Abstract
Background
The durability of first-line regimen is important to achieve long-term treatment success for the management of HIV infection. Our analysis describes the duration of sequential ART regimens and identifies the determinants leading to treatment change in HIV-positive patients initiating in Asia.
Methods
All HIV-positive adult patients initiating first-line ART in 2003–2013, from eight clinical sites among seven countries in Asia. Patient follow-up was to May 2014. Kaplan-Meier curves were used to estimate the time to second-line ART and third-line ART regimen. Factors associated with treatment durability were assessed using Cox proportional hazards model.
Results
A total of 16,962 patients initiated first-line ART. Of these, 4,336 patients initiated second-line ART over 38,798 person-years (pys), a crude rate of 11.2 (95% CI 10.8, 11.5) per 100 pys. The probability of being on first-line ART increased from 83.7% (95% CI 82.1, 85.1%) in 2003–2005 to 87.9% (95% CI 87.1, 88.6%) in 2010–2013. Third-line ART was initiated by 1,135 patients over 8,078 pys, a crude rate of 14.0 (95% CI 13.3, 14.9) per 100 pys. The probability of continuing second-line ART significantly increased from 64.9% (95% CI 58.5, 70.6%) in 2003–2005 to 86.2% (95% CI 84.7, 87.6%) in 2010–2013.
Conclusions
Rates of discontinuation of first- and second-line regimens have decreased over the last decade in Asia. Subsequent regimens were of shorter duration compared to the first-line regimen initiated in the same year period. Lower CD4+ T-cell count and the use of suboptimal regimens were important factors associated with higher risk of treatment switch.
Introduction
The introduction of combination antiretroviral treatment (ART) has dramatically changed the course of HIV infection, leading to a drastic reduction in morbidity and mortality in the HIV-positive population [1–3]. The benefits of using ART have been well described including immunologic repletion, durable virological suppression and a substantial decline in the incidence of AIDS-related diseases [4–7]. However, ART is not curative and HIV has become a chronic disease which demands lifelong treatment.
The durability of first-line regimen is particularly important to achieve long-term treatment success for the management of HIV infection. Subsequent regimens are more complex, costly and have exhibited progressively shorter durability [8,9]. Factors associated with regimen longevity include adherence [10,11], patient profile [12], regimen complexity [13], drug tolerance and toxicity [14], and country income or HIV monitoring levels [15]. In resource-rich settings, ART regimens are individualized in order to minimize these factors, increasing the chance for a successful long term HIV outcome.
In the Asia-Pacific region, with an estimated 4.9 million people living with HIV and an overall treatment coverage of 51% [16], access to treatment options beyond first-line and to virological and immunological monitoring remains limited [17]. WHO has proposed a public-health approach to ART based on standardized simplified treatment protocols, tools and approaches to clinical decision-making [18]. Most countries within the region have implemented these strategies [19]. ART durability experienced in clinical practice is useful for predicting lifelong treatment requirements and examining future needs of second- and third-line ART regimens.
However, there are few data published on the durability of ART regimens and the factors that limit their success in the Asia-Pacific region. The aim of this study is to examine the time until second- and third-line ART regimens from first-line ART regimen and to identify the determinants leading to treatment change in HIV-positive patients attending care in a regional research network in the Asia-Pacific region between 2003 and 2013.
Methods
The study population consisted of patients enrolled in the TREAT Asia HIV Observational Database-Low Intensity TransfEr (TAHOD-LITE) sub study of the TREAT Asia HIV Observational Database (TAHOD), part of the IeDEA (International Epidemiology Databases to Evaluate AIDS) global cohort consortium. While TAHOD collects rich clinical data on a subset of patients seen at 20 clinical sites in Asia [20], TAHOD-LITE collects data on all patients seen at eight sites from Cambodia, Hong Kong, India, Indonesia, Singapore, South Korea and Vietnam. A more detailed description of TAHOD-LITE has been described previously [21]. Briefly, patient data routinely collected at clinic visits, including patient demographics, hepatitis serology, HIV-related laboratory test results and ART history, are anonymized and then electronically transferred to the Kirby Institute, UNSW Australia. Patients are followed-up as they attend routine clinic visits. Currently, patient data are available to May 2014. Ethical approvals were obtained for TAHOD-LITE from Institutional Review boards (IRBs) at each participating site, the University of New South Wales and the coordinating center at TREAT Asia/amfAR. Written consent is collected only if required by the site-specific IRB.
The primary objective was to analyse and describe the trends in, and factors associated with, treatment durability on first- and second-line ART regimens as well as to assess the need for second-line ART regimen for each country and overall. Secondary study objectives were to describe the temporal changes in first-, second- and third-line ART regimens for each country and overall.
Patients were included in this analysis if they were aged over 18 years, initiated an ART regimen consisting of three or more antiretroviral (ARV) drugs from 01 January 2003 to 31 December 2013, and had at least one subsequent visit after ART initiation. The first ART regimen was defined as initiating a regimen consisting of three or more ARV drugs for ≥14 days. Second-line ART regimen was defined as a subsequent regimen which had at least one drug class change or two individual drug changes within NRTI drug class that was undertaken for ≥14 days. Third-line ART regimen was the next regimen which had at least one drug class change or two individual drug changes within a drug class that was undertaken for ≥14 days. Changes in drug regimens could have been made for any reason (for example, failure, toxicity, simplification), and may have not been correlated with CD4+ T-cell count, viral load, or reasons for change. Alterations to dosage were not considered.
Statistical analyses
First-line ART durability was considered as the time from first-line ART initiation to second-line ART initiation. Second-line ART durability was considered as the time from second-line ART initiation to third-line ART initiation. If a patient had a treatment break between ART regimens, then the date of ceasing the prior ART was taken as the end date for the previous regimen and the initiation date of the subsequent regimen was taken as the start date of the next regimen (that is, time during treatment breaks was not included). Patients who were lost to follow up (LTFU) or had ceased a regimen without starting another regimen during the follow-up period were censored. The censor date was defined as the date of death, most recent clinic visit or date of ceasing ART treatment, whichever occurred first. Kaplan-Meier curves were used to estimate the probability of remaining on the given regimen, by the year of ART initiation, for each country and overall. Log-rank tests were used to determine whether regimen switching was significantly different between the years of ART initiation.
A Cox proportional hazards model, stratified by clinical site, was used to evaluate the risk factors associated with treatment durability. The predictor variables, selected a priori, included year of ART initiation, age (years), sex, mode of HIV exposure, baseline HIV viral load, baseline CD4+ T-cell count, ART regimen, previous mono/duo antiretroviral exposure, and hepatitis B co-infection (surface antigen; HBV) and hepatitis C co-infection (antibody; HCV). Year of ART initiation was categorized into three groups (2003–2005, 2006–2009, 2010–2013), which aligned with major modifications to WHO recommendations including ART scale-up from 2003, no longer recommended stavudine (d4T) for first-line regimen in 2006 and earlier ART initiation at higher CD4+ T-cell counts in 2010–2013 [22–25]. Baseline laboratory results were considered the result closest to, and within 6 months, of initiation of the given ART regimen. The ART regimens were also described, by year of initiation, using histograms.
Data were analysed using Stata version 14 (Stata Corporation, College Station, Texas, USA) and SAS software (Version 9.4 for Windows; SAS Institute Inc., Cary, NC, USA).
Results
A total of 16,962 patients, aged 18 years or older, had initiated a first-line ART regimen from 1 January 2003 to 31 December 2013, and had subsequent follow-up visits. Of these, 4,336 had initiated a second-line ART and 1,135 a third-line ART.
Patient Characteristics
A summary of patient demographics by first-, second- and third-line ART regimen is given in Table 1. The majority of the patients were male (69%) aged between 31–40 years (45%), had heterosexual mode of HIV exposure (80%), and initiated ART in recent years (46% in 2010–2013). Most patients had not been tested for hepatitis B (45%) or hepatitis C (50%) during follow-up. The median CD4+ T-cell count prior to first-line ART regimen initiation was 136 cells/μl (IQR 50–231). The median HIV viral load prior to first-line ART regimen initiation was 110,782 copies/ml (IQR 30,500–372,000).
Table 1.
Demographics of patients by first-, second- and third-line ART regimen
| First-line regimen (n= 16,962) | Second-line regimen (n= 4,336) | Third-line regimen (n= 1,135) | |
|---|---|---|---|
|
| |||
| Year of ART initiation | |||
| 2003–2005 | 2,874 (17) | 274 (6) | 72 (6) |
| 2006–2009 | 6,248 (37) | 1,473 (34) | 381 (34) |
| 2010–2013 | 7,840 (46) | 2,589 (60) | 682 (60) |
| Age at ART initiation | |||
| ≤30 years | 4,328 (25) | 630 (14) | 139 (12) |
| 31–40 years | 7,648 (45) | 1,981 (46) | 482 (42) |
| 41–50 years | 3,335 (20) | 1,161 (27) | 361 (32) |
| 51+ years | 1,651 (10) | 564 (13) | 153 (14) |
| Median (IQR) | 36 (30–42) | 38 (33–45) | 40 (34–46) |
| Sex | |||
| Male | 11,637 (69) | 3,155 (73) | 843 (74) |
| Female | 5,309 (31) | 1,176 (27) | 292 (26) |
| Transgender | 16 (<1) | 5 (<0.2) | 0 (-) |
| Mode of HIV exposure | |||
| Heterosexual | 13,549 (80) | 3,445 (79) | 917 (81) |
| Homosexual | 1,300 (8) | 407 (9) | 102 (9) |
| Injecting drug user | 780 (4) | 132 (3) | 28 (2) |
| Other/Unknown | 1,333 (8) | 352 (8) | 88 (8) |
| Hepatitis B (surface antigen) co-infection (ever) | |||
| Positive | 886 (5) | 227 (5) | 42 (4) |
| Negative | 8,510 (50) | 2,192 (51) | 562 (49) |
| Not tested | 7,566 (45) | 1,917 (44) | 531 (47) |
| Hepatitis C (antibody) co-infection (ever) | |||
| Positive | 1,027 (6) | 187 (4) | 40 (3) |
| Negative | 7,456 (44) | 1,924 (45) | 464 (41) |
| Not tested | 8,479 (50) | 2,225 (51) | 631 (56) |
| CD4+ T-cell count at ART initiation | |||
| ≤50 cells/μl | 3,623 (21) | 532 (12) | 149 (13) |
| 51–100 cells/μl | 2,291 (13) | 424 (10) | 106 (9) |
| 101–200 cells/μl | 3,848 (23) | 802 (18) | 199 (18) |
| >200 cells/μl | 4,692 (28) | 1,844 (43) | 485 (43) |
| Not tested | 2,508 (15) | 734 (17) | 196 (17) |
| Median (IQR) | 136 (50–231) | 207 (93–371) | 214 (88–390) |
| HIV viral load at ART initiation | |||
| <1,000 copies/ml | 141 (1) | 618 (14) | 196 (17) |
| 1,000–10,000 copies/ml | 310 (2) | 137 (3) | 34 (3) |
| ≥10,001 copies/ml | 3,147 (18) | 692 (16) | 172 (15) |
| Not tested | 13,364 (79) | 2,889 (67) | 733 (65) |
| Median (IQR) | 110,782 (30,500–372,000) | 6,080 (49–106,000) | 1,628 (49–103,046) |
| ART regimen | |||
| NRTI+NNRTI | 16,030 (94) | 2,287 (53) | 412 (36) |
| NRTI+PI | 819 (5) | 1,504 (35) | 509 (45) |
| Other | 113 (1) | 545 (12) | 214 (19) |
| Previous mono/dual therapy | |||
| No | 16,365 (96) | 4,061 (94) | 1,022 (90) |
| Yes | 597 (4) | 275 (6) | 113 (10) |
Data are n (%) unless otherwise indicated. ART, antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
ART regimens over time
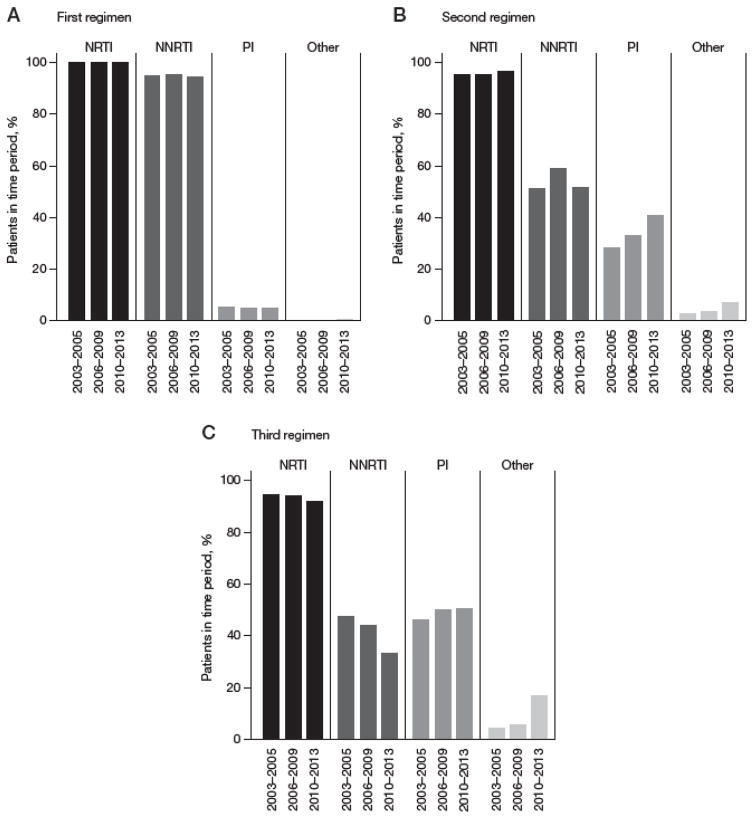
NRTI and non-nucleoside reverse transcriptase inhibitors (NNRTI) use in the first-line ART regimen remained high (>95%) while protease inhibitor (PI) use was minimal (<5%), regardless of the year of ART initiation (Figure 1A). NNRTI use in the second-line ART regimen decreased to less than 60% of patients, remaining relatively stable across year of ART initiation while PI use in the second-line ART regimen steadily increased over time (Figure 1B). In the third-line regimen, NNRTI use steadily decreased from 47% in 2003–2005 to 33% in 2010–2013 and PI use has remained stable. In 2010–2013, there was an increase in the use of other classes, which was mainly attributed to greater raltegravir use at some clinics (8.5% of patients overall; Figure 1C).
Figure 1. Proportion of patients initiating in each drug class, by year of ART initiation.
(A) First-line regimen (drugs initiated in other drug class for first-line regimen included: raltegravir [0.4% overall]; clinical trial drug [<0.1%]). (B) Second-line regimen (drugs initiated in other drug class for second regimen included: raltegravir [3.9%]; clinical trial drug [1.8%]). (C) Third-line regimen (drugs initiated in other drug class for third regimen included: raltegravir [8.5%]; cobicistat [0.5%]; elvitegravir [0.5%]; clinical trial drug [3.8%]). NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
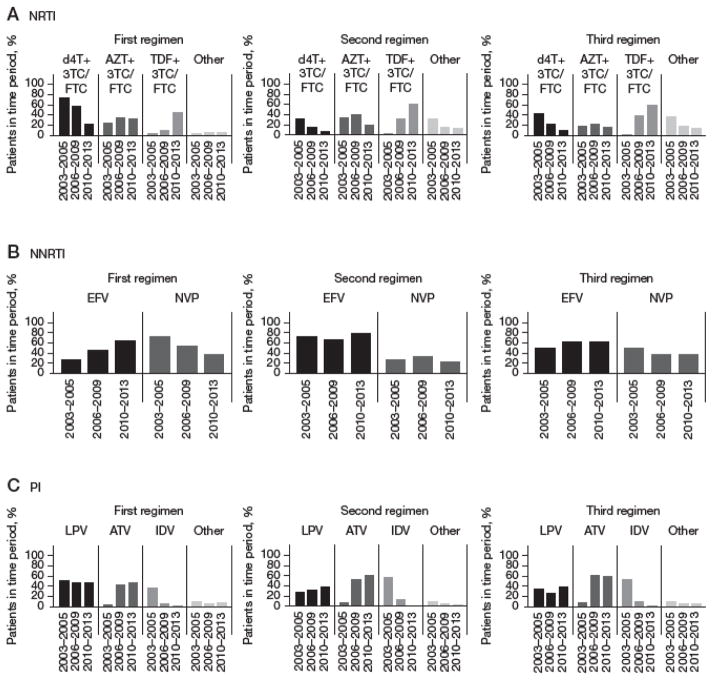
Nearly all NRTI drugs were combined with lamivudine (3TC) or emtricitabine (FTC). Stavudine (d4T) + 3TC/FTC use has been decreasing while tenofovir (TDF) + 3TC/FTC use has been increasing, over time, in first-, second- and third-line ART regimens (Figure 2A). In the first-line ART, efavirenz (EFV) use had increased from 27% in 2003–2005 to 62% in 2010–2013 while nevirapine (NVP) use decreased from 73% in 2003–2005 to 38% in 2010–2013. EFV and NVP use remained stable over time in second- and third-line ART (Figure 2B). Patterns of PI drug use were similar in first-, second- and third-line regimens. There was a decrease in indinavir (IDV) use to <1% in 2010–2013 and concurrent increases in atazanavir/ritonavir (ATV) use to 40–60% in recent years. Lopinavir/ritonavir (LPV) was more prominently used in the first-line ART regimen (45–50%) than the second- (30–40%) or third-line (30–40%) regimens (Figure 2C).
Figure 2. Proportion of patients initiating ARV drugs within each drug class for first-, second- and third-line regimen, by year of ART initiation.
(A) Nucleoside reverse transcriptase inhibitor (NRTI) drug combinations (other NRTI drugs initiated included: abacavir or abacavir +3TC/FTC [first-line regimen: 2.8% overall; second-line regimen: 5.4%; third-line regimen: 7.4%]; didanosine or didanosine +3TC/FTC [first-line regimen: 1.0%; second-line regimen: 4.9%; third-line regimen: 5.4%]; stavudine [first-line regimen: 0.1%; second-line regimen: 0.4%; third-line regimen: 0.8%]; zalcitabine [first-line regimen: <0.1%]; zidovudine [first-line regimen: 0.1%; second-line regimen: 1.1%; third-line regimen: 1.2%]; tenofovir [first-line regimen: <0.1%; second-line regimen: 1.2%; third-line regimen: 2.0%]; adefovir [second-line regimen: <0.1%]). (B) Non-nucleoside reverse transcriptase inhibitor (NNRTI) drugs (not represented NNRTI drugs initiated included: DPC 083 [first-line regimen: <0.1%; second-line regimen: <0.1%]; etravirine [first-line regimen: <0.1%; third-line regimen: 0.4%]; rilpivirine [first-line regimen: <0.1%; second-line regimen: 0.1%; third-line regimen: 0.4%]). (C) Protease inhibitor (PI) drugs (other PI drugs initiated included: nelfinavir [first-line regimen: 1.6%; second-line regimen: 0.7%; third-line regimen: 0.9%]; ritonavir [first-line regimen: 0.4%; second-line regimen: 0.2%; third-line regimen: 0.2%]; saquinavir [first-line regimen: 1.8%; second-line regimen 1.5%; third-line regimen: 0.9%]; darunavir [first-line regimen: 3.2%; second-line regimen: 1.4%; third-line regimen: 3.3%]; amprenavir [second-line regimen: <0.1%; third-line regimen: 0.2%]). ATV, atazanavir/ritonavir; AZT, zidovudine; d4T, stavudine; EFV, efavirenz; IDV, indinavir; LPV, lopinavir/ritonavir; NVP, nevirapine; TDF, tenofovir; 3TC/FTC, lamivudine/emtricitabine.
Treatment durability over time
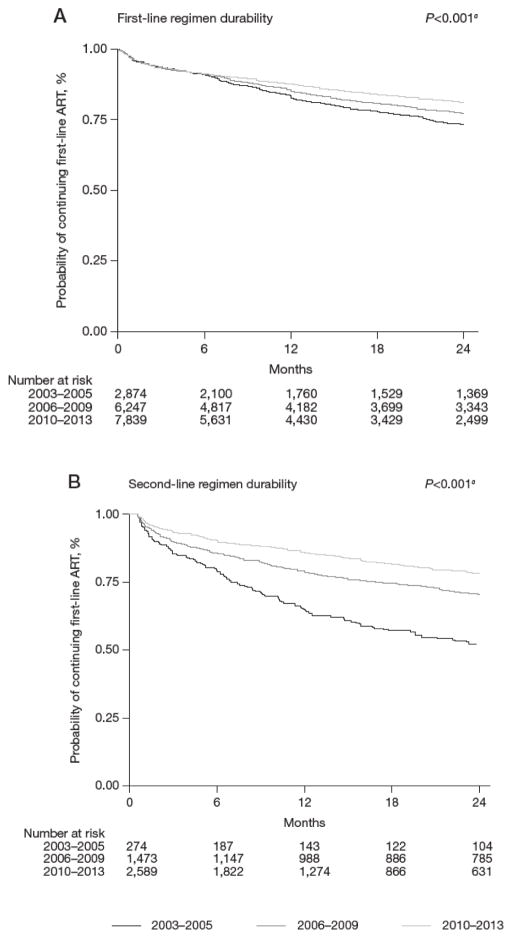
The median duration of follow-up for those on their first-line ART regimen was 19 months (IQR: 6–41 months). Of these 16,962 patients, 4,336 patients had initiated a second-line ART regimen over 38,798.7 person-years (pys), a crude rate of 11.2 (95% CI: 10.8, 11.5) per 100 pys. Overall, at 1 year of follow-up, 4,598 patients (27%) had been censored (2003–2005, n=723; 2006–2009, n=1,279; 2010–2013, n=2,596). The probability of continuing a first-line ART regimen at 1 year of follow-up when initiating in 2003–2005 was 83.7% (95% CI: 82.1, 85.1%), in 2006–2009 was 85.7% (95% CI: 84.8, 86.6%), in 2010–2013 was 87.9% (95% CI: 87.1, 88.6%; Figure 3A). Overall, the probability of continuing a first-line ART regimen was significantly higher in more recent time periods (P-value <0.001). This trend was also apparent when stratified by country (Additional file 1).
Figure 3. Treatment durability or time to treatment switch for all countries, by year of ART initiation.
(A) Time to second-line regimen from first-line regimen initiation. (B) Time to third-line regimen from second regimen initiation. ART, antiretroviral therapy. aLog rank test for trend.
The median duration of follow-up for those on the second-line ART regimen was 15 months (IQR: 5–33 months). Of these 4,336 patients, 1,135 patients had initiated a third-line ART regimen over 8,078.5 pys, a crude rate of 14.0 (95% CI: 13.3, 14.9) per 100 pys. Overall, at 1 year of follow-up, 1,257 patients (29%) had been censored (2003–2005, n=45; 2006–2009, n=197; 2010–2013, n=1,015). The probability of continuing a second-line ART regimen at 1 year of follow-up when initiating in 2003–2005 was 64.9% (95% CI: 58.5, 70.6%), in 2006–2009 was 79.0% (95% CI 76.7, 81.1%), in 2010–2013 was 86.2% (95% CI 84.7, 87.6% Figure 3B). The overall probability of continuing a second-line ART regimen was significantly higher for those initiating in recent years (P-value <0.001). When stratified by country, this trend was also apparent (Additional file 2).
As a small proportion of the total number of patients initiated a third-line ART regimen (6.6%), the multivariate analysis of risk factors associated with treatment durability was limited to first-line ART regimen durability. In the univariate model, stratified by clinical site, factors associated with lower first-line ART durability included male sex, low CD4+ T-cell count, first-line ART regimen and previous mono/dual therapy exposure (Table 2). Year of ART initiation was also borderline significantly associated with first-line ART treatment durability. However, this effect did not remain in the stratified multivariate model (Table 2). In the multivariate model, other factors significantly associated with a greater hazard risk of changing from a first-line ART to a second-line ART regimen included being aged 31–40 vs ≤31 years (HR: 1.08, 95% CI 1.00, 1.17), initiating a regimen containing NRTI+PI (HR: 1.45, 95% CI 1.27, 1.65) or other (HR: 2.06, 95% CI 1.52, 2.81) compared to NRTI+NNRTI and previous mono/dual antiretroviral therapy (HR: 1.76, 95% CI 1.55, 2.00). While factors associated with a lower hazard of changing from a first-line ART to a second-line ART regimen included female vs male sex (HR: 0.90, 95% CI 0.84, 0.97), higher CD4+ T-cell count (≥201 vs ≤50: HR: 0.72, 95% CI 0.65, 0.79).
Table 2.
Risk factors associated with first-line ART regimen durability, or time to second-line ART regimen, from first-line ART regimen initiation
| Number of treatment discontinuations | pys | Rate per 100 pys | 95% CI | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |||||
| Total | 4,336 | 38,798.7 | 11.2 | 10.8, 11.5 | ||||||
|
| ||||||||||
| Year of ART Initiation | 0.057 | 0.635 | ||||||||
| 2003–2005 | 1,113 | 9,150.8 | 12.2 | 11.5, 12.9 | 1.00 | 1.00 | ||||
| 2006–2009 | 1,929 | 18,327.2 | 10.5 | 10.1, 11 | 0.94 | 0.87, 1.02 | 0.149 | 1.00 | 0.92, 1.08 | 0.983 |
| 2010–2013 | 1,294 | 11,320.6 | 11.4 | 10.8, 12.1 | 0.91 | 0.83, 1.00 | 0.056 | 1.02 | 0.93, 1.13 | 0.631 |
|
| ||||||||||
| Age at ART initiation | 0.096 | 0.285 | ||||||||
| ≤30 years | 1,003 | 10,131.5 | 9.9 | 9.3, 10.5 | 1.00 | 1.00 | ||||
| 31–40 years | 2,001 | 17,541.6 | 11.4 | 10.9, 11.9 | 1.10 | 1.02, 1.19 | 0.015 | 1.08 | 1.00, 1.17 | 0.042 |
| 41–50 years | 885 | 7,461.2 | 11.9 | 11.1, 12.7 | 1.11 | 1.01, 1.21 | 0.031 | 1.08 | 0.98, 1.18 | 0.116 |
| 51+ years | 447 | 3,664.4 | 12.2 | 11.1, 13.4 | 1.07 | 0.96, 1.20 | 0.228 | 1.05 | 0.93, 1.18 | 0.420 |
|
| ||||||||||
| Sex | ||||||||||
| Male/transgender | 3,160 | 26,057.0 | 12.1 | 11.7, 12.6 | 1.00 | 1.00 | ||||
| Female | 1,176 | 12,741.6 | 9.2 | 8.7, 9.8 | 0.88 | 0.82, 0.94 | <0.001 | 0.90 | 0.84, 0.97 | 0.007 |
|
| ||||||||||
| Mode of HIV Exposure | 0.817 | 0.614 | ||||||||
| Heterosexual contact | 3,445 | 31,286.9 | 11.0 | 10.6, 11.4 | 1.00 | 1.00 | ||||
| Homosexual contact | 407 | 3,023.6 | 13.5 | 12.2, 14.8 | 0.96 | 0.85, 1.09 | 0.550 | 1.03 | 0.91, 1.17 | 0.648 |
| Injecting drug use | 132 | 1,664.2 | 7.9 | 6.7, 9.4 | 1.00 | 0.82, 1.21 | 0.964 | 0.92 | 0.74, 1.14 | 0.422 |
| Other/unknown | 352 | 2,824.0 | 12.5 | 11.2, 13.8 | 0.95 | 0.84, 1.07 | 0.373 | 0.95 | 0.84, 1.07 | 0.404 |
|
| ||||||||||
| HIV viral load at ART initiation | 0.012 | 0.020 | ||||||||
| ≤999 copies/ml | 27 | 287.8 | 9.4 | 6.4, 13.7 | 1.00 | 1.00 | ||||
| 1 000–10 000 copies/ml | 71 | 664.3 | 10.7 | 8.5, 13.5 | 1.01 | 0.65, 1.58 | 0.951 | 1.11 | 0.71, 1.74 | 0.633 |
| ≥10 001 copies/ml | 841 | 6,799.3 | 12.4 | 11.6, 13.2 | 1.36 | 0.92, 1.99 | 0.119 | 1.37 | 0.93, 2.01 | 0.110 |
| Not tested | 3,397 | 31,047.4 | 10.9 | 10.6, 11.3 | 1.58 | 1.08, 2.32 | 0.018 | 1.49 | 1.01, 2.18 | 0.043 |
|
| ||||||||||
| CD4+ T-cell count at ART initiation | <0.001 | <0.001 | ||||||||
| ≤50 cells/μl | 937 | 8,525.0 | 11.0 | 10.3, 11.7 | 1.00 | 1.00 | ||||
| 51–100 cells/μl | 605 | 5,342.0 | 11.3 | 10.5, 12.3 | 0.89 | 0.80, 0.98 | 0.023 | 0.89 | 0.80, 0.99 | 0.028 |
| 101–200 cells/μl | 990 | 9,641.2 | 10.3 | 9.6, 10.9 | 0.78 | 0.71, 0.86 | <0.001 | 0.79 | 0.72, 0.87 | <0.001 |
| 201+ cells/μl | 970 | 9,811.7 | 9.9 | 9.3, 10.5 | 0.71 | 0.65, 0.78 | <0.001 | 0.72 | 0.65, 0.79 | <0.001 |
| Not tested | 834 | 5,478.7 | 15.2 | 14.2, 16.3 | 1.11 | 1.01, 1.23 | 0.035 | 1.12 | 1.02, 1.24 | 0.024 |
|
| ||||||||||
| First-line ART regimen | <0.001 | <0.001 | ||||||||
| NRTI+NNRTI | 3,943 | 36,882.5 | 10.7 | 10.4, 11.0 | 1.00 | 1.00 | ||||
| NRTI+PI | 349 | 1,773.6 | 19.7 | 17.7, 21.9 | 1.52 | 1.33, 1.73 | <0.001 | 1.45 | 1.27, 1.65 | <0.001 |
| Other | 44 | 142.6 | 30.9 | 23.0, 41.5 | 2.14 | 1.58, 2.91 | <0.001 | 2.06 | 1.52, 2.81 | <0.001 |
|
| ||||||||||
| Previous mono/duo exposure | ||||||||||
| No | 4,061 | 37,413.3 | 10.9 | 10.5, 11.2 | 1.00 | 1.00 | ||||
| Yes | 275 | 1,385.4 | 19.9 | 17.6, 22.3 | 1.79 | 1.58, 2.02 | <0.001 | 1.76 | 1.55, 2.00 | <0.001 |
|
| ||||||||||
| Hepatitis B (surface antigen) co-infection | 0.146 | 0.012 | ||||||||
| Negative | 2,192 | 21,576.7 | 10.2 | 9.7, 10.6 | 1.00 | 1.00 | ||||
| Positive | 227 | 2,265.6 | 10.0 | 8.8, 11.4 | 1.11 | 0.96, 1.27 | 0.148 | 1.07 | 0.93, 1.23 | 0.332 |
| Not tested | 1,917 | 14,956.3 | 12.8 | 12.3, 13.4 | 0.95 | 0.88, 1.03 | 0.260 | 0.85 | 0.76, 0.96 | 0.007 |
|
| ||||||||||
| Hepatitis C (antibody) co-infection | 0.551 | 0.703 | ||||||||
| Negative | 1,924 | 19,521.9 | 9.9 | 9.4, 10.3 | 1.00 | 1.00 | ||||
| Positive | 187 | 2,170.4 | 8.6 | 7.5, 9.9 | 1.05 | 0.89, 1.23 | 0.584 | 1.03 | 0.85, 1.23 | 0.785 |
| Not tested | 2,225 | 17,106.4 | 13.0 | 12.5, 13.6 | 0.96 | 0.87, 1.05 | 0.379 | 1.06 | 0.93, 1.20 | 0.416 |
Bold represents P-values <0.05. Global P-values for year of antiretroviral therapy (ART) initiation, age and pre-ART CD4+ T-cell count are test for trend. Other global P-values are test for heterogeneity. HR, hazard ratio; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; pys, person-years.
As there were large proportions of missing data for some covariates, including pre-ART HIV viral load, pre-ART CD4+ T-cell count, HBV and HCV, we performed a sensitivity analysis for the multivariate model examining risk factors associated with treatment durability. We excluded patients who did not have a pre-ART CD4+ T-cell count, assumed patients not tested for HBV or HCV were negative and excluded pre-ART HIV viral load from the model. The model included 14,454 patients of which 3,502 initiated a second-line ART regimen over 33,320.0 pys of follow-up. We found similar risk factors to the main analysis where a greater hazard risk of changing from first-line ART regimen to second-line ART regimen was associated with lower pre-ART CD4+ T-cell count, initiating a regimen containing NRTI+PI or other, and previous exposure to mono/dual therapy (Additional file 3). Unlike the main analysis, age at ART initiation and sex were not significantly associated with first-line ART regimen durability. However, HR estimates were relatively similar for the main analysis (age 31–40 years, HR: 1.08, 95% CI 1.00, 1.17; female sex, HR: 0.90, 95% CI 0.84, 0.97) and the sensitivity analysis (age 31–40 years, HR: 1.05, 95% CI 0.96, 1.14; female sex, HR: 0.96, 95% CI 0.88, 1.04).
Discussion
In this study of 16,962 HIV-positive patients enrolled from eight clinical sites in Asia we found that, the 12-month probability of continuing a first-line ART regimen increased over time from 83.7% in 2003–2005 to 87.9% in 2010–2013. Sequential regimens were of progressively shorter duration, supporting that first-line treatment is the best opportunity for obtaining durable viral suppression. Other factors associated with increased risk of treatment switch included lower CD4+ T-cell cell count at ART initiation, male sex, initiating any regimen combination besides NRTI+NNRTI and previous mono/dual antiretroviral therapy.
The probability of continuing first-line ART regimens has increased over the past decade in the Asia-Pacific region, confirming previous findings [26–28]. This was likely a result of several factors. First, the changes in the composition of first-line ART regimens that reflect the availability of stronger, newer and better-tolerated antiretroviral drugs. For instance, during the earlier period of our cohort (2003–2005), more than 70% of prescribed first-line ART regimens included d4T, whose use is associated with several treatment limiting side effects [29,30]. During the most recent time period analyzed (2010–2013) and following the WHO recommendations of transitioning away from the use of d4T, this agent was received by less than 20% of our cohort contrasting with an increase of TDF use, which has a more favorable safety profile [29,31]. Second, an increase in the average CD4+ T-cell cell counts at ART initiation in the last decade that reflects recent changes in the guidelines for treatment initiation [21,32,33]. Finally, patients in the earlier period were more likely to receive prior suboptimal mono or dual therapy before commencing combination ART, which has been related to drug resistance and subsequent virological and treatment failure [34].
The rate of discontinuation of a first-line regimen in our study was substantially lower than those in other observational cohorts from developed countries where treatment modifications rates were higher, 36% to 54% [14,35,36]. The limited access to alternative regimens in our region may affect the clinicians’ decision to initiate new regimens. Patients in developed countries are also followed more closely making it easier to identify potential intolerance or toxicities, both consistently described as main reasons affecting treatment durability [12,35,37].
As previously reported [28,38,39]a low CD4+ T-cell cell count when starting ART is an important predictor for first-line regimen discontinuation. Patients initiating therapy at higher CD4+ T-cell count are less likely to suffer from other HIV-related illnesses that require treatment with other drugs that could interact with the current regimen (for example, anti-tuberculosis medicines) [40]. Those results indicate that prompt ART initiation not only reduces the high mortality during the initial months of ART [40,41] but also can help to reduce the risk of early failure and thus preserve first-line regimens use. Initial therapies other than NRTIs+NNRTI combinations were also associated with discontinuation. Similar observations were reported by Palella et al. [8] where durable treatment success was associated with being antiretroviral naive before initiation of a first-line HAART regimen. Men were more likely to discontinue than women, a finding which has not been seen in other studies, and may be related to the minimal referral into our ART clinics from prevention of mother-to-child transmission programs [14,28].
PIs and integrase inhibitors are less commonly used in developing countries as a first-line regimen due to high cost and limited availability. The 2013 WHO guideline recommends a combination of ATV/r or LPV/r with two NRTIs as the preferred strategy after first-line therapy with NNRTIs has failed [42,43]. In our cohort, the inclusion of PIs in initial ART regimens was minimal. The same results were reported in a previous analysis of our cohort, which found that the use of PI-based first-line ART that was more common in the 1990s was taken over by the scale-up of NNRTI-based regimens across the region [44]. First-generation PI use (for example, indinavir) in second-line ART regimens then increased over time, consistent with WHO guidelines. Indinavir use decreased substantially in recent years in our cohort following the increase in ATV/r use, as would be expected due to treatment-limiting adverse effects.
There were limitations to our analysis. First, we used observational data from six countries in the Asia-Pacific region where most had only one self-selected contributing clinical site. Hence, our findings should not be interpreted as representative of a specific country or the entire region. In addition, there were large proportions of missing data for some patient data collected. In particular, HIV viral load is likely to be targeted rather than routine, so these data could not be considered completely missing at random (CMAR). Thus, we advise caution to not overly interpret findings where data is not CMAR as findings may be bias. However, our sensitivity analysis including patients with complete data at baseline found similar risk factors associated with first -line ART regimen durability. Second, we were limited in describing the durability of first-line regimen for those initiating in recent years because of reduced follow-up on these patients. Third, we had few patients receiving second- and third-line ART regimens and thus, the estimates for second-line regimen durability should be cautiously interpreted. Fourth, patients LTFU were censored, so we were unable to determine whether they had actually stopped their treatment or not. However, as previously reported in a study of TAHOD-LITE [21], the LTFU rate in this cohort was relatively low and consistent across periods of ART initiation. Fifth, although the countries in our cohort have adopted the WHO ART guidelines, the limited number of data variables collected did not allow for investigation of other widely accepted reasons for poor regimen durability or shifting to second- and third-line, such as adverse events, or poor adherence. Last, HIV viral load testing were not widely available in all sites and thus further regimens were guided based on clinical and CD4+ T-cell count criteria.
In summary, our study describes trends of durability of ART regimens in an Asia-Pacific observational cohort over the past decade. Discontinuation rates have decreased over time, likely related to improvements in the individual drugs used in first-line ART regimens. Lower CD4+ T-cell count and the use of suboptimal regimens were associated with a higher risk of treatment switch.
Supplementary Material
Additional file 1. First regimen durability or time to second regimen by country and year of first ART initiation.
Additional file 2. Second regimen durability or time to third regimen by country and year of second ART initiation.
Additional file 3. Risk factors associated with first ART regimen durability, or time to second ART regimen, from first ART regimen initiation, excluding patients who did not have a baseline CD4
Acknowledgments
TAHOD-LITE study members:
PS Ly and V Khol, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia;
MP Lee, PCK Li, W Lam and YT Chan, Queen Elizabeth Hospital, Hong Kong SAR, China;
N Kumarasamy, S Saghayam and C Ezhilarasi, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), YRGCARE Medical Centre, VHS, Chennai, India;
TP Merati, DN Wirawan and F Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia;
OT Ng, PL Lim, LS Lee and R Martinez-Vega, Tan Tock Seng Hospital, Singapore;
JY Choi, Na S and JM Kim, Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea;
TT Pham, DD Cuong and HL Ha, Bach Mai Hospital, Hanoi, Vietnam;
KV Nguyen, HV Bui, DTH Nguyen and DT Nguyen, National Hospital for Tropical Diseases, Hanoi, Vietnam;
AH Sohn, JL Ross and B Petersen, TREAT Asia, amfAR - The Foundation for AIDS Research, Bangkok, Thailand;
NL De La Mata, A Jiamsakul, DC Boettiger and MG Law, The Kirby Institute, UNSW Australia, Sydney, Australia.
Footnotes
Authors’ contributions
RM-V and OTN contributed to the concept development. NK, PSL, KVN, TPM, TTP, MPL, JYC and OTN contributed data for the analysis. NLDLM performed the statistical analysis and wrote the methods and results section. RM-V wrote the remainder of the manuscript for the first draft. All authors commented on the draft manuscript and approved of the final manuscript for submission.
Disclosure statement
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- 1.Sterne JAC, Hernán MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366:378–384. doi: 10.1016/S0140-6736(05)67022-5. [DOI] [PubMed] [Google Scholar]
- 2.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 3.Madec Y, Laureillard D, Pinoges L, et al. Response to highly active antiretroviral therapy among severely immunocompromised HIV-infected patients in Cambodia. AIDS. 2007;21:351–359. doi: 10.1097/QAD.0b013e328012c54f. [DOI] [PubMed] [Google Scholar]
- 4.Spacek LA, Shihab HM, Kamya MR, et al. Response to antiretroviral therapy in HIV-infected patients attending a public, urban clinic in Kampala, Uganda. Clin Infect Dis. 2006;42:252–259. doi: 10.1086/499044. [DOI] [PubMed] [Google Scholar]
- 5.May MT, Sterne JAC, Costagliola D, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006;368:451–458. doi: 10.1016/S0140-6736(06)69152-6. [DOI] [PubMed] [Google Scholar]
- 6.Bendavid E, Holmes CB, Bhattacharya J, Miller G. HIV development assistance and adult mortality in Africa. JAMA. 2012;307:2060–2067. doi: 10.1001/jama.2012.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toure S, Kouadio B, Seyler C, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Côte d’Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–882. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palella FJ, Jr, Chmiel JS, Moorman AC, Holmberg SD HIV Outpatient Study Investigators. Durability and predictors of success of highly active antiretroviral therapy for ambulatory HIV-infected patients. AIDS. 2002;16:1617–1626. doi: 10.1097/00002030-200208160-00007. [DOI] [PubMed] [Google Scholar]
- 9.Pursuing Later Treatment Option II (PLATO II) project team, Observational HIV Epidemiological Research Europe (COHERE) Group. Costagliola D, et al. Trends in virological and clinical outcomes in individuals with HIV-1 infection and virological failure of drugs from three antiretroviral drug classes: a cohort study. Lancet Infect Dis. 2012;12:119–127. doi: 10.1016/S1473-3099(11)70248-1. [DOI] [PubMed] [Google Scholar]
- 10.Parienti J-J, Massari V, Descamps D, et al. Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis. 2004;38:1311–1316. doi: 10.1086/383572. [DOI] [PubMed] [Google Scholar]
- 11.Bangsberg DR, Acosta EP, Gupta R, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20:223–231. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- 12.Chen RY, Westfall AO, Mugavero MJ, et al. Duration of highly active antiretroviral therapy regimens. Clin Infect Dis. 2003;37:714–722. doi: 10.1086/377271. [DOI] [PubMed] [Google Scholar]
- 13.Abgrall S, Ingle SM, May MT, et al. Durability of first ART regimen and risk factors for modification, interruption or death in HIV-positive patients starting ART in Europe and North America 2002–2009. AIDS. 2013;27:803–813. doi: 10.1097/QAD.0b013e32835cb997. [DOI] [PubMed] [Google Scholar]
- 14.Elzi L, Marzolini C, Furrer H, et al. Treatment modification in human immunodeficiency virus-infected individuals starting combination antiretroviral therapy between 2005 and 2008. Arch Intern Med. 2010;170:57–65. doi: 10.1001/archinternmed.2009.432. [DOI] [PubMed] [Google Scholar]
- 15.Wright S, Boyd MA, Yunihastuti E, et al. Rates and factors associated with major modifications to first-line combination antiretroviral therapy: results from the Asia-Pacific region. PLoS One. 2013;8:e64902. doi: 10.1371/journal.pone.0064902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.HIV in Asia and the Pacific. [Accessed 12 June 2017];UNAIDS report 2013. 2013 Available from http://www.unaids.org/sites/default/files/media_asset/2013_HIV-Asia-Pacific_en_0.pdf.
- 17.Srikantiah P, Ghidinelli M, Bachani D, et al. Scale-up of national antiretroviral therapy programs: progress and challenges in the Asia Pacific region. AIDS. 2010;24(Suppl 3):S62–S71. doi: 10.1097/01.aids.0000390091.45435.ea. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach (2003 revision) Geneva: WHO; 2004. [Accessed 12 June 2017]. 2003. Available from http://www.who.int/hiv/pub/prev_care/en/arvrevision2003en.pdf. [Google Scholar]
- 19.Phuphuakrat A, Kiertiburanakul S, Sungkanuparph S. Current status of HIV treatment in Asia and the Pacific region. Sex Health. 2014;11:119–125. doi: 10.1071/SH13045. [DOI] [PubMed] [Google Scholar]
- 20.Zhou J, Li P, Kumarasamy N, et al. Deferred modification of antiretroviral regimen following documented treatment failure in Asia: results from the TREAT Asia HIV Observational Database (TAHOD) HIV Med. 2010;11:31–39. doi: 10.1111/j.1468-1293.2009.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De La Mata NL, Kumarasamy N, Khol V, et al. Improved survival in HIV treatment programmes in Asia. Antivir Ther. 2016;21:517–527. doi: 10.3851/IMP3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. UNAIDS. Treating 3 million by 2005. [Accessed 12 June 2017];Making it happen. 2003 Available from http://www.who.int/3by5/publications/documents/isbn9241591129/en/
- 23. [Accessed 15 May 2017];Antiretroviral Therapy for HIV Infection in Adults and Adolescents: recommendations for a Public Health Approach. 2006 :1–134. Available from http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. [PubMed]
- 24.Makinson A, Moing VL, Kouanfack C, Laurent C, Delaporte E. Safety of stavudine in the treatment of HIV infection with a special focus on resource-limited settings. Expert Opin Drug Saf. 2008;7:283–293. doi: 10.1517/14740338.7.3.283. [DOI] [PubMed] [Google Scholar]
- 25. [Accessed 15 May 2017];Antiretroviral therapy for HIV infection in Adults and Adolescents: recommendations for a public health approach. 2011 :1–156. Available from http://apps.who.int/iris/bitstream/10665/44379/1/9789241599764_eng.pdf. [PubMed]
- 26.Boettiger DC, Kerr S, Ditangco R, et al. Trends in first-line antiretroviral therapy in Asia: results from the TREAT Asia HIV observational database. PLoS One. 2014;9:e106525. doi: 10.1371/journal.pone.0106525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slama L, Li X, Brown T, et al. Increases in duration of first highly active antiretroviral therapy over time (1996–2009)and associated factors in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2014;65:57–64. doi: 10.1097/QAI.0b013e3182a99a0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez-Serna A, Chan K, Yip B, et al. Temporal trends in the discontinuation of first-line antiretroviral therapy. J Antimicrob Chemother. 2014;69:2202–2209. doi: 10.1093/jac/dku112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 30.Saag MS, Cahn P, Raffi F, et al. Efficacy and safety of emtricitabine vs stavudine in combination therapy in antiretroviral-naive patients: a randomized trial. JAMA. 2004;292:180–189. doi: 10.1001/jama.292.2.180. [DOI] [PubMed] [Google Scholar]
- 31.Madruga JRV, Cassetti I, Suleiman JMAH, et al. The safety and efficacy of switching stavudine to tenofovir df in combination with lamivudine and efavirenz in hiv-1-infected patients: three-year follow-up after switching therapy. HIV Clin Trials. 2007;8:381–390. doi: 10.1310/hct0806-381. [DOI] [PubMed] [Google Scholar]
- 32.Günthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA. 2014;312:410–425. doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- 33. [Accessed 20 July 2016];Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2016 Available from https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
- 34.Delfraissy J-F, Flandre P, Delaugerre C, et al. Lopinavir/ritonavir monotherapy or plus zidovudine and lamivudine in antiretroviral-naive HIV-infected patients. AIDS. 2008;22:385–393. doi: 10.1097/QAD.0b013e3282f3f16d. [DOI] [PubMed] [Google Scholar]
- 35.Vo TTN, Ledergerber B, Keiser O, et al. Durability and outcome of initial antiretroviral treatments received during 2000--2005 by patients in the Swiss HIV Cohort Study. J Infect Dis. 2008;197:1685–1694. doi: 10.1086/588141. [DOI] [PubMed] [Google Scholar]
- 36.Cicconi P, Cozzi-Lepri A, Castagna A, et al. Insights into reasons for discontinuation according to year of starting first regimen of highly active antiretroviral therapy in a cohort of antiretroviral-naive patients. HIV Med. 2010;11:104–113. doi: 10.1111/j.1468-1293.2009.00750.x. [DOI] [PubMed] [Google Scholar]
- 37.Palladino C, Briz V, Bellón JM, et al. Determinants of highly active antiretroviral therapy duration in HIV-1-infected children and adolescents in Madrid, Spain, from 1996 to 2012. PLoS One. 2014;9:e96307. doi: 10.1371/journal.pone.0096307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palombi L, Marazzi MC, Guidotti G, et al. Incidence and predictors of death, retention, and switch to second-line regimens in antiretroviral-treated patients in sub-Saharan African Sites with comprehensive monitoring availability. Clin Infect Dis. 2009;48:115–122. doi: 10.1086/593312. [DOI] [PubMed] [Google Scholar]
- 39.ART-LINC of IeDEA Study Group. Keiser O, Tweya H, et al. Switching to second-line antiretroviral therapy in resourcelimited settings: comparison of programmes with and without viral load monitoring. AIDS. 2009;23:1867–1874. doi: 10.1097/QAD.0b013e32832e05b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.INSIGHT START Study Group. Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.TEMPRANO ANRS 12136 Study Group. Danel C, Moh R, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–822. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 42.Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. [Accessed 20 July 2016];2014 Available from http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [PubMed]
- 43.Gatell J, Salmon-Ceron D, Lazzarin A, et al. Efficacy and safety of atazanavir-based highly active antiretroviral therapy in patients with virologic suppression switched from a stable, boosted or unboosted protease inhibitor treatment regimen: the SWAN Study (AI424-097) 48-week results. Clin Infect Dis. 2007;44:1484–1492. doi: 10.1086/517497. [DOI] [PubMed] [Google Scholar]
- 44.Boettiger DC, Nguyen VK, Durier N, et al. Efficacy of second-line antiretroviral therapy among people living with HIV/AIDS in Asia: results from the TREAT Asia HIV observational database. J Acquir Immune Defic Syndr. 2015;68:186–195. doi: 10.1097/QAI.0000000000000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. First regimen durability or time to second regimen by country and year of first ART initiation.
Additional file 2. Second regimen durability or time to third regimen by country and year of second ART initiation.
Additional file 3. Risk factors associated with first ART regimen durability, or time to second ART regimen, from first ART regimen initiation, excluding patients who did not have a baseline CD4