Abstract
The imaginal discs of the genetically tractable model organism Drosophila melanogaster have been used to study cell-fate specification and plasticity, including homeotic changes and regeneration-induced transdetermination. The identity of the reprogramming mechanisms that induce plasticity has been of great interest in the field. Here we identify a change from antennal fate to eye fate induced by a Distal-less-GAL4 (DllGAL4) P-element insertion that is a mutant allele of Dll and expresses GAL4 in the antennal imaginal disc. While this fate change is not induced by tissue damage, it appears to be a hybrid of transdetermination and homeosis as the GAL4 expression causes upregulation of Wingless, and the Dll mutation is required for the fate change. Neither GAL4 expression nor a Dll mutation on its own is able to induce antenna-to-eye fate changes. This plasticity appears to be unique to the DllGAL4 line, possibly due to cellular stress induced by the high GAL4 expression combined with the severity of the Dll mutation. Thus, we propose that even in the absence of tissue damage, other forms of cellular stress caused by high GAL4 expression can induce determined cell fates to change, and selector gene mutations can sensitize the tissue to these transformations.
Introduction
Normal development requires that cells become progressively restricted in their potential as they become determined and differentiate toward specific fates. This determination of fate is regulated by homeotic or selector genes1. Drosophila imaginal discs, precursors of adult fly appendages, are an important system for studying fate determination and developmental plasticity. In Drosophila, the imaginal disc primordia are established and acquire a disc-specific determined state during embryogenesis2–4. The discs grow and maintain their determined state through the larval stages and then proceed through differentiation during metamorphosis. This state of determination with respect to disc type usually stays fixed. However, fate determination is not completely irreversible and two processes can produce changes in disc identity: homeosis and transdetermination.
Homeosis occurs when mutations are generated in hox or selector genes, which are important for establishing cell, tissue and segment identities. These mutations result in replacement of one body part by another5. Transdetermination occurs when cells or tissues switch from one determined state to another due to experimental manipulation. Mechanically fragmented imaginal discs cultured in adult hosts have the ability to regenerate and can faithfully replace the lost structures over many generations of serial fragmentation and culture, indicating that the determined state is maintained6,7. However, in specific locations in each disc, called the weak point, a few cells become plastic and undergo transdetermination8. While all imaginal discs are capable of transdetermination, this fate change is not a random event, occurring only in particular reproducible directions with characteristic probabilities. The restricted nature of these transformations indicates that while disc determination is plastic, disc cells prefer certain developmental pathways over others.
In addition to tissue damage, ectopic activation of different signaling pathways, such as Wingless (Wg), Decapentaplegic (Dpp), and Jun N-terminal Kinase (JNK) signaling, can also induce transdetermination events. Misexpression of wingless in the foreleg imaginal discs induces transdetermination to wing cells in a manner very similar to fragmentation9. Dpp signaling has an important role in defining the weak point, and high levels of endogenous dpp expression enable transdetermination in response to both damage-induced and transgene-induced ectopic wingless expression10. Maves and Schubiger have proposed that the ectopic interaction of Wg and Dpp signaling in wounded imaginal discs induces transdetermination at the points where they overlap11. Furthermore, JNK signaling, which is activated upon wounding12,13 can induce transdetermination through the suppression of Polycomb group (PcG) proteins14.
There has been much debate in the literature over whether homeotic transformations and transdetermination are different aspects of the same phenomenon or are distinct processes15,16. Despite the different causes of the two phenomena, tissue damage and mutations, they share similarities. Transdetermination was predicted to alter expression of genes that act as developmental switches17, and subsequent work showed that homeotic gene expression is altered in transdetermining tissue18. Additionally, misexpression of selector genes can result in transformations that phenotypically resemble transdetermination5,19. It remains to be seen whether cell fate plasticity can be induced through mechanisms other than tissue damage, such as general cellular stress, mechanical tension, or activation of alternative signaling pathways.
To support the possibility of damage-independent plasticity, here we describe an antenna-to-eye fate change that occurs in response to GAL4 expression. We found a Distal-less (Dll) GAL4 line, which is also a mutant for the Dll gene, that causes apoptosis in the Distal-less domain in a temperature-dependent manner. This apoptosis induced many aspects of the damage response, including expansion of the Wg expression domain, as well as upregulation of JNK signaling and compensatory proliferation. The GAL4 expression also led to certain cells in the third antennal segment changing fate to produce pigmented eye tissue. Surprisingly, caspase-mediated cell death was not required for the fate change, suggesting it was not induced by tissue damage. The fate change was also not due to just the Dll mutation or just GAL4 expression, because neither Dll mutations nor GAL4 expression alone were able to produce this particular fate change. Furthermore, other Dll mutations in combination with a different GAL4 transgene that was expressed in the same cells (ssGAL4) caused some tissue disruption, but did not induce the fate change. Thus, the fate change appears to be specific to the DllGAL4 line, where the unique combination of the strength of DllGAL4 expression and severity of the Dll mutation may cause enough cellular stress to perturb cell fates. Therefore, cellular stress, independent of cell death, could play a role in perturbing cell fate in a sensitized mutant background. This stress-induced plasticity seems to be a hybrid of transdetermination and homeosis, and may confound interpretation of experiments conducted using this DllGAL4 line for analysis of different developmental processes.
Results
High expression of GAL4 induces cell death at elevated temperatures
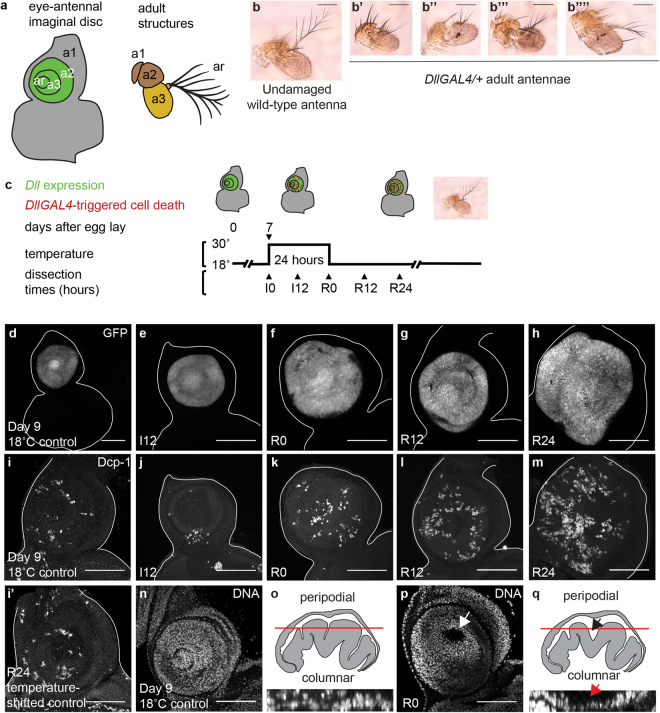
To express transgenes in the antennal imaginal disc for experimental purposes, we used a GAL4 enhancer trap in the Distal-less (Dll) locus20, which is expressed in the arista and the second and third antennal segments (Fig. 1a). In the course of these experiments, we examined the DllGAL4 line independently as a control. Remarkably, the DllGAL4 alone produced antennal phenotypes. DllGAL4 animals that were maintained at 18 °C rarely showed antennal defects, while animals maintained at 25 °C or shifted to 30 °C during early third instar for 24 hours showed a high frequency of defects, including altered morphology in the arista and the third antennal segment (Fig. 1b–b””). Importantly, GAL4 activity is increased at higher temperatures, which could explain the morphological defects at 25 °C and 30 °C21. DllGAL4 animals kept at 18 °C throughout development were used as controls (18 °C controls), as were animals that did not contain DllGAL4 but had gone through the temperature shift to rule out temperature as a causative agent (temperature-shifted controls).
Figure 1.
High DllGAL4 expression induces cell death in the antennal disc. (a) Diagram showing regions of the eye-antennal imaginal disc and corresponding adult structures. a1, a2, a3 and ar are first, second, and third antennal segments and the arista, respectively. The green region shows Distal-less expression. (b) Undamaged wild-type adult antenna. (b’–b””) Adult antennae from DllGAL4/+ animals raised at 25 °C. The antennae showed a range of altered morphology. The aristae and the third antennal segments were the most affected. (c) The protocol used to observe DllGAL4-induced phenotypes. Animals were raised at 18 °C and shifted to 30 °C for 24 hours during early third-instar larval development (day 7 AEL). Larvae were returned to 18 °C and allowed to pupariate and eclose or were dissected at the time points noted during damage induction (I) or recovery (R). (d–h) DllGAL4 expression marked by UAS-EGFP in a day 9 18 °C control disc (DllGAL4/+), which is developmentally similar to R0 (d), and temperature-shifted DllGAL4/+ discs at I12 (e), R0 (f), R12 (g), and R24 (h). (i and i’) Dying cells marked using an antibody for the cleaved form of the effector caspase Dcp-1 in a day 9 18 °C control disc (DllGAL4/+) (i) and a temperature-shifted control disc (w1118; +/SM6.TM6B) at R24 (i’). Note that there was no temperature-induced increase in cleaved Dcp-1 positive cells in these control discs. (j–m) A progressive increase in dying cells was seen during induction and recovery times as marked by cleaved Dcp-1 immunostaining. Note that I12 discs had less apoptosis compared to control discs, due to I12 discs being developmentally younger. (n and p) Disc morphology was affected, as visualized with nuclear staining of the antennal epithelium using TO-PRO-3 in day 9 18 °C control discs (n) and R0 discs (p). In (p) the arrow marks the gap between the peripodial and columnar epithelia. (o) Orthogonal view of part of the disc shown in (n) and diagram of a cross-section of an undamaged antennal disc. The red line shows the approximate level of the confocal image of the undamaged epithelium shown in (n). (q) Orthogonal view of part of the disc shown in (p) and diagram of a cross-section of an R0 disc. The arrows indicate the gap between the epithelial layers visible in (p). Scale bars are all 100 μm.
To determine why GAL4 expression in the imaginal disc caused defects in the adult antenna, we examined the antennal imaginal discs after induction of GAL4 expression. DllGAL4 animals were raised at 18 °C and shifted to 30 °C on day 7 after egg lay (AEL), when they entered the early third larval instar stage. The larvae were kept at 30 °C for a 24-hour period, and examined at different Induction times denoted as I0 and I12 (Fig. 1c), after which they were brought back to 18 °C. The imaginal discs were also observed at multiple points after the temperature shift, over the course of 24 hours, denoted as Recovery times R0-R24 (Fig. 1c).
We confirmed GAL4 expression by marking the DllGAL4 expression domain using UAS-EGFP (Fig. 1d–h). High GAL4 expression causes cell death and can lead to developmental defects, often in a temperature-dependent manner22,23. To determine whether the DllGAL4 expression induced cell death, apoptotic cells were marked by immunostaining for the cleaved form of the effector caspase Dcp-124. 18 °C control discs showed a basal level of apoptosis (Fig. 1i), which was similar to the levels of apoptosis observed in temperature-shifted control discs (Fig. 1i’). Congruent with previous reports22, we observed that shifting DllGAL4 animals to 30 °C resulted in increased apoptosis in the Dll-expressing domain, likely due to higher GAL4 expression. Apoptosis progressively increased in discs from I12-R24 (Fig. 1j–m), continuing even after the animals were returned to 18 °C. In the third-instar disc, the apoptosis resulted in altered morphology such that the gap between the peripodial epithelium and the columnar epithelium became more pronounced at R0 (Fig. 1n–q). The gap was the most prominent in the arista region (Fig. 1p–q), which experienced the highest DllGAL4 expression levels (Fig. 1d). The increased apoptosis was sufficient to cause defects in the arista, and, to a lesser extent, the third antennal segment in the adult animals. The defects were temperature-dependent, as DllGAL4 animals maintained at 18 °C throughout development almost always had perfectly formed antennae. Six in 156 antennae of animals maintained at 18 °C had very slight deformities of the arista. Even in these antennae the rest of the antennal segments were perfectly formed.
GAL4-triggered cell death induces a limited damage response
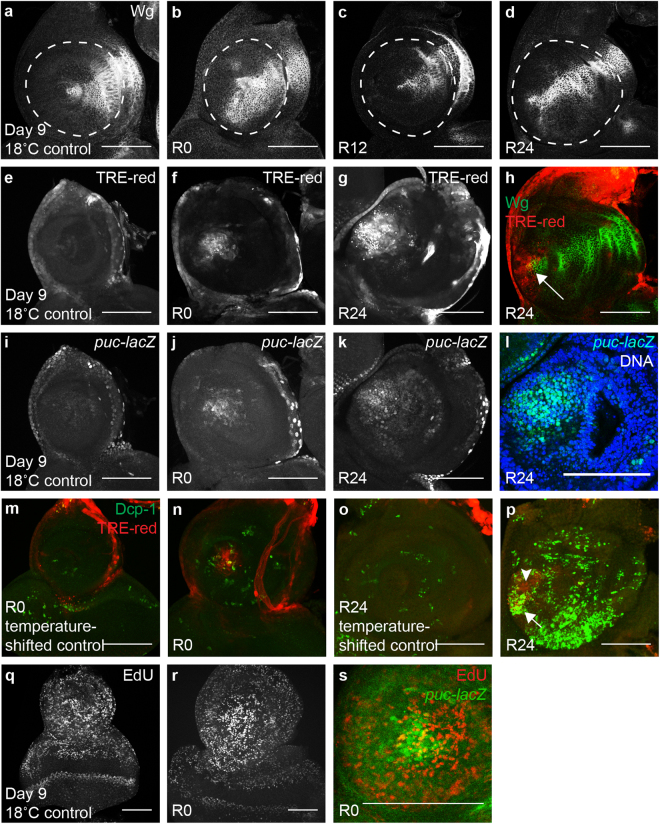
To determine whether the moderate amount of DllGAL4-triggered apoptosis could elicit a tissue-wide damage response, we looked for features characteristic of imaginal disc regeneration or apoptosis-induced proliferation. Wg upregulation near the wound is an early response following damage in the wing and leg imaginal discs25–27. In the antennal disc, Wg expression normally occurs in a wedge shape on the dorsal side during early second larval instar, and is maintained in that shape through the second and third instars and into the pupal stages28. Wg was present in this characteristic pattern in 18 °C control discs (Fig. 2a). However, after GAL4 activation at higher temperature, the wedge had expanded by R0 such that Wg was present in almost half of the Dll-expressing region (Fig. 2b). Wg expression was restored to the characteristic wedge pattern by R12 and remained in the wedge through R24 and into the pupal stage (Fig. 2c–d). Thus, ectopic expression of Wg was observed in response to GAL4-triggered apoptosis.
Figure 2.
GAL4-triggered cell death elicits a damage response. (a–d) Anti-Wg immunostaining in a day 9 18 °C control disc (DllGAL4/+) (a), an R0 disc (b), an R12 disc (c), and an R24 disc (d). Dashed circle in (a–d) marks approximate Dll-expressing region. (e–g) TRE-red reporter expression indicating JNK signaling in a day 9 18 °C control disc (e), and discs at R0 (f) and R24 (g). (h) Wg (green) co-expression with the TRE-red reporter (red) at R24. Arrow shows JNK signaling co-localized with Wg expression at the center of the disc. (i–k) puc-lacZ expression indicating JNK signaling in a day 9 18 °C control disc (i), and discs at R0 (j) and R24 (k). (l) Higher magnification image of (k) showing puc-lacZ (green) co-localization with epithelial nuclei (blue) marked with TO-PRO-3 at the center of the disc, showing puc expression in living cells. (m–p) Co-expression of Dcp-1 (green) and TRE-red reporter (red) in a temperature-shifted control disc (+; +/SM6.TM6B) at R0 (m), an (DllGAL4/+) R0 disc (n), a temperature-shifted control disc at R24 (o) and an R24 disc (p). (m and o) TRE-red expression is absent in temperature-shifted control discs. (n and p) Some JNK-expressing cells co-localized with Dcp-1 positive cells (arrow in p) while others did not (arrow head in p) showing that not all JNK-expressing cells were apoptotic. (q and r) EdU incorporation marks cells in S phase in a day 9 18 °C control disc (q) and an R0 disc (r). (s) puc-lacZ (green) co-localization with EdU (red) at R0, showing puckered expression in and near proliferating cells. Scale bars are 100 μm.
JNK signaling is triggered after injury and is important for wound healing and regeneration after both surgical and genetic ablation12,13,27. Activation of JNK signaling can be monitored through an expression reporter for its transcriptional target puckered29 or through a transgenic reporter construct controlled by AP-1 binding sites (TRE-red)30. Using these reporters, we found that JNK activity was absent in the Dll domain in 18 °C control discs (Fig. 2e,i). However, with elevated GAL4 expression at 30 °C, both reporters were expressed at R0 (Fig. 2f,j) and R24 (Fig. 2g,k). The AP-1 reporter was upregulated at the center of the disc (arrow in Fig. 2h). Likewise, puckered-lacZ expression showed clear co-localization with epithelial nuclei in the center of the disc, demonstrating puckered expression in live cells rather than cellular debris (Fig. 2l). While JNK signaling was upregulated in some cells that were also dying, most of the reporter activity was not associated with apoptosis (Fig. 2m–p).
To examine whether GAL4-triggered apoptosis was inducing proliferation, we visualized proliferating cells by EdU incorporation, which marks the S phase of the cell cycle. EdU incorporation was enriched at the center of the antennal disc in response to GAL4 expression (Fig. 2q–r). Some puckered-lacZ expressing cells were also EdU-positive, indicating that JNK was activated in some of the proliferating cells (Fig. 2s).
Myc is important for regenerative growth in wing imaginal discs26. To determine whether Myc was elevated during the damage response in the antennal disc, Myc protein levels were observed by immunostaining. Myc was present at low levels throughout the eye-antennal disc in temperature-shifted control discs (Supplemental Fig. 1a). Myc was not upregulated in response to the cell death caused by DllGAL4 in the antennal imaginal disc (Supplemental Fig. 1b–d). Thus, GAL4-triggered apoptosis in the antennal disc reproduces some elements of a damage response including Wg expression, JNK signaling, and proliferation, but not others, such as elevated Myc expression.
GAL4-expressing discs experience segment-specific patterning changes
From proximal to distal, the Drosophila antenna is made of segments a1, a2, a3 and the arista (ar) (Fig. 1a). We examined gene expression that marks segment identity in the antenna to determine whether GAL4-triggered cell death perturbed disc patterning. For these experiments, we examined the discs at R12 to observe immediate changes in patterning, and we used the temperature-shifted controls to eliminate the possibility of temperature changes causing the patterning defects.
In third-instar larvae, homothorax (hth) is expressed in a1 and a2, and weakly in a3 (Fig. 3a)31. The gene cut (ct) is also expressed throughout a1 and a2 (Fig. 3d)32. The pattern of these broadly expressed proximal genes remained unchanged at R0 and R12 after DllGAL4 expression (Fig. 3b,c,e,f), suggesting that the proximal segments, a1 and a2, remained predominantly unaffected, consistent with the adult phenotype. To examine a2 more closely, we observed spalt major (salm), which is expressed in a ring only in a2 (Fig. 3g)33. While disc morphology appeared more folded at R12, salm expression was not altered at R0 and R12 (Fig. 3h,i), again indicating that a2 was unaffected by DllGAL4 expression.
Figure 3.
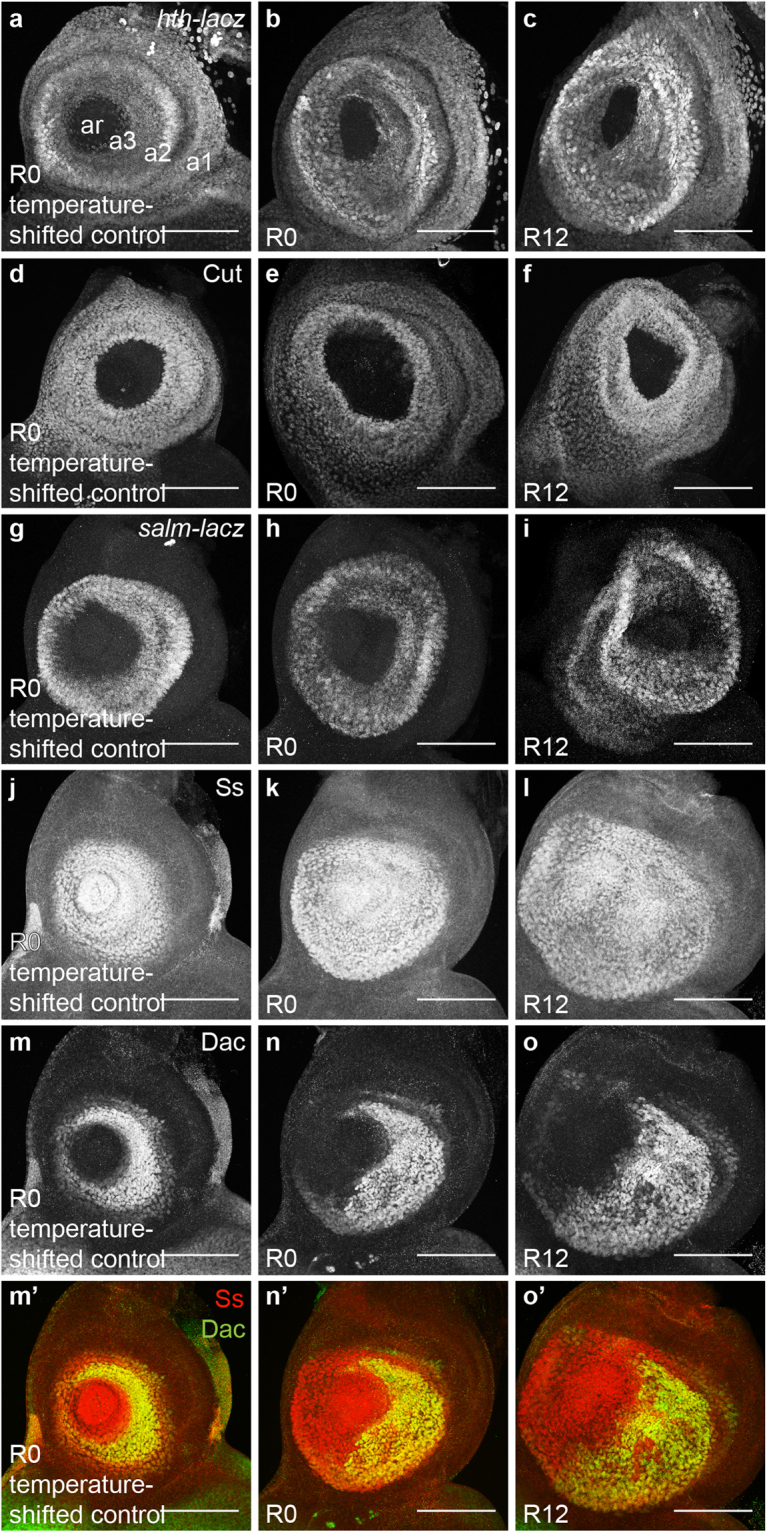
Patterning changes occur in the antennal discs after GAL4-induced cell death. (a–l) Proximal segment-specific gene expression was not perturbed at R0 and R12. (a–c) hth-lacZ expression in a temperature-shifted control disc (+; hth-lacZ/SM6.TM6B) at R0 (a) and (DllGAL4/+; hth-lacZ/+) discs at R0 (b) and R12 (c). (d–f) Anti-Cut immunostaining in a temperature-shifted control disc (+; +/SM6.TM6B) at R0 (d) and (DllGAL4/+) discs at R0 (e) and R12 (f). (g–i) salm-lacZ expression in a temperature-shifted control disc (salm-lacZ; +/SM6.TM6B) at R0 (g) and (DllGAL4/salm-lacZ) discs at R0 (h) and R12 (i). (j–l) Anti-Spineless (Ss) immunostaining in a temperature-shifted control disc (+; +/SM6.TM6B) at R0 (j) and (DllGAL4/+) discs at R0 (k) and R12 (l). (m–o) Distal segment-specific gene expression was perturbed as early as R0. Anti-Dachshund (Dac) immunostaining in a temperature-shifted control disc (+; +/SM6.TM6B) at R0 (m) and (DllGAL4/+) discs at R0 (n) and R12 (o). (m’–o’) Merge of Anti-Ss (j–l) and Anti-Dac (m–o) at different time points. Note that Dac expression was perturbed and ventral expression was lost, indicating third segment-specific damage and mispatterning. Scale bars are 100 μm.
To examine more distal antennal segments, we visualized the expression of spineless (ss), which is present in a circular pattern from a3 to ar (Fig. 3j)34, and dachshund (dac), which is present in a ring only in a3 (Fig. 3m)35. ss expression was unchanged and remained within a3-ar at both R0 and R12 (Fig. 3k–l). However, a large region of dac expression was lost as early as R0, specifically on the ventral side (Fig. 3n) and remained missing at R12 (Fig. 3o). dac expression remained within the ss domain, indicating that it did not expand into other antennal segments (Fig. 3m’–o’). Consistent with the adult phenotype, the loss of dac expression suggests damage to the third antennal segment.
GAL4 expression induces antenna-to-eye fate changes
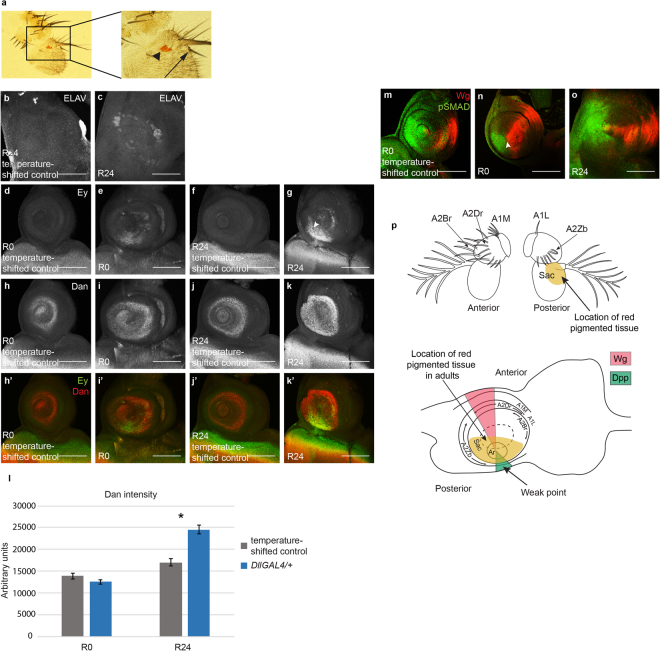
The DllGAL4-expressing animals that were maintained at 25 °C rather than taken through the 30 °C temperature shift showed more severe damage than shown in Fig. 1b’–b””, as well as outgrowths that appeared similar to leg tissue. Intrigued by these observations, we examined the antennae of animals that were maintained at 25 °C more closely. We found evidence of fate changes in these antennae, such as the presence of bracted bristles that are normally only found on the distal segments of the legs36 and the proximal costa of the wings37 (Fig. 4a). Antenna-to-leg and antenna-to-wing transdetermination events have been observed before38. Incredibly, we also found what appeared to be pigmented eye tissue in some of the affected antennae (Fig. 4a), a fate change that has not been previously reported as occurring after tissue damage. We also observed the antenna-to-eye fate change in antennae of animals that underwent the 30 °C temperature shift. These fate changes were dependent on GAL4 expression, as they were completely suppressed by expression of GAL8039 in the DllGAL4 animals maintained at 25 °C (0 in 488 antennae showed any defects, 2 independent experiments) (Supplemental Table 1).
Figure 4.
GAL4 expression induces antenna-to-eye fate changes. (a) Adult antenna from a DllGAL4/+ animal raised at 25 °C. Arrowhead marks the red pigmented tissue in the third antennal segment. Arrow shows the presence of a bracted bristle also on the third antennal segment. (b) Anti-ELAV staining was absent in the antennal segments in a temperature-shifted control disc (+; +/SM6.TM6B) at R24. (c) Ectopic ELAV staining occurred in a ring pattern in an (DllGAL4/+) R24 disc. (d–g) Anti-Eyeless (Ey) staining was absent in temperature-shifted control discs at both R0 (d) and R24 (f). Ey was ectopically expressed as early as R0 (e) and increased in area and intensity by R24 (g). Note that Ey expression was present near the weak point in the antennal disc, arrowhead in (g). (h–k) Anti-Dan expression in a temperature-shifted control disc at R0 (h), an R0 disc (i), a temperature-shifted control disc at R24 (j) and an R24 disc (k). (h’–k’) Merge of Anti-Ey (d–g) and Anti-Dan (h–k). (d–k’) Images were taken with the same confocal settings to enable comparison of fluorescence levels. (l) Graph showing Dan immunofluorescence intensity in temperature-shifted control discs at R0 (n = 16), R0 discs (n = 16), temperature-shifted control discs at R24 (n = 16) and R24 discs (n = 17). *p = 0.000001. (m–o) Anti-Wg (red) and Anti-pSMAD (green) immunostaining in a temperature-shifted control disc at R0 (m) and in discs at R0 (n) and R24 (o). Arrowhead in (n) shows the region of overlap of Wg and pSMAD. (p) Fate map of the antennal imaginal disc adapted from Haynie and Bryant52. Adult structures of the antenna are labelled showing anterior and posterior views. A1L = antennal segment 1: isolated dorsal lateral bristle, A1M = antennal segment 1: dorsal medial bristles, A2Br = antennal segment 2: large anterior bristles, A2Dr = antennal segment 2: small anterior row bristles, A2Zb = antennal segment 2: posterior tooth bristles, Sac = antennal segment 3: sacculus. Light brown region shows the fate change zone that acquired red pigmented tissue in the adults. The corresponding locations of these structures are labelled on the antennal imaginal disc. Pink and green regions mark Wg and Dpp expression, respectively. Error bars represent SEM. Student’s T-test used for statistical analysis. Scale bars are 100 μm.
To confirm that the red-pigmented tissue in the antenna was indeed aberrant eye tissue, we examined expression of the proneural marker ELAV in the antennal discs of animals that underwent the 30 °C temperature shift to identify photoreceptor precursors. ELAV immunostaining was never observed in temperature-shifted control discs (Fig. 4b), while aberrant ELAV expression was observed in a ring in GAL4-expressing discs at R24 (Fig. 4c). We also examined Eyeless (Ey) expression, as ey is an eye selector gene and can ectopically induce eye tissue40. Ey immunostaining was indeed observed as early as R0, and expression increased in area and intensity by R24 (Fig. 4d–g). Interestingly, Ey was expressed in an asymmetric ring, with much wider and intense expression on the ventral posterior side, which may coincide with the weak point (Fig. 4p). The presence of Ey in this particular domain could explain why red-pigmented tissue was only observed on the posterior side of the third antennal segment and not in a ring (Fig. 4a). Ey expression could be conferring competence to the cells in this domain, enabling ELAV-expressing cells to recruit pigment cells and other eye cell types to form rudimentary eyes, while ELAV-expressing cells in other portions of the imaginal disc either did not survive to adulthood, did not remain neuronal precursors, or were not visible due to lack of red pigment cells.
The genes distal antenna (dan) and distal antenna related (danr) are effector genes that are involved in antennal fate specification41. However, over-expression of dan and danr induces the formation of eye tissue in the third antennal segment42,43. dan expression is normally present in the antennal disc from a3-ar (Fig. 4h,j). Interestingly, expression of DllGAL4 caused upregulation of dan expression in R24 discs (Fig. 4h–l), consistent with a change to eye fate. These data suggest that GAL4 expression can cause gene expression changes in the antennal disc, resulting in a subset of cells adopting eye fate.
It was unclear whether the disc region that produced the visible eye tissue in the adult constituted a classic weak point as defined in the transdetermination literature. Interaction between Wg and Dpp signaling is able to induce transdetermination at the weak points, and the weak point in the antennal disc is the region of Dpp expression on the ventral side of the disc11. To determine whether any ectopic interaction of Wg and Dpp was occurring in the DllGAL4-expressing discs, we examined Wg expression and Dpp signaling, as marked by pSMAD immunostaining. Wg and pSMAD are present in two opposing wedges that overlap at the center of a temperature-shifted control antennal disc (Fig. 4m)28. Upon DllGAL4 expression, the Wg expression domain expanded radially right up to the Dpp signaling domain at R0 (Fig. 4n), and a very slight overlap of Wg and pSMAD was seen where the two domains met (arrowhead in Fig. 4n). Subsequently, the Wg and Dpp domains separated44, and the Wg expression domain was restored to the characteristic wedge pattern by R12 (Fig. 2e). By R24, Dpp signaling had diminished and no overlap with Wg was observed except at the center (Fig. 4o). Importantly, the observed region of ectopic expression of Ey includes the portion of the disc in which Wg and Dpp overlap at R0.
Distinguishing between transdetermination and homeosis as the cause for the GAL4-induced fate changes
There are several possible ways in which the DllGAL4 line could be causing fate changes. The DllGAL4 enhancer trap is also a mutant for the Dll gene. Thus, the Dll mutation alone could cause homeotic fate changes in the antennae, although this possibility is unlikely as GAL80 suppressed the fate changes, indicating a requirement for GAL4 activity. Alternatively, GAL4 could perturb gene expression, leading to fate changes, or the cell death and damage response induced by the GAL4 expression could cause the fate changes. Finally, the combination of GAL4 expression in the Dll mutant background could be causing the aberrant cell plasticity.
To rule out the possibility that the fate changes were solely a result of a homeotic mutation in the Dll locus, we examined three other Dll mutants: Dll5 45, Dll01092 46 and Dll9 47. None of these mutant alleles showed any red-pigmented tissue in the adult antennae when heterozygous (0 in 206, 0 in 224 and 0 in 258 antennae, respectively) (Supplemental Table 1). These results indicate that homeosis alone is unable to produce the antenna-to-eye fate change in Dll heterozygotes.
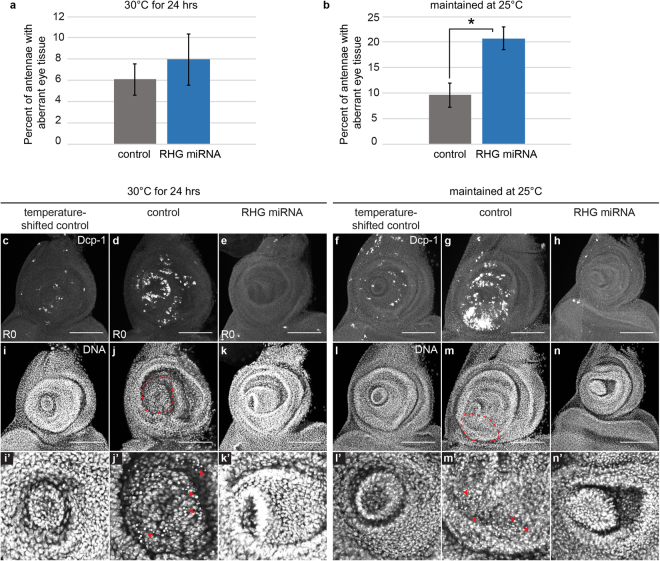
While we have shown that GAL4 activity is required for the antenna-to-eye fate changes in the DllGAL4/+ animals, it was unclear whether the fate changes were due to direct activity of GAL4 on gene expression, cellular stress caused by high expression of an exogenous protein, or a transdetermination event induced by the apoptotic cells. To determine whether the fate changes were caused by the DllGAL4-triggered apoptosis, we blocked caspase-mediated cell death by expressing a miRNA targeting reaper, hid and grim48 under DllGAL4 control either for a 24-hour period at 30 °C or by continuously maintaining the animals at 25 °C. Controls were DllGAL4 animals not expressing the miRNA. Strikingly, in both these experiments, the frequency of fate change was not decreased as would be expected if apoptosis in nearby cells or the damage response were the cause of the fate change (Fig. 5a–b). On the contrary, the fate change frequency was significantly increased upon block of apoptosis in animals always maintained at 25 °C (Fig. 5b). Absence of apoptotic cells was confirmed by immunostaining for the cleaved form of the effector caspase Dcp-1 (Fig. 5c–h). We also examined the rpr, hid, grim miRNA-expressing imaginal discs for pyknotic nuclei to rule out other forms of cell death. Our examination did not show any clear evidence of dying cells (Fig. 5i–n). These results suggest that high DllGAL4 expression might result in aberrant signaling or cellular stress independent of cell death, making this fate change different from classical transdetermination events. Interestingly, these results also suggest that caspase activation and/or apoptosis play important roles in restricting the frequency of fate changes in the antenna.
Figure 5.
Regulation of fate changes. (a and b) Frequency of red-pigmented tissue in adult antennae of animals expressing miRNAs targeting reaper, hid and grim (RHG miRNA) under DllGAL4 control, compared to controls. (a) Animals underwent the 30 °C temperature shift. 4 independent experiments, control (DllGAL4/+) n = 258 antennae, RHG miRNA (DllGAL4/UASRHG miRNA) n = 116 antennae. (b) Animals were maintained at 25 °C. 3 independent experiments, control (DllGAL4/+) n = 476 antennae, RHG miRNA (DllGAL4/UASRHG miRNA) n = 294 antennae. *p = 0.02. (c–e and i–k’) Examination of cell death by cleaved Dcp-1 immunostaining and pyknotic nuclei identified by DAPI-marked DNA in animals that underwent the 30 °C temperature shift. (i’–k’) Magnified view of (i–k). (c and i–i’) Cleaved Dcp-1 and DAPI in a temperature-shifted control disc (+; +/SM6.TM6B) at R0. (d and j–j’) Cleaved Dcp-1 and DAPI in a DllGAL4/+ disc at R0. Red outlined area (j) and arrowheads (j’) mark location of pyknotic nuclei indicating dying cells. (e and k–k’) Cleaved Dcp-1 and DAPI in a DllGAL4 disc also expressing the RHG miRNA (DllGAL4/UASRHG miRNA) at R0. Note the absence of dying cells as detected by molecular marker or by morphology. (f–h and l–n’) Examination of cell death by cleaved Dcp-1 immunostaining and TO-PRO3-marked DNA for observing pyknotic nuclei in animals that were maintained at 25 °C. All discs were dissected from third-instar larvae. (l’–n’) Magnified view of (l–n). (f and l–l’) Cleaved Dcp-1 and TO-PRO-3 in a temperature-shifted control disc. (g and m–m’) Cleaved Dcp-1 and TO-PRO-3 in a DllGAL4/+ disc. Red outlined area (m) and arrowheads (m’) mark location of pyknotic nuclei. (h and n–n’) Cleaved Dcp-1 and TO-PRO-3 in a DllGAL4 disc also expressing the RHG miRNA (DllGAL4/UASRHG miRNA). Note the absence of dying cells as detected by molecular marker or by morphology. Error bars represent SEM. Student’s T-test used for statistical analysis. Scale bars are 100 μm.
If the fate changes were a result of GAL4-induced aberrant gene expression or cellular stress, GAL4 driven by another promoter in the same domain should also produce the same phenotype. To assess this hypothesis, we used a transgenic ssGAL4, as ss is expressed from a3 to ar (Fig. 3j). This GAL4 transgene does not affect the endogenous ss gene and hence is not a mutant. We confirmed GAL4 expression by marking the ssGAL4 expression domain using UAS-EGFP in heterozygous ssGAL4 animals (Supplemental Fig. 2b). ssGAL4 expression was slightly lower than DllGAL4 expression (Supplemental Fig. 2a–c). Adult antennae of homozygous ssGAL4 animals showed defects similar to DllGAL4 animals, specifically altered morphology in the arista and the third antennal segment (Supplemental Fig. 2d–d””). Compared to wild-type controls, homozygous ssGAL4 animals showed much higher levels of cell death, which could explain the altered morphology of the adults (Supplemental Fig. 2e–f). While heterozygous ssGAL4 activity increased with temperature as observed by GFP expression (Supplemental Fig. 2g,j), there was no apparent increase in cell death (Supplemental Fig. 2h,k). Interestingly, we did not observe any red-pigmented tissue in adult antennae of homozygous ssGAL4 animals maintained at 25 °C (0 in 334 antennae) (Supplemental Table 1).
We also examined homozygous ssGAL4 antennal discs for Ey and ELAV expression to see if the cells were undergoing fate changes that were not apparent at the adult stage. No Ey or ELAV expression was observed in the antennal portion of the wild-type control discs (Supplemental Fig. 2m). By contrast, while ELAV staining was not observed, Ey expression was seen in homozygous ssGAL4 discs (Supplemental Fig. 2n). However, the Ey expression was not present near the weak point as observed for the DllGAL4 discs. These results suggest that low levels of Ey may be expressed in the homozygous ssGAL4 discs, but it not enough and not in the right place to lead to fate changes observable in the adult antenna.
It is possible that GAL4 alone may not be sufficient to drive fate changes unless it is expressed in a Dll heterozygous mutant background. To test this hypothesis, we examined adult antennae of ssGAL4 animals crossed to multiple Dll mutants. However, we did not detect any antennae with red-pigmented tissue: 0 in 290 antennae for Dll01092/+; ssGAL4/+ animals, 0 in 302 antennae for Dll9/+; ssGAL4/+ animals, and 0 in 470 antennae for Dll5/+; ssGAL4/+ animals (Supplemental Table 1). Dll5/+ animals showed antenna-to-leg transformations, and the frequency of these transformation events increased from 5.2% in Dll5/+ animals to 19.4% in Dll5/+; ssGAL4/+ animals (Supplemental Table 2). Thus, while ssGAL4 expression can enhance the homeosis observed in Dll5/+, it does not induce the eye fates observed in the DllGAL4 line. We also examined adult antennae of homozygous ssGAL4 animals crossed to multiple Dll mutants. Again, we did not observe any antennae with red-pigmented tissue: 0 in 260 antennae for Dll9/+; ssGAL4/ssGAL4 animals and 0 in 232 antennae in Dll5/+; ssGAL4/ssGAL4 animals (Supplemental Table 1). However, we did observe necrotic tissue that was present in a region similar to where the red-pigmented tissue was observed (Supplemental Fig. 2o–o’). The necrotic tissue appeared with a frequency of 3.07% in Dll9/+; ssGAL4/ssGAL4 animals and 1.29% in Dll5/+; ssGAL4/ssGAL4 animals (Supplemental Table 3). These data support our hypothesis that fate changes may be initiated in ssGAL4 animals but do not go through the full differentiation process to result in red-pigmented tissue in the adults.
Heterozygous DllGAL4 animals have slightly higher GAL4 activity as compared to heterozygous ssGAL4 animals (Supplemental Fig. 2a–c). They also have much lower levels of Dll protein as compared to controls, but Dll is still expressed in the correct domain (Supplemental Fig. 3a–c). To test whether the frequency of fate changes in the Dll heterozygous background depends on the dose of GAL4 activity, we combined the DllGAL4 and ssGAL4 constructs and quantified the frequency of pigmented eye tissue in the antennae. Surprisingly, the combination of the two GAL4s resulted in complete loss of pigmented eye tissue in the antennae (0 in 442 antennae for DllGAL4/+; ssGAL4/+ animals, 2 independent experiments) (Supplemental Table 1). The adult antennae of these animals still showed defects in the third antennal segment and the arista. We examined Dcp-1 levels in DllGAL4 and DllGAL4/+; ssGAL4/+ animals to investigate the differences in levels of cell death in these two genotypes. We found that DllGAL4/+; ssGAL4/+ antennal discs showed higher levels of cell death as compared to DllGAL4 discs (Supplemental Fig. 3d–e). Thus, it is possible that high levels of cell death caused by the extremely high GAL4 activity led to loss of the cells that would have changed fate. This finding is consistent with the observed increase in frequency of fate changes when cell death was blocked (Fig. 5), and is also consistent with a recent study describing notum-to-wing transformations where increasing apoptosis suppressed the formation of ectopic wings49.
To rule out the possibility that the fate change may be caused by the background of the DllGAL4 line (+; DllGAL4/CyO) that is maintained in the Bloomington Drosophila Stock Center, possibly due to an enhancer mutation that has arisen recently in the stock, we obtained y, w, hsFLP; DllGAL4/CyO from a different source, further altered the background by outcrossing to a w1118 line for two generations, and confirmed the antenna-to-eye fate change in that line as well. We were also unable to detect the presence of any UAS elements by PCR in our DllGAL4 stock, ruling out the possibility of contamination with an unknown transgene that induces overexpression of eye fate genes under DllGAL4 control. While it remains possible that an enhancer is present that is tightly linked to the DllGAL4, we propose that some unique combination of the strength of GAL4 expression and severity of the Dll mutation in the DllGAL4 line enable this unprecedented plasticity in the antennal disc.
Discussion
Given the results presented here, we propose a model in which the high GAL4 expression in the DllGAL4 mutant line perturbs normal gene expression and induces cell fate changes in the antennal imaginal disc. We do not yet know the mechanism through which GAL4 induces the fate change to eye tissue, but it is independent of GAL4-triggered apoptosis. Regardless, this study highlights the functional impact that GAL4 expression can have on Drosophila tissues that are being manipulated genetically, and researchers must be aware of the perturbations that GAL4 expression may cause. Interestingly, DllGAL4 is usually balanced over a CyO chromosome, which we found suppresses the frequency of the fate change (Supplemental Table 1). This balancer-induced suppression may explain why this fate change has not been previously reported. Here the fate change appears to be specific to the DllGAL4 line, in which high GAL4 expression, possibly combined with this particular Dll mutation, produces the phenotype in this unique context. It is possible that in other contexts, GAL4 expression may cause perturbations that lead to different kinds of developmental phenotypes.
The genetic utility of the GAL4/UAS system has been of immense importance in Drosophila and has been employed to control gene expression in specific spatial domains50. Since GAL4 is a yeast transcription factor, it is believed to have little or no effect on the endogenous promoters/enhancers in Drosophila. However, there have been recent reports where high GAL4 expression causes cell death and can lead to developmental defects. Increased GAL4 dosage in a subset of neurons affects neuronal physiology and behavior in the animals, which was associated with apoptotic neuronal loss in the GAL4-expressing neurons. Introduction of either chaperone proteins or inhibition of cell death, but not expression of GAL80, was able to rescue these defects, supporting the hypothesis that increased GAL4 dosage in the neurons causes protein misfolding, resulting in protein aggregates leading to cellular stress and cell death23. GMR-GAL4 in the Drosophila eye induces apoptosis and leads to the formation of irregular ommatidial arrays in a dosage- and temperature-dependent manner22. In this case, the GAL4-associated defects can be rescued by constitutive expression of GAL80, suggesting that GAL4 may be activating the misexpression of certain endogenous genes that leads to developmental defects23. These findings suggest that the effects of high GAL4 expression can, in some cases, be independent of cell death. Therefore, because high GAL4 expression can stress cells and change them, care should be taken, and proper controls included when designing Drosophila experiments.
We found that neither Dll mutations nor GAL4 expression on their own is able to transform antennal tissue to eye tissue such that red pigment is visible in the adult antenna. We found that the combination of Dll mutations with ssGAL4 expression was also unable to cause the transformation. However, we did observe ectopic Ey expression in ssGAL4 animals, suggesting perturbations in the normal development of these cells. Finally, we observed that GAL80 completely rescued the fate transformation phenotype. From this result, we can conclude that GAL4 activity is required for the antenna-to-eye transformation.
Sporadic fate changes in a small part of an imaginal disc are usually a consequence of transdetermination, which could be induced by damage or by ectopic induction of Wg signaling. Another way that fate changes can be induced is through homeotic mutations. This particular antenna-to-eye fate change may be caused by a combination of both processes, as we observed ectopic Wg expression, but the fate change was specific to the mutant DllGAL4 background. However, neither transdetermination nor homeosis on its own is able to explain the phenotype, since cell death does not contribute to this fate change, and other mutations in the Dll locus do not lead to eye tissue formation in the antennae. Furthermore, overexpression of Wg using dppGAL4 or patchedGAL4 cannot by itself cause antenna-to-eye transformations18. It is possible that high GAL4 activity perturbs the expression of genes in the eye-specification network such as eyeless and dan, and the Distal-less heterozygous mutant background sensitizes the antennae to these perturbations, allowing the fate change to eye.
The location of the transformed tissue is broadly consistent with previous literature. Classical transdetermination studies have shown that presumptive cells of the third antennal segment and the arista can change fate to other organs. The second antennal segment, which proliferates at a lower rate, rarely ever forms transdetermined structures38. Our results show that the DllGAL4-induced fate change occurred in the third antennal segment. While the classical tissue damage work by Gehring identified antennal disc transdetermination to portions of the eye disc that form head structures, it did not identify transdetermination to eye tissue marked by red pigment or photoreceptors16,38,51. To our knowledge, this study is the first report of antennal tissue transforming to pigmented eye tissue without the use of hox gene mutants or ectopic expression of eye fate genes.
The weak point in the antennal disc has been identified as the region of Dpp expression on the ventral side of the disc11 (Fig. 4h). In our experiments, the ectopic eye tissue was always seen on the posterior side of the third antennal segment in the adult animals. Using the fate map of the antennal disc developed by Haynie and Bryant, we determined that the transformation zone extends radially from the arista/third segment border to some distance in the posterior half of the third antennal segment52 (Fig. 4p). While we observed ELAV-expressing cells present in a ring pattern in the antennal imaginal disc, Ey was only primarily expressed in a region overlapping the antennal weak point. It is possible that the ELAV-expressing cells outside of the weak spot are in tissue that is not competent to form pigment cells. We also observed loss of Dac expression in this zone in the antennal imaginal disc (Fig. 3n–o). Interestingly, Dac downregulation is necessary but not sufficient for leg-to-wing transdetermination53. Thus, the observed loss of Dac may also be partly defining the transformation-competent zone in the antenna.
Our findings emphasize the need for further work to understand the circumstances under which fate changes can be induced. The antenna-to-eye fate change that we report here differs from previously reported transformations in that it is not induced by damage or homeotic mutations. We conclude that it is possible for determined cell fates to change in the absence of tissue damage but in the presence of other forms of cellular stress caused by high GAL4 activity, and that selector gene mutations sensitize the tissue to these forms of transformations.
Materials and Methods
GAL4-induced damage protocol
DllGAL4 females were crossed to w1118 males or males harboring specific mutations or transgenes. Eggs were collected at room temperature in 5-hour intervals on grape juice plates and subsequently maintained at 18 °C or 25 °C. 50 newly hatched larvae were collected into each vial 48 or 24 hours later, respectively. The vials contained food prepared according to the standard medium recipe provided by the Bloomington Drosophila Stock Center, which was churned and supplemented with yeast paste. Vials containing larvae that had been maintained at 18 °C were placed in a 30 °C circulating water bath on day 7 after egg lay. The vials were kept at 30 °C for a 24-hour period after which they were cooled in an ice-water bath for 60 seconds and returned to 18 °C. In some noted experiments, larvae were maintained constantly at 25 °C. Controls were always maintained at 18 °C. Temperature-shifted controls were the siblings of damaged animals, with the genotype w1118; +/SM6.TM6B, which were taken through the 30 °C temperature shift or were maintained at 25 °C.
Fly stocks
The following Drosophila stocks were used: Dllmd23 (called DllGAL4 in the text)20, Dll545, Dll01092 46, Dll947, w1118; P{GMR14C05-GAL4}attP2 (called ssGAL4 in the text)54, w1118 (Wild-type)55, Cyo, tubGAL8039, UASEGFP56, the AP-1 reporter TRE-red30 (a gift from Dirk Bohmann, University of Rochester), hth05745 57, salm03602 57, pucE69 29, UASRHG miRNA48 (a gift from Sarah Siegrist, University of Virginia) and y, w, hsFLP; DllGAL4/CyO (obtained from Xin Li, University of Illinois Urbana-Champaign). All fly stocks are available from the Bloomington Drosophila Stock Center unless stated otherwise.
Immunohistochemistry
Immunostaining was carried out as previously described26. Primary antibodies were rabbit anti-Cleaved Drosophila Dcp-1 (1:250) (Cell Signaling), mouse anti-Wingless (1:100) (The Developmental Studies Hybridoma Bank [DSHB]), rabbit anti-dMyc (1:500) (Santa Cruz Biotechnologies), mouse anti-βgal (1:100) (DSHB), mouse anti-Cut (1:10) (DSHB), mouse anti-Dac (1:5) (DSHB), guinea pig anti-Spineless (1:200) (a gift from Michael Kim, University of Miami), rat anti-ELAV (1:30) (DSHB), mouse anti-Ey (1:100) (DSHB), rat anti-Dan41 (1:200) (obtained from Xin Li, University of Illinois Urbana-Champaign), rabbit anti-phospho-Mad (1:100) (Cell Signaling), rabbit anti-Distal-less (1:200) (a gift from Sean Carroll, University of Wisconsin-Madison). The Developmental Studies Hybridoma Bank (DSHB) was created by the NICHD of the NIH and is maintained at the University of Iowa, Department of Biology, Iowa City, IA 52242.
Secondary antibodies were AlexaFluor probes (1:1000) (Life Technologies). DNA was marked using TO-PRO3 (1:500) (Life Technologies) or DAPI (1:5000 of 0.5 mg/mL stock) (Sigma). Discs were mounted in Vectashield mounting medium (Vector Laboratories).
EdU labeling was carried out as previously described58 using the Click-It EdU kit (Life Technologies). Tissue was incubated in EdU for 20 minutes at room temperature.
Discs were imaged on a Zeiss LSM 510 or a Zeiss LSM 700 confocal microscope. Parameters for imaging as well as brightness and contrast settings were set to optimize for expression and location rather than for quantitative comparison unless stated specifically. Images were processed using ZEN lite (Zeiss), ImageJ (NIH) and Photoshop (Adobe).
Adult antenna microscopy
Adult antennae were mounted in Gary’s Magic Mount (Canada balsam [Sigma] dissolved in methyl salicylate [Sigma]). Images were taken with an Olympus SZX10 microscope and a Leica DM RXA2 microscope with an Olympus DP21 camera using the CellSens Dimension software (Olympus).
Data availability
The data and images generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
The authors would like to thank L. Setiawan for help with translating articles that were published in German; A. R. Brock and S. J. Khan for critical reading of the manuscript and helpful discussions; D. Bohmann, S. Siegrist, M. Kim, X. Li, S. Carroll, the Bloomington Drosophila Stock Center (NIH P40OD018537) and the Developmental Studies Hybridoma Bank for reagents. This work was supported by a Young Investigator Award from the Roy J. Carver Charitable Trust (#12-4041) and a grant from the NIH (NIGMS R01GM107140).
Author Contributions
S.N.F.A. and R.K.S.-B. designed and interpreted the experiments. S.N.F.A. carried out all experiments. S.N.F.A. and R.K.S.-B. wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-23093-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lawrence PA, Morata G. Homeobox genes: their function in Drosophila segmentation and pattern formation. Cell. 1994;78:181–189. doi: 10.1016/0092-8674(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 2.Simcox AA, Sang JH. When does determination occur in Drosophila embryos? Dev. Biol. 1983;97:212–221. doi: 10.1016/0012-1606(83)90078-7. [DOI] [PubMed] [Google Scholar]
- 3.Meise M, Janning W. Cell lineage of larval and imaginal thoracic anlagen cells of Drosophila melanogaster, as revealed by single-cell transplantations. Development. 1993;118:1107–1121. [Google Scholar]
- 4.Fuse N, Hirose S, Hayashi S. Determination of wing cell fate by the escargot and snail genes in Drosophila. Development. 1996;122:1059–1067. doi: 10.1242/dev.122.4.1059. [DOI] [PubMed] [Google Scholar]
- 5.Kauffman, S. A. Pattern formation in the Drosophila embryo. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 567–594 (1981). [DOI] [PubMed]
- 6.Hadorn E. Differenzierungsleistungen wiederholt fragmentierter Teilstücke männlicher Genitalscheiben von Drosophila melanogaster nach Kultur in vivo. Dev. Biol. 1963;7:617–629. doi: 10.1016/0012-1606(63)90146-5. [DOI] [Google Scholar]
- 7.Schubiger G. Regeneration, duplication and transdetermination in fragments of the leg disc of Drosophila melanogaster. Dev. Biol. 1971;26:277–295. doi: 10.1016/0012-1606(71)90127-8. [DOI] [PubMed] [Google Scholar]
- 8.Hadorn E. Problems of determination and transdetermination. Brookhaven Symp. Biol. 1965;18:148–161. [Google Scholar]
- 9.Maves L, Schubiger G. Wingless induces transdetermination in developing Drosophila imaginal discs. Development. 1995;121:1263–1272. doi: 10.1242/dev.121.5.1263. [DOI] [PubMed] [Google Scholar]
- 10.Maves L, Schubiger G. A molecular basis for transdetermination in Drosophila imaginal discs: interactions between wingless and decapentaplegic signaling. Development. 1998;125:115–124. doi: 10.1242/dev.125.1.115. [DOI] [PubMed] [Google Scholar]
- 11.Maves L, Schubiger G. Transdetermination in Drosophila imaginal discs: a model for understanding pluripotency and selector gene maintenance. Curr. Opin. Genet. Dev. 2003;13:472–479. doi: 10.1016/j.gde.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Bosch M, Serras F, Martín-Blanco E, Baguñà J. JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev. Biol. 2005;280:73–86. doi: 10.1016/j.ydbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Bergantinos C, Corominas M, Serras F. Cell death-induced regeneration in wing imaginal discs requires JNK signalling. Development. 2010;137:1169–1179. doi: 10.1242/dev.045559. [DOI] [PubMed] [Google Scholar]
- 14.Lee N, Maurange C, Ringrose L, Paro R. Suppression of Polycomb group proteins by JNK signalling induces transdetermination in Drosophila imaginal discs. Nature. 2005;438:234–237. doi: 10.1038/nature04120. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson J. A major difference bwteen transdetermination and homeosis. Nature. 1979;279:426–428. doi: 10.1038/279426a0. [DOI] [PubMed] [Google Scholar]
- 16.Schmid H. Transdetermination in the homeotic eye-antenna imaginal disc of Drosophila melanogaster. Dev. Biol. 1985;107:28–37. doi: 10.1016/0012-1606(85)90372-0. [DOI] [PubMed] [Google Scholar]
- 17.Kauffman SA. Control Circuits for Determination and Transdetermination. Science. 1973;181:310–318. doi: 10.1126/science.181.4097.310. [DOI] [PubMed] [Google Scholar]
- 18.Johnston LA, Schubiger G. Ectopic expression of wingless in imaginal discs interferes with decapentaplegic expression and alters cell determination. Development. 1996;122:3519–3529. doi: 10.1242/dev.122.11.3519. [DOI] [PubMed] [Google Scholar]
- 19.McClure KD, Schubiger G. Transdetermination: Drosophila imaginal disc cells exhibit stem cell-like potency. Int. J. Biochem. Cell Biol. 2007;39:1105–1118. doi: 10.1016/j.biocel.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calleja M, Moreno E, Pelaz S, Morata G. Visualization of Gene Expression in Living Adult Drosophila. Science. 1996;274:252–255. doi: 10.1126/science.274.5285.252. [DOI] [PubMed] [Google Scholar]
- 21.Brand, A. H., Manoukian, A. S. & Perrimon, N. Chapter 33 Ectopic Expression in Drosophila. in Methods in Cell Biology (eds Goldstein, L. S. B. & Fyrberg, E. A.) 44, 635–654 (Academic Press, 1994). [DOI] [PubMed]
- 22.Kramer JM, Staveley BE. GAL4 causes developmental defects and apoptosis when expressed in the developing eye of Drosophila melanogaster. Genet Mol Res. 2003;2:43–47. [PubMed] [Google Scholar]
- 23.Rezával C, Werbajh S, Ceriani MF. Neuronal death in Drosophila triggered by GAL4 accumulation: Neuronal death by protein overload. Eur. J. Neurosci. 2007;25:683–694. doi: 10.1111/j.1460-9568.2007.05317.x. [DOI] [PubMed] [Google Scholar]
- 24.Song Z, McCall K, Steller H. DCP-1, a Drosophila Cell Death Protease Essential for Development. Science. 1997;275:536–540. doi: 10.1126/science.275.5299.536. [DOI] [PubMed] [Google Scholar]
- 25.Schubiger M, Sustar A, Schubiger G. Regeneration and transdetermination: The role of wingless and its regulation. Dev. Biol. 2010;347:315–324. doi: 10.1016/j.ydbio.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith-Bolton RK, Worley MI, Kanda H, Hariharan IK. Regenerative Growth in Drosophila Imaginal Discs Is Regulated by Wingless and Myc. Dev. Cell. 2009;16:797–809. doi: 10.1016/j.devcel.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryoo HD, Gorenc T, Steller H. Apoptotic Cells Can Induce Compensatory Cell Proliferation through the JNK and the Wingless Signaling Pathways. Dev. Cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Lebreton G, Faucher C, Cribbs DL, Benassayag C. Timing of Wingless signalling distinguishes maxillary and antennal identities in Drosophila melanogaster. Development. 2008;135:2301–2309. doi: 10.1242/dev.017053. [DOI] [PubMed] [Google Scholar]
- 29.Martín-Blanco E, et al. Puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee N, Bohmann D. A Versatile ΦC31 Based Reporter System for Measuring AP-1 and Nrf2 Signaling in Drosophila and in Tissue Culture. PloS One. 2012;7:e34063. doi: 10.1371/journal.pone.0034063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong PD, Chu J, Panganiban G. Coexpression of the homeobox genes Distal-less and homothorax determines Drosophila antennal identity. Development. 2000;127:209–216. doi: 10.1242/dev.127.2.209. [DOI] [PubMed] [Google Scholar]
- 32.Dong PS, Dicks JS, Panganiban G. Distal-less and homothorax regulate multiple targets to pattern the Drosophila antenna. Development. 2002;129:1967–1974. doi: 10.1242/dev.129.8.1967. [DOI] [PubMed] [Google Scholar]
- 33.Barrio R, de Celis JF, Bolshakov S, Kafatos FC. Identification of Regulatory Regions Driving the Expression of the Drosophila spalt Complex at Different Developmental Stages. Dev. Biol. 1999;215:33–47. doi: 10.1006/dbio.1999.9434. [DOI] [PubMed] [Google Scholar]
- 34.Duncan DM, Burgess EA, Duncan I. Control of distal antennal identity and tarsal development inDrosophila by spineless–aristapedia, a homolog of the mammalian dioxin receptor. Genes Dev. 1998;12:1290–1303. doi: 10.1101/gad.12.9.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong PS, Chu J, Panganiban G. Proximodistal domain specification and interactions in developing Drosophila appendages. Development. 2001;128:2365–2372. doi: 10.1242/dev.128.12.2365. [DOI] [PubMed] [Google Scholar]
- 36.Hannah-Alava A. Morphology and chaetotaxy of the legs of Drosophila melanogaster. J. Morphol. 1958;103:281–310. doi: 10.1002/jmor.1051030205. [DOI] [Google Scholar]
- 37.Bryant PJ. Pattern formation in the imaginal wing disc of Drosophila melanogaster: fate map, regeneration and duplication. J. Exp. Zool. 1975;193:49–77. doi: 10.1002/jez.1401930106. [DOI] [PubMed] [Google Scholar]
- 38.Gehring W. Clonal analysis of determination dynamics in cultures of imaginal disks in Drosophila melanogaster. Dev. Biol. 1967;16:438–456. doi: 10.1016/0012-1606(67)90058-9. [DOI] [PubMed] [Google Scholar]
- 39.Vef O, Cleppien D, Löffler T, Altenhein B, Technau GM. A new strategy for efficient in vivo screening of mutagenized Drosophila embryos. Dev. Genes Evol. 2006;216:105–108. doi: 10.1007/s00427-005-0036-5. [DOI] [PubMed] [Google Scholar]
- 40.Halder, G., Callaerts, P., Gehring, W. J. & others. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Sci.-N. Y. Then Wash.- 1788–1788 (1995). [DOI] [PubMed]
- 41.Emerald BS. Distal antenna and distal antenna related encode nuclear proteins containing pipsqueak motifs involved in antenna development in Drosophila. Development. 2003;130:1171–1180. doi: 10.1242/dev.00323. [DOI] [PubMed] [Google Scholar]
- 42.Suzanne M, Estella C, Calleja M, Sánchez-Herrero E. The hernandez and fernandez genes of Drosophila specify eye and antenna. Dev. Biol. 2003;260:465–483. doi: 10.1016/S0012-1606(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 43.Curtiss J, Burnett M, Mlodzik M. distal antenna and distal antenna-related function in the retinal determination network during eye development in Drosophila. Dev. Biol. 2007;306:685–702. doi: 10.1016/j.ydbio.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Domínguez M, Casares F. Organ specification-growth control connection: New in- sights from the Drosophila eye-antennal disc: Drosophila Eye-Antennal Disc. Dev. Dyn. 2005;232:673–684. doi: 10.1002/dvdy.20311. [DOI] [PubMed] [Google Scholar]
- 45.Sunkel CE, Whittle JRS. Brista: a gene involved in the specification and differentiation of distal cephalic and thoracic structures in Drosophila melanogaster. Dev. Genes Evol. 1987;196:124–132. doi: 10.1007/BF00402034. [DOI] [PubMed] [Google Scholar]
- 46.Goto S, Hayashi S. Specification of the embryonic limb primordium by graded activity of Decapentaplegic. Development. 1997;124:125–132. doi: 10.1242/dev.124.1.125. [DOI] [PubMed] [Google Scholar]
- 47.Sato T. A new homoeotic mutation affecting antennae and legs. Drosoph. Inf. Serv. 1984;60:180–182. [Google Scholar]
- 48.Siegrist SE, Haque NS, Chen C-H, Hay BA, Hariharan IK. Inactivation of Both foxo and reaper Promotes Long-Term Adult Neurogenesis in Drosophila. Curr. Biol. 2010;20:643–648. doi: 10.1016/j.cub.2010.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Worley MI, Alexander LA, Hariharan IK. CtBP impedes JNK- and Upd/STAT-driven cell fate misspecifications in regenerating Drosophila imaginal discs. eLife. 2018;7:e30391. doi: 10.7554/eLife.30391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 51.Gehring W. Übertragung und Änderung der Determinations qualitäten in Antennenscheiben-Kulturen von Drosophila melanogaster. Development. 1966;15:77–111. [PubMed] [Google Scholar]
- 52.Haynie JL, Bryant PJ. Development of the eye-antenna imaginal disc and morphogenesis of the adult head in Drosophila melanogaster. J. Exp. Zool. 1986;237:293–308. doi: 10.1002/jez.1402370302. [DOI] [PubMed] [Google Scholar]
- 53.Ing T, Tseng A, Sustar A, Schubiger G. Sp1 modifies leg-to-wing transdetermination in Drosophila. Dev. Biol. 2013;373:290–299. doi: 10.1016/j.ydbio.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfeiffer BD, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hazelrigg T, Levis R, Rubin GM. Transformation of white locus DNA in Drosophila: Dosage compensation, zeste interaction, and position effects. Cell. 1984;36:469–481. doi: 10.1016/0092-8674(84)90240-X. [DOI] [PubMed] [Google Scholar]
- 56.Halfon MS, et al. New fluorescent protein reporters for use with the drosophila gal4 expression system and for vital detection of balancer chromosomes. genesis. 2002;34:135–138. doi: 10.1002/gene.10136. [DOI] [PubMed] [Google Scholar]
- 57.Spradling AC, et al. The Berkeley Drosophila Genome Project gene disruption project: Single P-element insertions mutating 25% of vital Drosophila genes. Genetics. 1999;153:135–177. doi: 10.1093/genetics/153.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gouge, C. A. & Christensen, T. W. Detection of S Phase in multiple Drosophila tissues utilizing the EdU labeling technique. Dros. Inf. Serv. 93, 203–212 (2010).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and images generated during and/or analysed during the current study are available from the corresponding author on reasonable request.