Abstract
Therapeutic outcomes for adoptive cell transfer (ACT) therapy are constrained by the quality of the infused T cells. The rapid expansion necessary to obtain large numbers of cells results in a more terminally differentiated phenotype with decreased durability and functionality. N-acetyl cysteine (NAC) protects against activation-induced cell death (AICD) and improves anti-tumor efficacy of Pmel-1 T cells in vivo. Here, we show that these benefits of NAC can be extended to engineered T cells and significantly increases T-cell survival within the tumor microenvironment. The addition of NAC to the expansion protocol of human TIL13838I TCR-transduced T cells that are under evaluation in a Phase I clinical trial, demonstrated that findings in murine cells extend to human cells. Expansion of TIL13838I TCR-transduced T cells in NAC also increased their ability to kill target cells in vitro. Interestingly, NAC did not affect memory subsets, but diminished up-regulation of senescence (CD57) and exhaustion (PD-1) markers and significantly decreased expression of the transcription factors EOMES and Foxo1. Pharmacological inhibition of the PI3K/Akt pathway ablates the decrease in Foxo1 induced by NAC treatment of activated T cells. This suggests a model in which NAC through PI3K/Akt activation suppresses Foxo1 expression, thereby impacting its transcriptional targets EOMES, PD-1, and granzyme B. Taken together, our results indicate that NAC exerts pleiotropic effects that impact the quality of TCR-transduced T cells and suggest that the addition of NAC to current clinical protocols should be considered.
Electronic supplementary material
The online version of this article (10.1007/s00262-018-2120-5) contains supplementary material, which is available to authorized users.
Keywords: Adoptive T-cell therapy, Cell death, N-acetyl-cysteine, Akt, Foxo1, PD1
Introduction
The adoptive cell transfer (ACT) of tumor-infiltrating lymphocytes (TILs) which recognize tumor associated antigens has shown great promise in clinical trials elevating the 5-year survival rate of metastatic melanoma patients from 15.2% in standard-of-care regimens to upwards of 40% [1, 2]. However, not all tumors are resectable or yield functional lymphocytes [3]. As an alternative approach, the TCR sequences of tumor reactive TILs have been cloned and transduced into patient autologous peripheral blood mononuclear cells (PBMCs). These TCR-engineered T cells have demonstrated the ability to elicit regression in melanoma, synovial sarcoma, and multiple myeloma. Current clinical trials are expanding the use of TCR-engineered cells into breast, lung, esophageal, ovarian, and bladder cancers as well as neuroblastoma [3].
While adoptive transfer therapy is promising, the majority of patients do not exhibit a durable complete response. Transfer of cells that are younger and less differentiated correlates with positive patient responses [4]. However, the intense polyclonal stimulation during the rapid expansion protocol (REP) drives cells to a more differentiated phenotype characterized by an increase in effector memory cells over central-memory cells [5], a decline in the co-stimulatory receptor CD28, and shortening of telomeres [6]. In addition, post-REP cells exhibit an increase in the exhaustion marker PD-1 and the senescence marker CD57 [6] both of which are associated with impaired T-cell functionality [7]. Post-REP cells are also more susceptible to activation-induced cell death (AICD) [8]. Therefore, strategies to counteract these negative consequences of the REP may have the potential to improve clinical outcomes.
Previously, we have shown that Pmel-1 cells expanded in the glutathione pro-drug N-acetyl cysteine (NAC) were more efficacious at controlling transplanted B16F10 murine melanoma tumors and enhanced the survival of recipient mice. Moreover, these cells were more resistant to ex vivo peptide-induced AICD up to 6 days after transfer, suggesting that NAC exerted a durable change in the cells that persisted in the absence of the drug [9]. The goal of this study was to determine whether NAC has a similar benefit in TCR-engineered T cells. Our results show that the benefit of improved tumor control extends to transduced murine T cells. The addition of NAC to an expansion protocol used to prepare TCR-transduced T cells in the clinic revealed pleiotropic effects of the drug on senescence, exhaustion, cytolytic capacity, and levels of transcription factors EOMES and Foxo1.
Materials and methods
Cell lines
Melanoma cell lines were obtained from Drs. Michael Nishimura (Loyola University, Maywood IL, USA) and Mark Rubinstein (MUSC, Charleston SC, USA). All cells were periodically verified to be free of mycoplasma contamination. In addition, B16F10 cells were confirmed to be free of rodent pathogens.
TRP-1 TCR transduction of murine T cells
Mouse splenocytes were enriched for CD3+ T cells via column purification (R&D Systems) and activated with αCD3/αCD28-coated beads (Dynabeads, Life Technologies). In parallel, 5 × 106 Platinum-E ecotropic packaging cells (Cell Biolabs) were transfected with retroviral plasmid DNA encoding the MSGV-1 TRP-1 TCR and the helper plasmid pCL-Eco using Lipofectamine 2000 (Invitrogen). After 24 h, medium was replaced and cells were incubated an additional 24 h. The viral supernatant was spun (2000 g, 2 h, 32 °C) onto non-tissue-culture-treated 24-well plates (USA Scientific) coated with Retronectin (Takara Bio). Following centrifugation, the viral supernatant was removed and activated T cells and fresh virus were added into the same well and the plate was centrifuged again. Next, 1 ml of media was replaced with fresh media containing 200 IU/mL IL-2, and cells incubated overnight. The following day the cells were collected, washed, and cultured for further expansion for 6 more days (−/+ 10 mM NAC). For restimulation of the TCR, cells were exposed overnight to TRP-1 peptide (4 µg/mL) pulsed onto irradiated splenocytes.
Adoptive cell transfer and biodistribution
Experiments were performed as previously described [9]. Briefly, 8-week-old female C57BL/6 wild-type mice were injected subcutaneously with 3 × 105 B16–F10 murine melanoma cells. Mice were randomized into treatment groups and lymphodepleted through total body irradiation (5 Gy,) 1 day prior to ACT. TRP-1 TCR-transduced T cells (2 × 106) cultured −/+ 10 mM NAC were adoptively transferred via retro-orbital injection and tumor size recorded until endpoints were reached. For ex vivo analysis of transferred cells, a subset of mice was sacrificed 9 days after adoptive transfer. Spleens and tumors were processed into single cell suspensions by mechanical dissociation. Tumors were further digested in 1 mg/mL Collagenase II (Sigma) for 30 min and TILs were isolated by density gradient separation with Histopaque 1083 (Sigma). Cells were restimulated overnight with TRP-1 peptide (4 µg/mL) to assess basal and peptide-induced cell death and γH2AX staining.
Activation and culture of human PBMC (and transduction of TIL1383I)
Normal healthy donor apheresis cells were purchased from Key Biologics, Inc. or Research Blood Components. Apheresis and cells from melanoma patients were obtained as part of a clinical trial (NCT01586403). Cell cultures were maintained in either AIM-V (Life Technologies) supplemented with 5% human AB Serum (Gemini Bio) or RPMI 1640 (Mediatech) supplemented with 10% FBS (Rocky Mountain Bio). All cell cultures contained 300 IU/ml rh-IL2 and 100 ng/ml rh-IL15. The transduction of TIL1383I TCR into human T cells has been previously described [10]. The viral construct co-expressed a truncated CD34 as a marker of expression [11]. Following transductions, cells were divided and expanded in the absence or the presence of 2 mM NAC. On day 6, transduced cells were purified by magnetic selection on the CliniMACs based on CD34 staining. On day 10, enriched transduced T cells underwent an REP by co-culturing at a 1:200 ratio with irradiated feeder cells supplemented with 30 ng/mL anti-CD3 until day 20. Experiments with AktX (Cayman Chemical), LY294002 (Calbiochem) or CAL-101 (Selleckchem) were performed with TIL1383I TCR-transduced cells that had been REPed in the absence of NAC.
Flow cytometry
Cells were surface stained with fluorochrome-conjugated antibodies to allow for gating of specific populations. TIL1383I TCR-transduced cells were gated on CD34 and TRP-1 TCR-transduced murine cells were gated on Vβ14 prior to subsequent gating on CD8 and CD4 populations as indicated in figure legends. Annexin V, surface thiol, and intracellular staining was performed as described [9]. The following reagents were used: Annexin V-Cy5 (BioVision), Alexa Fluor 488-conjugated C2Maleimide reagent (Anaspec), PD-1, and CD57 (BioLegend), and Foxo1-PE (Cell Signal), T-Bet, Eomes (eBioscience), and γH2AX (EMD Millipore). TRP-1 TCR-transduced mouse cells were stained with anti-mouse γH2AX obtained from BioLegend. Samples were acquired using the BD LSRFortessa cell analyzer (BD Biosciences) and analyzed using the FlowJo software (Tree Star Inc.).
In vitro cytotoxicity
MEL624 and MEL624-28 cells were labeled with 0.1 and 0.01 µM CFSE (BioLegend), respectively, according to vendor protocol. Labeled melanoma cells (2 × 104 each) were co-cultured with TIL1383I TCR-transduced T cells at various effector:target ratios achieved through serial dilution of the T cells. Cells were incubated overnight, stained with Annexin V & 7AAD, and acquired via flow cytometry. Populations within these co-culture experiments were identified as follows: CFSE− CD34+ for TIL1383I-transduced T cells, CFSEHI for MEL624, and CFSELO for MEL624-28 cells. Within each cell population, cell death was quantified as the percentage of Annexin V+/7AAD+ cells.
Statistical methods
Graphical displays are used to demonstrate empirical means and standard errors for in vivo data. Analysis of in vivo data was performed using longitudinal linear regression to model tumor size over time with log of tumor size as the outcome and a quadratic model for time. Interactions between time and group were included to evaluate differences in growth trajectories. The log-likelihoods of nested models (one model assuming different slopes per group and the other model assuming that the two groups being compared have the same slope coefficients) were tested using a likelihood ratio test with 2 degrees of freedom. A simpler approach (e.g., a Wald test) is not possible due to the quadratic model. Differences in time of survival were analyzed using a log rank test and displayed using Kaplan–Meier curves. In vitro data were compared using two-sample t tests assuming unequal variances. For comparison of patient samples cultured with or without NAC, a paired t test was used.
Results
NAC increases the antioxidant capacity of TCR-engineered T cells and improves in vivo tumor control and survival
We recently reported that NAC inhibits AICD and improves the ability of Pmel-1 T cells to control tumor growth in vivo [9]. While T cells from Pmel-1 mice express a native TCR [12], many clinical trials are performed with TCR-engineered cells, which prompted us to investigate the effect of NAC in splenocytes that had been transduced with the TRP-1 TCR [13]. In vitro analysis prior to ACT showed that expansion in the presence of NAC increased surface thiols of TRP-1 TCR-transduced T cells and reduced γH2AX staining (Suppl. Figure 1a). NAC also protected cells from AICD following restimulation of the transduced TCR with TRP-1 peptide (Suppl. Figure 1b). These results are consistent with our previous results in the Pmel-1 model and suggest that the benefit of NAC extends to TCR-engineered cells.
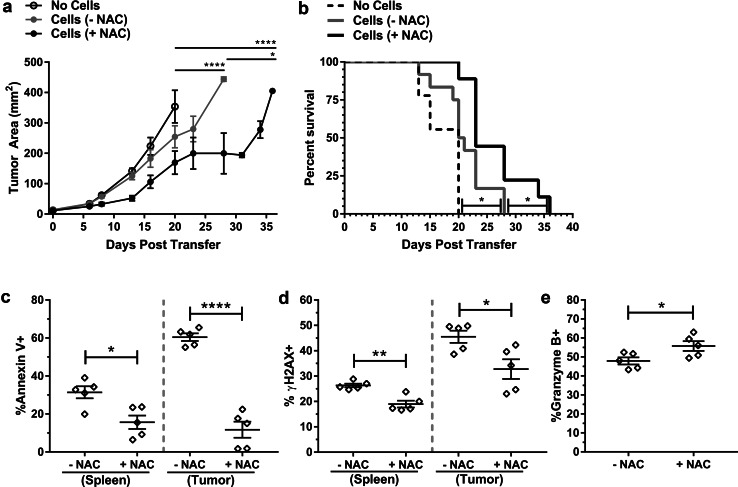
To determine if NAC conferred an in vivo anti-melanoma benefit, we adoptively transferred TRP-1 TCR-transduced T cells into tumor-bearing recipients. TRP-1 TCR-transduced cells cultured in the absence of NAC significantly delayed tumor growth (Fig. 1a) and enhanced the survival of mice (Fig. 1b). However, expansion of TRP-1 TCR-transduced cells in the presence of NAC prior to transfer resulted in a significant improvement in both tumor control (Fig. 1a) and survival (Fig. 1b). On day 9 post-ACT, we analyzed adoptively transferred cells recovered from spleens and tumors in a subset of mice. Expansion in NAC afforded TRP-1 TCR-transduced cells isolated from the spleen or tumor protection from AICD (Fig. 1c) and DNA damage (Fig. 1d). Notably, Annexin V staining was approximately twice as high in TRP-1 TCR-transduced cells recovered from the tumor (~ 60%) compared to the spleen (~ 30%) (p < 0.0001), yet expansion of cells in NAC was able to reduce the Annexin V level to below 20% in both populations (Fig. 1c). The expression of granzyme B, a key molecule in killing target cells, was elevated in NAC-cultured cells (Fig. 1e). Together, these data demonstrate that expansion of TCR-engineered T cells in NAC imparts a durable DNA damage and AICD resistant phenotype that is maintained in vivo despite the absence of the drug.
Fig. 1.
Murine TCR-engineered T cells expanded in NAC exhibit enhanced tumor control when transferred in vivo and maintain a DNA damage and AICD-resistant phenotype. a Tumor growth and b survival was determined in untreated mice (no cells, n = 9) or mice receiving TRP-1 TCR-transduced T cells that were expanded in the absence or the presence of NAC (n = 12). c, d Splenocytes and TILs were isolated from a subset of mice (n = 5) 9-day post-transfer and restimulated as described in “Materials and methods”. Vβ14+CD8+ TRP-1 TCR-transduced cells analyzed for c Annexin V staining and d γH2AX expression. e Granzyme B expression in Vβ14+CD8+ splenocytes. *p < 0.05; **p < 0.01; ****p < 0.0001
Culturing TIL1383I TCR-transduced human T cells in NAC improves antioxidant capacity, reduces DNA damage, and enhances their ability to kill melanoma cells in vitro
To determine the potential clinical relevance of our observations, we investigated whether NAC affected the quality of human TCR-transduced T cells. NAC has been reported to increase (0.4–3.1 mM) or decrease (> 12.5 mM) proliferation of human T cells [14]. In a pilot experiment, we observed that 5 mM NAC inhibited the proliferation of human T cells, whereas concentrations of 2 mM or less had no effect. Therefore, experiments with human T cells were performed using 2 mM NAC.
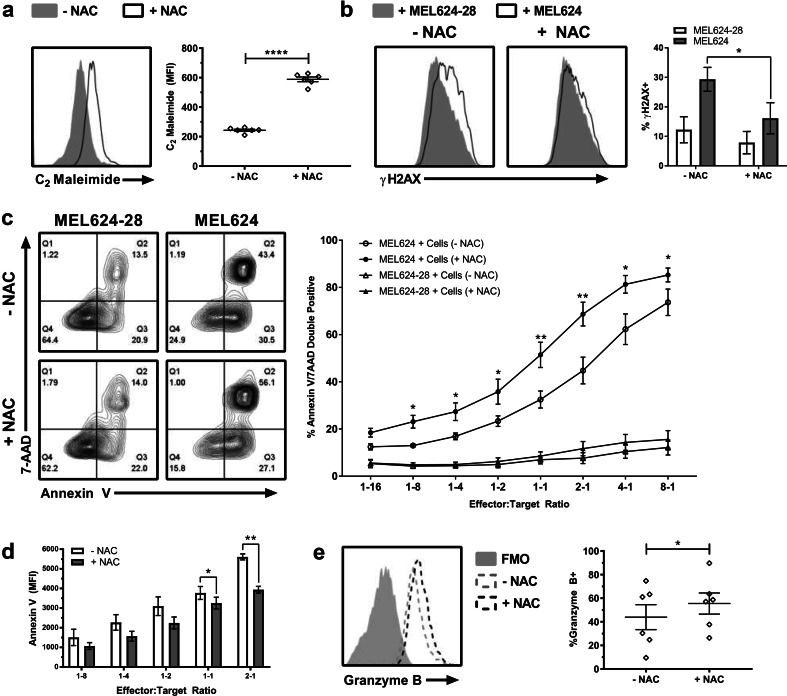
The TIL1383I TCR, which recognizes the melanoma associated antigen tyrosinase in an HLA-A2 restricted manner [10], is currently being used in a Phase I clinical study (NCT01586403) [15]. In the clinical protocol, activated TIL1383I TCR-transduced cells are rapidly expanded from day 10 to day 20. We investigated how the addition of NAC on day 3 (following activation and transduction) impacted T cell phenotype. Consistent with observations in the pilot experiment, NAC did not have a significant effect on proliferation, as the cellular yield of post-REP cultures on day 20 was comparable in three sets of experiments (each performed with a healthy donor and a melanoma patient, p = 0.3796, n = 6). Analysis of day 20 cultures demonstrated that surface thiols in NAC-expanded TIL1383I TCR-transduced human T cells were ~ 2.4-fold higher than controls (Fig. 2a). Co-culture of T cells with melanoma cells demonstrated that TCR restimulation by target cells presenting the cognate antigen in the context of HLA-A2 (MEL624 cells) resulted in an increase in γH2AX staining compared to MEL624-28 cells that lack HLA-A2 (Fig. 2b). This increase in γH2AX staining was reduced by ~ 1.8-fold in NAC-cultured cells (Fig. 2b). A three-way co-culture assay that included melanoma cells (both MEL624 and MEL624-28) and T cells showed that TIL1383I TCR NAC-expanded transduced human T cells were more efficacious at killing MEL624 melanoma cells than control-cultured cells (Fig. 2c) while being less susceptible to tumor-cell-induced AICD (Fig. 2d). In addition, a modest but significant elevation in granzyme B expression was observed in TIL1383I TCR-transduced T cells expanded in NAC (Fig. 2e). Thus, similar to the murine models, human TCR-transduced cells expanded in NAC have increased surface thiols, decreased γH2AX and Annexin V staining, and increased granzyme B expression.
Fig. 2.
Human TIL1383I TCR-transduced T cells cultured in NAC have enhanced antioxidant capacity, reduced evidence of DNA damage, and superior cytotoxic activity against melanoma cells in vitro. TIL1383I TCR-transduced human T cells were collected on day 20 and CD34+CD8+ cells analyzed for surface thiols (a) and for γH2AX following co-culture with melanoma target cells (b). Representative histograms and quantification (mean ± SEM) are shown. c, d In vitro cytotoxicity assay as described in “Materials and methods”. c Representative contour plot of Annexin V & 7AAD expression in melanoma cells at a 2:1 E:T ratio (left) and quantification (mean ± SEM) of Annexin V+/7AAD+ cells over all E:T ratios (right). d Quantification (mean ± SEM) of Annexin V MFI of CD34+ gated cells used in “c”. e Representative histogram overlay and quantification (mean ± SEM) of granzyme B expression in CD34+ CD8+ cells after rapid expansion (± NAC). n = 5–6; *p < 0.05; **p < 0.01; ****p < 0.0001
NAC does not impact of memory subset development, but diminishes the up-regulation of PD-1 and CD57 caused by rapid expansion in TIL1383I TCR-transduced human T cells
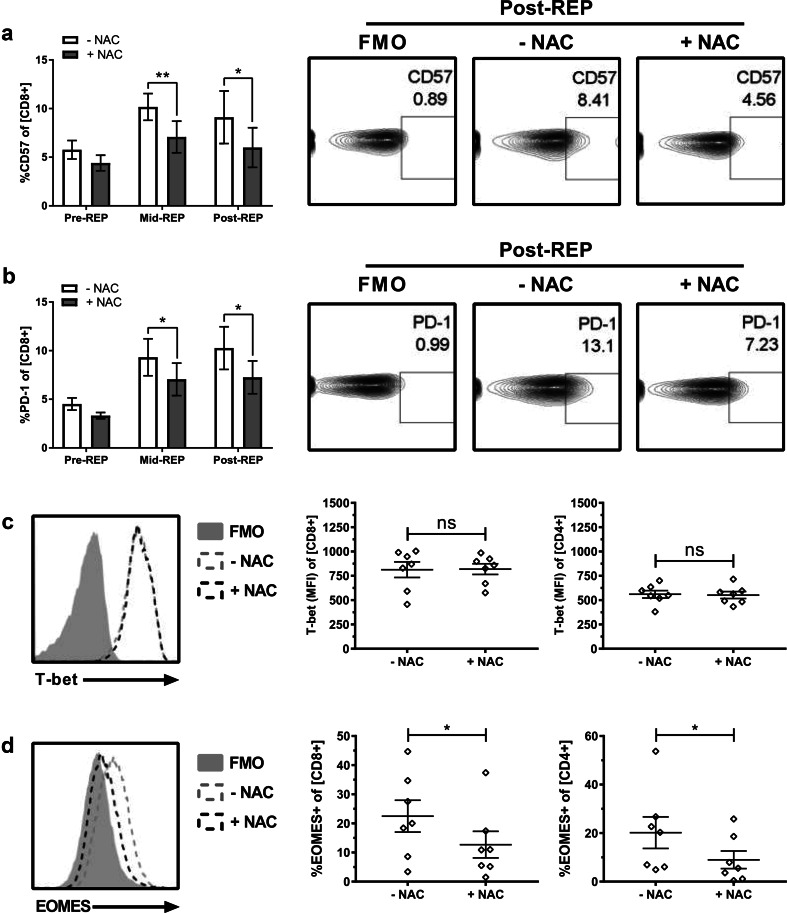
The negative effects of polyclonal stimulation during the REP not only increases susceptibility to AICD [8] but has also been reported to increase the CD57 senescence marker [6], promote a more differentiated phenotype characterized by an increase effector memory cells (TEM) over central-memory cells (TCM) [5], and increase PD-1 expression, which can be associated with exhaustion [7]. Analysis of these parameters revealed that supplementation with NAC during the REP significantly reduced up-regulation of senescence marker CD57 (Fig. 3a). As TCM possess superior antioxidant capacity, are more resistant to AICD, and exhibit improved tumor control, we hypothesized that NAC promotes a shift in memory phenotype [16, 17]. However, cultures expanded in the absence or the presence of NAC did not differ in their proportion of TEM and TCM cells (Suppl. Figure 2). We also evaluated the cells for expression of the transcription factors T-bet and EOMES that regulate differentiation into TEM and TCM phenotypes [18]. However, there was no decrease in T-bet expression or increase in EOMES expression that would be associated with the TCM phenotype (Fig. 3c, d). Interestingly, there was a small but significant decrease in the expression of EOMES that was observed in both CD8 and CD4 populations (Fig. 3d).
Fig. 3.
NAC impedes expression of senescence and exhaustion markers during rapid expansion. After 10 days in culture, TIL1383I TCR-transduced T cells were rapid expanded for an additional 10 days (± 2 mM NAC). Samples were cryopreserved before (Pre), on day 5 (Mid), and at the conclusion (Post) of the REP. Samples were subsequently analyzed for expression of a CD57 or b PD-1. Right panels display representative contour plots of post-REP cells. Left panels show quantification (mean ± SEM) of each indicated marker in CD34+ CD8+ cells over the course of the REP. n = 4 (2 melanoma patients, 2 healthy controls); *p < 0.05; **p < 0.01. TIL1383I TCR-transduced T cells rapidly expanded (± 2 mM NAC) were analyzed for the expression of c T-bet and d EOMES in CD34+CD8+-gated cells. Left panels display representative histogram overlay. Right panels are quantification (mean ± SEM) of n = 7. *p < 0.05; ns not significant
While EOMES can promote a more anti-tumor efficacious TCM phenotype, increased EOMES expression has also been associated with T-cell exhaustion [19] and a decline in T-cell effector functionality [7]. Exhausted T cells express elevated levels of PD-1 [20, 21] and rapid expansion can increase PD-1 levels in therapeutic T cells [6]. We found that the REP significantly elevated PD-1 expression (p < 0.0001 pre-REP versus post-REP). This increase was significantly reduced when cultures were expanded in NAC (Fig. 3b). Overall, these data suggest that supplementation with NAC does not alter memory phenotype, but significantly reduces CD57 and PD-1 expressions.
TIL1383I TCR-transduced human T cells expanded in NAC have reduced expression of the transcription factor Foxo1
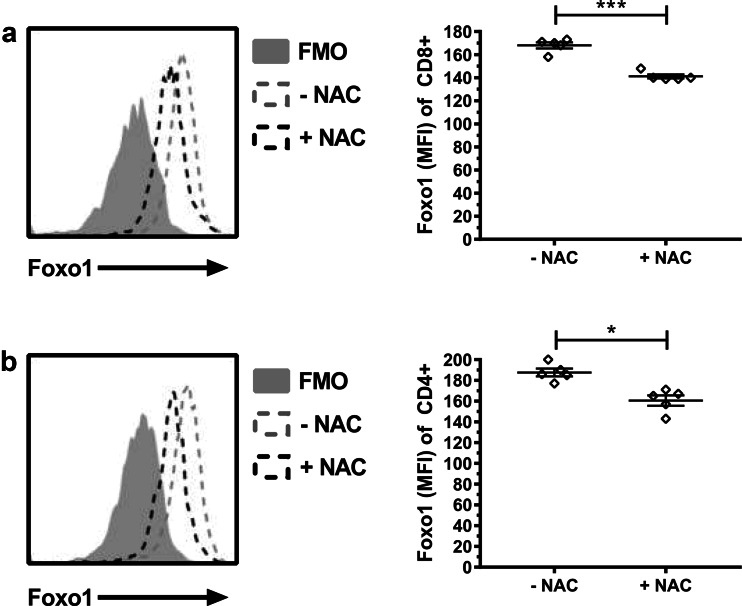
Having observed that NAC reduces the expression of EOMES and PD-1 while increasing the expression of granzyme B led us to hypothesize that expansion of T cells in NAC might repress the expression of Foxo1. Foxo1 has recently been identified as a critical transcription factor responsible for the induction of EOMES and expression of PD-1 [22]. Foxo1 has also been shown to repress the expression of the cytolytic molecule granzyme B [23]. Analysis of Foxo1 in our cultures revealed that TIL1383I TCR-transduced cells expanded in NAC had significantly reduced Foxo1 MFI intensity in both CD8+ and CD4+ T cells (Fig. 4).
Fig. 4.
TIL1383I TCR-transduced human T cells expanded in NAC have reduced expression of the transcription factor Foxo1. Rapidly expanded TIL1383I TCR-transduced human T cells were analyzed for the expression of Foxo1 in a CD34+CD8+ and b CD34+CD4+ T cells. Left panels display representative histogram overlay of T cells expanded in the absence or the presence of NAC compared to FMO control. Right panels display quantification (mean ± SEM) of Foxo1 MFI (n = 5). *p < 0.05, ***p < 0.001
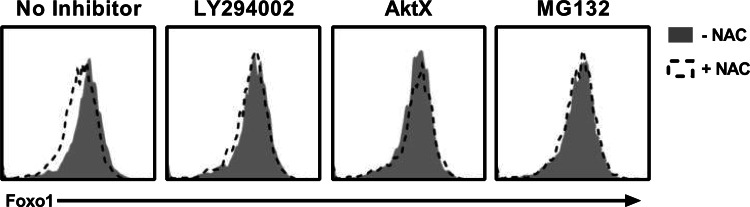
Regulation of Foxo1 expression and subcellular localization is complex. In its unphosphorylated state, Foxo1 resides in the nucleus, where it controls the expression of numerous genes. Upon phosphorylation by Akt, Foxo1 is excluded from the nucleus, ubiquitinated, and targeted for proteasomal degradation [24]. To determine a possible direct effect of NAC on Foxo1 expression and dependence on Akt signaling, TIL1383I TCR-transduced T cells that had been REPed were treated with pharmacological inhibitors and then exposed them to NAC. As shown in Fig. 5, exposure of activated T cells to NAC significantly decreased Foxo1 expression (p = 0.0004). The NAC-mediated decrease in Foxo1 was completely prevented when cells were pretreated with the PI3K inhibitor LY294002, the Akt inhibitor AktX, or the proteasomal inhibitor MG132 (Fig. 5). Similar results were observed in a total of 4 donors. Furthermore, the inhibitory effect was observed only with the broad-spectrum PI3K inhibitor LY294002 but not with CAL-101, which preferentially inhibits p110δ relative to other PI3K class I enzymes (Suppl. Figure 3). These results suggest that NAC, in the absence of TCR restimulation, can diminish Foxo1 expression through a PI3K/Akt-dependent mechanism involving proteasomal degradation.
Fig. 5.
Acute Treatment of T cells with NAC rapidly degrades Foxo1 in an Akt-dependent manner. T cells were pretreated with LY294002 (20 µm), AktX (5 µm), or MG132 (20 µm) for 60 min prior to adding NAC (25 mM) for an additional 60 min. Display is a representative histogram overlay of CD8+ cells. Similar results were obtained from four individual donors treated with NAC versus untreated controls (p = 0.0004)
Discussion
Previously, we demonstrated that NAC protects against AICD and that NAC supplementation during expansion of murine transgenic Pmel-1 T cell cultures increased their ability control tumor and enhanced survival in recipient mice [9]. Moreover, adoptively transferred cells recovered from recipient mice were less susceptible to peptide-induced DNA damage and AICD [9]. In this study, we expanded these observations to engineered T cells and further investigated how NAC impacts the phenotype of human-transduced T cells that are currently under evaluation in a Phase I clinical trial.
Our results suggest that the benefits of NAC extend to TCR-engineered cells. Adoptive transfer of TRP-1 TCR-transduced T cells cultured in NAC significantly delayed tumor growth and improved the median survival time in mice (Fig. 1). Similar to the Pmel-1 model, enhanced antioxidant capacity alongside reduced susceptibility to DNA damage and resistance to AICD likely contributed to this outcome (Suppl. Figure 1). The NAC-induced phenotype was relatively durable as it was maintained for at least 9 days after in vivo transfer (Fig. 1). In the Pmel-1 model, insufficient cell recovery from tumors in the control group limited comparisons between experimental groups [9]. Here, we show that TRP-1 TCR-transduced cells expanded in the absence of NAC that had trafficked to the tumor were significantly more susceptible to AICD than cells recovered from the spleen (Fig. 1c). This observation is consistent with multiple reports that have demonstrated the tumor microenvironment to be extraordinarily hostile towards recruited T cells, inducing them to undergo AICD or become anergic [25–27]. We, therefore, found it significant that expansion of engineered T cells in NAC resulted in comparable protection from AICD in both compartments (Fig. 1c). These results suggest that while NAC has an overall survival benefit for adoptively transferred T cells, the protection afforded by NAC may be particularly crucial to T cells when they enter the tumor microenvironment.
We have previously shown that NAC protects human T cells from AICD by reducing activation of the DNA damage response pathway [9], but how the addition of NAC affects the phenotype during expansion for adoptive transfer to patients has not been investigated. Similar to both murine models, expansion of TIL1383I TCR-transduced human cells in NAC enhanced surface thiols and reduced γH2AX and AICD (Fig. 2a, b). These results suggest that NAC similarly impacts antioxidant capacity, DNA damage, and AICD resistance in murine and human T cells. Notably, the dosage required for these effects in human cells was fivefold lower than in mouse cells, suggesting that optimal concentrations of NAC differ between species.
Persistence and maintenance of anti-tumor efficacy are key factors in the efficacy of ACT [28]. The ability of a cell to persist once transferred may even be paramount to its cytolytic ability. In the context of ACT, although TEM have an enhanced capacity to secrete effector molecules such as granzyme B and IFNγ, TCM have a greater capacity for self-renewal and persistence than TEM and are superior at tumor control [17, 29]. In our hands, NAC did not influence the memory phenotype of the cells, yet NAC supplemented T cells were more efficacious in killing MEL624 melanoma cells, implicating that NAC improves the effector functionality of T cells (Suppl. Figure 2; Fig. 2). One possibility is that the elevated expression of the cytotoxic effector molecule granzyme B, which was increased following expansion in NAC, contributes to the enhanced cytolytic phenotype of the TIL1383I TCR-transduced cells (Fig. 2e). The increase in granzyme B expression following expansion of T cells in NAC has been consistently observed in all models we tested (Pmel-1 transgenic mouse T cells, TPR-1-transduced mouse cells, and TIL1383I TCR-transduced human cells). Despite the increase in granzyme B levels, we observed greater efficacy in tumor control in vivo (Fig. 1a, Ref [9]). These results are in contrast to earlier studies in Pmel-1 cells in which increased levels of granzyme B in terminally differentiated cells rendered them highly cytolytic in vitro but impaired their ability to persist in vivo [30]. Thus, the expansion of T cells in NAC appears to increase levels of granzyme B in parallel with increasing resistance to cell death, suggesting that granzyme B expression and ability to persist may be regulated independently.
The use of younger, less differentiated cells that are more durable after transfer is associated with better tumor control [4, 30]. However, rapid expansion of T cells for adoptive transfer promotes a terminally differentiated phenotype characterized by a loss in CD28 expression and the up-regulation in both the senescence marker CD57 and exhaustion marker PD-1 [6]. Our data confirm that CD57 levels increase during the REP and that NAC can significantly diminish this up-regulation (Fig. 3a). The expression of CD57 is indicative of cells reaching the “Hayflick limit” of replicative senescence caused by maximal telomere erosion following cell division [31]. Consequently, T cells with longer telomeres, indicative of a shorter replicative history, have superior long-term anti-tumor efficacy for ACT [32–34]. Although NAC can affect T-cell proliferation [14], our data argue against NAC decreasing the number of replications as there was no difference in the REP yield between experimental groups. Several studies across multiple cell types have demonstrated that NAC induces the activity of telomerase, an enzyme that rebuilds telomere caps, by constraining telomerase to the nucleus [35–37]. An increase in γH2AX is associated with shorter telomeres [38]. Therefore, while the effect of NAC on telomerase in T cells has not been investigated, it is reasonable to postulate that NAC has a telomere-protective effect, since NAC reduces γH2AX levels.
The transcription factor EOMES and PD-1 were also significantly decreased with NAC supplementation during the REP (Fig. 3), suggesting the possibility of a common upstream regulator. We identified Foxo1 as a potential candidate as it promotes the expression of both EOMES and PD-1 while suppressing granzyme B expression. Indeed, TIL1383I TCR-transduced cells expanded in NAC had significantly decreased Foxo1 expression (Fig. 4). Studies with Foxo1-deficient cells have demonstrated that this transcription factor acts as a chief regulator of central-memory development with CD62L, CCR7, and IL-7α as direct target genes [23, 39]. However, we observed no modulation of CD62L or CCR7 in NAC-cultured cells (Suppl. Figure 2), which suggests that the modest, yet significant, decrease in Foxo1 expression following expansion in NAC is insufficient to impact memory development. As a critical upstream factor of EOMES, Foxo1 promotes the exhausted phenotype, including the induction of PD-1 [22]. As both EOMES and PD-1 were significantly decreased in NAC-cultured cells (Suppl. Figure 3; Fig. 3), our data suggest that NAC supplementation diminishes exhaustion.
Foxo1 is negatively regulated by Akt which facilitates its nuclear exclusion and proteasomal degradation in the cytosol [24, 40]. The NAC-mediated degradation of Foxo1 in activated T cells was ablated by pharmacological inhibition of the PI3K/Akt pathway (Fig. 5), which is consistent with numerous studies in various cell types such as neurons [41], cardiomyocytes [42], hepatocytes [43], and pancreatic islet cells [44], in which NAC can activate Akt. In T cells, initial TCR stimulation activates Akt and promotes the development into effector T cells. However, the repetitive TCR stimulation diminishes Akt activity and increases Foxo1 transcriptional activity and expression of PD-1, resulting in an exhausted phenotype [22]. Our data support a model in which NAC maintains Akt activity and suppresses Foxo1 thereby allowing maintenance of an effector phenotype despite repetitive TCR stimulation. It has been established that Akt can be reversibly activated and inactivated by intracellular redox status [45, 46]. A speculative mechanism by which NAC may impact Akt activity comes from the field of neuroscience. In neurodegenerative diseases such as Parkinson’s disease, Akt activity decreases following oxidative modification and dephosphorylation [47]. Mutation of the two cysteine residues (296/310) flanking the Thr308 phosphorylation site while not affecting basal kinase activity prevented oxidative stress mediated inactivation of Akt, thereby increasing cell survival [47]. Thus, oxidative stress induced by repetitive TCR stimulation may lead to modification of Cys296/310 and reduced Akt activity, which then promotes expression of Foxo1 (and PD-1). By reducing oxidative stress, NAC may prevent or reverse inactivation of Akt.
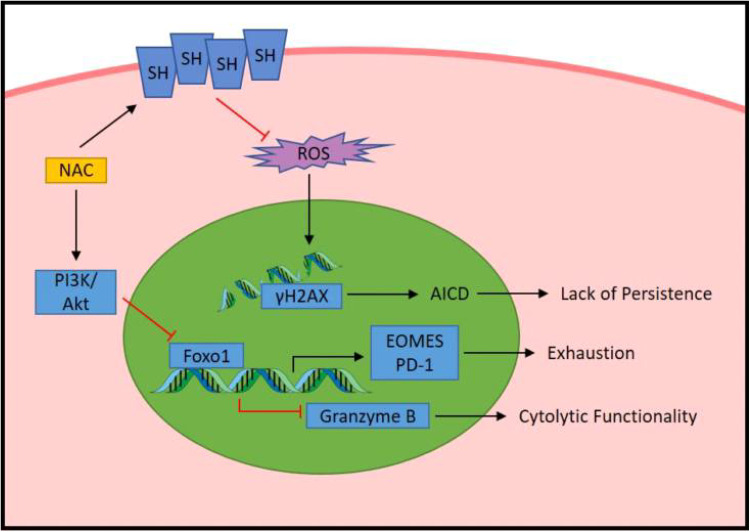
In conclusion, we report that the addition of NAC during expansion of cancer-specific T cells enhances their durability via resistance to AICD, delays the onset of REP induced exhaustion, and bolsters the overall cytolytic functionality of the cell (Fig. 6). Typically, enhancement of effector functionality associates with an increase in T-cell differentiation and a decline in durability [48]. However, our study demonstrates that NAC improves the phenotype of T cells by uncoupling the concomitant increase in effector functionality with increased differentiation. Our data suggest that NAC promotes a “hybrid” cell with enhanced functional capacity of a TEM cell yet the durability of a TCM cell. Thus, supplementation with NAC during the REP results in therapeutic T cells with a “younger” phenotype without compromising their ability to expand logarithmically or the cytolytic ability of a more differentiated cell. As such, we contend that further investigations into the addition of NAC to current clinical ACT protocols are merited.
Fig. 6.
Proposed model by which NAC enhances anti-tumor functionality of T cells
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The retroviral plasmid DNA encoding the MSGV-1 TRP-1 TCR was a kind gift from N. Restifo to C. Paulos.
Abbreviations
- 7-AAD
7-Aminoactinomycin D
- AICD
Activation-induced cell death
- EOMES
Eomesodermin
- NAC
N-acetyl cysteine
- REP
Rapid expansion protocol
- TRP-1
Tyrosinase related protein 1
Author contributions
MJS and CV-J conceived and designed the study. CV-J directed the project and edited the final draft of the manuscript. MJS carried out all experiments except for the aspects described for co-authors below and drafted the manuscript. GS was responsible for transduction and REP of human T cells. MMW transduced murine T cells. EG-M was responsible for the biostatistical analysis of animal experiments. GS, MMW, and EG-M drafted the corresponding method sections. CMP provided oversight for murine T-cell transduction and contributed towards the design of the study. MIN was responsible for direction and oversight of the clinical trial and contributed towards the design of the study. All authors read and approved the final manuscript.
Funding
This work was supported by the National Institutes of Health Grant P01CA154778. The Cell Evaluation and Therapy Shared Resource of the Hollings Cancer Center, Medical University of South Carolina was in part supported by the National Institutes of Health Grant P30 CA138313.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval and ethical standards
Apheresis and cells from melanoma patients were obtained as part of clinical trial that is registered with clinicaltrials.gov NCT01586403 and was approved by the Institutional Review Board at Loyola Medical University Center (LU 203732). All procedures performed in studies using human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All animal experiments were performed with approval by the Institutional Animal Care and Use Committee at the Medical University of South Carolina to ensure that ethical regulatory and policy mandates governing the use of animals in research are met (Animal Welfare Assurance #A3428-01). All methods were performed in accordance with the relevant guidelines and regulations.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Animal source
TRP-1 TCR transgenic mice, originally obtained from Dr. Nicolas Restifo, were provided by Dr. Chrystal Paulos. Recipient C57BL6 mice were purchased from Jackson Laboratories.
Cell line authentication
The tumor-cell lines in this study are used to determine T-cell reactivity and specificity, so the critical feature is their HLA and antigen expression. Cells are, therefore, routinely checked to ensure their antigen (tyrosinase) and expression HLA (HLA-A2 for MEL624) is correct.
References
- 1.DeSantis C, Lin C, Mariotto A, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg S. Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med. 2012;4:127ps8. doi: 10.1126/scitranslmed.3003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson LA, June CH (2016) Driving gene-engineered T cell immunotherapy of cancer. Cell Res 38–58 [DOI] [PMC free article] [PubMed]
- 4.Tran KQ, Zhou J, Durflinger KH, et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31:742–751. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell DJ, Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241–250. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Liu S, Hernandez J, et al. MART-1-specific melanoma tumor-infiltrating lymphocytes maintaining CD28 expression have improved survival and expansion capability following antigenic restimulation in vitro. J Immunol. 2010;184:452–465. doi: 10.4049/jimmunol.0901101. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez-Chacon JA, Li Y, Wu RC, et al. Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. J Immunother. 2011;34:236–250. doi: 10.1097/CJI.0b013e318209e7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheffel MJ, Scurti G, Simms P, et al. Efficacy of adoptive T-cell therapy is improved by treatment with the antioxidant N-acetyl cysteine, which limits activation-induced T-cell death. Cancer Res. 2016;76:6006–6016. doi: 10.1158/0008-5472.CAN-16-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roszkowski JJ, Lyons GE, Kast WM, et al. Simultaneous generation of CD8+ and CD4+ melanoma-reactive T cells by retroviral-mediated transfer of a single T-cell receptor. Cancer Res. 2005;65:1570–1576. doi: 10.1158/0008-5472.CAN-04-2076. [DOI] [PubMed] [Google Scholar]
- 11.Norell H, Zhang Y, McCracken J, et al. CD34-based enrichment of genetically engineered human T cells for clinical use results in dramatically enhanced tumor targeting. Cancer Immunol Immunother. 2010;59:851–862. doi: 10.1007/s00262-009-0810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerkar SP, Sanchez-Perez L, Borman Z, et al. Genetic engineering of murine CD8+ and CD4+ T cells for preclinical adoptive immunotherapy studies. J Immunother. 2011;34:343–352. doi: 10.1097/CJI.0b013e3182187600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karlsson H, Nava S, Remberger M, et al. N-acetyl-l-cysteine increases acute graft-versus-host disease and promotes T-cell-mediated immunity in vitro. Eur J Immunol. 2011;41:1143–1153. doi: 10.1002/eji.201040589. [DOI] [PubMed] [Google Scholar]
- 15.Moore T, Wagner CR, Scurti GM et al (2017) Clinical and immunologic evaluation of three metastatic melanoma patients treated with autologous melanoma-reactive TCR-transduced T cells. Cancer Immunol Immunother. 10.1007/s00262-017-2073-0 [DOI] [PMC free article] [PubMed]
- 16.Kesarwani P, Al-Khami AA, Scurti G, et al. Promoting thiol expression increases the durability of antitumor T-cell functions. Cancer Res. 2014;74:6036–6047. doi: 10.1158/0008-5472.CAN-14-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLane LM, Banerjee PP, Cosma GL, et al. Differential localization of T-bet and Eomes in CD8 T-cell memory populations. J Immunol. 2013;190:3207–3215. doi: 10.4049/jimmunol.1201556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawford A, Wherry EJ. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr Opin Immunol. 2009;21:179–186. doi: 10.1016/j.coi.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 22.Staron MM, Gray SM, Marshall HD, et al. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8+ T cells during chronic infection. Immunity. 2014;41:802–814. doi: 10.1016/j.immuni.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao RR, Li Q, Bupp MRG, Shrikant PA. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8+ T cell differentiation. Immunity. 2012;36:374–387. doi: 10.1016/j.immuni.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoki M, Jiang H, Vogt PK. Proteasomal degradation of the FoxO1 transcriptional regulator in cells transformed by the P3k and Akt oncoproteins. Proc Natl Acad Sci USA. 2004;101:13613–13617. doi: 10.1073/pnas.0405454101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaks TZ, Chappell DB, Rosenberg SA, Restifo NP. Fas-mediated suicide of tumor-reactive T cells following activation by specific tumor: selective rescue by caspase inhibition. J Immunol. 1999;162:3273–3279. [PMC free article] [PubMed] [Google Scholar]
- 26.Lu B, Finn OJ. T-cell death and cancer immune tolerance. Cell Death Differ. 2008;15:70–79. doi: 10.1038/sj.cdd.4402274. [DOI] [PubMed] [Google Scholar]
- 27.Saff RR, Spanjaard ES, Hohlbaum AM, Marshak-Rothstein A. Activation-induced cell death limits effector function of CD4 tumor-specific T cells. J Immunol. 2004;172:6598–6606. doi: 10.4049/jimmunol.172.11.6598. [DOI] [PubMed] [Google Scholar]
- 28.Robbins PF, Dudley ME, Wunderlich J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother. 2012;35:651–660. doi: 10.1097/CJI.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenchley JM, Karandikar NJ, Betts MR, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 32.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen X, Zhou J, Hathcock KS, et al. Persistence of tumor infiltrating lymphocytes in adoptive immunotherapy correlates with telomere length. J Immunother. 2007;30:123–129. doi: 10.1097/01.cji.0000211321.07654.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J, Shen X, Huang J, et al. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsin IL, Sheu GT, Chen HH, et al. N-acetyl cysteine mitigates curcumin-mediated telomerase inhibition through rescuing of Sp1 reduction in A549 cells. Mutat Res. 2010;688:72–77. doi: 10.1016/j.mrfmmm.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Liu M, Ye X, et al. Delay in oocyte aging in mice by the antioxidant N-acetyl-l-cysteine (NAC) Hum Reprod. 2012;27:1411–1420. doi: 10.1093/humrep/des019. [DOI] [PubMed] [Google Scholar]
- 37.Haendeler J, Hoffmann J, Diehl JF, et al. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ Res. 2004;94:768–775. doi: 10.1161/01.RES.0000121104.05977.F3. [DOI] [PubMed] [Google Scholar]
- 38.Hao LY, Strong MA, Greider CW. Phosphorylation of H2AX at short telomeres in T cells and fibroblasts. J Biol Chem. 2004;279:45148–45154. doi: 10.1074/jbc.M403924200. [DOI] [PubMed] [Google Scholar]
- 39.Kerdiles YM, Beisner DR, Tinoco R, et al. Foxo1 links homing and survival of naive T cells by regulating l-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuzaki H, Daitoku H, Hatta M, et al. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA. 2003;100:11285–11290. doi: 10.1073/pnas.1934283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noh YH, Chob HS, Kim DH, et al. N-acetylcysteine enhances neuronal differentiation of P19 embryonic stem cells via Akt and N-cadherin activation. Mol Biol (Mosk) 2012;46:741–746. doi: 10.1134/S0026893312040085. [DOI] [PubMed] [Google Scholar]
- 42.Wang T, Mao X, Li H, et al. N-Acetylcysteine and allopurinol up-regulated the Jak/STAT3 and PI3K/Akt pathways via adiponectin and attenuated myocardial postischemic injury in diabetes. Free Radic Biol Med. 2013;63:291–303. doi: 10.1016/j.freeradbiomed.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 43.Wang C, Xia Y, Zheng Y, et al. Protective effects of N-acetylcysteine in concanavalin a-induced hepatitis in mice. Mediators Inflamm. 2015;2015:189785. doi: 10.1155/2015/189785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin HM, Zhou DC, Gu HF, et al. Antioxidant N-acetylcysteine protects pancreatic β-cells against aldosterone-induced oxidative stress and apoptosis in female db/db mice and insulin-producing MIN6 cells. Endocrinology. 2013;154:4068–4077. doi: 10.1210/en.2013-1115. [DOI] [PubMed] [Google Scholar]
- 45.Leslie NR. The redox regulation of PI 3-kinase-dependent signaling. Antioxid Redox Signal. 2006;8:1765–1774. doi: 10.1089/ars.2006.8.1765. [DOI] [PubMed] [Google Scholar]
- 46.Leslie NR, Bennett D, Lindsay YE, et al. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmad F, Nidadavolu P, Durgadoss L, et al. Critical cysteines in Akt1 regulate its activity and proteasomal degradation: implications for neurodegenerative diseases. Free Radic Biol Med. 2014;74:118–28. doi: 10.1016/j.freeradbiomed.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.