Abstract
The authors investigated relations between obesity, age, and sex and the availabilities of striatal dopamine transporter (DAT) and extrastriatal serotonin transporter (SERT) by 123I-FP-CIT single-photon emission computed tomography. The study population consisted of 192 healthy controls with screening 123I-FP-CIT scans. Specific bindings of 123I-FP-CIT to DAT and SERT were calculated using regions of interest. Specific binding ratios (SBRs) of DAT and SERT except pons (r = 0.2217, p = 0.0026), were not correlated with body mass index (BMI). SBRs of midbrains correlated negatively with the BMIs of obese subjects (r = −0.3126, p = 0.0496), and positively with the those of non-obese subjects (r = 0.2327, p = 0.0053). SBRs of caudate nucleus (r = −0.3175, p < 0.0001), striatum (r = −0.226, p = 0.0022), and thalamus (r = −0.1978, p = 0.0074) reduced with age, and SERT availability was higher in males. However, DAT availability was similar in males and females. In conclusion, obesity has an effect on midbrain SERT availability. In addition, BMI was correlated with pontine SERT availability but not with striatal DAT availability. SERT availability was higher in males, but DAT availability showed no gender predilection.
Introduction
Over half of adults are overweight and 19.5% of the adult population are obese in Organisation for Economic Co-operation and Development member countries1. Furthermore, obesity is a risk factor for several malignancies including colon2, pancreatic3, thyroid4, hepatic5, and uterine6 cancer and for cardiovascular diseases7, and diabetes mellitus8. Obesity is due to a loss of the balance between energy intake and expenditure over long periods of time9, and the brain plays a critical role in controlling and inhibiting the pre-potent responses to foods9,10.
Dopamine and serotonin are neurotransmitters involved in the regulation of food intake and body weight11,12. Previous studies have used 123I-FP-CIT to investigate the role of dopamine transporter (DAT) in striatum13 and of serotonin transporter (SERT) in midbrain14, pons15, thalamus15, and hypothalamus16. 123I-FP-CIT shows high affinity for DAT, and slightly less affinity for SERT17. However, as DAT and SERT display nonoverlapping distributions in subcortical structures14, 123I-FP-CIT enables the co-evaluations of DAT and SERT distributions in a single scan15.
The aim of this study was to explore the relations between obesity, age, and sex and the availabilities of striatal DAT and extrastriatal SERT as determined by 123I-FP-CIT single-photon emission computed tomography (SPECT) using data obtained from the Parkinson’s Progression Markers Initiative (PPMI).
Material and Methods
Subjects
Data used for this article were obtained from the PPMI database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org18. The study population consisted of 192 healthy controls that underwent screening 123I-FP-CIT SPECT. According to PPMI criteria, males or females aged ≥ 30 years at screening were included. The exclusion criteria applied were; neurological disorder, first degree relative with idiopathic Parkinson’s disease, Montreal Cognitive Assessment score of ≤ 26, medication that might interfere with DAT SPECT scans, anticoagulants that might preclude safe completion of lumbar puncture, investigational drugs, and a condition that precluded the safe performance of routine lumbar puncture. Medical histories, results of neurological examinations (motor and non-motor assessments), and 123I-FP-CIT SPECT scans were downloaded.
123I-FP-CIT SPECT
123I-FP-CIT SPECT was performed during screening visits. SPECT scans were acquired 4 ± 0.5 hrs after injecting 111–185 MBq of 123I-FP-CIT. Subjects were pretreated with iodine solution or perchlorate prior to injection to block thyroid uptake. Raw data were acquired into a 128 × 128 matrix stepping each 3 or 4 degrees for total projections. Raw projection data were reconstructed using the iterative ordered subset expectation maximization and HERMES (Hermes Medical Solutions, Stockholm, Sweden). Reconstructed images were transferred to pmod (PMOD Technologies LLC, Zürich, Switzerland) for subsequent processing, including attenuation correction.
Image analysis
Downloaded scans were loaded using pmod v3.6 (PMOD Technologies LLC, Zürich, Switzerland) using a single subject MRI template in Montreal Neurological Institute space19. Specific bindings of 123I-FP-CIT to DAT and SERT were calculated by region of interest (ROI) analysis. A standard set of volumes of interest (VOIs) defining putamen, caudate nucleus, striatum (putamen + caudate nucleus), and thalamus as described by the Automated Anatomical Labeling (AAL) atlas20, and spherical VOIs for pons and midbrain were defined. The cerebellum was chosen as a reference region. A VOI template was applied to measure specific binding ratios (SBRs) of caudate nucleus, putamen, striatum, thalamus, pons, and midbrain as follows; SBR = (target– cerebellum)/cerebellum.
Statistical analysis
Pearson correlation was used to measure the linear dependences between SBRs and body mass indices (BMIs). For comparisons between obese and non-obese subjects, analyses of covariance was performed using SBR as a dependent variable, BMI as an independent variable, and age as a covariate. The T-test was used to compare the SBRs of males and females. The analysis was performed using GraphPad Prism 7 for Mac OS X (GraphPimad Software Inc, San Diego, CA, USA).
Data availability
Data used in the preparation of this article were obtained from PPMI database (www.ppmi-info.org/data).
Results
182 healthy subjects with 123I-FP-CIT SPECT from PPMI data were included in this study.
SBR with BMI
DAT and SERT SBRs were not correlated with subject BMIs, except in pons (r = 0.2217, p = 0.0026, Fig. 1). Subjects were divided into two groups using a BMI cut-off of 30 kg/m2: an obese (n = 40) or a non-obese (n = 142) (Table 1). SBRs of midbrain correlated negatively with BMIs of obese subjects (r = −0.3126, p = 0.0496), and positively with the BMIs of non-obese subjects (r = 0.2327, p = 0.0053), and these slopes were significantly different (F = 9.204, p = 0.0028) (Fig. 2). SBRs of pons showed a positive correlation with BMI (r = 0.1968, p = 0.0189) in non-obese subjects. (Table 2). When comparing obese and non-obese subjects, SBR of pons showed significant difference (p = 0.025) with an effect of sex (p = 0.010) and without an age effect (p = 0.153). However, no group difference was found in SBRs of caudate nucleus (p = 0.296), putamen (p = 0.305), striatum (p = 0.293), midbrain (p = 0.847), and thalamus (p = 0.920).
Figure 1.
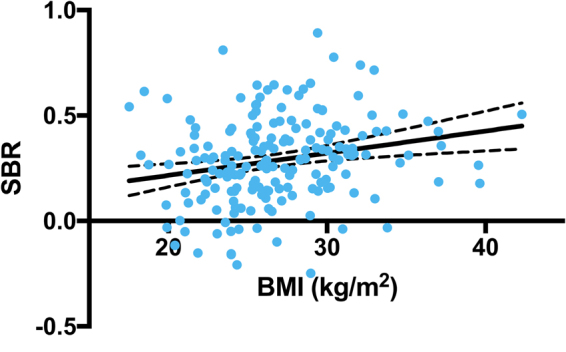
Correlation between SERT availabilities in pons and BMIs (r = 0.2217, p = 0.0026).
Table 1.
Subjects’ characteristics.
| Obese (n = 40) | Non-obese (n = 142) | p value | |
|---|---|---|---|
| Sex (Male/Female) | 27/13 | 91/51 | 0.8515 |
| Age | 58.2 ± 10.1 | 61.9 ± 11.4 | 0.0686 |
| BMI (kg/m2) | 32.9 ± 3.0 | 25.1 ± 2.8 | <0.0001 |
| Benton test | 25.9 ± 5.1 | 26.2 ± 3.7 | 0.7123 |
| MOANS score | 12.2 ± 3.3 | 12.4 ± 2.7 | 0.7661 |
| Epworth Sleepiness Scale | 4.9 ± 2.9 | 5.8 ± 3.5 | 0.1544 |
| Geriatric Depression Scale | 5.0 ± 1.4 | 5.3 ± 1.4 | 0.2132 |
| Derived-Letter Number Sequencing | 11.4 ± 2.3 | 11.9 ± 2.9 | 0.2818 |
| Shared decision making score | 47.2 ± 10.4 | 46.9 ± 10.5 | 0.8536 |
| UPSIT score | 34.1 ± 5.3 | 33.9 ± 4.9 | 0.8435 |
*BMI, body mass index; MOANS, Mayo’s Older Americans Normative Studies; UPSIT, University of Pennsylvania Smell Identification Test.
Figure 2.
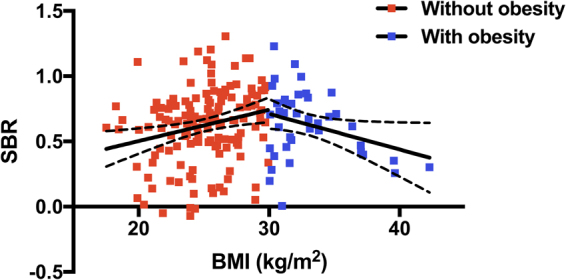
Correlation between SERT availabilities in midbrain and the BMIs of non-obese (r = 0.2327, p = 0.0053) and obese subjects (r = −0.3126, p = 0.0496).
Table 2.
Correlations between Specific binding ratios and Body mass indices.
| SBR | Obese | Non-obese | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r | 95% CI | p value | r | 95% CI | p value | r | 95% CI | p value | |
| Dopamine transporter | |||||||||
| Caudate nucleus | 0.0154 | −0.2976~0.3253 | 0.9251 | 0.0301 | −0.1353~0.1939 | 0.7220 | 0.1079 | −0.0382~0.2494 | 0.1472 |
| Putamen | −0.06 | −0.3647~0.2563 | 0.7131 | 0.0643 | −0.1015~0.2266 | 0.4472 | 0.1008 | −0.0453~0.2427 | 0.1755 |
| Striatum | −0.0231 | −0.3322~0.2905 | 0.8877 | 0.0490 | −0.1167~0.212 | 0.5625 | 0.1061 | −0.0400~0.2477 | 0.1540 |
| Serotonin transporter | |||||||||
| Midbrain | −0.3126 | −0.5687~−0.0012 | 0.0496 | 0.2327 | 0.0707~0.3827 | 0.0053 | 0.0828 | −0.0634~0.2255 | 0.2655 |
| Pons | 0.0176 | −0.2955~0.3273 | 0.9140 | 0.1968 | 0.0332~0.3502 | 0.0189 | 0.2217 | 0.0788~0.3557 | 0.0026 |
| Thalamus | −0.1381 | −0.431~0.1812 | 0.3955 | 0.0666 | −0.0992~0.2288 | 0.4309 | 0.0343 | −0.1117~0.1789 | 0.6454 |
*SBR, Specific binding ratio; CI, confidence interval.
SBR with Age, and Sex
SBRs of caudate nucleus (r = −0.3175, p < 0.0001), striatum (r = −0.226, p = 0.0022), and thalamus (r = −0.1978, p = 0.0074) showed a reduction with aging (Fig. 3). When the 182 study subjects were divided according to sex, SBRs of DAT in caudate nucleus (p = 0.3949), putamen (p = 0.2403), and striatum (p = 0.2987) were no different, but SBRs of SERT in midbrain (p < 0.0001), pons (p = 0.0068), and thalamus (p = 0.0113) were significantly higher in men than in women.
Figure 3.
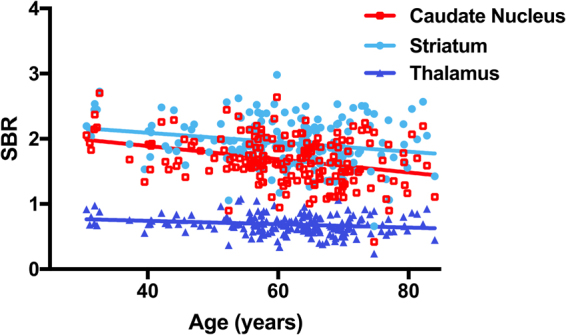
Correlations between DAT availabilities in caudate nucleus (r = −0.3175, p < 0.0001), striatum (r = −0.226, p = 0.0022) and between SERT availabilities in thalamus (r = −0.1978, p = 0.0074) and age.
Discussion
To the best of our knowledge, this is the largest study undertaken to investigate the effect of obesity on the availabilities of DAT and SERT in healthy controls. In this study, obesity has an effect on midbrain SERT availability. Striatal DAT availability was not correlated with BMI, but pontine SERT availability was found to be positively correlated with BMI. SERT availability was higher in men, but DAT availability was not.
Obesity arises from energy imbalance, whereby energy intake exceeds energy expenditure21. This imbalance can be triggered by the internal state of the caloric equation (homeostasis) and by non-homeostatic factors, such as, social, cultural, psychological, environmental factors, food type and the amount consumed22–24. In industrialized countries where foods are plentiful, food palatability in increases food intake via a reward mechanism25. Dopamine is a neurotransmitter that modulates reward9. Repeated exposure to a food reward, reduces the activation of dopamine and induces habituation26, as this blunted activation can trigger compensatory overeating27. Therefore, decreased sensitivity to the rewarding effects of food consumption due to reduced dopaminergic neuron activation develops in obesity28. In this regard, previous studies have focused on the role of DAT and SERT in obesity. Of three studies that investigated the correlation between BMI and DAT29–31, one study, in which 99mTc-TRODAT was used, reported a significant correlation coefficient of −0.4429. However, in most studies no association was found between BMI and DAT availability30,31, which is consistent with our observations. DAT controls extracellular dopamine levels by selectively uptaking dopamine into presynaptic neurons32. However, we observed no significant correlation between DAT and BMI. It has been suggested postsynaptically located dopamine receptor might play a dominant role in the reward system33. Although previous researches have mainly focused on the role played by dopamine in the reward system, serotonin is also known to play an important role in reward processing34. In a previous study, a negative correlation was observed between SERT and BMI was reported using global neocortex, midbrain, and striatum as target regions and the SERT selective radiotracer, 11C-DASB35. However, a voxel-based analysis of 123I-FP-CIT found a positive correlation between BMI and SERT availability in thalamus36, and Versteeg RI et al.31 and Hesses S et al.37 found no significant association between SERT availability and BMI. In the present study, SERT availability in pons was positively correlated with BMI. Higher SERT recruitment may be a consequence of higher serotonin recruitment due to food overload or overactive reward and homeostatic circuits38. Interestingly, SERT availabilities in midbrain were negatively and positively correlated with BMIs in obese and non-obese subjects, respectively. BMI might reflect a different role in predicting serotonergic tonus in midbrain. Thus, we hypothesized that SERT availability increases with BMI in response to serotonin in non-obese subjects. Hinderberger P et al.39 found SERT availability in the nucleus accumbens was negatively correlated in non-obese subjects and positively correlated in obese subjects with serum brain-derived neurotrophic factor levels, which are negatively correlated with BMI40. Therefore, SERT availability in the nucleus accumbens might present similar slopes to those observed in the present study. Serotonin receptor availability is negative correlated with serotonin levels41, and SERT availabilities in severely obese subjects were reported to be no different from those of lean subjects37, but those of overweight/moderately obese subjects were higher than those of lean subjects42. van Galen et al. suggested an inversed parabolic relationship between SERT availability and serotonin levels43, which is consistent with our findings. However, no previous study has reported a significant link between BMI and SERT availability42,43, and thus, this is the first study to describe the effect of obesity on midbrain SERT availability.
Because there is no direct means of measuring dopamine or serotonin concentrations in human brain, we chose to examine DAT and SERT availabilities as determined by 123I-FP-CIT SPECT44. DAT and SERT availabilities are influenced by radioligand affinity and are sensitive to changes in neurotransmitter concentration, and thus, these factors must be taken into account when interpreting neuroimages44. Furthermore, some controversy exists regarding the interpretations of results obtained using radiopharmaceuticals44. For example, an increase in SERT can be interpreted as an increase in serotonin level in synapses, or as increased serotonin clearance from extracellular regions in the brain, and thus, interpretations of the opposite effect of obesity on midbrain SERT availability may be less than straightforward.
Age related decreases in DAT45 and SERT45,46 have been well documented, and were also observed in the present study. However, DAT and SERT availability differences are controversial. Previous studies have reported higher SBRs in striatum47 and thalamus15 in females, but higher SBRs in pons in males15. In the present study, SERT availability was higher in males, but DAT availability was similar in men and women. Therefore, although sex hormones affect serotonin48 and dopamine49, serotonin neurotransmission may be more susceptible to gender.
This study has several limitations that warrant consideration. First, although this is the largest 123I-FP-CIT SPECT study conducted on the topic, data acquisition procedures at multiple sites may have been differed. Second, for normal subjects included in PPMI, 123I-FP-CIT SPECT was performed once at baseline enrollment, and thus, further longitudinal studies are needed to investigate the association between the availabilities of DAT and SERT and subject weight changes. Third, we investigated the associations between neuroimaging findings and BMI, and thus, additional studies are needed to investigate the effects of food intake, food stimulation, and glucose loading in obese and lean subjects. Forth we used BMI to define obesity and although BMI is the most commonly used parameter, it might not be (is not?) directly related to body fat levels50.
In conclusion, obesity has an effect on midbrain SERT availability. In addition, BMIs were not found to be correlated with striatal DAT availability, but rather with pontine SERT availability. Furthermore, SERT availability was greater in men, whereas DAT availability was similar in men and women.
Acknowledgements
PPMI – is a public-private partnership is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including abbVie, Avid, Biogen, Bristol-Myers Squibb, COVANCE, GE Healthcare, Genentech, GlaxoSmithKline, Lundbeck, Lilly, Merck, MesoScaleDiscovery, Pfizer, Piramal, Roche, Sanofi Genzyme, Servier, TEVA, and UCB. This study was supported by a Biomedical Research Institute Grant from Pusan National University Hospital (grant no. 2016–03).
Author Contributions
Su Bong Nam, Kyoungjune Pak: study design and writing of the manuscript Seung Hun Lee: study design Hyung-Jun Im, Kyoungjune Pak: image analysis Keunyoung Kim, Bum Soo Kim, Seong Jang Kim: data analysis In Joo Kim: study design and image analysis
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.OECD. Obesity Update 2017, http://www.oecd.org/health/health-systems/Obesity-Update-2017.pdf (2017).
- 2.Na SY, Myung SJ. [Obesity and colorectal cancer] Korean J Gastroenterol. 2012;59:16–26. doi: 10.4166/kjg.2012.59.1.16. [DOI] [PubMed] [Google Scholar]
- 3.Gukovsky I, Li N, Todoric J, Gukovskaya A, Karin M. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1199–1209. doi: 10.1053/j.gastro.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mijovic T, How J, Payne RJ. Obesity and thyroid cancer. Front Biosci (Schol Ed) 2011;3:555–564. doi: 10.2741/s171. [DOI] [PubMed] [Google Scholar]
- 5.Alzahrani B, Iseli TJ, Hebbard LW. Non-viral causes of liver cancer: does obesity led inflammation play a role? Cancer Lett. 2014;345:223–229. doi: 10.1016/j.canlet.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 6.Gu W, Chen C, Zhao KN. Obesity-associated endometrial and cervical cancers. Front Biosci (Elite Ed) 2013;5:109–118. doi: 10.2741/E600. [DOI] [PubMed] [Google Scholar]
- 7.Burke GL, et al. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:928–935. doi: 10.1001/archinte.168.9.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mokdad AH, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 9.Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15:367–378. doi: 10.1038/nrn3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravussin E, Bogardus C. Energy balance and weight regulation: genetics versus environment. Br J Nutr. 2000;83(Suppl 1):S17–20. doi: 10.1017/s0007114500000908. [DOI] [PubMed] [Google Scholar]
- 12.Lam DD, Garfield AS, Marston OJ, Shaw J, Heisler LK. Brain serotonin system in the coordination of food intake and body weight. Pharmacol Biochem Behav. 2010;97:84–91. doi: 10.1016/j.pbb.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Booij J, et al. Quantification of striatal dopamine transporters with 123I-FP-CIT SPECT is influenced by the selective serotonin reuptake inhibitor paroxetine: a double-blind, placebo-controlled, crossover study in healthy control subjects. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2007;48:359–366. [PubMed] [Google Scholar]
- 14.Roselli F, et al. Midbrain SERT in degenerative parkinsonisms: a 123I-FP-CIT SPECT study. Mov Disord. 2010;25:1853–1859. doi: 10.1002/mds.23179. [DOI] [PubMed] [Google Scholar]
- 15.Koch W, et al. Extrastriatal binding of [(1)(2)(3)I]FP-CIT in the thalamus and pons: gender and age dependencies assessed in a European multicentre database of healthy controls. Eur J Nucl Med Mol Imaging. 2014;41:1938–1946. doi: 10.1007/s00259-014-2785-8. [DOI] [PubMed] [Google Scholar]
- 16.Borgers AJ, et al. Imaging of serotonin transporters with [123I]FP-CIT SPECT in the human hypothalamus. EJNMMI Res. 2013;3:34. doi: 10.1186/2191-219X-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joutsa J, Johansson J, Seppanen M, Noponen T, Kaasinen V. Dorsal-to-Ventral Shift in Midbrain Dopaminergic Projections and Increased Thalamic/Raphe Serotonergic Function in Early Parkinson Disease. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2015;56:1036–1041. doi: 10.2967/jnumed.115.153734. [DOI] [PubMed] [Google Scholar]
- 18.Parkinson Progression Marker I. The Parkinson Progression Marker Initiative (PPMI) Prog Neurobiol. 2011;95:629–6355. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins DL, et al. Design and construction of a realistic digital brain phantom. IEEE Trans Med Imaging. 1998;17:463–468. doi: 10.1109/42.712135. [DOI] [PubMed] [Google Scholar]
- 20.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 21.Bellisle F, Drewnowski A, Anderson GH, Westerterp-Plantenga M, Martin CK. Sweetness, satiation, and satiety. J Nutr. 2012;142:1149S–1154S. doi: 10.3945/jn.111.149583. [DOI] [PubMed] [Google Scholar]
- 22.Volkow ND, Wang GJ, Tomasi D, Baler RD. The addictive dimensionality of obesity. Biol Psychiatry. 2013;73:811–818. doi: 10.1016/j.biopsych.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-B. [DOI] [PubMed] [Google Scholar]
- 24.Pecina S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12:500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- 25.Levin BE, Routh VH. Role of the brain in energy balance and obesity. Am J Physiol. 1996;271:R491–500. doi: 10.1152/ajpregu.1996.271.3.R491. [DOI] [PubMed] [Google Scholar]
- 26.Schultz W. Subjective neuronal coding of reward: temporal value discounting and risk. Eur J Neurosci. 2010;31:2124–2135. doi: 10.1111/j.1460-9568.2010.07282.x. [DOI] [PubMed] [Google Scholar]
- 27.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh PC, et al. Correlation between errors on the Wisconsin Card Sorting Test and the availability of striatal dopamine transporters in healthy volunteers. J Psychiatry Neurosci. 2010;35:90–94. doi: 10.1503/jpn.090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomsen G, et al. No correlation between body mass index and striatal dopamine transporter availability in healthy volunteers using SPECT and [123I]PE2I. Obesity (Silver Spring) 2013;21:1803–1806. doi: 10.1002/oby.20225. [DOI] [PubMed] [Google Scholar]
- 31.Versteeg, R. I. et al. Serotonin Transporter Binding in the Diencephalon is Reduced in Insulin Resistant Obese Humans. Neuroendocrinology (2016). [DOI] [PMC free article] [PubMed]
- 32.van de Giessen E, et al. No association between striatal dopamine transporter binding and body mass index: a multi-center European study in healthy volunteers. Neuroimage. 2013;64:61–67. doi: 10.1016/j.neuroimage.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Eisenstein SA, et al. A comparison of D2 receptor specific binding in obese and normal-weight individuals using PET with (N-[(11)C]methyl)benperidol. Synapse. 2013;67:748–756. doi: 10.1002/syn.21680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kranz GS, Kasper S, Lanzenberger R. Reward and the serotonergic system. Neuroscience. 2010;166:1023–1035. doi: 10.1016/j.neuroscience.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 35.Erritzoe D, et al. Cerebral serotonin transporter binding is inversely related to body mass index. Neuroimage. 2010;52:284–289. doi: 10.1016/j.neuroimage.2010.03.086. [DOI] [PubMed] [Google Scholar]
- 36.Hesse S, et al. Association of central serotonin transporter availability and body mass index in healthy Europeans. Eur Neuropsychopharmacol. 2014;24:1240–1247. doi: 10.1016/j.euroneuro.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Hesse S, et al. Central serotonin transporter availability in highly obese individuals compared with non-obese controls: A [(11)C] DASB positron emission tomography study. Eur J Nucl Med Mol Imaging. 2016;43:1096–1104. doi: 10.1007/s00259-015-3243-y. [DOI] [PubMed] [Google Scholar]
- 38.Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol Ther. 2011;129:220–238. doi: 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hinderberger P, et al. The effect of serum BDNF levels on central serotonin transporter availability in obese versus non-obese adults: A [(11)C]DASB positron emission tomography study. Neuropharmacology. 2016;110:530–536. doi: 10.1016/j.neuropharm.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 40.Lommatzsch M, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Gunther L, Liebscher S, Jahkel M, Oehler J. Effects of chronic citalopram treatment on 5-HT1A and 5-HT2A receptors in group- and isolation-housed mice. Eur J Pharmacol. 2008;593:49–61. doi: 10.1016/j.ejphar.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Koskela AK, et al. Serotonin transporter binding and acquired obesity–an imaging study of monozygotic twin pairs. Physiol Behav. 2008;93:724–732. doi: 10.1016/j.physbeh.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 43.Haahr ME, et al. Central 5-HT neurotransmission modulates weight loss following gastric bypass surgery in obese individuals. J Neurosci. 2015;35:5884–5889. doi: 10.1523/JNEUROSCI.3348-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Galen, K. A., Ter Horst, K. W., Booij, J., la Fleur, S. E. & Serlie, M. J. The role of central dopamine and serotonin in human obesity: lessons learned from molecular neuroimaging studies. Metabolism (2017). [DOI] [PubMed]
- 45.Pirker W, et al. Imaging serotonin and dopamine transporters with 123I-beta-CIT SPECT: binding kinetics and effects of normal aging. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2000;41:36–44. [PubMed] [Google Scholar]
- 46.van Dyck CH, et al. Age-related decline in central serotonin transporter availability with [(123)I]beta-CIT SPECT. Neurobiol Aging. 2000;21:497–501. doi: 10.1016/S0197-4580(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 47.Lavalaye J, Booij J, Reneman L, Habraken JB, van Royen EA. Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. Eur J Nucl Med. 2000;27:867–869. doi: 10.1007/s002590000279. [DOI] [PubMed] [Google Scholar]
- 48.France M, Skorich E, Kadrofske M, Swain GM, Galligan JJ. Sex-related differences in small intestinal transit and serotonin dynamics in high-fat-diet-induced obesity in mice. Exp Physiol. 2016;101:81–99. doi: 10.1113/EP085427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinclair D, Purves-Tyson TD, Allen KM, Weickert CS. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology (Berl) 2014;231:1581–1599. doi: 10.1007/s00213-013-3415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pak K, Lee SH, Lee JG, Seok JW, Kim IJ. Comparison of Visceral Fat Measures with Cardiometabolic Risk Factors in Healthy Adults. PLoS One. 2016;11:e0153031. doi: 10.1371/journal.pone.0153031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in the preparation of this article were obtained from PPMI database (www.ppmi-info.org/data).


