Abstract
The effect of aspirin on the risk of hepatocellular carcinoma (HCC) remains unclear. We investigated the association between aspirin use and HCC development in a region where viral hepatitis prevails. We conducted a population-based cohort study including a total of 460,755 participants who were tracked to identify incidents of HCC since 2007. The use of drug before the index date was assessed and standardized by the Defined Daily Dose system. We calculated the hazard ratios (HRs) and their 95% confidence intervals (CIs) for the association between aspirin use and HCC occurrence, using Cox proportional hazard regression models. There were 2,336 cases of HCC during the period of 2,965,500 person-years. Overall, aspirin users had a lower HCC risk (HR, 0.87; 95% CI, 0.77–0.98) than non-users in a dose-response manner (Ptrend = 0.002). The protective effect of aspirin was amplified when combined with those of non-aspirin non-steroidal anti-inflammatory drugs (HR, 0.65; 95% CI, 0.50–0.85). Subgroup analyses revealed a significant chemopreventive effect of aspirin in individuals who were young, were male, or had viral hepatitis, whereas no protective effect was observed in patients with liver cirrhosis. Our results, suggesting different carcinogenic pathways between viral and non-viral etiologies, may validate the design of future intervention trials of aspirin for HCC prevention in eligible populations.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most commonly diagnosed cancer worldwide and its incidence is anticipated to increase further in the next decade1. HCC continues to lead to unsatisfactory patient outcomes, even after curative treatment, because of its aggressive growth and high rates of recurrence and metastasis, which cause it to be the second most common cause of cancer death2. Moreover, because early detection methods for HCC are ineffective, the implementation of preventive measures is of considerable importance3.
Chemoprevention to reduce the risk of HCC is greatly appealing. Universal hepatitis B-virus (HBV) vaccination has substantially reduced the risk of HBV-associated HCC by decreasing the rate of new HBV infections4. Furthermore, antiviral therapies are consistently effective in preventing or delaying HCC5,6; however, their limited efficacy, dose-limiting side effects, high costs, and vulnerability to the emergence of drug-resistant mutants restrict their widespread use. Hence, there is a pressing need for new drugs that reduce the risk of HCC. Several medications commonly prescribed in primary practice, such as aspirin, have recently gained attention as promising protectors against HCC7. These non-etiology-specific drugs are inexpensive, have favorable rates of adverse events, and might have several extra-hepatic benefits.
Aspirin, because of its antiplatelet effect due to cyclooxygenase (COX) inhibition, is frequently prescribed to decrease the risk of cardiovascular disease-related mortality. Because chronic inflammation plays a pivotal role in the pathogenesis of HCC, chemoprevention of HCC by aspirin has been suggested8. Besides the inflammatory process, platelets have effects on immune modulation in the liver, facilitating immune-mediated liver injury and carcinogenesis9. In a murine model of chronic hepatitis B, treatment with aspirin reduced hepatic inflammation, fibrosis, and HCC development10.
A few epidemiologic studies investigated the effect of aspirin on the primary prevention of HCC, but the results were inconsistent11–13. Those studies, specifically designed to address effects of aspirin on HCC prevention, had major flaws: (i) too few HCC cases14,15, (ii) the use of self-reporting to identify previous aspirin intake with only time point of exposure12,13, (iii) no confirmation of dose-dependent or duration-dependent effects12, and (iv) no consideration of potential confounders such as other putative agents (e.g., statin and metformin) and underlying liver disease (e.g., chronic viral hepatitis and/or liver cirrhosis)12.
HCC is prevalent in East Asia, including Korea, where chronic HBV infection is endemic. To clarify the relationship between aspirin use and HCC risk in Korea, we performed a cohort study of a high-risk population using nationwide pharmaceutical claims data.
Results
Demographic characteristics of the cohort
Of the 460,755 participants included in the final cohort, 14.1% used aspirin (≥30 Daily Defined Doses [DDDs]). Table 1 lists the demographics, medical conditions, and medication use of the study cohort stratified by aspirin use. The median age of the aspirin users and non-users was 58 and 49 years, respectively. Compared with the aspirin non-users, the aspirin users were more likely to have healthy habits (smoked or drankless and had more physical activity) but were generally at higher risk for HCC, because they were more obese, had higher blood pressure/serum glucose/serum cholesterol levels, and had more comorbidities.
Table 1.
Characteristics of the study population by aspirin use.
| Characteristic | All subjects (N = 460,755) | Aspirin user (n = 64,782) | Aspirin non-user (n = 395,973) | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Age, years | ||||||
| 40–49 | 222,481 | 48.29 | 14,428 | 22.27 | 208,053 | 52.54 |
| 50–59 | 129,866 | 28.19 | 21,402 | 33.04 | 108,464 | 27.39 |
| 60–69 | 84,481 | 18.34 | 21,830 | 33.70 | 62,651 | 15.82 |
| ≥70 | 23,927 | 5.19 | 7,122 | 10.99 | 16,805 | 4.24 |
| Median (IQR) | 50 (44–59) | 58 (50–65) | 49 (44–57) | |||
| Sex | ||||||
| Men | 247,008 | 53.61 | 33,230 | 51.30 | 213,778 | 53.99 |
| Women | 213,747 | 46.39 | 31,552 | 48.70 | 182,195 | 46.01 |
| Body mass index, kg/m2 | ||||||
| <18.5 | 10,913 | 2.37 | 888 | 1.37 | 10,025 | 2.53 |
| 18.5–22.9 | 161,184 | 34.98 | 15,635 | 24.13 | 145,549 | 36.76 |
| 23–24.9 | 127,696 | 27.71 | 17,627 | 27.21 | 110,069 | 27.80 |
| 25–29.9 | 147,825 | 32.08 | 27,173 | 41.95 | 120,652 | 30.47 |
| ≥30 | 13,137 | 2.85 | 3,459 | 5.34 | 9,678 | 2.44 |
| Median(IQR) | 23.9 (22.0–25.8) | 24.8 (23.0–26.8) | 23.7 (21.9–25.7) | |||
| Smoking status | ||||||
| Never | 320,104 | 69.47 | 47,758 | 73.72 | 272,346 | 68.78 |
| Former | 39,990 | 8.68 | 5,780 | 8.92 | 34,210 | 8.64 |
| Current | 95,325 | 20.69 | 10,403 | 16.06 | 84,922 | 21.45 |
| N/A | 5,336 | 1.16 | 841 | 1.30 | 4,495 | 1.14 |
| Alcohol consumption/week | ||||||
| None | 269,203 | 58.43 | 41,875 | 64.64 | 227,328 | 57.41 |
| <1 drinks | 65,886 | 14.30 | 7,443 | 11.49 | 58,443 | 14.76 |
| 1–2 drinks | 74,640 | 16.20 | 8,688 | 13.41 | 65,952 | 16.66 |
| ≥3 drinks | 48,300 | 10.48 | 6,318 | 9.75 | 41,982 | 10.60 |
| N/A | 2,726 | 0.59 | 458 | 0.71 | 2,268 | 0.57 |
| Physical activity/week | ||||||
| None | 241,823 | 52.48 | 33,826 | 52.22 | 207,997 | 52.53 |
| 1–2 | 116,948 | 25.38 | 14,364 | 22.17 | 102,584 | 25.91 |
| ≥3 | 98,020 | 21.27 | 15,948 | 24.62 | 82,072 | 20.73 |
| N/A | 3,964 | 0.86 | 644 | 0.99 | 3,320 | 0.84 |
| Blood pressure category* | ||||||
| Normal | 126,031 | 27.35 | 9,179 | 14.17 | 116,852 | 29.51 |
| Prehypertension | 207,200 | 44.97 | 26,785 | 41.35 | 180,415 | 45.56 |
| Hypertension | 127,524 | 27.68 | 28,818 | 44.48 | 98,706 | 24.93 |
| Fasting plasma glucose, mg/dL | ||||||
| <100 | 307,813 | 66.81 | 35,388 | 54.63 | 272,425 | 68.80 |
| 100–125.9 | 115,967 | 25.17 | 19,309 | 29.81 | 96,658 | 24.41 |
| ≥126 | 36,975 | 8.02 | 10,085 | 15.57 | 26,890 | 6.79 |
| Total cholesterol, mg/dL | ||||||
| <200 | 242,966 | 52.73 | 32,713 | 50.50 | 210,253 | 53.10 |
| 200–239 | 154,876 | 33.61 | 21,721 | 33.53 | 133,155 | 33.63 |
| ≥240 | 62,913 | 13.65 | 10,348 | 15.97 | 52,565 | 13.27 |
| CCI†, mean (SD) | 1.55 (1.47) | 2.64 (1.79) | 1.37 (1.33) | |||
| Socioeconomic status | ||||||
| Quartile1 | 139,040 | 30.18 | 20,103 | 31.03 | 118,937 | 30.04 |
| Quartile2 | 116,893 | 25.37 | 16,097 | 24.85 | 100,796 | 25.46 |
| Quartile3 | 123,034 | 26.70 | 16,311 | 25.18 | 106,723 | 26.95 |
| Quartile4 | 81,788 | 17.75 | 12,271 | 18.94 | 69,517 | 17.56 |
| Statin, DDD | ||||||
| <30 | 421,235 | 91.42 | 45,361 | 70.02 | 375,874 | 94.92 |
| 30–365 | 31,701 | 6.88 | 14,363 | 22.17 | 17,338 | 4.38 |
| >365 | 7,819 | 1.70 | 5,058 | 7.81 | 2,761 | 0.70 |
| Median‡ (IQR) | 140 (65–305) | 183 (81–376) | 108 (60–236) | |||
| Metformin, DDD | ||||||
| <30 | 439,872 | 95.47 | 55,471 | 85.63 | 384,401 | 97.08 |
| 30–365 | 13,948 | 3.03 | 6,055 | 9.35 | 7,893 | 1.99 |
| >365 | 6,935 | 1.51 | 3,256 | 5.03 | 3,679 | 0.93 |
| Median‡ (IQR) | 233 (101–453) | 249 (114–468) | 221 (93–440) | |||
| Non-aspirin NSAIDs, DDD | ||||||
| <30 | 347,182 | 75.35 | 41,175 | 63.56 | 306,007 | 77.28 |
| 30–90 | 76,141 | 16.53 | 14,043 | 21.68 | 62,098 | 15.68 |
| >90 | 37,432 | 8.12 | 9,564 | 14.76 | 27,868 | 7.04 |
| Median‡ (IQR) | 61 (41–115) | 71 (44–146) | 59 (40–108) | |||
| Aspirin, DDD | ||||||
| <30 | 395,973 | 85.94 | — | 395,973 | 100.00 | |
| 30–365 | 31,188 | 6.77 | 31,188 | 48.14 | — | |
| 365–730 | 13,781 | 2.99 | 13,781 | 21.27 | — | |
| ≥730 | 19,813 | 4.30 | 19,813 | 30.58 | — | |
| Median‡ (IQR) | 390 (127–858) | 390 (127–858) | — | |||
Abbreviations: IQR = interquartile range; CCI = Charlson comorbidity index; SD = standard deviation; DDD = defined daily dose; NSAIDs = non-steroidal anti-inflammatory drugs.
*Normal, SBP < 120 mmHg and DBP < 80 mmHg; prehypertension, 120 mmHg ≤ SBP < 140 mmHg or 80 mmHg ≤ DBP < 90 mmHg; hypertension, SBP ≥ 140 mmHg or DBP ≥ 90 mmHg.
†Including acute myocardial infarction, congestive heart failure, peripheral vascular disease, cerebral vascular accident, dementia, pulmonary disease, connective tissue disorder, peptic ulcer, liver disease, diabetes, diabetes complications, paraplegia, renal disease, severe liver disease, and HIV infection based on ICD-10 codes of hospital visits during years 2003 through 2006.
‡The median prescribed number of DDDs for every study drug used (≥30 DDDs) in the cohort.
Aspirin use and HCC risk
There were 2,336 cases of HCC in the entire cohort during the observation period of 2,965,500 person-years; the overall incidence was 78.8 HCCs per 100,000 person-years (95% CI, 75.6–82.0). The HCC incidence was 76.2, 103.7, 88.1, and 87.5 among participants with aspirin use of <30, 30–365, 365–730, and ≥730 DDDs, respectively (Table 2). There was a dose-dependent relationship between aspirin use and the risk of HCC (P for trend = 0.002). The adjusted hazard ratios [HRs] (95% confidence intervals [CIs]) were 0.98 (0.84–1.15), 0.79 (0.62–1.00), and 0.75 (0.60–0.91) for individuals with aspirin use of 30–365, 365–730, and ≥730 DDDs, respectively. A propensity score matching analysis also revealed the similar result with original multivariate analysis (Supplemental Table 1). No difference in mortality between aspirin users and aspirin non-users was noted during the observation period (Supplemental Table 2).
Table 2.
Incidence and HRs of HCC associated with aspirin use.
| Incidence rate of HCC | Risk* of HCC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of subjects | No. of person-years | No. of HCC | per 105 person-years | 95% CI | Adjusted HR | 95% CI | P for trend | |||
| All subjects | 460,755 | 2,965,500 | 2,336 | 78.77 | 75.64 | 82.03 | ||||
| Women | 213,747 | 1,394,642 | 697 | 49.98 | 46.40 | 53.83 | 1 | |||
| Men | 247,008 | 1,570,858 | 1,639 | 104.34 | 99.41 | 109.51 | 2.17 | 1.95 | 2.41 | |
| Aspirin use | ||||||||||
| Non-user (<30 DDDs) | 395,973 | 2,565,103 | 1,954 | 76.18 | 72.87 | 79.63 | 1 | 0.002 | ||
| User (≥30 DDDs) | 64,782 | 400,397 | 382 | 95.41 | 86.30 | 105.47 | 0.87 | 0.77 | 0.98 | |
| 30–365 DDDs | 31,188 | 192,907 | 200 | 103.68 | 90.26 | 119.09 | 0.98 | 0.84 | 1.15 | |
| 365–730 DDDs | 13,781 | 85,143 | 75 | 88.09 | 70.25 | 110.46 | 0.79 | 0.62 | 1.00 | |
| >730 DDDs | 19,813 | 122,346 | 107 | 87.46 | 72.36 | 105.70 | 0.75 | 0.60 | 0.91 | |
| Concurrent non-aspirin NSAID use (≥30 DDDs) | ||||||||||
| Neither | 325,136 | 2,114,601 | 1667 | 78.83 | 75.14 | 82.71 | 1 | |||
| Aspirin only user (≥365 DDDs) | 22,046 | 137,237 | 123 | 89.63 | 75.11 | 106.95 | 0.76 | 0.62 | 0.92 | |
| Non-aspirin NSAID only user | 102,025 | 643,410 | 487 | 75.69 | 69.26 | 82.72 | 0.81 | 0.73 | 0.91 | |
| Both user | 11,548 | 70,253 | 59 | 83.98 | 65.07 | 108.39 | 0.65 | 0.50 | 0.85 | |
Abbreviations: HR = hazard ratio; HCC = hepatocellular carcinoma; DDD = defined daily dose; NSAIDs = non-steroidal anti-inflammatory drugs; CI = confidence interval.
*Cox proportional hazards regression models were used to calculate the adjusted hazard rate ratios and two-sided 95% confidence intervals, with adjustment for age, sex, body mass index, health behaviors (cigarette smoking, alcohol consumption, and physical activity), concurrent medication, category of blood pressure, fasting plasma glucose and total cholesterol, socioeconomic status, and Charlson comorbidity index score.
To sight the potential contribution of the COX enzyme, we further analyzed the additive effects of aspirin use in combination with non-aspirin non-steroidal anti-inflammatory drug (NSAID) use. In this analysis, individuals who used more than 365 DDDs of aspirin were considered aspirin users, considering both the robust effect of aspirin and the sufficient number of HCC cases for analysis. Compared with participants who used neither aspirin nor any other NSAID, users of aspirin only (HR, 0.76; 95% CI, 0.62–0.92) or of non-aspirin NSAIDs only (HR, 0.81; 95% CI, 0.73–0.91) had a reduced risk of developing HCC. The risk of developing HCC was far lower among participants who used both aspirin and other NSAIDs (HR, 0.65; 95% CI, 0.50–0.85).
In the sensitivity analysis, the shifting of the index date had little effect on the association between aspirin use and the incidence of HCC (Table 3). The statistical significance might have depended on the follow-up duration, with a longer follow up more likely to result in statistical significance. Table 3 shows whether the effects of aspirin were significant when the data were stratified according to age, sex, and underlying liver disease. The protective effect of aspirin against HCC development was significant among males who had more than 365 DDDs of aspirin, participants younger than 60 years of age who had more than 365 DDDs of aspirin, and patients with viral hepatitis who had more than 730 DDDs of aspirin. Aspirin had no protective effect in patients with liver cirrhosis.
Table 3.
Sensitivity analysis of adjusted* HRs of aspirin use for risk reduction of hepatocellular carcinoma.
| No. of HCC/total subjects | 30–365 DDDs | 365–730 DDDs | ≥730 DDDs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |||||
| Shifting the index date, year | ||||||||||
| 2005 | 2,938/480,193 | 0.92 | 0.78 | 1.08 | 0.75 | 0.59 | 0.96 | 0.54 | 0.38 | 0.75 |
| 2007 (main) | 2,336/460,755 | 0.98 | 0.84 | 1.15 | 0.79 | 0.62 | 1.00 | 0.75 | 0.60 | 0.91 |
| 2009 | 1,606/439,865 | 0.89 | 0.74 | 1.08 | 1.03 | 0.81 | 1.31 | 0.78 | 0.64 | 0.94 |
| Subgroup effect | ||||||||||
| Age, years | ||||||||||
| <60 | 1,640/382,013 | 0.99 | 0.80 | 1.21 | 0.63 | 0.44 | 0.91 | 0.66 | 0.48 | 0.90 |
| ≥60 | 716/132,853 | 1.04 | 0.83 | 1.31 | 1.03 | 0.75 | 1.41 | 0.89 | 0.68 | 1.18 |
| Sex | ||||||||||
| Women | 697/235,741 | 1.02 | 0.78 | 1.33 | 1.03 | 0.70 | 1.50 | 0.84 | 0.59 | 1.21 |
| Men | 1,639/279,125 | 0.97 | 0.80 | 1.17 | 0.69 | 0.51 | 0.93 | 0.71 | 0.55 | 0.91 |
| Liver disease | ||||||||||
| No liver disease | 1,203/389,256 | 1.19 | 0.96 | 1.46 | 0.84 | 0.59 | 1.18 | 1.02 | 0.78 | 1.34 |
| Viral hepatitis | 773/31,528 | 0.68 | 0.51 | 0.91 | 0.76 | 0.50 | 1.16 | 0.51 | 0.33 | 0.79 |
| Alcoholic or toxic liver disease | 461/40,720 | 0.91 | 0.65 | 1.27 | 1.20 | 0.76 | 1.89 | 0.59 | 0.33 | 1.06 |
| Liver cirrhosis or hepatic failure | 490/10,174 | 0.88 | 0.64 | 1.21 | 0.71 | 0.43 | 1.18 | 0.78 | 0.50 | 1.20 |
Abbreviations: HR = hazard ratio; DDD = defined daily dose; CI = confidence interval.
*Adjustment for age, sex, body mass index, health behaviors (cigarette smoking, alcohol consumption, and physical activity), concurrent medication, blood pressure category, fasting plasma glucose category, total cholesterol category, socioeconomic status, and Charlson comorbidity index score.
Discussion
To firmly establish the efficacy of aspirin against HCC, randomized controlled trials (RCTs) are needed. However, given the low incidence of HCC and the slow rate of progression to HCC in the general population, it would be logically and ethically questionable to perform an RCT focused primarily on that issue7. Therefore, a well-designed, population-based cohort study, especially of a high-risk population, might be the best alternatives to address the question. In this large study with substantial duration, we found a dose-dependent association between aspirin use and the reduction of HCC risk, which was robust in the general population and in subpopulations including individuals who are young, are male, or have a history of viral hepatitis. Within patients with liver cirrhosis, we did not found any protective effect of aspirin.
Our study has a number of strengths, besides being conducted with large-scale, nationwide data from an area with a high HCC incidence. First, we used data collected prospectively. To avoid a “survivor bias” or an “immortal time bias”, our follow up started at the same calendar date for aspirin users and non-users, and aspirin use was treated as a time-dependent variable in Cox proportional hazard ratio models. Further, to examine the effect of aspirin use after the index date, we performed sensitivity analyses with different time frames, which produced similar overall trends. Second, we used the DDD system together with data from the prescription database to reliably assess drug exposure. The DDD system has been validated by the World Organization Collaborating Center for Drug Statistics Methodology, the membership of which covers most of the world and represents a compromise among countries that recommend different indications and doses. In addition, we verified all hospital prescriptions based on visits to the pharmacy within 14 days of the date of issue, after which time the prescriptions would become invalid. Third, we directly investigated the association between aspirin use and HCC risk, stratified according to underlying liver disease.
The two previous cohort studies12,13 that reported a preventive effect of aspirin on HCC development used questionnaire-based information about self-reported aspirin use and were subject to recall bias. Those studies did not report a cumulative dose-response relationship; the HCC risk reduction was similar between patients who reported monthly aspirin use and those who reported daily aspirin use12. To complement that defect, Yang et al. conducted a nested case-control study using the prescription database from the UK’s Clinical Practice Research Datalink11. Possibly because of potential confounders such as underlying liver disease and the possible use of other drugs, they did not find any efficacy of aspirin for the reduction of HCC risk (HR, 1.11; 95% CI, 0.86–1.44)11. The discrepancy between our results and those of that study11 might be partially explained by the prevalence of chronic viral infection (6.8% in our study vs. 1.4% in the previous study). It is well known that chronic viral infection is the most crucial cause of HCC: the HCC risk is increased 5–100-fold with HBV infection and 15–20-fold with HCV infection16.
The mechanism by which aspirin reduces HCC risk is not well understood. Aspirin may, however, differentially impact the risks of viral and non-viral hepatocarcinogenesis. Our study documents differential protective effects of aspirin according to the underlying liver disease. In the immune-mediated inflammatory process resulting from chronic viral hepatitis, platelets facilitate liver injury by promoting the accumulation of CD8+ T cells17. Sitia et al. showed that aspirin reduced T-cell-mediated inflammation and HCC progression in an HBV transgenic mouse model of chronic immune-mediated liver disease; aspirin failed to produce the same protective effect in a non-immunologically mediated, toxin-induced model of HCC10. Seemingly in line with our results are those of recent secondary prevention studies. Aspirin use was associated with better outcomes among patients with HBV-related HCC18 but did not reduce the recurrence risk of all-cause HCC (HR, 0.82; 95% CI, 0.64–1.06)19. Taken together, our findings highlight the need for future clinical trials to evaluate the impact of aspirin in patients with chronic viral hepatitis.
The finding that aspirin had no protective effect in patients with liver cirrhosis was unexpected, because the majority of HCC cases involve underlying viral cirrhosis. Aspirin does not appear to be effective for all individuals who have a high risk of developing HCC, considering that aspirin had no protective effect in elderly people despite the explicitly higher risk for HCC in that subpopulation (adjusted HR per 10 years, 1.25; 95% CI, 1.20–1.32; data not shown). A functionally inefficient, virus-specific CD8+ T-cell response causes continuous, low-level hepatocellular injury, which promotes hepatocellular proliferation and exposure to inflammatory mutagens20. With the repair functions compromised, the repetitive inflammatory cycle of necrosis and regeneration is thought to trigger random genetic alterations, ultimately leading to HCC development20. That cycle may happen less, however, under uncompromised conditions (e.g., immunosenescence)21. In addition, the potential confounding of the data by the indication is of concern, but it would rather overestimate the association in this population. Because of the high risk of gastrointestinal bleeding, aspirin might be given less to patients with more progressed cirrhosis and HCC. Although a recent review pointed out that excessive bleeding in some patients with advanced cirrhosis might be a lesser risk factor than thrombosis22, from the clinical perspective, aspirin prescription for patients with advanced liver disease should be minimized because of its diminished protective effect against HCC.
The inhibition of COX enzyme, especially COX-2, has been suggested as another potential mechanism to explain the chemoprotective effect of aspirin23. A large body of basic evidence supports an inverse association of COX-2 inhibition with HCC development. Celecoxib, a selective COX-2 inhibitor, inhibited hepatocellular growth by potentiating apoptosis24,25, which is similar to the effects of conventional NSAIDs26. Epidemiologic studies of the association between the use of non-aspirin NSAIDs and the risk of HCC have been inconclusive, however11–14. In our study, subgroup analyses revealed that the combined use of non-aspirin NSAIDs and aspirin was related to greater reduction of HCC risk, which is comparable to the result of a study of HCC postoperative outcomes18. Those suggest that aspirin has a chemopreventive mechanism that is distinct from those of other NSAIDs (i.e., an immunologic effect through anti-platelet properties).
Our results should be interpreted with caution. Efforts to advance from observational studies into clinical trials sometimes fail to reproduce associations27. Our results do not necessarily signify that giving aspirin to patients will reduce their likelihood of developing HCC. A fundamental flaw in our observational study arises from the non-randomized allocation of aspirin. An imbalance in unobserved covariates between aspirin users and non-users could influence the results, although we fully adjusted statistically for all of the observed covariates. Individuals who consistently engage in beneficial activities (e.g., using aspirin daily to reduce cardiovascular disease) may have a number of characteristics distinguishing them from individuals who do not consistently engage in such activities. Additionally, only 65.3% of all eligible Koreans participate in national health examinations, which might limit the generalizability of our results because of selection bias. Another inherent limitation is that prescription databases do not contain information about over-the-counter (OTC) aspirin use. There is no evidence, however, of massive OTC purchases of aspirin in Korea, and one simulation study indicated that, under many circumstances, missed OTC exposure may not invalidate results based on prescription data28. Finally, we could not assess clinical information about the treatment and extent of liver disease (i.e., the stage, viral load, and mutation), which could influence the risk of HCC.
In conclusion, long-term aspirin use may reduce the risk of HCC in patients with viral hepatitis. Our study was conducted with a unique cohort located where chronic viral hepatitis is common, using a large, longitudinal database containing complete prescription pharmaceutical information and health-examination data. Future RCTs and additional experimental studies are needed to clarify the mechanism linking aspirin use and HCC.
Methods
Data source and study population
We collected data from the medical insurance claims and biennial health examinations of a standardized cohort sampled from the Korean National Health Insurance Corporation (NHIC) claims database, which were provided for research purposes by the Korean NHIC with strict confidentiality guidelines.
Since 1995 in South Korea, the NHIC, the single insurer of the Korean public health-insurance sector, has provided compulsory universal health insurance covering all forms of health care services, in which 97% of the population is obliged to enroll. Clinics and hospitals submit claims for patient care; including electronic resources with demographic information, diagnoses, procedures, and prescriptions; to be reimbursed for 70% of the total medical costs. NHIC also provides biennial health examinations, conducted by medical staff at local hospitals, to individuals over 40 years of age. The NHIC databases have been used for epidemiologic research in the past, and the information about prescription use, diagnoses, and hospitalizations is of high quality29.
From the NHIC records, we obtained the following information about individuals who were 40 years of age or older and went to the Korean national health-examination service at least once between January 1, 2002 and December 31, 2006 (N = 514,866): sex, date of birth, socioeconomic status based on average insurance premium per month, details on admissions and outpatient visits, and comorbid conditions. We also obtained information about patient prescriptions, including the names of drugs, dosage, duration, and total expenditure. We verified that patient actually received the drugs prescribed by cross checking pharmacy visits.
We selected the examination nearest to the index date (January 1, 2007) and extracted the following information: height, weight, blood pressure, self-reported health-related habits (tobacco use, alcohol consumption, and physical activity), and blood chemistries (serum glucose and total cholesterol). Blood chemistries were measured under fasting conditions using clinical laboratories with a standard procedure.
We excluded from the study cohort individuals (n = 53,786) who had been diagnosed with any cancer, indicated by the International Classification of Diseases code–10th Revision (ICD-10) “C” code, had a medical history of cancer according to health check-up survey data, died before the index date, or were missing any non-survey health check-up variables. We allowed a latent period of 1 year, excluding HCC cases within 1 year after the index date (n = 325). Thus, we selected a total of 460,755 participants for the final analysis. The study design and recruitment of participants are depicted in Fig. 1.
Figure 1.
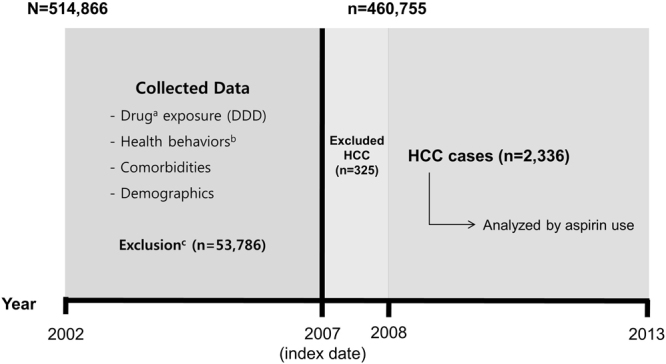
Study design and recruitment of participants. Abbreviations: DDD, defined daily dose; HCC, hepatocellular carcinoma; NHIC, National Health Insurance Corporation. aUsing the claims database of the NHIC, including non-steroidal anti-inflammatory drugs, statin, and metformin. bFrom national health examinations, including smoking status, drinking habit, and physical activity. cPatients with any cancer diagnosis with the ICD-10 “C” code, past medical history of cancer according to health-check survey data, or missing non-survey health check-up variables and those who died before the index date were excluded from the study.
The Institutional Review Board of Seoul National University Hospital (IRB number: E-1509–004–699) approved the study protocol. The ethics committee waived the need for participant consent, because the study involved routinely collected medical data that were anonymized at all stages, including during the data cleaning and statistical analysis. The methods were carried out in accordance with the relevant guidelines and regulations.
Data collection
We observed the participants from the index date until December 31, 2013. The primary outcome was a newly diagnosis of HCC, represented by the ICD-10 code in the nationwide claims database. To reduce the effects of coding errors in the database, we defined HCC cases as those in which patients who visited the hospital at least once and received ICD-10 code “C220” met any of the following additional criteria: (i) made at least three outpatient visits associated with code “C220”, (ii) had a 3 or more days of admission with code “C220”, (iii) received radiation therapy, systemic chemotherapy, or therapy without chemotherapy or radiation claimed via Korean Diagnosis-Related Group (HDRG) code “H61-Malignacy of Liver”, (iv) died of causes related to code “C220”. In cases meeting those criteria, the first date of diagnosis under code “C220” was defined as the date of the event. The validity of using claims codes of the NHIC dataset for HCC as compared with the Korea Central Cancer Registry has been tested through comparing incidence rates. The accuracy of the HCC incidence in the NHIC dataset is above 95%30. Cases that met the criteria but involved a diagnosis of any other cancer prior to the date of the event were not considered HCC cases for the purpose of our analysis.
We extracted from the data all exposures and covariates recorded during the 5 years prior to the index date. The primary exposure of interest was the cumulative use of aspirin. We gathered data pertaining to aspirin prescriptions such as the date of the prescription, the daily dose, the number of days supplied, and the number of pills. We collected similar information about prescriptions for non-aspirin NSAIDs, statins, and metformin, which might influence the risk of HCC. To indicate the drug exposure, we used the DDD system, which assumes the average daily maintenance dose of an individual drug used for the drug’s main indication in adults. The DDD is a unit of measurement; it does not indicate a recommended dose or a real dose. We calculated the average daily dose (in units of DDD per day) of each prescription dispensed, weighted by the intended duration of each prescription. To examine the dose-response relationship, we categorized the cumulative aspirin use during the 5 years prior to the index date into four groups (<30, 30–365, 365–730, and >730 DDDs). We also categorized other drugs based on their median DDD, as appropriate. Patients who used less than 30 DDDs of a given drug were defined as non-users.
We expressed comorbid conditions as a Charlson comorbidity score, which was derived from ICD-10 codes in the claims database, using the sum of the weighted scores of all comorbidities (e.g., cardiovascular disease, pulmonary disease, renal disease, and liver disease, etc.)31.
We calculated body mass index (BMI) as the weight divided by the adult height squared (kg/m2). For analysis, we classified the participants into the following categories: BMI (<18.5, 18.6–22.9, 23–24.9, 25–29.9, or ≥30 kg/m2); blood pressure (normal, prehypertension, or hypertension); fasting glucose level (<100, 100–125.9, or ≥126 mg/dL or history of diabetes); cholesterol level (<200, 200–239, or ≥240 mg/dL or history of hyperlipidemia); frequency of physical activity (none, 1–2, or ≥3 times/week); smoking status (never, former, or current smoker); frequency of alcohol drinking (none, <1, 1–2, or ≥3 times/week).
Statistical analysis
We calculated the incidence per 100,000 person-years by dividing the number of HCC events by the total number of person-years at risk and multiplying the result by 100,000. We calculated the 95% CI assuming a Poisson distribution.
We used Cox proportional hazards models to estimate the HRs and 95% CIs of HCC development with or without aspirin use. We calculated the accumulated person-years of risk, beginning with the index date and ending with the date of HCC diagnosis, diagnosis of any other cancer, death, or December 31, 2013, whichever came first—HCC diagnosis was the primary endpoint. To investigate the independent effect of aspirin on HCC risk, we conducted multivariable survival analyses using the Breslow method after adjusting for all potential confounders such as demographics, health risk behaviors, concurrent medications, and medical conditions. Furthermore, for a more unbiased result, we created a 1:1 propensity score matched cohort to compare the risk of HCC between aspirin users and aspirin non-users using the following covariates: age, sex, BMI, cigarette smoking, alcohol consumption, physical activity, blood pressure, fasting plasma glucose, Charlson comorbidity index, statin use, and metformin use.
Because drug consumption after the index date might confound the results, we performed a sensitivity analysis, shifting the index date forwards and backwards. We also conducted subgroup analyses stratified by age, sex, and underlying liver disease; in the stratified multivariable analyses, the association between aspirin use and the risk of HCC was reexamined in different subgroups. All analyses were performed using the STATA statistical software (version 11.0 for Windows; STATA Corp., Inc.). All statistical test results were considered statistically significant when two-tailed p-values were < 0.05.
Electronic supplementary material
Acknowledgements
The authors thank the staff of the Korean National Health Insurance Corporation for their cooperation. This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant no. 2017R1D1A1B03033721). Kyuwoong Kim received a scholarship form the BK21-plus education program provided by the NRF.
Author Contributions
Prof. Park had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: all authors. Acquisition of data: all authors. Analysis and interpretation of data: all authors.
Competing Interests
The authors declare no competing interests.
Footnotes
In Cheol Hwang and Jooyoung Chang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-23343-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McGlynn, K. A. & London, W. T. The global epidemiology of hepatocellular carcinoma: present and future. Clinics in liver disease15, 223–243, vii–x, 10.1016/j.cld.2011.03.006 (2011). [DOI] [PMC free article] [PubMed]
- 2.Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992-2008. Hepatology (Baltimore, Md.) 2012;55:476–482. doi: 10.1002/hep.24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma. The New England journal of medicine. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 4.Chang MH, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. Journal of the National Cancer Institute. 2009;101:1348–1355. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 5.Lai CL, Yuen MF. Prevention of hepatitis B virus-related hepatocellular carcinoma with antiviral therapy. Hepatology (Baltimore, Md.) 2013;57:399–408. doi: 10.1002/hep.25937. [DOI] [PubMed] [Google Scholar]
- 6.Morgan RL, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Singh PP, Roberts LR, Sanchez W. Chemopreventive strategies in hepatocellular carcinoma. Nature reviews. Gastroenterology & hepatology. 2014;11:45–54. doi: 10.1038/nrgastro.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar M, Zhao X, Wang XW. Molecular carcinogenesis of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: one step closer to personalized medicine? Cell & bioscience. 2011;1:5. doi: 10.1186/2045-3701-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 10.Sitia G, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2165–2172. doi: 10.1073/pnas.1209182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang B, et al. Associations of NSAID and paracetamol use with risk of primary liver cancer in the Clinical Practice Research Datalink. Cancer epidemiology. 2016;43:105–111. doi: 10.1016/j.canep.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahasrabuddhe VV, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. Journal of the National Cancer Institute. 2012;104:1808–1814. doi: 10.1093/jnci/djs452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrick JL, et al. NSAID Use and Risk of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma: The Liver Cancer Pooling Project. Cancer prevention research (Philadelphia, Pa.) 2015;8:1156–1162. doi: 10.1158/1940-6207.CAPR-15-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coogan PF, et al. Nonsteroidal anti-inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2000;9:119–123. [PubMed] [Google Scholar]
- 15.Cibere J, Sibley J, Haga M. Rheumatoid arthritis and the risk of malignancy. Arthritis and rheumatism. 1997;40:1580–1586. doi: 10.1002/art.1780400906. [DOI] [PubMed] [Google Scholar]
- 16.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nature reviews. Gastroenterology & hepatology. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iannacone M, et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med. 2005;11:1167–1169. doi: 10.1038/nm1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee PC, et al. Antiplatelet Therapy is Associated with a Better Prognosis for Patients with Hepatitis B Virus-Related Hepatocellular Carcinoma after Liver Resection. Ann Surg Oncol. 2016 doi: 10.1245/s10434-016-5520-9. [DOI] [PubMed] [Google Scholar]
- 19.Yeh CC, et al. Nonsteroidal anti-inflammatory drugs are associated with reduced risk of early hepatocellular carcinoma recurrence after curative liver resection: a nationwide cohort study. Ann Surg. 2015;261:521–526. doi: 10.1097/SLA.0000000000000746. [DOI] [PubMed] [Google Scholar]
- 20.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 21.Pera A, et al. Immunosenescence: Implications for response to infection and vaccination in older people. Maturitas. 2015;82:50–55. doi: 10.1016/j.maturitas.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. The New England journal of medicine. 2011;365:147–156. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 23.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9:259–267. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 24.Cui W, Yu CH, Hu KQ. In vitro and in vivo effects and mechanisms of celecoxib-induced growth inhibition of human hepatocellular carcinoma cells. Clin Cancer Res. 2005;11:8213–8221. doi: 10.1158/1078-0432.CCR-05-1044. [DOI] [PubMed] [Google Scholar]
- 25.Kern MA, et al. Cyclooxygenase-2 inhibition induces apoptosis signaling via death receptors and mitochondria in hepatocellular carcinoma. Cancer Res. 2006;66:7059–7066. doi: 10.1158/0008-5472.CAN-06-0325. [DOI] [PubMed] [Google Scholar]
- 26.Fredriksson L, et al. Diclofenac inhibits tumor necrosis factor-alpha-induced nuclear factor-kappaB activation causing synergistic hepatocyte apoptosis. Hepatology (Baltimore, Md.) 2011;53:2027–2041. doi: 10.1002/hep.24314. [DOI] [PubMed] [Google Scholar]
- 27.Cook NR, et al. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 28.Yood MU, et al. Using prescription claims data for drugs available over-the-counter (OTC) Pharmacoepidemiol Drug Saf. 2007;16:961–968. doi: 10.1002/pds.1454. [DOI] [PubMed] [Google Scholar]
- 29.Shin JY, et al. Risk of intracranial haemorrhage in antidepressant users with concurrent use of non-steroidal anti-inflammatory drugs: nationwide propensity score matched study. BMJ. 2015;351:h3517. doi: 10.1136/bmj.h3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo HJ, Oh IH, Yoon SJ. A comparison of the cancer incidence rates between the national cancer registry and insurance claims data in Korea. Asian Pac J Cancer Prev. 2012;13:6163–6168. doi: 10.7314/APJCP.2012.13.12.6163. [DOI] [PubMed] [Google Scholar]
- 31.Quan H, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


