heart rate dynamics and adverse events in patients with implantable cardioverter-defibrillators. Am J Cardiol. 2001;88:17–22. doi: 10.1016/s0002-9149(01)01578-8. [DOI] [PubMed] [Google Scholar]
- Pickering L, Jennum P, Gammeltoft S, Poulsgaard L, Feldt-Rasmussen U, Klose M. Sleep-wake and melatonin pattern in craniopharyngioma patients. Eur J Endocrinol. 2014;170:873–84. doi: 10.1530/EJE-13-1025. [DOI] [PubMed] [Google Scholar]
- Pickering L, Klose M, Feldt-Rasmussen U, Jennum P. Polysomnographic findings in craniopharyngioma patients. Sleep Breath. 2017 doi: 10.1007/s11325-017-1574-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Pittman-Polletta BR, Scheer FA, Butler MP, Shea SA, Hu K. The role of the circadian system in fractal neurophysiological control. Biol Rev Camb Philos Soc. 2013;88:873–94. doi: 10.1111/brv.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets EM, Garssen B, Bonke B, De Haes JC. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–25. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- Stamm AC, Vellutini E, Balsalobre L. Craniopharyngioma. Otolaryngol Clin North Am. 2011;44:937–952viii. doi: 10.1016/j.otc.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- Ullrich NJ, Scott RM, Pomeroy SL. Craniopharyngioma therapy: Long-term effects on hypothalamic function. Neurologist. 2005;11:55–60. doi: 10.1097/01.nrl.0000149971.27684.d4. [DOI] [PubMed] [Google Scholar]
- Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB. Bright light therapy: Improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16:505–18. doi: 10.3109/07420529908998724. [DOI] [PubMed] [Google Scholar]
- Vikman S, Makikallio TH, Yli-Mayry S, Pikkujamsa S, Koivisto AM, Reinikainen P, Airaksinen KE, Huikuri HV. Altered complexity and correlation properties of R-R interval dynamics before the spontaneous onset of paroxysmal atrial fibrillation. Circulation. 1999;100:2079–84. doi: 10.1161/01.cir.100.20.2079. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: Cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–77. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YH, Fischer DF, Kalsbeek A, Garidou-Boof ML, Van Der Vliet J, Van Heijningen C, Ry L, Jn Z, Df S. Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the “master clock”. Faseb J. 2006;20:1874–76. doi: 10.1096/fj.05-4446fje. [DOI] [PubMed] [Google Scholar]
- Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol Aging. 1995;16:571–76. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.