Acute myeloid leukemia (AML) is a devastating blood cancer with 5-year survival of only 25%. Novel effective leukemia therapy is an urgent unmet need. Immunotherapy is a promising strategy for cancer treatment. Targeting inhibitory mechanisms, such as the programmed cell death protein 1 (PD-1) pathway to unleash the patient’s own anti-tumor immune response has achieved major success1. Several studies including ours have demonstrated an involvement of PD-1 and other T cell inhibitory pathways in AML progression2–7. Strategies of blocking immune suppression for leukemia treatment are attractive due to their relatively simple administration and better tolerability profile. In fact, clinical studies applying anti-PD-1 in AML therapy have been initiated and are currently in early phase trials8. AML is a highly heterogeneous disease with multiple steps involved in the treatments. Determining the status of the immune response at each disease stage in individual patients is crucial for decision making of subsequent clinical management. In addition, immune characterization has great potential to identify predictive biomarkers of responsiveness to immunotherapy, thus providing pivotal information to optimize clinical trial design using strategies modulating anti-leukemia immunity for AML treatment.
Due to limited accessibility, the majority of clinical studies in AML have been restricted to evaluation of peripheral blood samples. In contrast, the immune response in bone marrow of AML patients is poorly understood. AML is derived from myeloid hematopoietic progenitors and rapidly grows in bone marrow before mobilizing to peripheral blood in a majority of patients. Therefore the development and progression of AML largely relies on the bone marrow microenvironment9. A better understanding of the anti-leukemia immune response within the bone marrow of AML patients is likely to be a key to develop immune-based therapeutic approaches for leukemia. In this study, we investigated the T cell immune response within the bone marrow in AML.
Samples collected from a cohort of 22 patients with newly diagnosed AML were used in this study. Clinical and demographic information are summarized in Supplementary Table 1. Consistent with the heterogeneous nature of AML, there was wide variation in the white blood cell (WBC) count and percentage of blasts in both peripheral blood and bone marrow. The risk stratification based on cytogenetic features was defined by European Leukemia Net (ELN) 2017 recommendations10. Two patients (9.1%) were categorized with favorable risk, ten (45.5 %) with intermediate risk, and the other ten patients (45.5 %) were in the adverse risk category. Peripheral blood and bone marrow aspirates were collected from each patient prior to any leukemia treatment.
We first assessed the T cell composition of paired bone marrow and peripheral blood from the same patients. We observed that the frequency of CD3+ T cells among lymphocytes is significantly lower in bone marrow than in peripheral blood (Fig. 1a). Percentages of CD4 and CD8 T cell subsets among CD3+ T cells were comparable between bone marrow and peripheral blood. While the CD4/CD8 ratio was similar, the frequency of CD4 and CD8 T cell among lymphocytes was significantly lower in bone marrow (Fig. 1a). Our result is in line with reports that in healthy individuals, the bone marrow contains a lower percentage of CD3+ T lymphocytes compared with peripheral blood11. However, in contrast to the observations that healthy individuals contain comparable frequency of CD8 T cells among lymphocytes within the two anatomical locations11, our data demonstrate a diminishment of the CD8 T-cell subset in bone marrow of AML patients, suggesting a suppressed anti-leukemia CD8 activity in the marrow. Our further phenotypic analysis of T cells derived from AML patients showed that the majority of CD8 T cells in bone marrow are effector memory T cells (TEM), and the percentage of TEM in bone marrow is significantly higher than that of peripheral blood (Fig. 1b). Previous studies have demonstrated that bone marrow is a unique anatomical site with enrichment of memory T cells in healthy individuals and multiple disease conditions including viral infection, degenerative joint disease, and solid tumors12,13. Our finding is consistent with these observations and we similarly conclude that the bone marrow of patients with AML is also a reservoir for memory T cells.
Fig. 1. Bone marrow CD8 T cells express high frequency of PD-1 and contains more EomeshiT-betint cells than peripheral blood in AML.
Paired bone marrow and peripheral blood samples were collected from newly diagnose AML patients (n = 22). Flow cytometry analysis was performed on peripheral blood mononuclear cells (PBMCs) and bone marrow mononuclear cells (BMMCs). a The percentages of CD3+, CD4+, and CD8+ T cell within lymphocytes are shown. Histogram (left) displays the representative flow cytometry data. Panels on right are the summary plot of 22 patients. b Distribution of TN, TCM, TEM, and TEMRA among CD8 T cells in bone marrow and peripheral blood was evaluated based on the expression of CD45RA vs. CCR7. Representative flow data (top) and summary plot (bottom) are shown. c Flow cytometry analysis of the surface expression of PD-1, TIGIT, and TIM-3 was performed. Data of CD8 is shown. Representative histograms (left) and statistic summary plots (right) display the expression level of indicated inhibitory receptors. d Flow cytometry analysis of the intracellular expression of Eomes vs. T-bet among CD8 T cells was performed. Based on the levels of Eomes and T-bet expression, cells are divided into three fractions. The schema of each fraction is shown in representative flow data (top). Panels at the bottom display the summary of expression levels of I, II, and III among CD8 T cells. P values were obtained by the paired t test and Wilcoxon matched pairs signed rank test
Several studies including ours have demonstrated an involvement of the inhibitory receptors PD-1 and T cell immunoglobulin and mucin domain (TIM-3) in AML progression2–6. We also discovered a suppressive effect of T cell immunoglobulin and ITIM domain (TIGIT), a recently identified co-inhibitory receptor, in the CD8 T cell response in AML7. To evaluate whether the effect of inhibitory pathways is different between bone marrow and peripheral blood, we assessed the expression of PD-1, TIGIT, and TIM-3 on T cells derived from both sites. We observed a significantly increased frequency of PD-1-expressing CD8 T cells in bone marrow compared with that of peripheral blood (Fig. 1c). There were no significant differences in the proportion of CD8 T cells expressing TIGIT or TIM-3 (Fig. 1c). PD-1 is a key mediator for the development of T cell exhaustion, a state of T cell dysfunction that develops in response to persistent antigen stimulation, including cancer. Our observation that a higher proportion of bone marrow CD8 T cells express PD-1 suggests a more exhausted status of T cells and suppressive environment within bone marrow in AML patients. We further examined the expression of Eomesodermin (Eomes) and T-bet in CD8 T cells and found that the frequency of EomeshiT-betint CD8 T cells was significantly higher in bone marrow compared with that of peripheral blood (Fig. 1d). Eomes and T-bet are both T box transcription factors that are crucial in regulating T cell function. Recent studies demonstrated, in both a mouse model and human chronic viral infection, that the T-bethi subset of exhausted T cells retains some proliferative capacity and can be reinvigorated by PD-1 blockade. In contrast, Eomeshi T cells are terminally differentiated and irreversible14. Our observation that there are more Eomeshi CD8 T cells in bone marrow indicates that this microenvironment may skew these T cells toward the late stage of exhaustion.
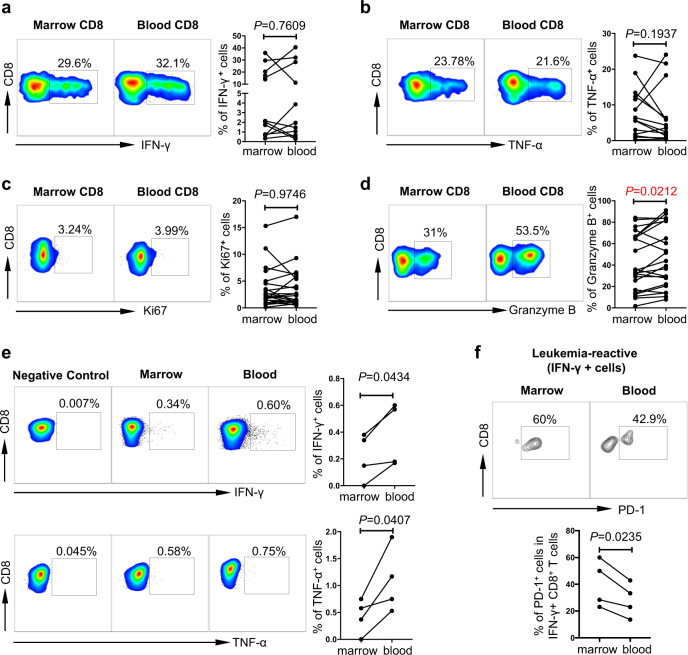
To evaluate the functional status of CD8 T cells in bone marrow. We performed multiple functional studies to assess the cytokine production, proliferative ability, and killing capacity. We found no significant difference in cytokine release (IFN-γ and TNF-α) upon in vitro stimulation with anti-CD3 and anti-CD28 (Fig. 2a, b). In addition, the expression of Ki67 on CD8 T cells was comparable between bone marrow and peripheral blood, demonstrating a similar proliferation (Fig. 2c). Strikingly, intracellular expression of Granzyme B in CD8 T cells from bone marrow was significantly lower compared with that of peripheral blood (Fig. 2d), suggestive of an impaired killing capacity of CD8 T cells in bone marrow. To further dissect the function of leukemia-reactive CD8 T cells in bone marrow vs. that in peripheral blood, we tested CD8 T cells for cytokine release in response to a WT-1 peptide. WT-1 is a well know tumor-associated antigen, in which HLA-A*0201 restricted WT-1126–134 is the most studied epitope in AML15. We found a significantly lower production of both IFN-γ and TNF-α by CD8 T cells from bone marrow compared with that from peripheral blood (Fig. 2e). Furthermore, when leukemia-reactive CD8 T cells were evaluated (gated on IFN- γ+), we observed a significantly higher expression of PD-1 on cells derived from bone marrow compared with that of peripheral blood (Fig. 2f). These important data demonstrate that in patients with AML, bone marrow leukemia-reactive CD8 T cells consist of a higher frequency of PD-1+ cells and are functionally deficient compared with that of peripheral blood. This novel finding provides a strong rationale for therapeutic strategies targeting inhibitory mechanisms including PD-1 to enhance the anti-leukemia response in AML patients.
Fig. 2. Functional status of total CD8 T cells and leukemia-reactive CD8 T cells in bone marrow vs. peripheral blood of AML.
a, b PBMCs and BBMCs collected from AML patients at initial diagnosis (n = 10) were stimulated in vitro with anti-CD3 and anti-CD28 before intracellular staining with IFN-γ and TNF-α. Flow cytometry analysis of the expression of IFN-γ (a) and TNF-α (b) are shown. Left panels, representative flow data; right panels, summary plots. c, d Expression of Ki67 (c) and Granzyme B (d) in CD8 T cells from bone marrow and peripheral blood of AML patients (n = 22) was assessed by flow cytometry. Representative flow data (left) and summary plot (right) are shown. e CD8 T cells purified from PBMCs or BMMCs were co-cultured with T2 cells (used as antigen presenting cells) pulsed with WT1 or SV40 peptide (used as negative control) for 6 days. After the co-culture, flow cytometry analysis of the intracellular expression of IFN-γ and TNF-α in CD8 T cells was performed (n = 4). Representative flow data (left) and statistic summary plot (right) are shown. f PD-1 expression on leukemia-reactive CD8 T cells (gated on IFN-γ+ cells, n = 4). Representative flow data (top) and statistic plot (bottom) are shown. P values were obtained by the paired t test
To evaluate the correlation between the PD-1 expression and clinical outcome, we defined high-PD-1 vs. low-PD-1 subgroups in the cohort of AML patients. The mean value of PD-1 expression on CD8 T cells in bone marrow of the 22 AML patients evaluated in our study was used as the cutoff here. Upon analyzing the rate of complete remission (CR) after induction treatment and the overall survival, we found no significant difference between the high-PD-1 and low-PD-1 subgroups (Supplementary Fig. 1). The small sample size in our study precludes a definite conclusion, further analysis with large cohort of patients is warranted to investigate this important question.
In summary, our study demonstrates a significant difference in the T cell immune response between bone marrow and peripheral blood in AML patients. An increased proportion of CD8 T cells within bone marrow expresses PD-1 and these T cells exhibit reduced anti-leukemia response. To our knowledge, this is the first study characterizing the phenotypic signature and anti-leukemia activity of CD8 T cells within bone marrow in AML patients. Our data highlight the importance of evaluating bone marrow specimens when defining the immune status in AML. Importantly our study demonstrates suppressive immune features in bone marrow and provides crucial information for clinical translation of immunotherapy for this devastating disease.
Electronic supplementary material
Acknowledgements
This work was supported by the American Society of Hematologist (ASH) Scholar Award, American Cancer Society Institutional Research Grant ACS IRG 124171-IRG-13-043-02 and the Kiesendahl Endowment funding. We thank all our patients for their trust, understanding, and willingness to provide their blood samples for our research.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41408-018-0069-4).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L, Gajewski TF, Kline J. Pd-1/pd-l1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114:1545–1552. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Q, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic t lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116:2484–2493. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norde WJ, et al. Pd-1/pd-l1 interactions contribute to functional T-cell impairment in patients who relapse with cancer after allogeneic stem cell transplantation. Cancer Res. 2011;71:5111–5122. doi: 10.1158/0008-5472.CAN-11-0108. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, et al. Expression of pd-l1, pd-l2, pd-1 and ctla4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28:1280–1288. doi: 10.1038/leu.2013.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong Y, et al. Pd-1(hi)tim-3(+) T cells associate with and predict leukemia relapse in aml patients post allogeneic stem cell transplantation. Blood Cancer J. 2015;5:e330. doi: 10.1038/bcj.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong Y, et al. T-cell immunoglobulin and itim domain (tigit) associates with cd8+T-cell exhaustion and poor clinical outcome in aml patients. Clin. Cancer Res. 2016;22:3057–3066. doi: 10.1158/1078-0432.CCR-15-2626. [DOI] [PubMed] [Google Scholar]
- 8.Lichtenegger FS, Krupka C, Haubner S, Kohnke T, Subklewe M. Recent developments in immunotherapy of acute myeloid leukemia. J. Hematol. Oncol. 2017;10:142. doi: 10.1186/s13045-017-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shafat MS, Gnaneswaran B, Bowles KM, Rushworth SA. The bone marrow microenvironment - home of the leukemic blasts. Blood Rev. 2017;31:277–286. doi: 10.1016/j.blre.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Dohner H, et al. Diagnosis and management of aml in adults: 2017 eln recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark P, Normansell DE, Innes DJ, Hess CE. Lymphocyte subsets in normal bone marrow. Blood. 1986;67:1600–1606. [PubMed] [Google Scholar]
- 12.Zhang X, et al. Human bone marrow: a reservoir for “enhanced effector memory” cd8+ T cells with potent recall function. J. Immunol. 2006;177:6730–6737. doi: 10.4049/jimmunol.177.10.6730. [DOI] [PubMed] [Google Scholar]
- 13.Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 2005;26:360–366. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Driessche A, Berneman ZN, Van Tendeloo VF. Active specific immunotherapy targeting the wilms’ tumor protein 1 (wt1) for patients with hematological malignancies and solid tumors: lessons from early clinical trials. Oncologist. 2012;17:250–259. doi: 10.1634/theoncologist.2011-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.