Abstract
Purpose
Positron emission tomography (PET) in non-human primates (NHP) is commonly performed under anesthesia, with sevoflurane being a widely used inhaled anesthetic. PET measurement in NHP can be repeated, and a difference in radioligand kinetics has previously been observed between the first and second PET measurement on the same day using sevoflurane anesthesia. In this study, we evaluated the effect of prolonged sevoflurane anesthesia on kinetics and binding potential (BPND) of [11C]raclopride in NHP.
Procedures
Three cynomolgus monkeys underwent two to three PET measurements with [11C]raclopride under continuous sevoflurane anesthesia on the same day. The concentration of sevoflurane was adjusted according to the general conditions and safety parameters of the NHP. Time to peak (TTP) radioactivity in the striatum was estimated from time-activity curves (TACs). The BPND in the striatum was calculated by the simplified reference tissue model using the cerebellum as reference region.
Results
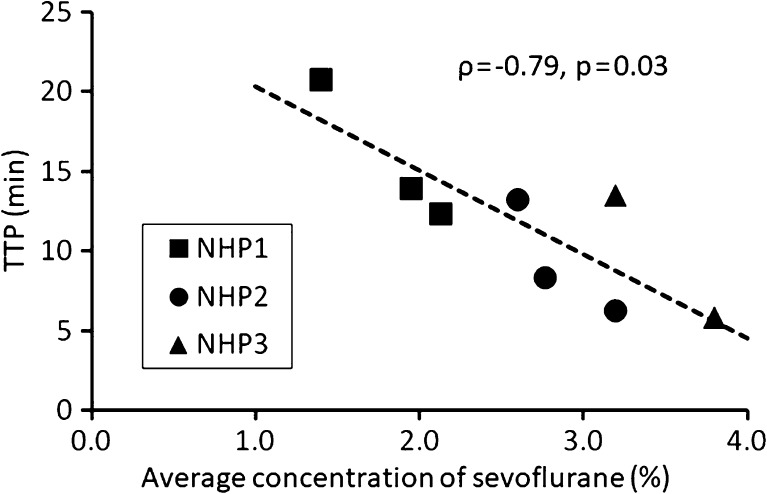
In each NHP, the TTP became shorter in the later PET measurements than in the first one. Across all measurements (n = 8), concentration of sevoflurane correlated with TTP (Spearman’s ρ = − 0.79, p = 0.03), but not with BPND (ρ = − 0.25, p = 0.55).
Conclusions
These data suggest that sevoflurane affects the shape of TACs but has no evident effect on BPND in consecutive PET measurements.
Key words: [11C]Raclopride, Binding potential, Positron emission tomography, Sevoflurane, Time-activity curve, Time to peak
Introduction
Positron emission tomography (PET) is widely used for examination of radioligand binding to receptors, enzymes, and transporters in the non-human primate (NHP) and human brain in vivo [1, 2]. A common approach is to use PET studies in NHP for the development of new radioligands, i.e., to assess whether the radioligand provides sufficient brain exposure and appropriate kinetic behavior. Another common approach is to use NHP to facilitate drug development by demonstration of brain exposure by microdosing or occupancy at the intended drug target.
PET studies in NHP are most often performed under anesthesia to maintain the position of the head during time of data acquisition. Inhalational anesthesia such as sevoflurane, isoflurane, and halothane is the most often used method for NHP study. Especially sevoflurane is currently widely used.
PET measurement in NHP can typically be performed up to three times in one experimental day [3–5]. Such series of measurements allows for estimation of the blocking or displacement effect of drugs, release of neurotransmitters in different conditions, or assessment of the test-retest reproducibility of a PET radioligand, in a single day. For optimal comparisons, experimental conditions should be identical among serial PET measurements. However, the concentration of anesthesia is practically adjusted to the condition of the individual NHP, and may consequently vary over time.
Importantly, in several studies using sevoflurane anesthesia, a difference in radioligand kinetics has been observed between the first and second PET measurement on the same day. For example, [11C]calbonyl-raclopride and [11C]methyl-raclopride, which are the same structure except radio-labeling position, showed different brain kinetics with the shift of time to peak (TTP) between first and second PET [6]. Other studies showed the different brain kinetics in initial part between first (for baseline) and second (for displacement) PET measurements of [11C]AZ10419369 for serotonin 1B receptor [7] and [11C]T-773 for phosphodiesterase 10A (PDE10A) [8]. Two PET measurements of these studies were conducted serially in one day. Whereas an effect of sevoflurane anesthesia on cerebral blood flow [9–12], cerebral vasodilation [13], and brain metabolic rate [10, 12] has been reported, the effect of sevoflurane anesthesia on radioligand kinetics has not been investigated in detail.
In the present study, we evaluated the change of brain kinetics of [11C]raclopride, especially the shift of TTP, in relation to the concentration or duration of sevoflurane using repeated PET measurements in NHPs on the same day. In addition to the change of brain kinetics, we also estimated how altered brain kinetics may propagate into changes in the binding potential (BPND) for [11C]raclopride in the striatum.
Methods
Subjects
Three cynomolgus monkeys (three females, body weight 4450, 4625, and 6065 g) were included in this study. The NHPs were housed in the Astrid Fagraeus Laboratory (AFL) of the Swedish Institute for Infectious Disease Control, Solna, Sweden. The study was approved by the Animal Ethics Committee of the Swedish Animal Welfare Agency and was performed according to “Guidelines for planning, conducting and documenting experimental research” (Dnr 4820/06-600) of Karolinska Institutet.
PET Measurements
Anesthesia was induced by intramuscular injection of ketamine hydrochloride (approximately 10 mg/kg) at AFL, and then NHPs were transported to the Karolinska Institutet PET center. Inhalation anesthesia by administration of a mixture of sevoflurane, oxygen, and medical air started 40 min after ketamine injection and maintained with endotracheal intubation. The concentration of sevoflurane was adjusted by certified registered nurse anesthetists (CRNAs) according to the general condition of the NHP, mainly heart rate and blood pressure. The concentration of sevoflurane was recorded throughout the experiments.
The head was immobilized with a fixation device. Body temperature was maintained by a Bair Hugger model 505 (Arizant Healthcare, MN, USA) and monitored by an esophageal thermometer. ECG, heart rate, blood pressure, respiratory rate, and oxygen saturation were continuously monitored throughout the experiments. Fluid balance was maintained by a continuous infusion of saline.
PET measurements were conducted using the High Resolution Research Tomograph (HRRT) (Siemens Molecular Imaging, TN, USA) [14]. A transmission scan of 6 min using a single 137Cs source was performed before the emission scan. List mode data were acquired continuously for 63 min immediately after intravenous injection of [11C]raclopride, which was prepared by reported method [15]. The injected radioactivity (n = 8) was 153.1 ± 6.7 (mean ± SD) (range 146–165) MBq. NHP1 and NHP2 were examined three times, and NHP3 was examined twice in one experimental day.
Data Analysis
The regions of interest (ROIs) were delineated manually on the MRI images of each NHP for striatum (combination of caudate and putamen) and cerebellum. The summed PET images of whole scanning were co-registered to the MRI image of the individual NHP. After applying the co-registration parameters to the dynamic PET data, the time-activity curves (TAC) of brain regions were generated for each PET measurement. BPND and R1 of the striatum were calculated by simplified reference tissue model (SRTM) [16] using the cerebellum as a reference region. The analyses of imaging data were performed using PMOD (version 3.6; PMOD Technologies, Zurich, Switzerland). To measure TTP of TAC in the striatum and cerebellum, a rational polynomial model was applied for noise reduction using MATLAB2013 (The Mathworks, MA, USA).
Statistics
The relation between the concentration of sevoflurane and TTP of TAC in the striatum or cerebellum was assessed by Spearman’s rank correlation coefficient. The average concentration from − 15 to + 15 min of radioligand injection was used for the analysis. The relation between the concentration of sevoflurane and BPND or R1 in the striatum was also assessed by Spearman’s rank correlation coefficient. Additionally, the relation between the duration of sevoflurane anesthesia and TTP in the striatum or cerebellum, BPND or R1 was assessed by Spearman’s rank correlation coefficient. In all tests, a p value of < 0.05 (two-tailed) was considered statistically significant.
Results
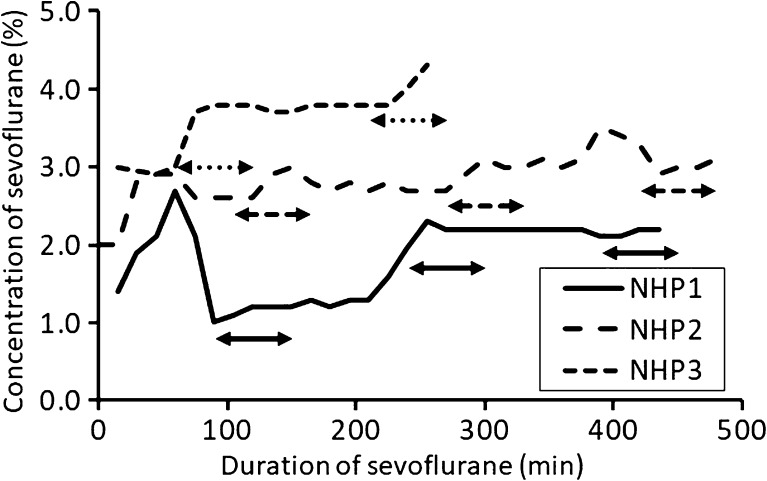
All PET measurements were run according to the protocol. The concentration and duration of sevoflurane anesthesia, TTP of the TAC in the striatum and cerebellum, and BPND and R1 for all measurements are shown in Table 1. The concentration of sevoflurane increased with time (Fig. 1).
Table 1.
The average concentration and duration of sevoflurane anesthesia, time to peak (TTP) of TAC in the striatum and cerebellum, and BPND and R1in the striatum
| PET1 | PET2 | PET3 | ||
|---|---|---|---|---|
| NHP1 | Conc | 1.40 | 1.95 | 2.13 |
| Dur | 111 | 254 | 400 | |
| TTP_str | 20.7 | 13.9 | 12.3 | |
| TTP_cer | 2.1 | 1.4 | 1.6 | |
| BPND | 4.56 | 4.28 | 4.59 | |
| R1 | 0.96 | 1.09 | 1.05 | |
| NHP2 | Conc | 2.60 | 2.77 | 3.20 |
| Dur | 120 | 286 | 437 | |
| TTP_str | 13.2 | 8.3 | 6.2 | |
| TTP_cer | 1.6 | 1.7 | 1.3 | |
| BPND | 4.55 | 4.56 | 4.93 | |
| R1 | 0.99 | 1.09 | 1.30 | |
| NHP3 | Conc | 3.20 | 3.80 | |
| Dur | 73 | 218 | ||
| TTP_str | 13.5 | 5.8 | ||
| TTP_cer | 1.3 | 1.3 | ||
| BPND | 4.54 | 4.09 | ||
| R1 | 0.85 | 0.98 | ||
Conc, average concentration of sevoflurane anesthesia (%); Dur, duration of sevoflurane anesthesia (min); TTP_str, time to peak (TTP) of TAC in the striatum (min); TTP_cer, time to peak (TTP) of TAC in the cerebellum (min); BP ND, binding potential in the striatum; R 1, R1 value in the striatum
Fig. 1.
The time-courses of concentration of sevoflurane anesthesia in three NHPs. The arrows indicate the period of PET measurement.
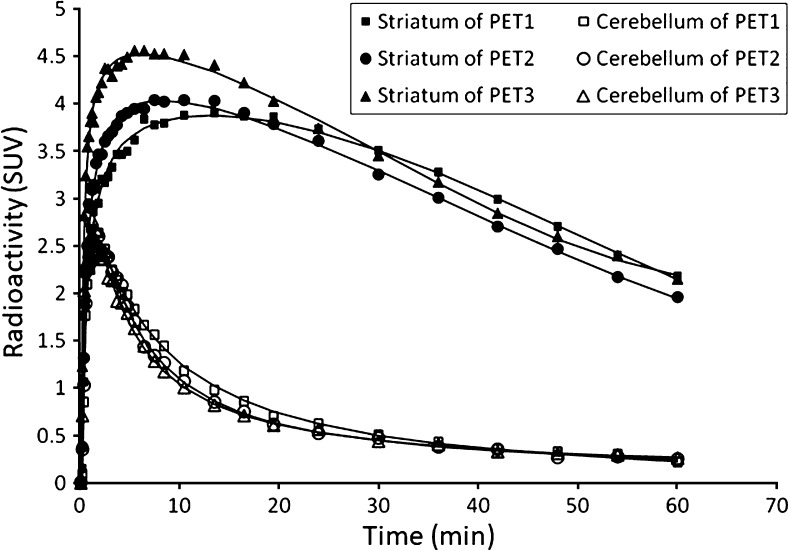
In all three NHPs, the TTP in the striatum became shorter in the later PET measurements than in the first one (Table 1, Fig. 2).When data for all three NHPs were combined, there was a statistically significant correlation (Spearman’s ρ = − 0.79, p = 0.03) between the concentration of sevoflurane and TTP of TAC in the striatum (Fig. 3). The correlation between the duration of sevoflurane anesthesia and TTP of TAC in the striatum was negative but not significant (ρ = − 0.57, p = 0.15). For TTP in the cerebellum, significant correlation was obtained for concentration (ρ = − 0.75, p = 0.04), but not duration (ρ = − 0.07, p = 0.87).
Fig. 2.
Typical time-activity curves (TACs) of NHP2.
Fig. 3.
The relation between the average concentration of sevoflurane anesthesia and time to peak (TTP) of TAC in the striatum.
There was no obvious intra-individual difference between the BPND values obtained in each NHP. Moreover, there were no significant correlations between BPND and concentration (ρ = − 0.25, p = 0.55) or duration (ρ = 0.52, p = 0.20) of sevoflurane anesthesia. For R1, significant correlation was obtained for duration (ρ = 0.88, p = 0.01), but not concentration (ρ = − 0.06, p = 0.90).
Discussion
In the present study, there was a significant negative correlation between the concentration of sevoflurane administered by inhalation and TTP of the brain TAC of [11C]raclopride. However, there was no significant correlation between the concentration of sevoflurane and binding potential of [11C]raclopride in the striatum.
The pharmacological characteristics of sevoflurane, such as the binding profile, are poorly described in the literature primarily since the binding of gases cannot be characterized in test tube experiments. Though the mechanism of action for sevoflurane is unclear, some functional effects may deserve attention. A change of cerebral blood flow (CBF) could be a possible reason for a change of brain kinetics, since the kinetic parameters across the blood-brain barrier, such as K1 in a compartment model, are dependent of CBF. In previous human studies, it has been reported that sevoflurane at about 1.0 minimum alveolar concentration (MAC) has an effect on CBF. However, the results are not consistent across the literature [9–13]. When the concentration of sevoflurane was higher than 1.5 MAC (approximately above 2 % inhaled), two studies [10, 13] have shown that sevoflurane increases CBF whereas another study [9] reported that sevoflurane decreases CBF. It is anyhow plausible that the relatively high concentration of sevoflurane in the present study may increase CBF, and induce the shift of TTP in radioligand kinetics.
Another factor which may change radioligand kinetics and the rate constant K1 is the blood-brain barrier (BBB) permeability. Some studies have indicated that sevoflurane has an effect on vascular endothelial cells thereby increasing brain permeability [17, 18]. Though this effect remains to be confirmed in intact tissue, it cannot be excluded that the more rapid brain kinetics observed at higher sevoflurane concentrations to some degree may be explained by a change in BBB permeability.
Despite the change in radioligand kinetics, there was no significant difference between the calculated BPND values of [11C]raclopride in consecutive PET measurements. Additionally, the BPND value did not correlate with the concentration of sevoflurane. TTP in the cerebellum became shorter in the latter PET measurements as well as the striatum. This shortening was correlated with sevoflurane concentration. The results suggested that the non-displaceable binding changed by anesthesia since cerebellum is devoid of specific binding. This change might contribute the change of the shape of TAC in the striatum. A small change of BPND induced by sevoflurane can anyhow not be excluded. [11C]Raclopride is sensitive to changes of endogenous dopamine, and sevoflurane has been reported to increase dopamine release in a microdialysis study in rodents [19]. However, the effect on dopamine release by sevoflurane (130–150 % of baseline) was much lower than the effect of methamphetamine (over 2000 % of baseline). Since it has been estimated that a 40 % increase in dopamine corresponds to a 1 % reduction of [11C]raclopride BPND [20, 21], a hypothetical change of BPND by sevoflurane should likely be negligible.
Some articles have reported regional difference in the effect of sevoflurane on CBF [9, 11]. The present study showed a significant correlation between duration of sevoflurane and R1. However, there was no significant correlation between concentration of sevoflurane and R1. These observations suggest that the prolonged effect of sevoflurane was greater in the striatum than in the cerebellum, resulting in increasing R1.
We used ketamine intramuscularly to induce anesthesia for the safety of animals. The decreasing concentration of ketamine could be a reason why we had to increase the sevoflurane concentration during the experimental session. In addition, ketamine might be another factor explaining the change of radioligand kinetics by having an effect on CBF [22]. A direct effect of ketamine on the dopamine system should also be considered. Some articles have reported that ketamine induces a decrease in [11C]raclopride binding [23, 24]. However, PET measurements in those studies started close to the time of ketamine infusion (just after or during ketamine infusion). This is different from the present study where PET started 2 h or more after ketamine administration. To rule out an effect of ketamine strictly, a study without initial administration of ketamine will be needed.
Besides anesthesia, a diurnal effect is another possible factor that might influence [11C]raclopride binding since several studies have suggested that the dopamine system may be affected by the circadian rhythm [25–27]. However, the interval between two PET measurements in this study was relatively short (2–3 h), compared to the interval in studies demonstrating a circadian rhythm, typically between morning and evening. Sevoflurane anesthesia is thus the most likely factor causing the demonstrated change of radioligand kinetics.
The present study suggests that these differences of radioligand kinetics were related to sevoflurane, and not caused by a change of specific binding. This means that NHP PET study with sevoflurane anesthesia is an appropriate method to evaluate the specific binding of radioligands. Ideally, the concentration of sevoflurane should be maintained throughout the experimental session. However, this maintenance of concentration is not always feasible since anesthesia levels have to be adjusted to the condition of the NHP. In studies where the changes of outcome measures are expected to be small, e.g., occupancy study of agonist compound [28], it may be worth to increase the number of measurements to strengthen the statistical power. Moreover, if the concentration of sevoflurane changed drastically during the PET measurement, the result should be interpreted with caution.
In this study, neither [15O]H2O PET to measure the CBF nor arterial blood sampling to obtain an input function for estimation of the kinetic parameters including K1 was performed. The relation between sevoflurane concentration and CBF or K1 could accordingly not be evaluated. These issues should be solved in a future study.
In conclusion, we found a negative correlation between TTP in the kinetics of [11C]raclopride and the concentration of sevoflurane. However, this effect is not to be viewed as a major confounder since it did not propagate to a significant effect on the calculated BPND values. In NHP PET study with sevoflurane anesthesia, the concentration of sevoflurane should be maintained as much as possible in particular if small changes in radioligand binding are expected.
Acknowledgements
We thank all members of the Karolinska Insitutet PET Center for excellent assistance with the PET experiments. The contribution of IY was supported by MindView (Multimodal Imaging of Neurological Disorders; project reference 603002).
Compliance with Ethical Standards
The study was approved by the Animal Ethics Committee of the Swedish Animal Welfare Agency and was performed according to “Guidelines for planning, conducting and documenting experimental research” (Dnr 4820/06-600) of Karolinska Institutet.
Conflict of Interest
Lars Farde is employed by AstraZeneca and affiliated with Karolinska Insitutet. All other authors declare that they have no conflict of interest.
References
- 1.Halldin C, Gulyás B, Farde L. PET studies with carbon-11 radioligands in neuropsychopharmacological drug development. Curr Pharm Des. 2001;7:1907–1929. doi: 10.2174/1381612013396871. [DOI] [PubMed] [Google Scholar]
- 2.Takano A, Varrone A, Gulyás B, et al. Guidelines to PET measurements of the target occupancy in the brain for drug development. Eur J Nucl Med Mol Imaging. 2016;43:2255–2262. doi: 10.1007/s00259-016-3476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finnema SJ, Stepanov V, Ettrup A, et al. Characterization of [11C]Cimbi-36 as an agonist PET radioligand for the 5-HT2A and 5-HT2C receptors in the nonhuman primate brain. NeuroImage. 2014;84:342–353. doi: 10.1016/j.neuroimage.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 4.Finnema SJ, Hughes ZA, Haaparanta-Solin M, et al. Amphetamine decreases α2C-adrenoceptor binding of [11C]ORM-13070: a PET study in the primate brain. Int J Neuropsychopharmacol. 2015;18:pyu081. doi: 10.1093/ijnp/pyu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finnema SJ, Halldin C, Bang-Andersen B, et al. Serotonin transporter occupancy by escitalopram and citalopram in the non-human primate brain: a [11C]MADAM PET study. Psychopharmacology. 2015;232:4159–4167. doi: 10.1007/s00213-015-3961-7. [DOI] [PubMed] [Google Scholar]
- 6.Rahman O, Takano A, Amini N, et al. Synthesis of ([11C]carbonyl)raclopride and a comparison with ([11C]methyl)raclopride in a monkey PET study. Nucl Med Biol. 2015;42:893–898. doi: 10.1016/j.nucmedbio.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Finnema SJ, Varrone A, Hwang T-J, et al. Confirmation of fenfluramine effect on 5-HT(1B) receptor binding of [11C]AZ10419369 using an equilibrium approach. J Cereb Blood Flow Metab. 2012;32:685–695. doi: 10.1038/jcbfm.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takano A, Stepanov V, Gulyás B, et al. Evaluation of a novel PDE10A PET radioligand, [11C]T-773, in nonhuman primates: brain and whole body PET and brain autoradiography. Synapse. 2015;69:345–355. doi: 10.1002/syn.21821. [DOI] [PubMed] [Google Scholar]
- 9.Kaisti KK, Metsähonkala L, Teräs M, et al. Effects of surgical levels of propofol and sevoflurane anesthesia on cerebral blood flow in healthy subjects studied with positron emission tomography. Anesthesiology. 2002;96:1358–1370. doi: 10.1097/00000542-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Kuroda Y, Murakami M, Tsuruta J, et al. Preservation of the ration of cerebral blood flow/metabolic rate for oxygen during prolonged anesthesia with isoflurane, sevoflurane, and halothane in humans. Anesthesiology. 1996;84:555–561. doi: 10.1097/00000542-199603000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Schlünzen L, Vafaee MS, Cold GE, et al. Effects of subanaesthetic and anaesthetic doses of sevoflurane on regional cerebral blood flow in healthy volunteers. A positron emission tomographic study. Acta Anaesthesiol Scand. 2004;48:1268–1276. doi: 10.1111/j.1399-6576.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- 12.Mielck F, Stephan H, Weyland A, Sonntag H. Effects of one minimum alveolar anesthetic concentration sevoflurane on cerebral metabolism, blood flow, and CO2 reactivity in cardiac patients. Anesth Analg. 1999;89:364–369. doi: 10.1097/00000539-199908000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Matta BF, Heath KJ, Tipping K, Summors AC. Direct cerebral vasodilatory effects of sevoflurane and isoflurane. Anesthesiology. 1999;91:677–680. doi: 10.1097/00000542-199909000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Varrone A, Sjöholm N, Eriksson L, et al. Advancement in PET quantification using 3D-OP-OSEM point spread function reconstruction with the HRRT. Eur J Nucl Med Mol Imaging. 2009;36:1639–1650. doi: 10.1007/s00259-009-1156-3. [DOI] [PubMed] [Google Scholar]
- 15.Andersson J, Truong P, Halldin C. In-target produced [11C]methane: increased specific radioactivity. Appl Radiat Isot. 2009;67:106–110. doi: 10.1016/j.apradiso.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 17.Thal SC, Luh C, Schaible E-V, et al. Volatile anesthetics influence blood-brain barrier integrity by modulation of tight junction protein expression in traumatic brain injury. PLoS One. 2012;7:e50752. doi: 10.1371/journal.pone.0050752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acharya NK, Goldwaser EL, Forsberg MM, et al. Sevoflurane and isoflurane induce structural changes in brain vascular endothelial cells and increase blood-brain barrier permeability: possible link to postoperative delirium and cognitive decline. Brain Res. 2015;1620:29–41. doi: 10.1016/j.brainres.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 19.Kimura-Kuroiwa K, Adachi YU, Mimuro S, et al. The effect of aging on dopamine release and metabolism during sevoflurane anesthesia in rat striatum: an in vivo microdialysis study. Brain Res Bull. 2012;89:223–230. doi: 10.1016/j.brainresbull.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Breier A, Su TP, Saunders R, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narendran R, Jedema HP, Lopresti BJ, et al. Imaging dopamine transmission in the frontal cortex: a simultaneous microdialysis and [11C]FLB 457 PET study. Mol Psychiatry. 2014;19:302–310. doi: 10.1038/mp.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeiler FA, Sader N, Gillman LM, et al. The cerebrovascular response to ketamine. J Neurosurg Anesthesiol. 2016;28:123–140. doi: 10.1097/ANA.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 23.Smith GS, Schloesser R, Brodie JD, et al. Glutamate modulation of dopamine measured in vivo with positron emission tomography (PET) and 11C-raclopride in normal human subjects. Neuropsychopharmacology. 1998;18:18–25. doi: 10.1016/S0893-133X(97)00092-4. [DOI] [PubMed] [Google Scholar]
- 24.Tsukada H, Harada N, Nishiyama S, et al. Ketamine decreased striatal [11C]raclopride binding with no alterations in static dopamine concentrations in the striatal extracellular fluid in the monkey brain: multiparametric PET studies combined with microdialysis analysis. Synapse. 2000;37:95–103. doi: 10.1002/1098-2396(200008)37:2<95::AID-SYN3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 25.Cervenka S, Halldin C, Farde L. Age-related diurnal effect on D2 receptor binding: a preliminary PET study. Int J Neuropsychopharmacol. 2008;11:671–678. doi: 10.1017/S1461145707008358. [DOI] [PubMed] [Google Scholar]
- 26.Minton GO, Young AH, McQuade R, et al. Profound changes in dopaminergic neurotransmission in the prefrontal cortex in response to flattening of the diurnal glucocorticoid rhythm: implications for bipolar disorder. Neuropsychopharmacology. 2009;34:2265–2274. doi: 10.1038/npp.2009.53. [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Zhong Z, Wang M, et al. Circadian modulation of dopamine levels and dopaminergic neuron development contributes to attention deficiency and hyperactive behavior. J Neurosci. 2015;35:2572–2587. doi: 10.1523/JNEUROSCI.2551-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deutschländer A, la Fougère C, Boetzel K, et al. Occupancy of pramipexole (Sifrol) at cerebral dopamine D2/3 receptors in Parkinson’s disease patients. NeuroImage Clin. 2016;12:41–46. doi: 10.1016/j.nicl.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]