Abstract
Little is known about the impact of comorbidities on multidrug resistant (MDR) and extensively drug resistant (XDR) tuberculosis (TB) treatment outcomes. We aimed to examine the effect of human immunodeficiency virus (HIV), diabetes, chronic kidney disease (CKD), alcohol misuse, and smoking on MDR/XDRTB treatment outcomes. We searched MEDLINE, EMBASE, Cochrane Central Registrar and Cochrane Database of Systematic Reviews as per PRISMA guidelines. Eligible studies were identified and treatment outcome data were extracted. We performed a meta-analysis to generate a pooled relative risk (RR) for unsuccessful outcome in MDR/XDRTB treatment by co-morbidity. From 2457 studies identified, 48 reported on 18,257 participants, which were included in the final analysis. Median study population was 235 (range 60–1768). Pooled RR of unsuccessful outcome was higher in people living with HIV (RR = 1.41 [95%CI: 1.15–1.73]) and in people with alcohol misuse (RR = 1.45 [95%CI: 1.21–1.74]). Outcomes were similar in people with diabetes or in people that smoked. Data was insufficient to examine outcomes in exclusive XDRTB or CKD cohorts. In this systematic review and meta-analysis, alcohol misuse and HIV were associated with higher pooled OR of an unsuccessful outcome in MDR/XDRTB treatment. Further research is required to understand the role of comorbidities in driving unsuccessful treatment outcomes.
Introduction
A major barrier to global tuberculosis (TB) elimination is the emergence of multidrug resistant TB (MDRTB) and extensively drug resistant TB (XDRTB)1–3. MDRTB is defined as resistance to at least rifampin and isoniazid1,3,4, while XDRTB is defined as resistance to rifampin, isoniazid, a fluoroquinolone and at least one second-line injectable agent2,3,5. MDR- and XDRTB require prolonged medical therapy and are associated with high rates of failure, loss to follow-up, relapse and death, largely the result of less effective and highly toxic TB treatment regimens3. In 2013, only 52% of MDRTB and 26% of XDRTB patients were successfully treated6. Understanding the drivers of unsuccessful treatment outcomes will be crucial in addressing the global MDR/XDRTB epidemic.
One driver of unsuccessful treatment outcomes may be comorbid conditions. The impact of comorbidities on drug sensitive TB treatment is well-described, with conditions such as human immunodeficiency virus (HIV) infection, diabetes mellitus (DM), chronic kidney disease (CKD) and alcohol misuse all associated with worse treatment outcomes3,7–10. MDR/XDRTB treatment programs often report high proportions of these comorbidities, with the prevalence of HIV, DM and alcohol misuse exceeding 10–20% in several large MDR/XDRTB cohort studies11–15. Unfortunately, the relationship between comorbid conditions and MDR/XDRTB treatment outcomes remains poorly described. We performed a systematic review of the published, peer-reviewed literature examining the association between specific comorbidities, including of HIV, diabetes, CKD, smoking and alcohol misuse, and MDR/XDRTB treatment outcomes3,7,8,16–19. We aimed to examine the relationship between comorbidities and standardized treatment outcomes including death, default, failure and a combined endpoint of unsuccessful treatment outcome.
Methods
This systematic review and meta-analysis conforms to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines20. Our research protocol is registered in PROSPERO (http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016039866, registration number CRD42016039866).
Objectives
Our primary objective was to estimate the association between individual comorbidities and risk of unsuccessful MDR/XDRTB treatment outcome (failure, death or default, as defined below). Our secondary objectives were to estimate the association between each comorbid condition with each specific treatment outcome.
Search strategy
Studies were identified by searching MEDLINE, EMBASE, Cochrane Central Registrar of Controlled Trials and Cochrane Database of Systematic Reviews for articles reporting MDRTB and XDRTB outcomes, published between January 1, 1980 and June 1, 2016. The full search strategy is provided in the online Appendix (Supp. Appendix). The database search was supplemented by reviewing bibliographies from all included full text articles and previous systematic reviews on MDRTB or XDRTB treatment outcomes3,5,18,19,21–24, as well as searching manually through all published titles from the International Journal of Tuberculosis and Lung Disease for relevant studies.
Eligibility Criteria and Study Selection
We included studies that enrolled at least 50 participants with microbiologically-confirmed MDRTB and/or XDRTB. Eligible studies included randomized control trials (RCTs), case-control (CC), retrospective cohort (RC) and prospective cohort (PC) studies. We examined studies reported in the peer-reviewed literature in English, French and Spanish.
Studies were excluded if they reported on exclusively surgical or non-medical therapy, exclusively used standardized first-line therapy or had non-consecutive enrolment. We also excluded studies with >30% loss to follow-up, default, or treatment outcomes otherwise unaccounted for. If two studies reported duplicate data, the publication with the more detailed reports on treatment outcomes was included for meta-analysis. Studies that did not report data necessary for calculating associations between comorbidities and outcomes were excluded from the meta-analysis but their data is reported in the Appendix (Supp. Tables 7–10)25–34.
Two authors (JS, AS) performed the search strategy. Titles then abstracts were reviewed; studies were excluded for lack of relevance or not meeting eligibility criteria. Articles identified by either reviewer based on title and abstract were included for full text review. In full text review, any discrepancies in eligibility were resolved by a third author (JCJ).
Treatment outcomes: definitions
Treatment outcome definitions reflected or approximated those published by Laserson et al.4.
Cure: completed MDRTB therapy with ≥5 negative cultures in the last 12 months of treatment; alternatively, a participant could have one positive culture followed by at least 3 negative cultures separated by 30 days with no clinical deterioration.
Treatment completion: completed MDRTB therapy without meeting the definition of cure.
Death: all-cause mortality during MDRTB therapy.
Default: interruption of therapy for ≥2 consecutive months for any reason.
Treatment failure: 2 of 5 cultures positive within the last 12 months of therapy or any culture positivity within the last 3 cultures; alternatively, failure was defined as treatment discontinuation due to lack of appropriate response or significant adverse events.
Defining co-morbid conditions
We accepted all studies’ original criteria for defining each comorbid condition.
Data Extraction
Data collection was performed in parallel by two authors (JS, AS), using a standardized data extraction tool, with discrepancies resolved by a third author (JCJ). Data collected included: study location, year, funding source, and design; participant characteristics (proportion with diabetes, HIV, smoking, CKD, alcohol misuse), as well as proportion with disease that was smear positive, cavitary, pulmonary, extra-pulmonary, XDRTB); treatment related variables (standardized vs individualized); and outcomes including treatment failure, default, death. When available in the original reports, effect estimates for the association between comorbidities and our outcomes of interest were extracted, along with their 95% confidence interval (95%CI).
Data Analysis
For our primary objective, we reported the pooled relative risk (RR) of the association between each comorbidity and unsuccessful treatment (a composite of failure, death, and default). For our secondary objectives, we reported the pooled RR for the association between each comorbidity and treatment failure, death, and default, as well as with the combined outcome of death and treatment failure. Pooled RR were calculated using Mantel-Haenszel random effects meta-analysis. The I-squared statistic (I2) was used to describe heterogeneity with values less than 33% being minor/no heterogeneity, 33–66% being moderate and values greater than 66% being significant heterogeneity. Outcomes were a composite of MDRTB and XDRTB data.
Statistical analyses were performed using Review Manager Software from the Cochrane Group. (Review Manager (RevMan) [Computer Program] Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration Group, 2014.)
Assessment of quality and bias
Study quality was assessed using the Newcastle-Ottawa Scale (NOS) for cohort studies35. Publication bias was assessed using visual inspection of funnel plots (Supp. Figs 1–4).
Results
Our literature search yielded 2457 titles; 2066 were excluded based on review of title and abstract, leaving 391 articles for full text review (Fig. 1). After full text review, 55 articles were eligible for analysis, with 7 articles reporting ≥30% of default, transfer out and loss to follow-up36–42, leaving 48 papers with 18,257 participants for final analysis10–15,28,34,43–82. There were no overlapping study populations in the final review.
Figure 1.
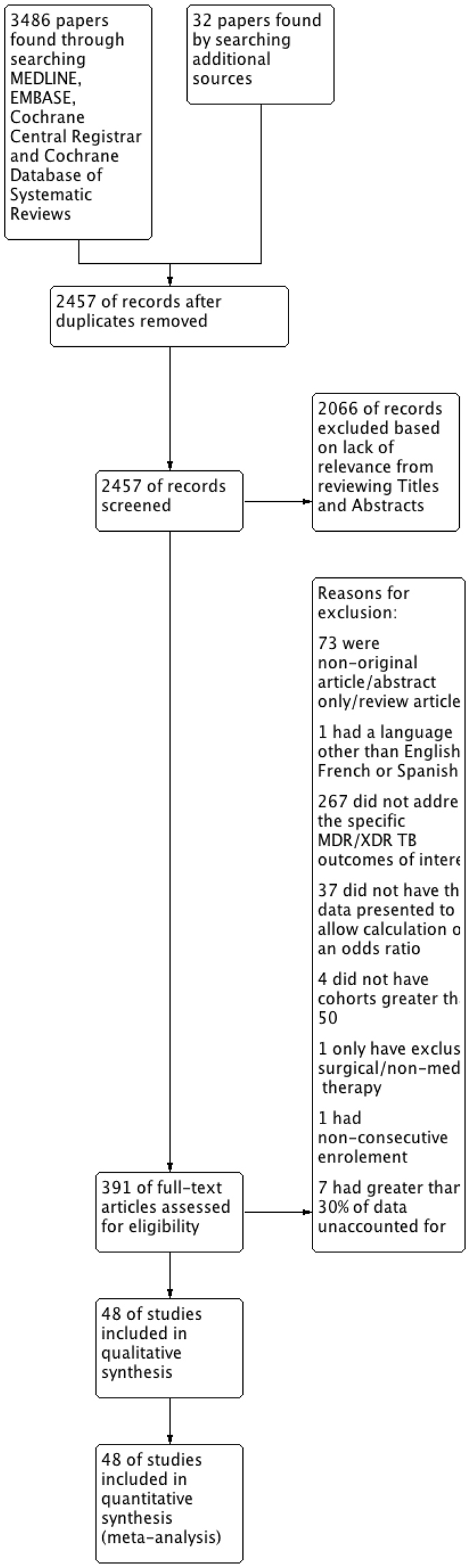
Flowchart showing Selection Process for Included Articles.
All 48 included studies were published between 1996 and 2016 with a median population of 235 (range 60–1768) (Tables 1 and 2). The majority (71.4%) of studies were retrospective cohorts10–13,15,28,34,44,46,48–50,54–57,59–61,63,65–71,73,74,76–79,81,82, while 26.5% of studies were prospective cohorts14,43,45,47,51–53,58,62,64,75,80 and one study was case-control in design72. There were no relevant RCTs that met our inclusion criteria. None of the included studies received direct funding from pharmaceutical companies.
Table 1.
Characteristics of Included Studies.
| Study | Country | Study Design | Quality (NOS) | Sample Size (n) | Age (median/IQRMean/SD) | Male n(%) |
Smear Positive n(%) | Cavitary Disease n(%) | XDR-TB n(%) | Length of Treatment (median/IQR) |
|---|---|---|---|---|---|---|---|---|---|---|
| Meressa et al.43 | Ethiopia | PC | 8 | 612 | 27 (22–36) |
325 (0.53) |
N/A | 306 (0.5) |
N/A | N/A |
| Kempker et al.44 | Georgia | RC | 5 | 141 | 34 (27–46) |
103 (0.73) |
46 (0.33) |
30 (0.21) |
9 (0.06) |
N/A |
| Gegia et al.45 | Georgia | PC | 7 | 380 | 38 (16–81) |
271 (0.71) |
N/A | N/A | N/A | >18 months |
| Miller et al.46 | Russia | RC | 9 | 407 | N/A | N/A | N/A | N/A | 20 (0.05) |
N/A |
| Kwak et al.10 | South Korea | RC | 7 | 123 | 37 (27–56) |
69 (0.56) |
N/A | 85 (0.69) |
26 (0.21) |
24.4 months (18.4–27.3) |
| Leimane et al.28 | Latvia | RC | 6 | 204 | N/A | 153 (0.75) |
90 (0.44) |
148 (0.73) |
N/A | N/A |
| Loveday et al.47 | South Africa | PC | 9 | 1549 | N/A | 746 (0.48) |
1048 (0.68) |
N/A | N/A | N/A |
| Seddon et al.11 | South Africa | RC | 9 | 111 | 50 (19–108) |
46 (0.41) |
53 (0.48) |
38 (0.34) |
5 (0.05) |
18 months |
| Duraisamy et al.12 | India | RC | 7 | 179 | N/A | 139 (0.78) |
N/A | 94 (0.53) |
N/A | N/A |
| Dheda et al.13 | South Africa | RC | 9 | 174 | N/A | 85 (0.49) |
N/A | N/A | 174 (1) |
6.9 months (3–12) |
| Kvasnovsky et al.48 | South Africa | RC | 7 | 206 | N/A | 101 (0.49) |
89 (0.43) |
50 (0.24) |
206 (1) |
N/A |
| Shah et al.49 | USA | RC | 9 | 83 | N/A | 53 (0.64) |
57 (0.69) |
24 (0.29) |
83 (1) |
N/A |
| Elliott et al.50 | South Africa | RC | 9 | 272 | N/A | 135 (0.50) |
131 (0.48) |
N/A | 27 (0.10) |
N/A |
| Vashakidze et al.51 | Georgia | PC | 8 | 75 | 30 (15–54) |
47 (0.63) |
12 (0.16) |
71 (0.95) |
24 (0.32) |
N/A |
| Kuaban et al.52 | Cameroon | PC | 6 | 150 | N/A | 77 (0.51) |
N/A | N/A | N/A | 368 days (363–375) |
| Brust et al.53 | South Africa | PC | 7 | 1209 | 33(26–41) | 737 (0.61) |
N/A | N/A | N/A | N/A |
| Van Altena et al.54 | Netherlands | RC | 9 | 113 | N/A | 69 (0.61) |
56 (0.50) |
N/A | 4 (0.04) |
445 days |
| O’Donnell et al.55 | South Africa | RC | 7 | 60 | N/A | 26 (0.43) |
N/A | N/A | 60 (1) |
N/A |
| Oladimeji et al.56 | Nigeria | RC | 6 | 162 | N/A | 105 (0.65) |
N/A | N/A | N/A | N/A |
| Hicks et al.57 | South Africa | RC | 7 | 84 | 8 (4–12) |
36 (0.43) |
40 (0.48) |
N/A | 6 (0.07) |
644 days (559–728) |
| Ahmad et al.58 | Pakistan | RC/PC | 8 | 196 | 31.5 (16.8–46.2) |
92 (0.47) |
178 (0.91) |
68 (0.35) |
N/A | 24 months (20–34) |
| Phuong et al.59 | Vietnam | RC | 9 | 1380 | N/A | 1074 (0.78) |
1167 (0.85) |
N/A | N/A | N/A |
| Akshata et al.82 | India | RC | 4 | 69 | N/A | 46 (0.67) |
69 (1) |
34 (0.49) |
2 (0.03) |
N/A |
| Kuksa et al.60 | Latvia | RC | 9 | 133 | N/A | 86 (0.65) |
79 (0.59) |
90 (0.68) |
133 (1) |
N/A |
| Eker et al.61 | Germany | RC | 8 | 184 | N/A | 139 (0.76) |
N/A | N/A | 7 (0.04) |
N/A |
| Cox et al.62 | Uzbekistan | PC | 7 | 87 | 34 (17–72) |
53 (0.61) |
N/A | 61 (0.70) |
N/A | 22 months (18–30) |
| Cegielski et al.14 | Multiple* | PC | 9 | 1244 | N/A | 771 (0.62) |
1034 (0.83) |
750 (0.60) |
N/A | 651 days (589–736) |
| Bendayan et al.63 | Israel | RC | 7 | 135 | 40 | 102 (0.76) |
93 (0.69) |
N/A | 11 (0.08) |
N/A |
| Park et al.15 | USA | RC | 7 | 173 | 40 | 159 (0.92) |
94 (0.54) |
54 (0.31) |
N/A | N/A |
| Farley et al.64 | South Africa | PC | 9 | 757 | N/A | 448 (0.59) |
434 (0.57) |
N/A | N/A | N/A |
| Satti et al.65 | South Africa | RC | 9 | 134 | N/A | 79 (0.59) |
15 (0.11) |
96 (0.72) |
N/A | 22.9 monhts (21.6–24) |
| Dolgusev et al.66 | Moldova | RC | 9 | 273 | N/A | N/A | N/A | N/A | N/A | N/A |
| Khaliaukin et al.67 | Belarus | RC | 9 | 439 | N/A | 367(0.84) | N/A | N/A | N/A | N/A |
| Kurbatova et al.68 | Multiple¶ | RC | 5 | 1768 | 36 | 1237 (0.70) |
N/A | 1085 (0.61) |
57 (0.03) |
21 months (17–25) |
| Oliveira et al.69 | Portugal | RC | 9 | 364 | N/A | 259 (0.71) |
N/A | 157 (0.43) |
107 (0.29) |
N/A |
| Franke et al.70 | Peru | RC | 8 | 671 | 31.4 (19.3–43.5) |
408 (0.61) |
N/A | N/A | N/A | N/A |
| Lalor et al.71 | Uzbekistan | RC | 7 | 710 | 29.4 (23.1–40.3) |
343 (0.48) |
N/A | N/A | N/A | N/A |
| Gandhi et al.72 | South Africa | cc | 6 | 262 | 34 (29–43) |
70 (0.27) |
161 (0.61) |
N/A | 139 (0.53) |
N/A |
| Tang et al.34 | China | RC | 9 | 586 | N/A | 395 (0.67) |
N/A | 389 (0.66) |
169 (0.29) |
N/A |
| Jeon et al.73 | South Korea | RC | 7 | 176 | 42.1 (28–56.2) |
127 (0.72) |
N/A | 132 (0.75) |
176 (1) |
N/A |
| Pazarli et al.74 | Turkey | RC | 6 | 103 | 40.5 (27–54) |
81 (0.79) |
N/A | N/A | N/A | N/A |
| Jain et al.75 | India | PC | 8 | 130 | N/A | 81 (0.62) |
N/A | N/A | N/A | N/A |
| Charles et al.76 | Haiti | RC | 7 | 110 | 28 (23–37) |
50 (0.45) |
N/A | N/A | N/A | 22.5 months (11.5–31) |
| Shin et a l.77 | Russia | RC | 8 | 244 | 32.3 (16–65) |
211(0.86) | N/A | N/A | N/A | N/A |
| Marais et al.78 | South Africa | RC | 7 | 324 | N/A | 170 (0.52) |
N/A | N/A | N/A | N/A |
| Van Der Walt et al.79 | South Africa | RC | 8 | 671 | N/A | N/A | N/A | N/A | N/A | N/A |
| Gler et al.80 | Philippines | RC/PC | 8 | 439 | N/A | 271 (0.62) |
N/A | 216 (0.49) |
N/A | N/A |
| Chung-Delgado et al.81 | Peru | RC | 7 | 201 | N/A | 127 (0.63) |
151 (0.75) |
N/A | N/A | N/A |
*Estonia, Latvia, Philippines, Peru, Russia, South Africa, South Korea, Taiwan, Thailand; ¶Estonia, Lativa, Philippines, Peru, Russia.
Table 2.
Overall Treatment outcomes.
| Study | DM n(%) |
HIV n(%) |
Smoker n(%) |
ETOH n(%) |
Regimen n(%)* |
Treatment Success n(%) |
Default, Death, Failure (%) |
Data unknown (%)¶ |
|---|---|---|---|---|---|---|---|---|
| Meressa et al.43 | 33 (0.05) |
133 (0.21) |
67 (0.11) |
N/A | S | 481 (0.79) |
95 (0.15) |
0.06 |
| Kempker et al.44 | 16 (0.10) |
6 (0.04) |
N/A | N/A | I | 79 (0.5) |
N/A | N/A |
| Gegia et al.45 | 35 (0.09) |
5 (0.01) |
152 (0.4) |
94 (0.24) |
I | 201 (0.53) |
179 (0.47) |
0.22 |
| Miller et al.46 | N/A | N/A | 343 (0.84) |
253 (0.62) |
N/A | 247 (0.61) |
160 (0.39) |
0.23 |
| Kwak et al.10 | 10 (0.08) |
N/A | 38 (0.31) |
N/A | I | 103 (0.83) |
18 (0.15) |
0.05 |
| Leimane et al.28 | 6 (0.03) |
1 (0.005) |
N/A | 125 (0.61) |
N/A | 135 (0.66) |
69 (0.34) |
0.13 |
| Loveday et al.47 | N/A | 1104 (0.71) |
N/A | N/A | S | 866 (0.56) |
661 (0.43) |
0.23 |
| Seddon et al.11 | N/A | 43 (0.39) |
N/A | N/A | I | 82 (0.74) |
14 (0.13) |
0.14 |
| Duraisamy et al.12 | 60 (0.33) |
1 (0.006) |
25 (0.14) |
16 (0.09) |
S | 112 (0.63) |
66 (0.37) |
0.15 |
| Dheda et al.13 | N/A | 82 (0.47) |
49(0.28) | N/A | S/I | N/A | 62 (0.36) |
N/A |
| Kvasnovsky et al.48 | N/A | 108(0.52) | N/A | N/A | I | N/A | 95 (0.46) |
N/A |
| Shah et al.49 | N/A | 31 (0.37) |
N/A | N/A | N/A | 33 (0.40) |
26 (0.31) |
0.28 |
| Elliott et al.50 | N/A | 93 (0.34) |
N/A | N/A | N/A | 113 (0.42) |
155 (0.57) |
N/A |
| Vashakidze et al.51 | 6 (0.08) |
1 (0.01) |
27 (0.36) |
11 (0.15) |
I | 59 (0.79) |
13 (0.17) |
0.16 |
| Kuaban et al.52 | N/A | 30 (0.2) |
N/A | N/A | S | N/A | N/A | 0.03 |
| Brust et al.53 | N/A | 362 (0.30) |
N/A | N/A | S | 526 (0.44) |
683 (0.56) |
0.21 |
| Van Altena et al.54 | N/A | 14 (0.12) |
N/A | N/A | N/A | 89 (0.79) |
17 (0.15) |
0.13 |
| O’Donnell et al.55 | N/A | 43 (0.72) |
N/A | N/A | N/A | N/A | 31 (0.52) |
0.10 |
| Oladimeji et al.56 | N/A | 28 (0.17) |
N/A | N/A | S | N/A | 24 (0.15) |
N/A |
| Hicks et al.57 | N/A | 64 (0.76) |
N/A | N/A | N/A | 66 (0.79) |
18 (0.21) |
0.06 |
| Ahmad et al.58 | 17 (0.09) |
N/A | 32 (0.16) |
N/A | I | 136 (0.69) |
45 (0.23) |
0.04 |
| Phuong et al.59 | N/A | 53 (0.04) |
N/A | N/A | S | 1008 (0.73) |
196 (0.41) |
0.13 |
| Akshata et al.82 | 8 (0.12) |
3 (0.04) |
N/A | N/A | S | 33 (0.48) |
34 (0.49) |
0.17 |
| Kuksa et al.60 | 3 (0.02) |
16 (0.12) |
N/A | 64 (0.48) |
I | 70 (0.53) |
63 (0.47) |
0.12 |
| Eker et al.61 | N/A | 7 (0.04) |
N/A | N/A | N/A | 109 (0.59) |
17 (0.09) |
0.14 |
| Cox et al.62 | N/A | N/A | 27 (0.31) |
20 (0.23) |
S/I | 54 (0.62) |
33 (0.38) |
0.14 |
| Cegielski et al.14 | 162 (0.13) |
159 (0.13) |
268 (0.22) |
179 (0.14) |
S/I | 722 (0.58) |
483 (0.39) |
0.22 |
| Bendayan et al.63 | 17 (0.13) |
8 (0.06) |
N/A | 34 (0.25) |
I | 70 (0.52) |
62 (0.46) |
0.09 |
| Park et al.15 | N/A | 90 (0.52) |
N/A | N/A | N/A | N/A | 100 (0.58) |
0.18 |
| Farley et al.64 | N/A | 287 (0.38) |
N/A | N/A | S | 348 (0.46) |
409 (0.54) |
0.21 |
| Satti et al.65 | N/A | 94 (0.70) |
N/A | N/A | S | 83 (0.62) |
48 (0.36) |
0.03 |
| Dolgusev et al.66 | N/A | 39 (0.14) |
N/A | N/A | I | 122 (0.45) |
95 (0.35) |
0.21 |
| Khaliaukin et al.67 | N/A | 67 (0.15) |
N/A | N/A | I | 148 (0.33) |
234 (0.53) |
0.13 |
| Kurbatova et al.68 | 68 (0.04) |
27 (0.015) |
272 (0.15) |
489 (0.28) |
I | 1156 (0.65) |
559 (0.32) |
0.17 |
| Oliveira et al.69 | N/A | 108 (0.30) |
N/A | 61 (0.17) |
N/A | 226 (0.62) |
138 (0.38) |
0.07 |
| Franke et al.70 | N/A | N/A | N/A | 28 (0.04) |
I | N/A | 67 (0.10) |
0.10 |
| Lalor et al.71 | N/A | N/A | 76 (0.11) |
90 (0.13) |
I | 438 (0.62) |
272 (0.38) |
0.20 |
| Gandhi et al.72 | N/A | 200 (0.76) |
N/A | N/A | S | N/A | 189 (0.72) |
N/A |
| Tang et al.34 | 80 (0.14) |
N/A | 165 (0.28) |
N/A | I | 240 (0.41) |
334 (0.57) |
0.08 |
| Jeon et al.73 | 26 (0.15) |
N/A | N/A | N/A | I | 28 (0.16) |
114 (0.65) |
0.28 |
| Pazarli et al.74 | 17 (0.17) |
N/A | 61 (0.59) |
N/A | I | 89 (0.86) |
14 (0.14) |
0.02 |
| Jain et al.75 | N/A | N/A | 46 (0.35) |
27 (0.21) |
S | 58 (0.45) |
72 (0.55) |
0.23 |
| Charles et al.76 | N/A | 27 (0.25) |
N/A | N/A | N/A | 43 (0.39) |
15 (0.14) |
0.04 |
| Shin et al.77 | 9 (0.04) |
N/A | 215 (0.88) |
86 (0.35) |
I | 188 (0.77) |
56 (0.23) |
0.11 |
| Marais et al.78 | N/A | 203 (0.58) |
N/A | N/A | S/I | 158 (0.45) |
166 (0.47) |
0.29 |
| Van Der Walt et al.79 | N/A | 393 (0.59) |
N/A | N/A | S | 404 (0.60) |
234 (0.35) |
0.09 |
| Gler et al.80 | 117 (0.27) |
N/A | N/A | N/A | I | 310 (0.71) |
129 (0.29) |
0.18 |
| Chung-Delgado et al.81 | N/A | 10 (0.05) |
N/A | N/A | S/I | 155 (0.77) |
46(0.23) | N/A |
*Default/Loss to follow up/data unavailable/Transfer Out; ¶I = individualized; S = standardized.
§Treatment Completion and/or cure.
Outcomes in people living with HIV
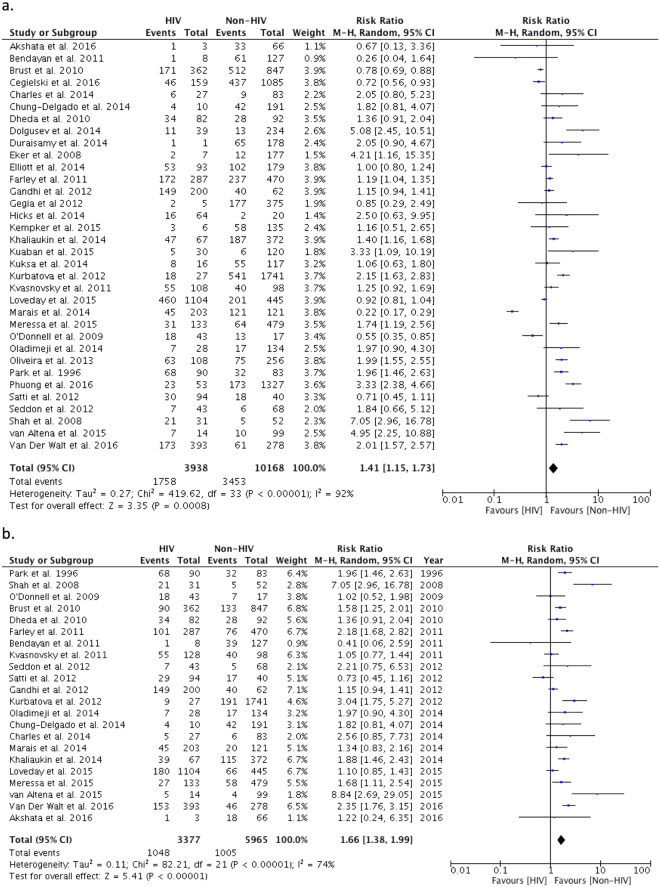
There were 34 studies with 14,106 participants reporting outcomes in people living with HIV (PLWH) compared with people without HIV infection (Fig. 2a)11–15,43–45,47–50,52–57,59–61,63–69,72,76,78,79,81,82. The proportion of study participants that were PLWH varied from 0.6 to 76%. The pooled RR for unsuccessful outcome in PLWH compared to those without HIV infection was 1.41 (95%CI: 1.15–1.73). Heterogeneity was significant (I2 = 92%, p < 0.001); funnel plots were not consistent with publication bias (Supp. Fig. 1).
Figure 2.
(a) Relative Risk of primary outcome in MDR/XDRTB patients living with HIV compared to those without HIV infection. (b) Relative Risk of mortality in MDR/XDRTB patients living with HIV compared to those without HIV infection.
We were able to analyze the outcome of mortality in 22 studies in 9342 PLWH (Fig. 2b)11,13,15,43,47–49,53–56,63–65,67,68,72,76,78,79,81,82; pooled RR for mortality in PLWH was 1.66 (95%CI: 1.38–1.99; I2 = 74%, p < 0.001). Treatment default was reported in 9 studies with 6311 participants; pooled RR for default was 1.05 (95%CI: 0.82–1.35; I2 = 52%, p = 0.04) (Supp. Fig. 8)47,53,54,64,65,68,76,79. There were 7 studies with 5930 participants reporting on treatment failure; pooled RR for treatment failure was 0.75 (95%CI: 0.44–1.29; I2 = 55%, p = 0.04) (Supp. Fig. 9)43,47,64,65,67,68,79. Finally, there were 28 studies with 12,999 participants compared the combined outcome of death and treatment failure with a pooled RR of 1.61 in PLWH (95% CI: 1.32–1.96; I2 = 86%, p < 0.00001) (Supp. Fig. 10)11–15,43,45,47–49,53–56,59,60,63–65,67–69,72,76,78,79,81,82.
We examined forest plots by study year, study quality, regional gross domestic product (GDP) and proportion of people using antiretroviral therapy (ART) (Supp. Figs 11–14). There was no obvious trend on visual inspection when comparing studies by year of publication or study quality. There was a greater effect of HIV on unsuccessful treatment outcomes in low-income regions (RR 2.23; 95%CI: 1.60–3.11) compared with high income regions (RR 1.22; 95%CI 0.97–1.53). On inspection of forest plots by stratified by ART usage, there was no clear visual trend towards improved outcomes amongst those with the highest proportion of ART usage (Supp. Fig. 14 and Supp. Table 5). Additionally there was only one study that reported outcomes according to whether or not participants were on ARTs67. Between-study heterogeneity did not decrease in any stratified analyses with the exception of study heterogeneity being reduced amongst in PLWH in low GDP countries (I2 = 41%, p = 0.12) (Supp. Figs 8–11). Unfortunately, data available to us was insufficient to perform meta-regression.
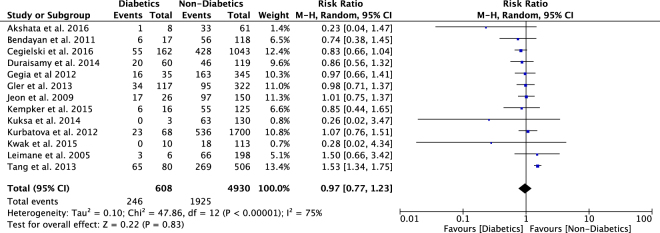
Outcomes in participants with diabetes
There were 13 studies with 5538 participants reported unsuccessful treatment outcomes in people with diabetes compared to people without diabetes10,12,14,28,34,44,45,60,63,68,73,80,82. The pooled RR for unsuccessful outcome was 0.97 (95%CI: 0.77–1.23) (Fig. 3), with significant heterogeneity observed (I2 = 75%, p < 0.001). Funnel plot inspection suggested some potential for publication bias with smaller studies demonstrating negative outcomes amongst those with diabetes (Supp. Fig. 2).
Figure 3.
Relative Risk of primary outcome in MDR/XDRTB patients with DM compared to those without DM.
Analyses of secondary outcomes were not feasible due to insufficient data; three studies reported data relevant for mortality63,68,82, one study reported default37 and one study reported treatment failure68. Further analysis by GDP and study quality did not significantly change outcomes. Heterogeneity was reduced when low GDP countries were analyzed (I2 = 19%, p = 0.29) although only three studies reported data from low GDP countries12,80,82 (Supp. Figs 5–7). Unfortunately, data available to us was insufficient to perform meta-regression.
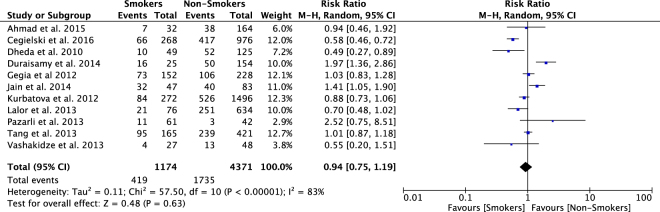
Outcomes in Smokers
There were 11 studies with 5545 participants reporting the primary outcome in smokers versus non-smokers12–14,34,45,51,58,68,71,74,75. The pooled RR for unsuccessful outcome was 0.94 (95%CI: 0.75–1.19) (Fig. 4). Heterogeneity was significant (I2 = 83%, p < 0.001), and no publication bias was noted on visual inspection of funnel plots (Supp. Fig. 3).
Figure 4.
Relative Risk of primary outcome in MDR/XDRTB patients that smoke compared to non-smokers.
Analyses of secondary outcomes was not feasible due to insufficient data, with only two studies reporting data relevant for mortality13,68, two studies reporting default68,71 and two reporting failure68,83. There was a visual trend towards improved outcomes in smokers in higher quality studies (Supp. Fig. 15). Analysis of the primary outcome according to regional GDP revealed a decrease in heterogeneity amongst high GDP countries (Supp. Figs 16–17). Unfortunately, data available to us was insufficient to perform meta-regression.
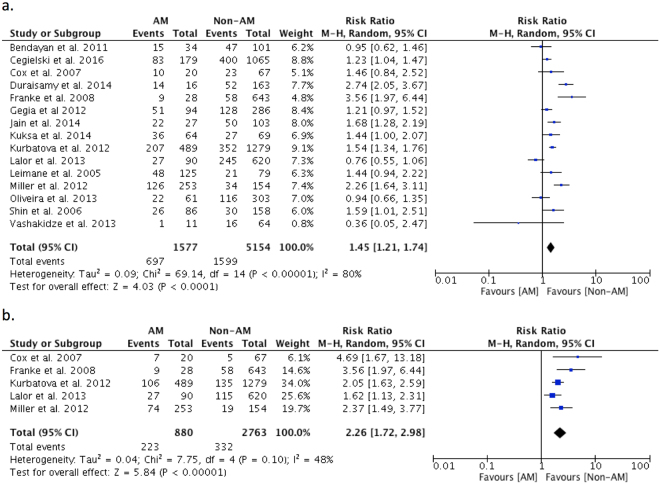
Outcomes in participants with Alcohol Misuse
There were 15 studies with 6731 participants reporting on the primary outcome in people with alcohol misuse compared to those without alcohol misuse12,14,28,45,46,51,60,62,63,68–71,75,77. The pooled RR for unsuccessful treatment outcome was 1.45 (95%CI: 1.21–1.74) (Fig. 5a) with significant heterogeneity (I2 = 80%, p < 0.001). There was no detectable publication bias (Supp. Fig. 4).
Figure 5.
(a) Relative Risk of primary outcome in MDR/XDRTB patients with alcohol misuse (AM) compared to those without alcohol misuse (Non-AM). (b) Relative Risk of default in MDR/XDRTB patients with alcohol misuse (AM) compared to those without alcohol misuse (Non-AM).
Five studies46,62,68,70,71 with 3643 participants enabled comparison of treatment default. The pooled RR for default was 2.26 in people with alcohol misuse (95%CI: 1.72–2.98) (Fig. 5b), with moderate heterogeneity (I2 = 48%, p = 0.10). Additional analyses of secondary outcomes were not feasible due to insufficient data with three studies reporting relevant data for mortality46,63,68, and two studies reporting on treatment failure46,68. There was no visual trend or change in heterogeneity observed when stratifying by study quality or country GDP (Supp. Figs 18–20). Unfortunately, data available to us was insufficient to perform meta-regression.
Chronic Kidney Disease Outcomes
Only two studies10,84 reported outcomes in participants with MDR/XDRTB and CKD. Analysis of primary and secondary outcomes was not possible due to insufficient data.
Discussion
In this systematic review examining the association between comorbidities and MDR/XDRTB treatment outcomes, we found that both HIV and alcohol misuse were associated with an increased pooled relative risk of unsuccessful treatment outcome in MDRTB patients. We found no clear association between unsuccessful treatment outcome with the comorbidities of smoking, diabetes or CKD.
To our knowledge, this is the first systematic review to comprehensively examine the relationship between comorbidities and MDR/XDRTB treatment outcomes. A previous systematic review in 2009 described the effect of HIV, DM, and alcohol misuse on MDRTB treatment outcomes, with the authors noting worse outcomes in patients with alcohol misuse, and no significant difference in outcomes in people with HIV or diabetes3. The data in this 2009 review, however, was quite limited, with only four studies reporting on the association between HIV- and DM-related MDRTB treatment outcomes3.
In contrast, this review reported on 34 studies with over 14,000 patients comparing outcomes by HIV status. We noted higher pooled relative risk of unsuccessful treatment outcome in PLWH. This appeared to be largely driven by an increase in mortality in PLWH. Further analysis suggested that the effect of HIV on mortality was increased in low income regions compared with high-income regions. Reasons for this remain unclear, as stratifying primary outcomes by study year, proportion with ART, and publication quality did not reveal any notable trends in study outcome. Given the preponderance of evidence demonstrating the mortality lowering effect of ART in co-infected patients85,86, we expected our results a trend towards improved outcomes in high ART settings. Surprisingly, however, there was no trend towards improved outcomes by study-level ART proportions. Further investigation into the drivers of mortality in the HIV-MDRTB co-infected populations is needed.
Those with alcohol misuse also had an increased pooled odds of unsuccessful treatment outcome. This appeared to be driven by default in people with alcohol misuse. Understanding the mechanisms behind the high default proportions will be critical in improving outcomes in MDR/XDRTB patients with a history of alcohol misuse. Interestingly, it seems that in the drug sensitive TB cohorts, alcohol misuse also predicts higher proportions of default87,88. This is thought to be primarily due to comorbid substance abuse, and socioeconomic conditions that prevent patients from accessing care reliably87–90. Programs have been developed to improve outcomes in people with alcohol use disorders in TB treatment and have been successful in decreasing loss to follow up in this population46,91.
We were somewhat surprised to find that people with diabetes had similar outcomes to people without diabetes in our analysis. Diabetes is associated with worse treatment outcomes in drug-susceptible TB, and is mentioned as a driver of poor TB treatment outcomes in several guidelines and reviews7. Unfortunately, due to lack of data, we were unable to explore the relationship between diabetes and individual MDR/XDRTB treatment outcomes. Similarly, the pooled primary outcome in smokers was not significantly different from non-smokers and we had insufficient data in secondary analysis to further investigate this relationship.
The strengths of this study include our broad search strategy, large sample size, and a priori study design. The studies included in this analysis reported treatment outcomes ranging over two decades and included studies from high, middle, and low-income regions. We also used clinically relevant variables consistent with accepted World Health Organization (WHO) treatment outcomes1,4.
There are also several notable weaknesses. First, we only estimated pooled effects on univariate analysis, and did not perform analysis that would control for potential confounding variables. Furthermore, in the vast majority of studies, there was not enough data available to assess potential confounding variables or perform meta-regression. In many comorbid conditions, confounding could potentially play a major role, as each of these comorbid conditions is associated with demographic, clinical, and socioeconomic characteristics that likely influence treatment outcomes. We explored some study-level variables and their influence on treatment outcomes, such as regional GDP, study quality, and publication year. These study-level variables, however, were of limited impact on pooled outcomes, and did not provide significant insight into the mechanisms through which comorbidities may impact treatment outcome. Ideally, an individual patient data meta-analysis (IPDMA) could be performed in an attempt to control for any confounding variables, however, this approach would likely limit sample size, and could potentially introduce bias, as better-resourced MDRTB/XDRTB treatment programs are more likely to compile, store and report such data for IPDMA.
Beyond confounding, other domains of bias may also be present and unaccounted for in studies included in our analysis. Specifically, some populations with co-morbidities may have had differential interventions (i.e. individuals with co-morbidities may receive more intensive treatment, monitoring, or support) which may bias outcomes in these populations. Additionally, we cannot exclude bias introduced from missing data, particularly given the relatively high proportions of default. Finally, bias introduced by selective reporting from better-resourced MDR-TB treatment programs may be present, as presumably well-resourced programs would be better equipped to treat individuals with MDR-TB and co-morbidities. We were unable to detect specific sources of bias when analyzing data by study quality, country income or ART coverage, but these sources of bias cannot be excluded.
The substantial between-study heterogeneity likely reflects a diversity of treatment conditions for MDR/XDRTB. Between-study heterogeneity was only partially reduced by analysis of study-level covariates (quality, country income category, and ART coverage). The presence of heterogeneity is not surprising, given the diversity of treatments, supports and approaches to MDR/XDRTB globally. Other likely sources of heterogeneity include programmatic factors such as treatment regimens, supports and infrastructure. Heterogeneity in study outcomes could also be attributed to the lack of standardized treatment outcomes in older studies. Unfortunately, we did not have the data to quantitatively assess each of these variables as potential sources of heterogeneity.
Recently, the World Health Organization (WHO) treatment guidelines for drug-resistant tuberculosis highlighted the need for “inclusion and separate reporting of outcomes for key subgroups…especially children and HIV-positive individuals on treatment”92. This systematic review highlights the need for improved reporting on a number of comorbid conditions in both MDRTB and XDRTB care. Other comorbidities not examined in this systematic review, such as mental illnesses and other substance use disorders, should also be considered for routine reporting in MDR/XDRTB treatment93. Improved reporting on outcomes related to specific comorbidities can help clarify the mechanisms that lead to unsuccessful treatment outcome for different subpopulations. This, in turn, would enable the development of new programs directed towards more individualized and appropriate MDRTB care and support.
Electronic supplementary material
Acknowledgements
There was no direct funding for this work. The Michael Smith Foundation for Health Research was involved indirectly with funding, but had no direct role or contribution to this project.
Author Contributions
J.S., F.A.K. and J.C.J. were involved in the conceptualization and formulation of the research protocol. J.S. piloted the data collection excel sheet. J.S. and A.S. collected and sorted titles, abstracts and full texts to determine which papers were included in this review. J.S. and A.S. collected the raw data from the studies used in this review. J.S. and J.R.C. performed the statistical analysis. J.S. created all figures and tables. J.S. wrote the manuscript and J.S., F.A.K. and J.C.J. were involved in reviewing and editing the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-23344-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joel Philip Samuels, Email: j.samuels@alumni.ubc.ca.
James Cameron Johnston, Email: James.Johnston@bccdc.ca.
References
- 1.Guidelines for the programmatic management of drug-resistant tuberculosis: Emergency update 2008.pdf [Internet]. [cited 2016 May 20]. Available from: http://apps.who.int/iris/bitstream/10665/43965/1/9789241547581_eng.pdf.
- 2.The Global MDR-TB & XDR-TB Response Plan 2007–2008.pdf [Internet]. [cited 2016 May 20]. Available from: http://www.who.int/tb/publications/2007/global_response_plan.pdf.
- 3.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS ONE Electron Resour. 2009;4:e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laserson KF, et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. J Tuberc. 2005;9:640–645. [PubMed] [Google Scholar]
- 5.Bastos ML, et al. Treatment outcomes of patients with multidrug-resistant and extensively drug-resistant tuberculosis according to drug susceptibility testing to first- and second-line drugs: an individual patient data meta-analysis. Clin Infect Dis. 2014;59:1364–1374. doi: 10.1093/cid/ciu619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Global Tuberculosis Report 2016.pdf [Internet]. [cited 2017 Jun 30]. Available from: http://www.who.int/tb/publications/global_report/gtbr2016_executive_summary.pdf?ua=1.
- 7.Baker MA, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan FA, et al. Treatment of active tuberculosis in HIV-coinfected patients: a systematic review and meta-analysis. Clin Infect Dis. 2010;50:1288–1299. doi: 10.1086/651686. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Gomez A, et al. Diabetes and Other Risk Factors for Multi-drug Resistant Tuberculosis in a Mexican Population with Pulmonary Tuberculosis: Case Control Study. Arch Med Res. 2015;46:142–148. doi: 10.1016/j.arcmed.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Kwak N, et al. Changes in treatment outcomes of multidrug-resistant tuberculosis. J Tuberc. 2015;19:525–530. doi: 10.5588/ijtld.14.0739. [DOI] [PubMed] [Google Scholar]
- 11.Seddon JA, Hesseling AC, Willemse M, Donald PR, Schaaf HS. Culture-confirmed multidrug-resistant tuberculosis in children: clinical features, treatment, and outcome. Clin Infect Dis. 2012;54:157–166. doi: 10.1093/cid/cir772. [DOI] [PubMed] [Google Scholar]
- 12.Duraisamy K, et al. Does Alcohol consumption during multidrug-resistant tuberculosis treatment affect outcome?. A population-based study in Kerala, India. Ann Am Thorac Soc. 2014;11:712–718. doi: 10.1513/AnnalsATS.201312-447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dheda K, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet. 2010;375:1798–1807. doi: 10.1016/S0140-6736(10)60492-8. [DOI] [PubMed] [Google Scholar]
- 14.Cegielski JP, et al. Multidrug-Resistant Tuberculosis Treatment Outcomes in Relation to Treatment and Initial Versus Acquired Second-Line Drug Resistance. Clin Infect Dis. 2016;62:418–430. doi: 10.1093/cid/civ910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park MM, Davis AL, Schluger NW, Cohen H, Rom WN. Outcome of MDR-TB patients, 1983–1993. Prolonged survival with appropriate therapy. J Respir. 1996;153:317–324. doi: 10.1164/ajrccm.153.1.8542137. [DOI] [PubMed] [Google Scholar]
- 16.Slama K, et al. Tobacco and tuberculosis: a qualitative systematic review and meta-analysis. Int J Tuberc Lung Dis. 2007;11:1049–1061. [PubMed] [Google Scholar]
- 17.Gegia M, et al. Tobacco smoking and tuberculosis treatment outcomes: A prospective cohort study in Georgia. Bull World Health Organ. 2015;93:390–399. doi: 10.2471/BLT.14.147439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isaakidis P, et al. Treatment outcomes for HIV and MDR-TB co-infected adults and children: systematic review and meta-analysis. J Tuberc. 2015;19:969–978. doi: 10.5588/ijtld.15.0123. [DOI] [PubMed] [Google Scholar]
- 19.Orenstein EW, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss P, Chen W, Cook VJ, Johnston JC. Treatment outcomes from community-based drug resistant tuberculosis treatment programs: a systematic review and meta-analysis. BMC Infect Dis. 2014;14:333. doi: 10.1186/1471-2334-14-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ettehad D, Schaaf HS, Seddon JA, Cooke GS, Ford N. Treatment outcomes for children with multidrug-resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:449–456. doi: 10.1016/S1473-3099(12)70033-6. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson KR, Tierney DB, Jeon CY, Mitnick CD, Murray MB. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis. 2010;51:6–14. doi: 10.1086/653115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahuja SD, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med Public Libr Sci. 2012;9:e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitnick CD, et al. Aggressive regimens for multidrug-resistant tuberculosis decrease all-cause mortality. PLoS ONE Electron Resour. 2013;8:e58664. doi: 10.1371/journal.pone.0058664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kendall EA, et al. Alcohol, hospital discharge, and socioeconomic risk factors for default from multidrug resistant tuberculosis treatment in rural South Africa: A retrospective cohort study. PLoS ONE. 2013;8:e83480. doi: 10.1371/journal.pone.0083480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu CH, et al. Characteristics and treatment outcomes of patients with MDR and XDR tuberculosis in a TB referral hospital in Beijing: a 13-year experience. PLoS ONE Electron Resour. 2011;6:e19399. doi: 10.1371/journal.pone.0019399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leimane V, et al. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005;365:318–326. doi: 10.1016/S0140-6736(05)70196-3. [DOI] [PubMed] [Google Scholar]
- 29.Seung KJ, et al. Early outcomes of MDR-TB treatment in a high HIV-prevalence setting in Southern Africa. PLoS ONE Electron Resour. 2009;4:e7186. doi: 10.1371/journal.pone.0007186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pietersen E, et al. High frequency of resistance, lack of clinical benefit, and poor outcomes in capreomycin treated South African patients with extensively drug-resistant tuberculosis. PLoS ONE Electron Resour. 2015;10:e0123655. doi: 10.1371/journal.pone.0123655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Velasquez GE, et al. Improving outcomes for multidrug-resistant tuberculosis: aggressive regimens prevent treatment failure and death. Clin Infect Dis. 2014;59:9–15. doi: 10.1093/cid/ciu209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung-Delgado K, Guillen-Bravo S, Revilla-Montag A, Bernabe-Ortiz A. Mortality among MDR-TB cases: comparison with drug-susceptible tuberculosis and associated factors. PLoS ONE Electron Resour. 2015;10:e0119332. doi: 10.1371/journal.pone.0119332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kliiman K, Altraja A. Predictors of poor treatment outcome in multi- and extensively drug-resistant pulmonary TB. Eur Respir J. 2009;33:1085–1094. doi: 10.1183/09031936.00155708. [DOI] [PubMed] [Google Scholar]
- 34.Tang S, et al. Risk factors for poor treatment outcomes in patients with MDR-TB and XDR-TB in China: retrospective multi-center investigation. PLoS ONE Electron Resour. 2013;8:e82943. doi: 10.1371/journal.pone.0082943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ottawa Hospital Research Institute [Internet]. [cited 2017 May 21]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 36.Magee MJ, et al. Diabetes mellitus, smoking status, and rate of sputum culture conversion in patients with multidrug-resistant tuberculosis: a cohort study from the country of Georgia. PLoS ONE Electron Resour. 2014;9:e94890. doi: 10.1371/journal.pone.0094890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang YA, et al. Impact of diabetes on treatment outcomes and long-term survival in multidrug-resistant tuberculosis. Respiration. 2013;86:472–478. doi: 10.1159/000348374. [DOI] [PubMed] [Google Scholar]
- 38.Lytvynenko N, et al. Management of multi- and extensively drug-resistant tuberculosis in Ukraine: How well are we doing? Public Health Action. 2014;4:S67–S72. doi: 10.5588/pha.14.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuchukhidze G, et al. Risk factors associated with loss to follow-up among multidrug-resistant tuberculosis patients in Georgia. Public Health Action. 2014;4:S41–S46. doi: 10.5588/pha.14.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez M, et al. Successful management of multidrug-resistant tuberculosis under programme conditions in the Dominican Republic. J Tuberc. 2013;17:520–525. doi: 10.5588/ijtld.12.0481. [DOI] [PubMed] [Google Scholar]
- 41.Shean KP, et al. Treatment outcome and follow-up of multidrug-resistant tuberculosis patients, West Coast/Winelands, South Africa, 1992–2002. [Erratum appears in Int J Tuberc Lung Dis. 2009 Jan; 13(1):150] J Tuberc. 2008;12:1182–1189. [PubMed] [Google Scholar]
- 42.Jeon DS, et al. Treatment outcome and mortality among patients with multidrug-resistant tuberculosis in tuberculosis hospitals of the public sector. J Korean Med Sci. 2011;26:33–41. doi: 10.3346/jkms.2011.26.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meressa D, et al. Achieving high treatment success for multidrug-resistant TB in Africa: initiation and scale-up of MDR TB care in Ethiopia–an observational cohort study. Thorax. 2015;70:1181–1188. doi: 10.1136/thoraxjnl-2015-207374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kempker RR, et al. Acquired Drug Resistance in Mycobacterium tuberculosis and Poor Outcomes among Patients with Multidrug-Resistant Tuberculosis. Emerg Infect Dis. 2015;21:992–1001. doi: 10.3201/eid2106.141873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gegia M, Kalandadze I, Kempker RR, Magee MJ, Blumberg HM. Adjunctive surgery improves treatment outcomes among patients with multidrug-resistant and extensively drug-resistant tuberculosis. J Infect Dis. 2012;16:391–396. doi: 10.1016/j.ijid.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller AC, et al. Alcohol use and the management of multidrug-resistant tuberculosis in Tomsk, Russian Federation. J Tuberc. 2012;16:891–896. doi: 10.5588/ijtld.11.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loveday M, et al. Community-based care vs. centralised hospitalisation for MDR-TB patients, KwaZulu-Natal, South Africa. J Tuberc. 2015;19:163–171. doi: 10.5588/ijtld.14.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kvasnovsky C, et al. Extensively drug-resistant TB in Eastern Cape, South Africa: High mortality in HIV-negative and HIV-positive patients. J Acquir Immune Defic Syndr. 2011;57:146–152. doi: 10.1097/QAI.0b013e31821190a3. [DOI] [PubMed] [Google Scholar]
- 49.Shah NS, et al. Extensively drug-resistant tuberculosis in the United States, 1993–2007. JAMA. 2008;300:2153–2160. doi: 10.1001/jama.300.18.2153. [DOI] [PubMed] [Google Scholar]
- 50.Elliott E, Draper HR, Baitsiwe P, Claassens M. Factors affecting treatment outcomes in drug-resistant tuberculosis cases in the Northern Cape, South Africa. Public Health Action. 2014;4:201–203. doi: 10.5588/pha.14.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vashakidze S, et al. Favorable outcomes for multidrug and extensively drug resistant tuberculosis patients undergoing surgery. Ann Thorac Surg. 2013;95:1892–1898. doi: 10.1016/j.athoracsur.2013.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuaban C, et al. High effectiveness of a 12-month regimen for MDR-TB patients in Cameroon. J Tuberc. 2015;19:517–524. doi: 10.5588/ijtld.14.0535. [DOI] [PubMed] [Google Scholar]
- 53.Brust JCM, Gandhi NR, Carrara H, Osburn G, Padayatchi N. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa, 2000–2003. J Tuberc. 2010;14:413–419. [PMC free article] [PubMed] [Google Scholar]
- 54.van Altena R, et al. Highly successful treatment outcome of multidrug-resistant tuberculosis in the Netherlands, 2000–2009. J Tuberc. 2015;19:406–412. doi: 10.5588/ijtld.14.0838. [DOI] [PubMed] [Google Scholar]
- 55.O’Donnell MR, Padayatchi N, Master I, Osburn G, Horsburgh CR. Improved early results for patients with extensively drug-resistant tuberculosis and HIV in South Africa. J Tuberc. 2009;13:855–861. [PMC free article] [PubMed] [Google Scholar]
- 56.Oladimeji O, et al. Intensive-phase treatment outcomes among hospitalized multidrug-resistant tuberculosis patients: results from a nationwide cohort in Nigeria. PLoS ONE Electron Resour. 2014;9:e94393. doi: 10.1371/journal.pone.0094393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hicks RM, et al. Malnutrition associated with unfavorable outcome and death among South African MDR-TB and HIV co-infected children. J Tuberc. 2014;18:1074–1083. doi: 10.5588/ijtld.14.0231. [DOI] [PubMed] [Google Scholar]
- 58.Ahmad N, et al. Management and treatment outcomes of MDR-TB: results from a setting with high rates of drug resistance. J Tuberc. 2015;19:1109–1114. doi: 10.5588/ijtld.15.0167. [DOI] [PubMed] [Google Scholar]
- 59.Phuong NT, et al. Management and treatment outcomes of patients enrolled in MDR-TB treatment in Viet Nam. Public Health Action. 2016;6:25–31. doi: 10.5588/pha.15.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuksa L, et al. Multi- and extensively drug-resistant tuberculosis in Latvia: Trends, characteristics and treatment outcomes. Public Health Action. 2014;4:S47–S53. doi: 10.5588/pha.14.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eker B, et al. Multidrug- and extensively drug-resistant tuberculosis, Germany. Emerg Infect Dis. 2008;14:1700–1706. doi: 10.3201/eid1411.080729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cox HS, et al. Multidrug-resistant tuberculosis treatment outcomes in Karakalpakstan, Uzbekistan: treatment complexity and XDR-TB among treatment failures. PLoS ONE Electron Resour. 2007;2:e1126. doi: 10.1371/journal.pone.0001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bendayan D, Hendler A, Polansky V, Weinberger M. Outcome of hospitalized MDR-TB patients: Israel 2000–2005. J Clin Microbiol. 2011;30:375–379. doi: 10.1007/s10096-010-1096-7. [DOI] [PubMed] [Google Scholar]
- 64.Farley JE, et al. Outcomes of multi-drug resistant tuberculosis (MDR-TB) among a cohort of South African patients with high HIV prevalence. PLoS ONE Electron Resour. 2011;6:e20436. doi: 10.1371/journal.pone.0020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Satti H, et al. Outcomes of multidrug-resistant tuberculosis treatment with early initiation of antiretroviral therapy for HIV co-infected patients in Lesotho. PLoS ONE Electron Resour. 2012;7:e46943. doi: 10.1371/journal.pone.0046943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dolgusev O, et al. Pattern of primary tuberculosis drug resistance and associated treatment outcomes in Transnistria, Moldova. Public Health Action. 2014;4:S64–S66. doi: 10.5588/pha.14.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khaliaukin A, et al. Poor treatment outcomes among multidrug-resistant tuberculosis patients in Gomel Region, Republic of Belarus. Public Health Action. 2014;4:S24–S28. doi: 10.5588/pha.14.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kurbatova EV, et al. Predictors of poor outcomes among patients treated for multidrug-resistant tuberculosis at DOTS-plus projects. Tuberculosis. 2012;92:397–403. doi: 10.1016/j.tube.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oliveira O, Gaio R, Villar M, Duarte R. Predictors of treatment outcome in multidrug-resistant tuberculosis in Portugal. Eur Respir J. 2013;42:1747–1749. doi: 10.1183/09031936.00197912. [DOI] [PubMed] [Google Scholar]
- 70.Franke MF, et al. Risk factors and mortality associated with default from multidrug-resistant tuberculosis treatment. Clin Infect Dis. 2008;46:1844–1851. doi: 10.1086/588292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lalor MK, et al. Risk factors associated with default from multi- and extensively drug-resistant tuberculosis treatment, Uzbekistan: a retrospective cohort analysis. PLoS ONE Electron Resour. 2013;8:e78364. doi: 10.1371/journal.pone.0078364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gandhi NR, et al. Risk factors for mortality among MDR- and XDR-TB patients in a high HIV prevalence setting. Int J Tuberc Lung Dis. 2012;16:90–97. doi: 10.5588/ijtld.11.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jeon DS, et al. Survival and predictors of outcomes in non-HIV-infected patients with extensively drug-resistant tuberculosis. J Tuberc. 2009;13:594–600. [PubMed] [Google Scholar]
- 74.Pazarli P, Duman DY, Mocin OY, Karagoz T. The effect of smoking on treatment outcome of multidrug-resistant tuberculosis. Turk Toraks Derg. 2013;14:93–97. [Google Scholar]
- 75.Jain K, Desai M, Solanki R, Dikshit RK. Treatment outcome of standardized regimen in patients with multidrug resistant tuberculosis. J Pharmacol Pharmacother. 2014;5:145–149. doi: 10.4103/0976-500X.130062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Charles M, et al. Treatment outcomes for patients with multidrug-resistant tuberculosis in post-earthquake Port-au-Prince, Haiti. J Trop Med. 2014;91:715–721. doi: 10.4269/ajtmh.14-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shin SS, et al. Treatment outcomes in an integrated civilian and prison MDR-TB treatment program in Russia. [Erratum appears in Int J Tuberc Lung Dis. 2006 Oct; 10(10):1183 Note: Mishustin, S [added]; Barnashov, A [added]; Karpeichik, Y [added]] J Tuberc. 2006;10:402–408. [PubMed] [Google Scholar]
- 78.Marais E, et al. Treatment outcomes of multidrug-resistant tuberculosis patients in Gauteng, South Africa. Infection. 2014;42:405–413. doi: 10.1007/s15010-013-0572-2. [DOI] [PubMed] [Google Scholar]
- 79.Van Der Walt M, Lancaster J, Shean K. Tuberculosis case fatality and other causes of death among multidrug-resistant tuberculosis patients in a high HIV prevalence setting, 2000–2008, South Africa. PLoS ONE. 2016;11:e0144249. doi: 10.1371/journal.pone.0144249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gler MT, et al. Weight gain and response to treatment for multidrug-resistant tuberculosis. J Trop Med. 2013;89:943–949. doi: 10.4269/ajtmh.13-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chung-Delgado K, Revilla-Montag A, Guillen-Bravo S, Bernabe-Ortiz A. Weight variation over time and its relevance among multidrug-resistant tuberculosis patients. J Infect Dis. 2014;23:20–24. doi: 10.1016/j.ijid.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 82.Akshata JS, Chakrabarthy A. Management of multidrug resistant tuberculosis (MDR-TB) - Monitoring is the key to successful outcome. J Chest Dis Tuberc. 2016;65:447–450. [Google Scholar]
- 83.Kim H-R, et al. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis. 2007;45:1290–1295. doi: 10.1086/522537. [DOI] [PubMed] [Google Scholar]
- 84.Anderson LF, et al. Treatment outcome of multi-drug resistant tuberculosis in the United Kingdom: retrospective-prospective cohort study from 2004 to 2007. Euro Surveill. 2013;18:20601. doi: 10.2807/1560-7917.ES2013.18.40.20601. [DOI] [PubMed] [Google Scholar]
- 85.Odone A, et al. The impact of antiretroviral therapy on mortality in HIV positive people during tuberculosis treatment: a systematic review and meta-analysis. PloS One. 2014;9:e112017. doi: 10.1371/journal.pone.0112017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abay SM, et al. The Effect of Early Initiation of Antiretroviral Therapy in TB/HIV-Coinfected Patients: A Systematic Review and Meta-Analysis. J Int Assoc Provid AIDS Care. 2015;14:560–570. doi: 10.1177/2325957415599210. [DOI] [PubMed] [Google Scholar]
- 87.Thapa P, Kamath R, Shetty BK, Monteiro A, Sekaran VC. Prevalence and Associated Factors of Alcoholism among Tuberculosis Patients in Udupi Taluk, Karnataka, India: A Cross Sectional Study. J Nepal Health Res Counc. 2014;12:177–181. [PubMed] [Google Scholar]
- 88.Roy N, et al. Risk factors associated with default among tuberculosis patients in Darjeeling district of West Bengal, India. J Fam Med Prim Care. 2015;4:388–394. doi: 10.4103/2249-4863.161330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gelmanova IY, et al. Barriers to successful tuberculosis treatment in Tomsk, Russian Federation: non-adherence, default and the acquisition of multidrug resistance. Bull World Health Organ. 2007;85:703–711. doi: 10.2471/BLT.06.038331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jakubowiak WM, et al. Social support and incentives programme for patients with tuberculosis: experience from the Russian Federation. Int J Tuberc Lung Dis. 2007;11:1210–1215. [PubMed] [Google Scholar]
- 91.Gelmanova IY, et al. “Sputnik”: a programmatic approach to improve tuberculosis treatment adherence and outcome among defaulters. J Tuberc. 2011;15:1373–1379. doi: 10.5588/ijtld.10.0531. [DOI] [PubMed] [Google Scholar]
- 92.WHO treatment guidelines for drug- resistant tuberculosis 2016 Update: October 2016 Revision.pdf [Internet]. [cited 2017 Jun 30]. Available from: http://apps.who.int/iris/bitstream/10665/250125/1/9789241549639-eng.pdf.
- 93.Walker IF, et al. Multidrug-resistant tuberculosis treatment programmes insufficiently consider comorbid mental disorders. Int J Tuberc Lung Dis. 2017;21:603–609. doi: 10.5588/ijtld.17.0135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.