Abstract
The recent advances in photocatalysis have opened a variety of new possibilities for energy and biomedical applications. In particular, plasmonic photocatalysis using hybridization of semiconductor materials and metal nanoparticles has recently facilitated the rapid progress in enhancing photocatalytic efficiency under visible or solar light. One critical underlying aspect of photocatalysis is that it generates and releases reactive oxygen species (ROS) as intermediate or final products upon light excitation or activation. Although plasmonic photocatalysis overcomes the limitation of UV irradiation, synthesized metal/semiconductor nanomaterial photocatalysts often bring up biohazardous and environmental issues. In this respect, this review article is centered in identifying natural photosensitizing organic materials that can generate similar types of ROS as those of plasmonic photocatalysis. In particular, we propose the idea of plasmonic photocatalyst-like fluorescent proteins for ROS generation under visible light irradiation. We recapitulate fluorescent proteins that have Type I and Type II photosensitization properties in a comparable manner to plasmonic photocatalysis. Plasmonic photocatalysis and protein photosensitization have not yet been compared systemically in terms of ROS photogeneration under visible light, although the phototoxicity and cytotoxicity of some fluorescent proteins are well recognized. A comprehensive understanding of plasmonic photocatalyst-like fluorescent proteins and their potential advantages will lead us to explore new environmental, biomedical, and defense applications.
Keywords: Plasmonic photocatalysis, Fluorescent proteins, Photosensitization, Reactive oxygen species, Visible light
Introduction
Photocatalysis has extensively been used in a variety of applications, including energy generation, environment remediation, and biomedicine, as mentioned in numerous review articles on photocatalysis [1–8]. Conventional photocatalysis requires three essential components of a semiconductor photocatalyst, a light source with appropriate wavelengths, and an oxidizing agent (e.g. water or oxygen molecules). In semiconductor photocatalysis, the wide bandgap energy (e.g. 3.0–3.2 eV) of semiconductor photocatalysts intrinsically limits light absorption to only the ultraviolet (UV) region (wavelength of light λ < 420 nm), which accounts for only about 4% of the total solar energy. Furthermore, the requirement of UV irradiation is commonly considered as a serious biohazard, potentially leading to premature aging of the skin, suppression of the immune system, damage to the eyes, and skin cancer [9–12]. Thus, to avoid the use of UV as an activation light source, plasmonic effects of metal nanoparticles (mNPs), such as Au, Ag, and Pt, have been successfully hybridized, resulting in broad and strong light absorption in the visible region [13–16], as summarized in several recent review articles [17–21].
One of the important aspects of photocatalysis is photoinduced production of reactive oxygen species (ROS), which often have direct applications for environment remediation and biomedicine, such as disinfection, water purification, and air purification. Typical semiconductor photocatalysts, such as titanium dioxide (TiO2) and zinc oxide (ZnO), were extensively studied for efficient and stable photogeneration of ROS [1–6, 22]. As intermediate or final products, semiconductor photocatalysis generates several different types of ROS, including superoxide anion (O•‒2), singlet oxygen (1O2), hydrogen peroxide (H2O2), and hydroxyl radical (–OH•). Regarding ROS produced by plasmonic photocatalysis, O•‒2 and 1O2 are typically generated via electron transfer under visible light excitation [13, 14]. Overall, O•‒2 and 1O2 play a key role in electrochemistry and photochemistry related to photocatalysis.
There is always an imperative need for cost-effective, eco-friendly, and nontoxic photocatalytic nanomaterials and their photoexcitation using visible (or solar) light. Although plasmonic photocatalysis overcomes the requirement of UV irradiation, it still has concerns with respect to environmental and biomedical utilizations. For example, nano-sized plasmonic photocatalysts (e.g. 1 − 100 nm) could potentially have hazardous and adverse (e.g. carcinogenic and cytotoxic) biological effects, which often result in the limited utilizations for environmental remediation and biomedicine [23, 24]. Noble metals (e.g. Ag, Au, and Pt) also have some drawbacks, including rarity, high cost, and easy dissolution (especially for Ag) upon exposure to air or humidity. In this respect, nontoxic organic photosensitizers (e.g. natural dyes or proteins) could potentially be an excellent alternative to noble mNP-based plasmonic photocatalysts, as photosensitization has a great similarity with visible light-driven plasmonic photocatalysis.
In this review article, we introduce plasmonic photocatalyst-like fluorescent proteins for ROS generation upon visible (or solar) light activation. Several recent review articles have extensively covered photosensitizing molecules found in nature (e.g. porphyrin and chlorophyll) [25–27] and genetically-encoded ROS-generating proteins for cellular functions and redox signaling pathways [28–30]. To the best of our knowledge, a systematic review on ROS photoproduction from fluorescent proteins has not yet been available, compared to plasmonic photocatalysis. First, we briefly describe the basic mechanisms of plasmonic photocatalysis and photosensitization in terms of ROS photogeneration. Second, we review selected photosensitizing proteins that can be compared with plasmonic photocatalytic nanomaterials in a parallel manner. Third, we discuss outlook based on the current state of understanding on ROS utilizations. An enhanced understanding of plasmonic photocatalysis and fluorescent protein photosensitization will allow us to take advantage of ROS generated from light-induced fluorescent proteins for unexplored environmental, biomedical, and defense applications.
Basic mechanisms of plasmonic photocatalysis and photosensitization
Visible light-driven plasmonic photocatalysis
In general, plasmonic photocatalytic activities involve several different underlying mechanisms of electron and energy transfer depending on excitation energy and light sources, as summarized in the recent review articles [17–21]. The current consensus in the community is that visible light-driven plasmonic photocatalysis is mainly associated with generating two types of ROS (O•‒2 and 1O2) [13, 14]. Specifically, ROS generation from visible light-activated plasmonic photocatalysis can be summarized as follows (Fig. 1): In plasmonic photocatalysis using plasmon resonance excited by visible light, an electron transfer process from mNP to the semiconductor occurs at the metal/semiconductor interface. In general, Schottky barrier, which interrupts the electron transfer from mNP to the semiconductor, is formed at the junction interface between mNP and the semiconductor in the hybrid nanostructures due to the Fermi level difference between the two different materials. However, an electron can travel to the adjacent semiconductor if the plasmonic excitation energy is higher than Schottky barrier. Such a highly energetic electron is often referred to as a ‘hot’ electron. As the energetic electron in mNP migrates to the conduction band (ECB) of the semiconductor, this process reduces molecular oxygen O2(3Σ−g) of triplet ground state (i.e. 3O2) to generate O•‒2 at the semiconductor surface. In the meantime, mNP can hold the positive hole. The positive hole remained in mNP further oxidizes the previously produced O•‒2 to generate additional ROS of 1O2 (i.e. O2(1Δg)) [13, 14]. As a result, plasmonic photocatalysis can generate and release O•‒2 and 1O2 under visible light irradiation.
Fig. 1.
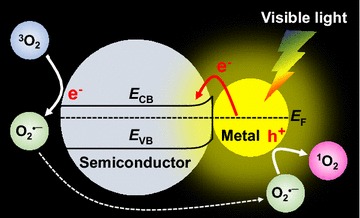
Schematic illustration of a plausible mechanism for generating O•‒2 and 1O2 (i.e. O2(1Δg)) on metal–semiconductor hybrid nanostructures via hot electron transfer caused by surface plasmon resonance upon visible light excitation. ECB and EVB represent the conduction and valence bands of the semiconductor photocatalyst, respectively. EF refers to the Fermi energy level
Type I and Type II reactions of photosensitization
Almost all photosensitizing molecules participate in Type I and/or Type II photoreactions involving the generation of ROS upon light activation (Fig. 2a) [31–34]. Predominant ROS generated by photosensitizers depends on a type of photosensitization reactions and a concentration of local electron acceptors. When light is incident on a photosensitizing molecule and light absorption occurs, the molecule is excited from the singlet ground state (S0) to the singlet excited state (S*1). The excited state loses the energy by returning back to S0 with fluorescent emission or through an intersystem crossing (ISC) process which involves conversion to the long-lived triplet excited state (T*1). T*1 can decay S0 via phosphorescent emission or can react with an electron donor molecule. In the latter case, O•‒2 is generated by electron transfer from the substrate in T*1 of the photosensitizer to 3O2 as Type I photoreaction. Because the most common electron acceptor is O2, O•‒2 can further interact with its surroundings to produce other reactive oxygenated products, such as H2O2 and -OH•. On the other hand, T*1 can also transfer the energy directly to 3O2, producing singlet oxygen of the first (i.e. lowest-energy) singlet excited state 1O2 (i.e. O2(1Δg)) as Type II photoreaction (Fig. 2b). O2(1Δg) has energy (E) of 0.98 eV (EΔ) and its second (higher energy) singlet excited state O2(1Σ+g) is 1.63 eV (EΔ + EΣ) above the triplet ground state (i.e. 3O2) [35–37]. O2(1Σ+g) decays extremely fast (~ picoseconds) to the first excited state 1O2 especially in aqueous media by its electronic-to-vibrational energy-transfer process [36–38]. Thus, the generation of O2(1Σ+g) in biology is often neglected. Similarly to visible light-activated plasmonic photocatalysis, Type I and Type II photoreactions of photosensitization can generate and release both O•‒2 and 1O2 under visible light activation.
Fig. 2.
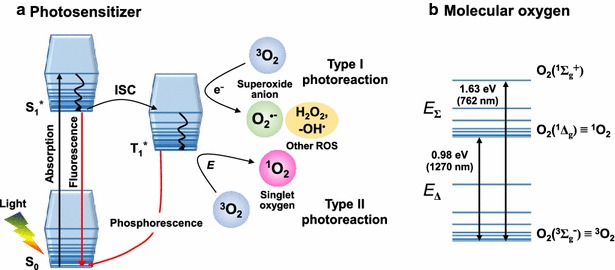
Jablonski diagram of photosensitizer and molecular oxygen (O2). a Photosensitization with the singlet ground (S0) and excited (S*1) states and their further interactions with O2. The triplet excited state (T*1) through an intersystem crossing (ISC) process can undergo electron (e−) transfer to the ground state of molecular oxygen O2(3Σ−g) (i.e. 3O2), generating superoxide anion (O•‒2) and other ROS products (e.g. H2O2 and –OH•) as Type I photoreaction. In addition, T*1 can undergo energy (E) transfer to 3O2, producing highly cytotoxic O2(1Δg), commonly known as singlet oxygen (1O2), as Type II photoreaction. b Electronic configuration of the triplet ground state molecular oxygen O2(3Σ−g), its first (i.e. lowest-energy) singlet excited state O2(1Δg), and its second (higher energy) singlet excited state O2(1Σ+g) [36, 37], where the superscripts 3 and 1 indicate triplet and singlet states, respectively. The energy gaps between the ground state and the two singlet excited states are shown in eV, including the corresponding luminescent wavelengths
ROS lifetime and migration distance in plasmonic photocatalysis and photosensitization
As explained above, both plasmonic photocatalysis and photosensitization under visible light activation can produce short-lived ROS, given that O•‒2 and 1O2 are highly unstable and reactive [39, 40]. ROS photogenerated from plasmonic photocatalysis and photosensitization is only effective in the vicinity to semiconductor photocatalyst nanomaterials or photosensitizing molecules. Typically, O•‒2 exhibits a lifetime of ~ 50 μs, depending on the local environments [41]. On the other hand, the typical lifetime of 1O2 is ~ 3.1–3.9 μs in H2O. The lifetime of 1O2 can be as long as 68 μs in deuterium oxide (D2O), because it is mainly determined by energy transfer to the vibrational energy levels of the surrounding molecules [38, 42]. Short-lived ROS from plasmonic photocatalysis and photosensitization allows the migration distance to be as long as ~ 320 and ~ 200 nm for O•‒2 and 1O2, respectively [41, 43]. Overall, the short lifetime and the relatively short migration (or damage) distance can be considered as a disadvantage requiring a high concentration for a prolonged effect or an advantage for a safeguard, given O•‒2 and 1O2 are extremely reactive and toxic.
Identification of phototoxic fluorescent proteins from biological studies
The phototoxicity and cytotoxicity of some fluorescent proteins are well known in different scientific communities. In cellular imaging, several nontoxic variants of phototoxic fluorescent proteins were successfully developed for cellular labeling and imaging in vivo [44–46]. In a contrary manner, phototoxic fluorescent proteins have extensively been employed as a means of selectively damaging target molecules in a localized region and at a particular time-point upon light activation [47–49]. Chromophore photoreduction in red fluorescent proteins (RFPs) is considered to be mainly responsible for photobleaching and phototoxicity, forming dianionic open-shell states of the chromophore in RFPs [50]. This method is known as chromophore-assisted light inactivation (CALI) that can be used to inactivate target cells and ablate tissue of interest. In particular, CALI using fluorescent proteins can allow for spatiotemporal knockdown or loss-of-function of targeted proteins, which can be microscopically controlled with light activation in situ [30, 47, 49, 51]. In addition, some fluorescent proteins can be used for photodynamic therapy (PDT) to destruct diseased tissue without affecting the surrounding healthy tissue [52–54].
CALI and PDT using fluorescent proteins can offer an initial overview to identify major ROS-generating fluorescent proteins. There are several studies on CALI using photosensitizing proteins, such as enhanced green fluorescent protein (EGFP) [55, 56], mini Singlet Oxygen Generator (miniSOG) [57], KillerRed [58], and SuperNova [59]. CALI with EGFP was used to inactivate α-actinin in fibroblasts, which resulted in stress fiber detachment [55]. EGFP variants, including enhanced yellow fluorescent protein (EYFP) and enhanced cyan fluorescent protein (ECFP), were used for CALI. In general, the efficiency followed an order of EGFP > EYFP > ECFP [56]. The use of miniSOG for CALI was demonstrated [57], in which miniSOG was fused with the succinate dehydrogenase complex subunit of the mitochondrial respiratory complex II to disrupt complex II activity. Mitochondrion-targeted miniSOG caused rapid and effective death of neurons in a cell-autonomous manner without detectable damages to the surrounding cells [52]. Immunophotosensitizer 4D5 single chain variable fragment (4D5scFv)-miniSOG was used to selectively recognize the extracellular domain of human epidermal growth factor receptor 2 (HER2/neu) [53]. KillerRed was used for CALI of Escherichia coli and eukaryotic cells [58, 60, 61]. KillerRed was also tested for PDT by fusing to an antibody to target tumor cells, resulting in tumor-specific cell death [54]. SuperNova, which is a monomeric variant of KillerRed, was used to suppress actin filament motility by illuminating orange light [59].
ROS photogeneration of phototoxic fluorescent proteins
The main underlying mechanism by which the aforementioned fluorescent proteins are phototoxic and cytotoxic is that these proteins are capable of generating and releasing several different types of ROS. The optical absorption and emission of phototoxic fluorescent proteins and their detected ROS types are summarized in Table 1. To the best of our knowledge, this table provides a comprehensive list of phototoxic fluorescent proteins that can generate and release ROS upon visible light excitation and activation in a comparable manner of visible light-activated plasmonic photocatalysis.
Table 1.
Optical excitation and emission of phototoxic fluorescent proteins and their detected ROS types
| Fluorescent protein variant | Excitation maximum (nm) | Emission maximum (nm) | Detected ROS type | References |
|---|---|---|---|---|
| GFP | 395/476 | 503/509 | 1O2 | [62] |
| EGFP | 488 | 507 | 1O2 | [63] |
| miniSOG | 448 | 500 | 1O2 | [64, 65] |
| SOPP | 439 | 488/515 | 1O2 | [66] |
| Pp2FbFP L30 M | 449 | 495 | 1O2 | [67] |
| KillerRed | 585 | 610 | O•‒2 and 1O2 | [59, 68, 69] |
| SuperNova | 579 | 610 | O•‒2 and 1O2 | [59] |
| TagRFP | 555 | 584 | 1O2 | [70] |
| mKate2 | 588 | 633 | O•‒2 and 1O2 | [71] |
GFP and EGFP
GFP was first discovered by Shimomura et al. [72] as a companion protein to the famous chemiluminescent protein (i.e. aequorin) from Aequorea jellyfish. Since then, GFP has revolutionized cell biology and cellular imaging [73]. As an electron donor, GFP was also utilized for converting light-to-electricity in photodetectors or photovoltaic cells [74–77]. GFP has a unique cylindrical (can)-like shape consisting of an 11-strand β-barrel with a single α-helical strand containing a chromophore. The GFP chromophore is p-hydroxybenzylidene-imidazolinone formed from residues 65–67 and is almost perfectly buried in the center of β-can (Fig. 3a) [78, 79]. As far as ROS is concerned, GFP and EGFP are typically known to produce 1O2 via Type II photoreaction under excitation at blue light of λ = 400–500 nm [62, 63]. In particular, 1O2 production ability of GFP is considered to attribute to the accessibility of molecular oxygen to the chromophore [80]. 1O2 was detected in GFP-expressing Escherichia coli bacteria and kidney cells by means of electron spin resonance (ESR); singlet oxygen spin-trap 2,2,6,6-tetramethyl-4-piperidinyloxy (TEMP) was bound to 1O2 to produce a stable secondary radical 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) and the TEMPO quantities were measured to correlate with the concentration of 1O2 by measuring ESR spectra [62]. 1O2 produced by EGFP in a solution was also measured by time-resolved near-infrared luminescence measurements at λ = 1275 nm (Fig. 3b) [63].
Fig. 3.
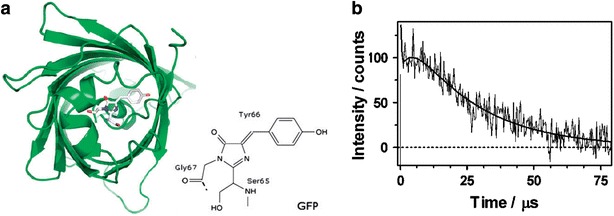
a 3D structure of GFP (PDB ID: 1GFL) obtained from X-ray crystallography and its mutant Ser65Thr (PDB ID: 1EMA). GFP has a β-barrel structure with the chromophore embedded in its core (Reproduced from [79] with the permission of Royal Society of Chemistry). The chromophore is shown in its neutral form with protonated phenolic oxygen. b Normalized time-resolved phosphorescent intensity. Emission signals of 1O2 generated from EGFP irradiated at λex = 532 nm in deuterated phosphate-buffered saline (d-PBS) (1:3) were detected at λ = 1275 nm (Reproduced from [63] with the permission of Elsevier)
miniSOG
By engineering the light-oxygen-voltage (LOV) domain of Arabidopsis thaliana phototropin 2 (AtPhot2), fluorescent flavoprotein miniSOG was originally developed to improve correlative light and electron microscopy [64, 65]. In terms of sizes (number of amino acids), miniSOG contains 106 amino acids, which is less than half the size of GFP (Fig. 4a). miniSOG is excited maximally at λex = 448 nm and emits green light with two peaks at λem = 500 and 528 nm [64]. Regarding 1O2 photogenerated from miniSOG, 1O2 was detected using anthracene-9,10-dipropionic acid (ADPA) as a turn-off sensor probe of 1O2 [64] and 1O2 phosphorescent signals (Fig. 4b, c) [65]. After ADPA reacted with 1O2, it was converted to an endoperoxide form, which led to a decrease in fluorescence at λem = 406 nm [81]. 1O2 photogeneration of miniSOG is also supported the idea that the chromophore is accessible to oxygen molecules [82]. In addition, as an improved mutant of miniSOG, singlet oxygen photosensitizing protein (SOPP) was developed to achieve more efficient photogeneration of 1O2 [66].
Fig. 4.
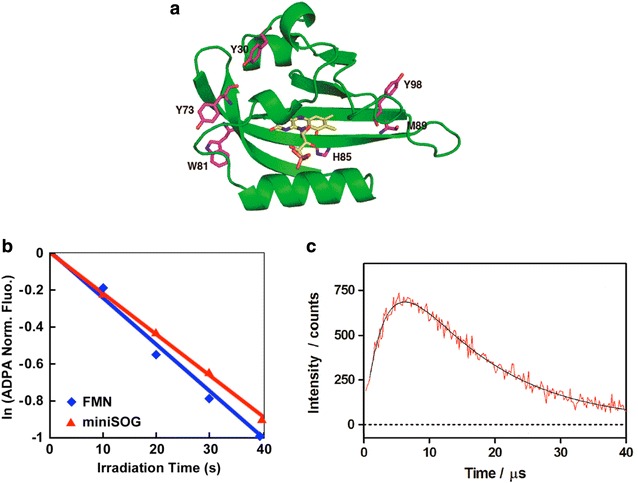
a 3D structure of miniSOG. This molecular model is based on the structure of the improved LOV protein (PBD ID: 4eet) using the Swiss-model server [83]. The backbone of miniSOG is shown as the green ribbon, flavin mononucleotide (FMN) as the orange sticks, and the amino acids as the magenta sticks (Reproduced from [65] with the permission of American Chemical Society). b Degradation of ADPA reacted with 1O2 photogenerated by miniSOG under light irradiation (red) (Figure from [64] and Creative Commons license). c Photosensitized 1O2 formation from miniSOG. Time-resolved 1O2 phosphorescent signals at λ = 1275 nm were recorded in d-PBS upon pulsed laser excitation at λex = 355 nm (Reproduced from [65] with the permission of American Chemical Society)
Pp2FbFP L30 M
Pp2FbFP L30 M was derived from Pseudomonas putida flavin-binding Pp2FbFP with a further mutation of L30 M, which was originated from blue-light photoreceptors of the LOV family [67]. Upon light excitation, 1O2 photoproduction of Pp2FbFP L30 M was detected by measuring phosphorescent emission of 1O2 at λem = 1275 nm (Fig. 5) [67].
Fig. 5.
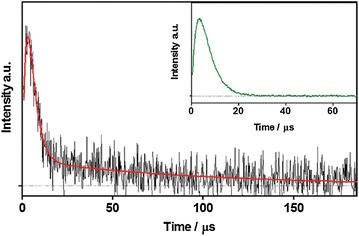
Time-resolved 1O2 phosphorescent signals for Pp2FbFP L30 M (λex = 355 nm) in an air-saturated PBS solution at λ = 1275 nm. The corresponding trace for FMN was included in the inset for comparison to show the absence of the long-lived tail (Reproduced from [67] with the permission of Royal Society of Chemistry)
KillerRed
KillerRed is one of the most studied RFPs for phototoxicity and ROS photogeneration. Regarding its origin, KillerRed was derived from the jellyfish chromoprotein anm2CP [84]. KillerRed is composed of 11 anti-parallel β-sheets that form a barrel structure with a central chromophore of Q65-Y66-G67 [85, 86]. Owing to its efficient ROS photogeneration, KillerRed was reported to be strongly phototoxic upon light illumination in a wavelength range of λex = 540–580 nm [50, 68], exceeding other fluorescent proteins at least 1000-fold [84]. The extremely high phototoxicity is considered to be mainly attributed from a unique (cleft-like) structural feature in the β-barrel frame between β7 and β10 sheets. This cleft-like structure has an opening channel filled with water (oxygen) molecules connecting the chromophore’s cavity with the exterior of the protein barrel [84–86] (Fig. 6a). The opening channel leading to the chromophore is considered to facilitate water and/or oxygen diffusion to/from the chromophore. Computational simulations also support the idea that the water channel can increase the chromophore’s accessibility to molecular oxygen (Fig. 6b) [86].
Fig. 6.
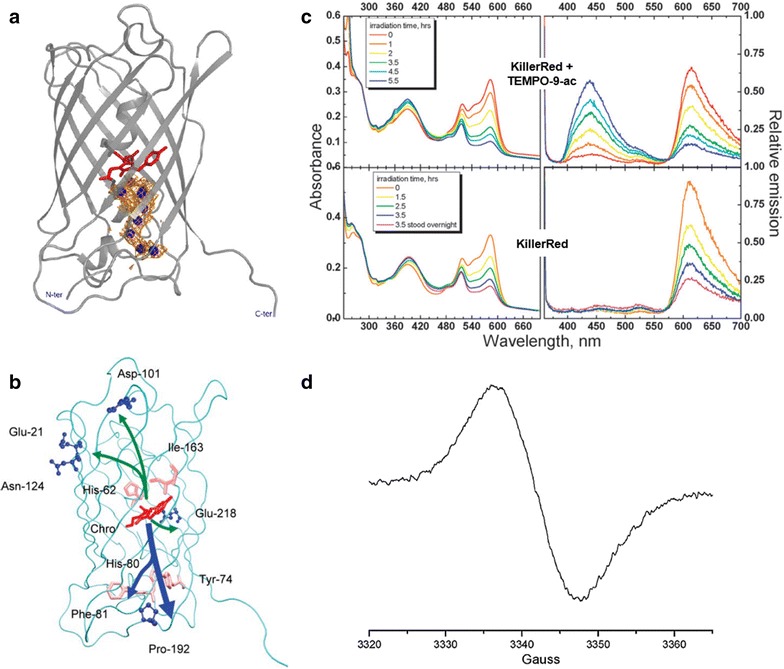
a 3D structure of a monomer of KillerRed obtained from X-ray crystallography (Reproduced from [85] with the permission of by John Wiley and Sons Inc.). Monomer A is shown with the backbone represented in gray and the chromophore in red. The cavity forming the channel is shown as the orange isomesh at 1 Å above the van der Walls radius and water molecules in the channel are depicted as the blue spheres. The cleft-like opening channel filled with water (oxygen) molecules is located in the β-barrel frame between β7 and β10 sheets. b Possible escape routes for molecular oxygen in the simulations (Reproduced from [86] with the permission of Royal Society of Chemistry). The chromophore is shown in red, the residues at the exits in blue, and other important residues along the pathways in pink. The escape pathways are highlighted by arrows (major channels in blue and minor channels in green). c, d Detection of O•‒2 photogenerated from KillerRed (Reproduced from [68] with the permission of Royal Society of Chemistry). c Light absorption (left panels) and emission (right panels) of KillerRed with and without the radical fluorescent probe TEMPO-9-ac (λex = 358 nm and λem = 440 nm). d Representative EPR spectrum of KillerRed with DMPO in a PBS solution under light irradiation at λex = 560 nm, supporting O•‒2 generation
As far as ROS photogeneration is concerned, KillerRed is known to undergo Type I photosensitization reaction to yield O•‒2 [68]. Irradiated KillerRed exhibited a tenfold increase in fluorescence signals (λem = 440 nm) of 4-((9-acridinecarbonyl)amino)-2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO-9-ac), compared to unchanged levels of controls (Fig. 6c) [68]. As a turn-on fluorescent free radical probe for sensing ROS related Type I photosensitization, the original status of TEMPO-9-ac is not fluorescent as the acridine moiety is initially quenched by the stable paramagnetic nitroxide moiety. ROS (mostly long-lived carbon- or sulfur-centered) can convert nitroxide to the corresponding piperidine, resulting in fluorescence turn-on (λex = 358 nm and λem = 440 nm) [68, 87, 88]. Irradiated KillerRed with 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) in PBS showed a broad singlet with a peak-to-trough width of 15 Gauss in the electron paramagnetic resonance (EPR) spectrum, supporting O•‒2 generation. (Fig. 6d). As controls, non-irradiated KillerRed and irradiated PBS did not produce EPR signals [68]. ROS associated with Type I photosensitization reaction was also detected with spin trapping of DMPO using steady-state EPR [89, 90]. In addition to O•‒2, 1O2 was detected in irradiated KillerRed using a radical scavenger (sodium azide, NaN3) and a fluorescent probe (ADPA) [59, 69]. Thus, ROS photoinduced by KillerRed is primarily associated to O•‒2 with a possibility of 1O2 photogeneration.
SuperNova
SuperNova is a monomeric mutant of KillerRed. When KillerRed is fused to a protein of interest, it usually disrupts function and localization of other proteins due to its larger size and functional dimerization. To overcome this limitation, a monomeric variant was derived from KillerRed via random mutagenesis [59]. Specifically, SuperNova was developed (Fig. 7a), following six mutations compared with KillerRed: G3V, N145S, L160T, F162T, L172 K, M204T [59]. As a result, SuperNova is considered to have the similar photochemical properties as the parental protein (i.e. KillerRed), showing the excitation and emission maxima at λex = 579 nm and λem = 610 nm, respectively. Both O•‒2 and 1O2 generated by SuperNova under orange light irradiation were detected using fluorescent probes of dihydroethidium (DHE) and ADPA, respectively [59]. In particular, enhanced photobleaching in DHE and ADPA supported photogeneration of O•‒2 and 1O2 from SuperNova, respectively (Fig. 7b, c) [59]. The original state of DHE exhibits blue fluorescent emission (λex = 365 nm and λem = 435 nm) until being oxidized primarily by O•‒2. Oxidation of DHE results in hydroxylation at 2-position forming 2-hydroxyethidium, showing reduced blue fluorescent emission (i.e. bleaching) and increased red fluorescent emission (λex = 490 nm and λem = 590 nm) [91, 92].
Fig. 7.
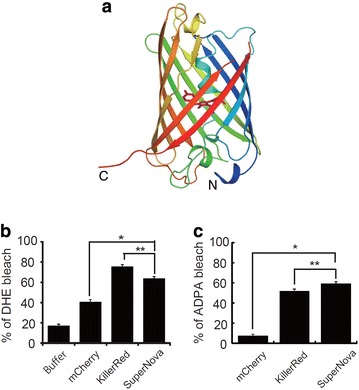
a 3D crystal structure of SuperNova (Reproduced from [59] with the permission of Springer Nature). SuperNova (monomer) is represented by the rainbow ribbon diagram and the chromophore is represented by the magenta stick model. b, c ROS detection generated from SuperNova (Reproduced from [59] with the permission of Springer Nature). b Detection of O•‒2 photogeneration of mCherry, KillerRed, and SuperNova by measuring bleaching of DHE fluorescence, including references (buffers). The irradiation condition was 0.73 W cm−2 for 10 min. c Detection of 1O2 photogeneration in mCherry, KillerRed, and SuperNova by measuring bleaching of ADPA fluorescence. The irradiation condition was 1.4 W cm−2 for 5 min. mCherry (λex = 587 nm and λem = 610 nm) is a monomeric fluorescent protein variant derived from the Discosoma red (DsRed) protein [93–95]
TagRFP
As a monomeric RFP, TagRFP was derived from the sea anemone Entacmaea quadricolor fluorescent protein TurboRFP (random mutant of eqFP578) (Fig. 8a) [96]. Unlike KillerRed [84, 85] and photosensitizing GFP mutants [80], TagRFP is known not to have a clear channel that connects the chromophore with the outside environment [97]. Thus, an alternative mechanism for oxygen diffusion in TagRFP was suggested such that transient protein permeability can play a role due to dynamical breathing [70]. This mechanism was also supported by the recent molecular dynamics simulations [86], in which the static picture offered by crystallography was explained by monitoring the triplet state. Regarding ROS photogeneration, TagRFP under green light (λex = 532 nm) produced 1O2, which was confirmed by both time-resolved phosphorescence of 1O2 and a turn-on fluorescent probe of singlet-oxygen sensor green (SOSG) (Fig. 8b, c), even though O•‒2 was not detected using a fluorescent probe of DHE [70]. Specifically, pulsed laser irradiation (λex = 532 nm) of TagRFP in an air-saturated mixture of PBS, glycerol, and d-PBS (1:1:20) allowed the detection of 1O2 phosphorescence at λ = 1270 nm (Fig. 8b), in which a fast spike in the earlier part of the signal (due to the scattered laser light and the sensitizer fluorescent emission) was followed by a slower rise and decay, corresponding to 1O2 kinetics. With the longer irradiation time, the enhanced SOSG fluorescence signals (λex = 480 nm and λem = 527 nm) were also detected (Fig. 8c). Green fluorescent emission of SOSG corresponds to endoperoxide generated by an interaction of 1O2 with the anthracene component of SOSG [98, 99].
Fig. 8.

a Chromophore and their environment in TagRFP (Reproduced from [97] with the permission of Elsevier). The chromophore backbone for TagRFP is shown in orange. The hydrogen bonds are indicated with the green dashed lines, the atoms are colored by the atom type, and the water molecules are shown as the red spheres. b, c Detection of 1O2 photogenerated from TagRFP (Reproduced from [70] with the permission of John Wiley and Sons). b 1O2 phosphorescence photosensitized by TagRFP in a PBS:Glycerol:d-PBS mixture (1:1:20) upon irradiation at λex = 532 nm. The luminescence signal at λ = 1270 nm was fit with a triexponential function. c Time course of fluorescent spectra of optically matched solutions of SOSG (λex = 480 nm) and TagRFP under light irradiation at λex = 532 nm. The increased amount of 1O2 was detected by the increase in the SOSG band (λem = 527 nm) with irradiation at λex = 532 nm. The concomitant decrease in the TagRFP band (λem = 590 nm) indicated photobleaching and/or photoconversion
mKate2
mKate2 is a far-red monomeric fluorescent protein with the maximal excitation wavelength (λex = 588 nm) and emission wavelength (λem = 633 nm), which was derived from mKate containing three mutations of S165A, V48A, and K238R [100]. The mutation of S165A increases the fluorescent brightness, while the mutations of V48A and K238R accelerate protein maturation, including enhanced pH stability and photostability. Both mKate and mKate2 are widely considered as one of the phototoxic fluorescent proteins [44, 101], because of a cleft-like opening channel filled with water (oxygen) molecules in the β-barrel frame between β7 and β10 (Fig. 9a) [102]. ROS generated from mKate2 embedded in silk (i.e. RFP fluorescent silk produced from silkworm transgenesis [71, 103]) under green light irradiation (λ = 532 nm) was detected using fluorescent probes of TEMPO-9-ac and 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABDA) for O•‒2 and 1O2, respectively (Fig. 9b, c) [71]. As the irradiation time of green light increased, the intensity of TEMPO-9-ac fluorescent peaks gradually enhanced and the ABDA fluorescent intensity decreased, compared to the baseline signals before light irradiation (controls). These result support the photogeneration of O•‒2 and 1O2 from mKate2. Similarly, ABDA was also widely used to detect the formation of 1O2 in solutions [71, 104, 105]; the original state of ABDA emits fluorescence at λem = 431 nm under photoexcitation at λex = 380 nm and the oxidation of ABDA by 1O2 creates an endoperoxide, resulting in reduced fluorescent intensity [104].
Fig. 9.
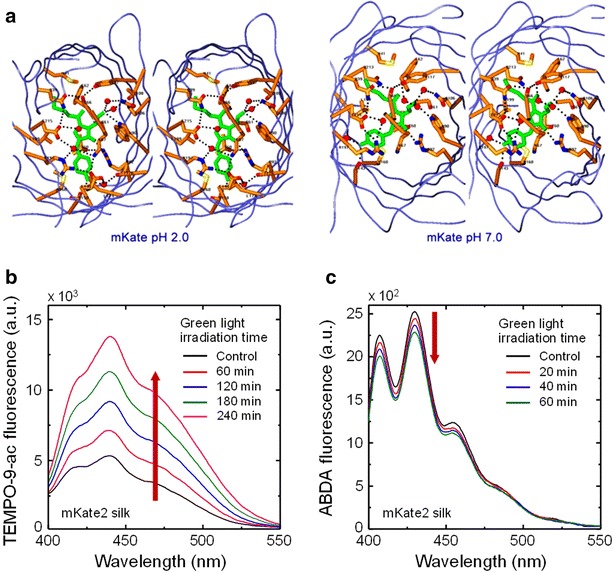
a 3D structure of the H-bond network in the vicinity of the trans chromophore (shown in green) for the mKate_pH2.0 structure (left) and the cis chromophore in the mKate_pH7.0 structure (right). Mediating waters are shown by red spheres (Reproduced from [102] with the permission of American Society for Biochemistry and Molecular Biology). b, c Detection of ROS generated by mKate2 embedded in silk (i.e. RFP fluorescent silk produced from silkworm transgenesis) using turn-on/off fluorescent probes upon green light irradiation at λex = 532 nm [71]. Fluorescent emission signals of the probes were monitored from solutions containing mKate2 fluorescent silk. b O•‒2 mediated by Type I photosensitization reaction, captured by turn-on fluorescent signals of TEMPO-9-ac (λex = 365 nm). c 1O2 mediated by Type II photosensitization reaction, detected by reduction in the original ABDA fluorescent intensity (λex = 365 nm)
Fluorescent proteins with a cleft-like structure in β-barrels
Besides the aforementioned fluorescent proteins including KillerRed [85, 86] and mKate/mKate2 [71, 102], other fluorescent proteins could potentially be efficient in generating and releasing ROS upon visible light irradiation. As excellent candidates, several fluorescent proteins are known to contain a cleft-like water-filled channel in the β-barrel frame. Excellent examples include DronPa [106], TurboGFP [107], mCherry [95], KillerOrange/mKillerOrange [108], and zGFP506, zYFP538, zRFP574 [109] (Fig. 10). A water channel inside these fluorescent protein is suggested to open up solvent access to methylene/imidazolinone moieties of chromophores, allowing for enhanced generation and release of ROS, which in turn results in photobleaching and phototoxicity [44, 85, 110]. The water channel may also facilitate oxygen transport to a premature chromophore. This may promote the dehydrogenation step of chromophore maturation and transport abstracted proton transport outside the β-barrel, speeding up the chromophore’s maturation [107]. Indeed, ROS release from the fluorescent protein’s β-barrel through the water-filled channel may explain some phototoxicity and adverse effects (e.g. inhibition of cell division) of other fluorescent proteins in cellular imaging. Overall, these phototoxic fluorescent proteins warrant further detailed detection studies on photoinduced ROS and their exact types.
Fig. 10.
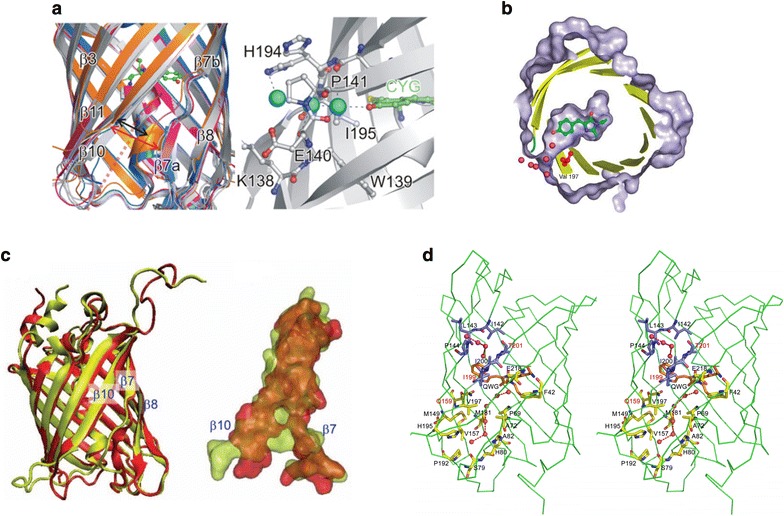
Cleft-like channels filled with water (oxygen) molecules in the β-barrel frame between β7 and β10 sheets of DronPa (a) (Reproduced from [106] with the permission of Portland Press Ltd.), TurboGFP (b) (Reproduced from [107] with the permission of John Wiley and Sons), mCherry (c) (Reproduced from [95] with the permission of AIP Publishing LLC.), and KillerOrange/mKillerOrange (d) (Figure from [108] and Creative Commons license). a DronPa: The cleft (indicated by the broken line) near to strand β7 is conserved. The red double headed arrow indicates the width of the cleft. Water molecules are shown as the cyan spheres, building hydrogen-bonded connection between the chromophore and the aqueous environment. The protein region around the opening and the chromophore is shown as the ball-and-stick model. b TurboGFP: A pore leading to the TurboGFP chromophore. The chromophore is highlighted in green and Val 197 in red. The protein surface (gray) is cut to show the pore and the chromophore cavity. The sections of secondary structure elements are shown as the yellow cartoons. Relevant water molecules are depicted as the magenta spheres. c mCherry: The superposition of ribbon structures of red (mCherry) and yellow (citrine) fluorescent proteins. The β7–β10 region is displayed with a space filling model to show that the gap in mCherry is larger than that in citrine. d KillerOrange (dimer) and mKillerOrange (monomer): The pore is filled by four water molecules connecting the indole moiety of the chromophore with the protein exterior. The water channel (residues shown in yellow) with a chain of seven water molecules (red spheres) and the pore (residues shown in blue) filled with four water molecules. The residues mutated in this work are labeled in red
Outlook and conclusion
We have discussed the similarities between plasmonic photocatalysis and phototoxic fluorescent proteins in terms of ROS generation under visible light activation. Like plasmonic photocatalysis, protein photosensitization requires three essential components of a fluorescent protein, a light source with appropriate wavelengths, and an oxidizing agent. A proper interaction of these elements leads to the photogeneration of ROS in the close vicinity. Among the current active applications in environment remediation and biomedicine, O•‒2 and/or 1O2 photogeneration from fluorescent proteins could highly be useful for inactivating harmful microorganisms and pathogens, such as bacteria, viruses, and fungi [111, 112], as well as contaminants and endocrine disrupting compounds [113]. In particular, ROS (i.e. 1O2) can be effective in inactivating viruses, impairing genome replication [114–117]. ROS could be useful for insect eradication [118, 119] and water disinfection for control of water-borne pathogens [120, 121].
Protein photosensitization can offer several pivotal advantages over conventional photocatalysis: (i) Fluorescent proteins can rule out biohazardous concerns on the byproducts and residuals of foreign synthesized metal/semiconductor nanomaterial photocatalysts. Thus, fluorescent proteins can overcome the limitation of hazardous and adverse (e.g. carcinogenic and cytotoxic) effects associated with photocatalytic nanoparticles [23, 24]. Fluorescent proteins are degradable and digestible, eliminating the potential risk of exposure and consumption. (ii) Without a need of additional nanoconjugations (e.g. mNPs, photosensitizers, and quantum dots), fluorescent proteins can generate selective ROS by being activated under solar (visible) light without UV irradiation. (iii) As ROS-generating nanomaterials, fluorescent proteins could potentially be mass-produced in an eco-friendly manner using biological reactors (e.g. microorganisms and insects) [71, 103, 122–124].
Authors’ contributions
JWL and YLK mainly wrote the manuscript. All of the authors participated in discussion. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by Cooperative Research Program for Agriculture Science & Technology Development (PJ0120892018) from Rural Development Administration, Republic of Korea and Air Force Office of Scientific Research (FA2386-17-1-4072), USA.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jung Woo Leem, Email: leem0@purdue.edu.
Seong-Ryul Kim, Email: ksr319@korea.kr.
Kwang-Ho Choi, Email: ckh@korea.kr.
Young L. Kim, Phone: 765-496-2445, Email: youngkim@purdue.edu
References
- 1.Schneider J, Matsuoka M, Takeuchi M, Zhang JL, Horiuchi Y, Anpo M, Bahnemann DW. Understanding TiO2 photocatalysis: mechanisms and materials. Chem. Rev. 2014;114:9919–9986. doi: 10.1021/cr5001892. [DOI] [PubMed] [Google Scholar]
- 2.Nakata K, Fujishima A. TiO2 photocatalysis: design and applications. J. Photochem. Photobiol. C. 2012;13:169–189. doi: 10.1016/j.jphotochemrev.2012.06.001. [DOI] [Google Scholar]
- 3.Hashimoto K, Irie H, Fujishima A. TiO2 photocatalysis: a historical overview and future prospects. Jpn. J. Appl. Phys. 2005;44:8269–8285. doi: 10.1143/JJAP.44.8269. [DOI] [Google Scholar]
- 4.Ni M, Leung MKH, Leung DYC, Sumathy K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sust. Energy Rev. 2007;11:401–425. doi: 10.1016/j.rser.2005.01.009. [DOI] [Google Scholar]
- 5.Maness PC, Smolinski S, Blake DM, Huang Z, Wolfrum EJ, Jacoby WA. Bactericidal activity of photocatalytic TiO2 reaction: toward an understanding of its killing mechanism. Appl. Environ. Microbiol. 1999;65:4094–4098. doi: 10.1128/aem.65.9.4094-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen XB, Shen SH, Guo LJ, Mao SS. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010;110:6503–6570. doi: 10.1021/cr1001645. [DOI] [PubMed] [Google Scholar]
- 7.Kisch H. Semiconductor photocatalysis: principles and applications. Weinheim: Wiley-VCH Verlag GmbH & Co; 2015. [Google Scholar]
- 8.Bhatkhande DS, Pangarkar VG, Beenackers AACM. Photocatalytic degradation for environmental applications—A review. J. Chem. Technol. Biotechnol. 2002;77:102–116. doi: 10.1002/jctb.532. [DOI] [Google Scholar]
- 9.Narayanan DL, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010;49:978–986. doi: 10.1111/j.1365-4632.2010.04474.x. [DOI] [PubMed] [Google Scholar]
- 10.de Gruijl FR. Skin cancer and solar UV radiation. Eur. J. Cancer. 1999;35:2003–2009. doi: 10.1016/S0959-8049(99)00283-X. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J. Photoch. Photobio. B. 2001;63:8–18. doi: 10.1016/S1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 12.D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013;14:12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito H, Nosaka Y. Mechanism of singlet oxygen generation in visible-light-induced photocatalysis of gold-nanoparticle-deposited titanium dioxide. J. Phys. Chem. C. 2014;118:15656–15663. doi: 10.1021/jp502440f. [DOI] [Google Scholar]
- 14.Saito H, Nosaka Y. Enhancement of the generation of photocatalytic active species by loading copper ions on gold-nanoparticle-deposited titanium dioxide. Catal. Commun. 2015;61:117–120. doi: 10.1016/j.catcom.2014.12.024. [DOI] [Google Scholar]
- 15.Bharat LK, Nagaraju G, Krishna KG, Yu JS. Controlled synthesis of yttrium gallium garnet spherical nanostructures modified by silver oxide nanoparticles for enhanced photocatalytic properties. CrystEngComm. 2016;18:8915–8925. doi: 10.1039/C6CE01988A. [DOI] [Google Scholar]
- 16.Dudem B, Bharat LK, Leem JW, Kim DH, Yu JS. Hierarchical Ag/TiO2/Si forest-like nano/micr-architectures as antireflective, plasmonic photocatalytic, and self-cleaning coatings. ACS Sustain. Chem. Eng. 2017;6:1580–1591. doi: 10.1021/acssuschemeng.7b02220. [DOI] [Google Scholar]
- 17.Linic S, Aslam U, Boerigter C, Morabito M. Photochemical transformations on plasmonic metal nanoparticles. Nat. Mater. 2015;14:567–576. doi: 10.1038/nmat4281. [DOI] [PubMed] [Google Scholar]
- 18.Boriskina SV, Ghasemi H, Chen G. Plasmonic materials for energy: from physics to applications. Mater. Today. 2013;16:375–386. doi: 10.1016/j.mattod.2013.09.003. [DOI] [Google Scholar]
- 19.Zhang XM, Chen YL, Liu RS, Tsai DP. Plasmonic photocatalysis. Rep. Prog. Phys. 2013;76:046401. doi: 10.1088/0034-4885/76/4/046401. [DOI] [PubMed] [Google Scholar]
- 20.Wang P, Huang BB, Dai Y, Whangbo MH. Plasmonic photocatalysts: harvesting visible light with noble metal nanoparticles. Phys. Chem. Chem. Phys. 2012;14:9813–9825. doi: 10.1039/c2cp40823f. [DOI] [PubMed] [Google Scholar]
- 21.Ma XC, Dai Y, Yu L, Huang BB. Energy transfer in plasmonic photocatalytic composites. Light-Sci. Appl. 2016;5:e16017. doi: 10.1038/lsa.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KM, Lai CW, Ngai KS, Juan JC. Recent developments of zinc oxide based photocatalyst in water treatment technology: a review. Water Res. 2016;88:428–448. doi: 10.1016/j.watres.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 23.Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009;69:8784–8789. doi: 10.1158/0008-5472.CAN-09-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma VK. Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment—A Review. J. Environ. Sci. Health A. 2009;44:1485–1495. doi: 10.1080/10934520903263231. [DOI] [PubMed] [Google Scholar]
- 25.Sternberg ED, Dolphin D, Bruckner C. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron. 1998;54:4151–4202. doi: 10.1016/S0040-4020(98)00015-5. [DOI] [Google Scholar]
- 26.Ormond AB, Freeman HS. Dye sensitizers for photodynamic therapy. Materials. 2013;6:817–840. doi: 10.3390/ma6030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacombe S, Pigot T. Materials for selective photo-oxygenation vs. photocatalysis: preparation, properties and applications in environmental and health fields. Catal. Sci. Technol. 2016;6:1571–1592. doi: 10.1039/C5CY01929J. [DOI] [Google Scholar]
- 28.Wojtovich AP, Foster TH. Optogenetic control of ROS production. Redox Biol. 2014;2:368–376. doi: 10.1016/j.redox.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson K, Rajfur Z, Vitriol E, Hahn K. Chromophore-assisted laser inactivation in cell biology. Trends Cell Biol. 2008;18:443–450. doi: 10.1016/j.tcb.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pryor WA. Oxy-radicals and related species: their formation, lifetimes, and reactions. Ann. Rev. Physiol. 1986;48:657–667. doi: 10.1146/annurev.ph.48.030186.003301. [DOI] [PubMed] [Google Scholar]
- 32.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 33.Baptista MS, Cadet J, Di Mascio P, Ghogare AA, Greer A, Hamblin MR, Lorente C, Nunez SC, Ribeiro MS, Thomas AH, Vignoni M, Yoshimura TM. Type I and Type II photosensitized oxidation reactions: guidelines and mechanistic pathways. Photochem. Photobiol. 2017;93:912–919. doi: 10.1111/php.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foote CS. Definition of type-I and type-II photosensitized oxidation. Photochem. Photobiol. 1991;54:659. doi: 10.1111/j.1751-1097.1991.tb02071.x. [DOI] [PubMed] [Google Scholar]
- 35.Behler J, Delley B, Lorenz S, Reuter K, Scheffler M. Dissociation of O2 at Al(111): the role of spin selection rules. Phys. Rev. Lett. 2005;94:036104. doi: 10.1103/PhysRevLett.94.036104. [DOI] [PubMed] [Google Scholar]
- 36.Blazquez-Castro A. Direct 1O2 optical excitation: a tool for redox biology. Redox Biol. 2017;13:39–59. doi: 10.1016/j.redox.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boix-Garriga E, Rodriguez-amigo B, Planas O, Nonell S. In: Singlet oxygen: applications in biosciences and nanosciences. Nonell S, Flors C, editors. Cambridge: Royal Society of Chemistry; 2016. p. 25. [Google Scholar]
- 38.Schweitzer C, Schmidt R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 2003;103:1685–1757. doi: 10.1021/cr010371d. [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Koo N, Min DB. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004;3:21–33. doi: 10.1111/j.1541-4337.2004.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 40.Hulten LM, Holmstrom M, Soussi B. Harmful singlet oxygen can be helpful. Free Radic. Biol. Med. 1999;27:1203–1207. doi: 10.1016/S0891-5849(99)00217-8. [DOI] [PubMed] [Google Scholar]
- 41.Lesser MP. Oxidative stress in marine environments: biochemistry and physiological ecology. Ann. Rev. Physiol. 2006;68:253–278. doi: 10.1146/annurev.physiol.68.040104.110001. [DOI] [PubMed] [Google Scholar]
- 42.Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trashin S, Rahemi V, Ramji K, Neven L, Gorun SM, De Wael K. Singlet oxygen-based electrosensing by molecular photosensitizers. Nat. Commun. 2017;8:16108. doi: 10.1038/ncomms16108. [DOI] [Google Scholar]
- 44.Strack RL, Strongin DE, Bhattacharyya D, Tao W, Berman A, Broxmeyer HE, Keenan RJ, Glick BS. A noncytotoxic DsRed variant for whole-cell labeling. Nat. Methods. 2008;5:955–957. doi: 10.1038/nmeth.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chudakov DM, Matz MV, Lukyanov S, Lukyanov KA. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol. Rev. 2010;90:1103–1163. doi: 10.1152/physrev.00038.2009. [DOI] [PubMed] [Google Scholar]
- 46.Smith BN, Banfield BW, Smeraski CA, Wilcox CL, Dudek FE, Enquist LW, Pickard GE. Pseudorabies virus expressing enhanced green fluorescent protein: a tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc. Nat. Acad. Sci. USA. 2000;97:9264–9269. doi: 10.1073/pnas.97.16.9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tour O, Meijer RM, Zacharias DA, Adams SR, Tsien RY. Genetically targeted chromophore-assisted light inactivation. Nat. Biotechnol. 2003;21:1505–1508. doi: 10.1038/nbt914. [DOI] [PubMed] [Google Scholar]
- 48.Zhou ZJ, Song JB, Nie LM, Chen XY. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016;45:6597–6626. doi: 10.1039/C6CS00271D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sano Y, Watanabe W, Matsunaga S. Chromophore-assisted laser inactivation—towards a spatiotemporal-functional analysis of proteins, and the ablation of chromatin, organelle and cell function. J. Cell Sci. 2014;127:1621–1629. doi: 10.1242/jcs.144527. [DOI] [PubMed] [Google Scholar]
- 50.Vegh RB, Bravaya KB, Bloch DA, Bommarius AS, Tolbert LM, Verkhovsky M, Krylov AI, Solntsev KM. Chromophore photoreduction in red fluorescent proteins is responsible for bleaching and phototoxicity. J. Phys. Chem. B. 2014;118:4527–4534. doi: 10.1021/jp500919a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Surrey T, Elowitz MB, Wolf PE, Yang F, Nedelec F, Shokat K, Leibler S. Chromophore-assisted light inactivation and self-organization of microtubules and motors. Proc. Nat. Acad. Sci. USA. 1998;95:4293–4298. doi: 10.1073/pnas.95.8.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qi YCB, Garren EJ, Shu XK, Tsien RY, Jin YS. Photo-inducible cell ablation in Caenorhabditis elegans using the genetically encoded singlet oxygen generating protein miniSOG. Proc. Nat. Acad. Sci. USA. 2012;109:7499–7504. doi: 10.1073/pnas.1204096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mironova KE, Proshkina GM, Ryabova AV, Stremovskiy OA, Lukyanov SA, Petrov RV, Deyev SM. Genetically encoded immunophotosensitizer 4D5scFv-miniSOG is a highly selective agent for targeted photokilling of tumor cells in vitro. Theranostics. 2013;3:831–840. doi: 10.7150/thno.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serebrovskaya EO, Edelweiss EF, Stremovskiy OA, Lukyanov KA, Chudakov DM, Deyev SM. Targeting cancer cells by using an antireceptor antibody-photosensitizer fusion protein. Proc. Nat. Acad. Sci. USA. 2009;106:9221–9225. doi: 10.1073/pnas.0904140106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajfur Z, Roy P, Otey C, Romer L, Jacobson K. Dissecting the link between stress fibres and focal adhesions by CALI with EGFP fusion proteins. Nat. Cell Biol. 2002;4:286–293. doi: 10.1038/ncb772. [DOI] [PubMed] [Google Scholar]
- 56.McLean MA, Rajfur Z, Chen ZZ, Humphrey D, Yang B, Sligar SG, Jacobson K. Mechanism of chromophore assisted laser inactivation employing fluorescent proteins. Anal. Chem. 2009;81:1755–1761. doi: 10.1021/ac801663y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wojtovich AP, Wei AY, Sherman TA, Foster TH, Nehrke K. Chromophore-assisted light inactivation of mitochondrial electron transport chain complex II in caenorhabditis elegans. Sci. Rep. 2016;6:29695. doi: 10.1038/srep29695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bulina ME, Lukyanov KA, Britanova OV, Onichtchouk D, Lukyanov S, Chudakov DM. Chromophore-assisted light inactivation (CALI) using the phototoxic fluorescent protein KillerRed. Nat. Protoc. 2006;1:947–953. doi: 10.1038/nprot.2006.89. [DOI] [PubMed] [Google Scholar]
- 59.Takemoto K, Matsuda T, Sakai N, Fu D, Noda M, Uchiyama S, Kotera I, Arai Y, Horiuchi M, Fukui K, Ayabe T, Inagaki F, Suzuki H, Nagai T. SuperNova, a monomeric photosensitizing fluorescent protein for chromophore-assisted light inactivation. Sci. Rep. 2013;3:2629. doi: 10.1038/srep02629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teh C, Chudakov DM, Poon KL, Mamedov IZ, Sek JY, Shidlovsky K, Lukyanov S, Korzh V. Optogenetic in vivo cell manipulation in KillerRed-expressing zebrafish transgenics. BMC Dev. Biol. 2010;10:110. doi: 10.1186/1471-213X-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jewhurst K, Levin M, Mclaughlin KA. Optogenetic control of apoptosis in targeted tissues of Xenopus laevis embryos. J. Cell Death. 2014;7:25–31. doi: 10.4137/JCD.S18368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greenbaum L, Rothmann C, Lavie R, Malik Z. Green fluorescent protein photobleaching: a model for protein damage by endogenous and exogenous singlet oxygen. Biol. Chem. 2000;381:1251–1258. doi: 10.1515/BC.2000.153. [DOI] [PubMed] [Google Scholar]
- 63.Jimenez-Banzo A, Nonell S, Hofkens J, Flors C. Singlet oxygen photosensitization by EGFP and its chromophore HBDI. Biophys. J. 2008;94:168–172. doi: 10.1529/biophysj.107.107128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shu XK, Lev-Ram V, Deerinck TJ, Qi YC, Ramko EB, Davidson MW, Jin YS, Ellisman MH, Tsien RY. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011;9:e1001041. doi: 10.1371/journal.pbio.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruiz-Gonzalez R, Cortajarena AL, Mejias SH, Agut M, Nonell S, Flors C. Singlet oxygen generation by the genetically encoded tag miniSOG. J. Am. Chem. Soc. 2013;135:9564–9567. doi: 10.1021/ja4020524. [DOI] [PubMed] [Google Scholar]
- 66.Westberg M, Bregnhoj M, Etzerodt M, Ogilby PR. No photon wasted: an efficient and selective singlet oxygen photosensitizing protein. J. Phys. Chem. B. 2017;121:9366–9371. doi: 10.1021/acs.jpcb.7b07831. [DOI] [PubMed] [Google Scholar]
- 67.Torra J, Burgos-Caminal A, Endres S, Wingen M, Drepper T, Gensch T, Ruiz-Gonzalez R, Nonell S. Singlet oxygen photosensitisation by the fluorescent protein Pp2FbFP L30 M, a novel derivative of Pseudomonas putida flavin-binding Pp2FbFP. Photochem. Photobiol. Sci. 2015;14:280–287. doi: 10.1039/C4PP00338A. [DOI] [PubMed] [Google Scholar]
- 68.Vegh RB, Solntsev KM, Kuimova MK, Cho S, Liang Y, Loo BLW, Tolbert LM, Bommarius AS. Reactive oxygen species in photochemistry of the red fluorescent protein “Killer Red”. Chem. Commun. 2011;47:4887–4889. doi: 10.1039/c0cc05713d. [DOI] [PubMed] [Google Scholar]
- 69.Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, Merzlyak EM, Shkrob MA, Lukyanov S, Lukyanov KA. A genetically encoded photosensitizer. Nat. Biotechnol. 2006;24:95–99. doi: 10.1038/nbt1175. [DOI] [PubMed] [Google Scholar]
- 70.Ragas X, Cooper LP, White JH, Nonell S, Flors C. Quantification of photosensitized singlet oxygen production by a fluorescent protein. ChemPhysChem. 2011;12:161–165. doi: 10.1002/cphc.201000919. [DOI] [PubMed] [Google Scholar]
- 71.Leem JW, Park J, Kim SW, Kim SR, Choi SH, Choi KH, Kim YL. Green light-activated photoreaction via genetic hybridization of far-red fluorescent protein and silk. Adv. Sci. 2018 doi: 10.1002/advs.201700863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimomura O. The discovery of aequorin and green fluorescent protein. J. Microsc. Oxf. 2005;217:3–15. doi: 10.1111/j.0022-2720.2005.01441.x. [DOI] [PubMed] [Google Scholar]
- 73.Day RN, Davidson MW. The fluorescent protein palette: tools for cellular imaging. Chem. Soc. Rev. 2009;38:2887–2921. doi: 10.1039/b901966a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi JW, Nam YS, Park SJ, Lee WH, Kim D, Fujihira M. Rectified photocurrent of molecular photodiode consisting of cytochrome c/GFP hetero thin films. Biosens. Bioelectron. 2001;16:819–825. doi: 10.1016/S0956-5663(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 75.Choi JW, Nam YS, Lee WH, Kim D, Fujihira M. Rectified photocurrent of the protein-based bio-photodiode. Appl. Phys. Lett. 2001;79:1570–1572. doi: 10.1063/1.1399308. [DOI] [Google Scholar]
- 76.Deepankumar K, George A, Priya GK, Ilamaran M, Kamini NR, Senthil TS, Easwaramoorthi S, Ayyadurai N. Next generation designed protein as a photosensitizer for biophotovoltaics prepared by expanding the genetic code. ACS Sustain. Chem. Eng. 2017;5:72–77. doi: 10.1021/acssuschemeng.6b01975. [DOI] [Google Scholar]
- 77.Chirgwandi ZG, Panas I, Johansson LG, Norden B, Willander M, Winkler D, Agren H. Properties of a biophotovoltaic nanodevice. J. Phys. Chem. C. 2008;112:18717–18721. doi: 10.1021/jp807925k. [DOI] [Google Scholar]
- 78.Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, Cormier MJ. Primary structure of the Aequorea victoria green-rluorescent protein. Gene. 1992;111:229–233. doi: 10.1016/0378-1119(92)90691-H. [DOI] [PubMed] [Google Scholar]
- 79.van Thor JJ. Photoreactions and dynamics of the green fluorescent protein. Chem. Soc. Rev. 2009;38:2935–2950. doi: 10.1039/b820275n. [DOI] [PubMed] [Google Scholar]
- 80.Jimenez-Banzo A, Ragas X, Abbruzzetti S, Viappiani C, Campanini B, Flors C, Nonell S. Singlet oxygen photosensitisation by GFP mutants: oxygen accessibility to the chromophore. Photochem. Photobiol. Sci. 2010;9:1336–1341. doi: 10.1039/c0pp00125b. [DOI] [PubMed] [Google Scholar]
- 81.Qin M, Hah HJ, Kim G, Nie GC, Lee YEK, Kopelman R. Methylene blue covalently loaded polyacrylamide nanoparticles for enhanced tumor-targeted photodynamic therapy. Photochem. Photobiol. Sci. 2011;10:832–841. doi: 10.1039/c1pp05022b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pimenta FM, Jensen RL, Breitenbach T, Etzerodt M, Ogilby PR. Oxygen-dependent photochemistry and photophysics of “MiniSOG,” a protein-encased flavin. Photochem. Photobiol. 2013;89:1116–1126. doi: 10.1111/php.12111. [DOI] [PubMed] [Google Scholar]
- 83.Swiss-model http://swissmodel.expasy.org. Accessed 1 Jan 2018
- 84.Pletnev S, Gurskaya NG, Pletneva NV, Lukyanov KA, Chudakov DM, Martynov VI, Popov VO, Kovalchuk MV, Wlodawer A, Dauter Z, Pletnev V. Structural basis for phototoxicity of the genetically encoded photosensitizer KillerRed. J. Biol. Chem. 2009;284:32028–32039. doi: 10.1074/jbc.M109.054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carpentier P, Violot S, Blanchoin L, Bourgeois D. Structural basis for the phototoxicity of the fluorescent protein KillerRed. FEBS Lett. 2009;583:2839–2842. doi: 10.1016/j.febslet.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 86.Roy A, Carpentier P, Bourgeois D, Field M. Diffusion pathways of oxygen species in the phototoxic fluorescent protein KillerRed. Photochem. Photobiol. Sci. 2010;9:1342–1350. doi: 10.1039/c0pp00141d. [DOI] [PubMed] [Google Scholar]
- 87.Blough NV, Simpson DJ. Chemically mediated fluorescence yield switching in nitroxide fluorophore adducts—Optical sensors of radical redox reactions. J. Am. Chem. Soc. 1988;110:1915–1917. doi: 10.1021/ja00214a041. [DOI] [Google Scholar]
- 88.Cohn CA, Simon SR, Schoonen MAA. Comparison of fluorescence-based techniques for the quantification of particle-induced hydroxyl radicals. Part. Fibre Toxicol. 2008;5:2. doi: 10.1186/1743-8977-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buettner GR. The spin-trapping of superoxide and hydroxyl free-radicals with DMPO (5,5-dimethylpyrroline-n-oxide)—more about iron. Free Rad. Res. Commun. 1993;19:S79–S87. doi: 10.3109/10715769309056s79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Menezes SL, Augusto O. EPR detection of glutathionyl and protein-tyrosyl radicals during the interaction of peroxynitrite with macrophages (J774) J. Biol. Chem. 2001;276:39879–39884. doi: 10.1074/jbc.M104012200. [DOI] [PubMed] [Google Scholar]
- 91.Bucana C, Saiki I, Nayar R. Uptake and accumulation of the vital dye hydroethidine in neoplastic cells. J. Histochem. Cytochem. 1986;34:1109–1115. doi: 10.1177/34.9.2426339. [DOI] [PubMed] [Google Scholar]
- 92.Lu JM, Lin PH, Yao QZ, Chen CY. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J. Cell Mol. Med. 2010;14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Zyl WF, Deane SM, Dicks LMT. Use of the mCherry fluorescent protein to study intestinal colonization by Enterococcus mundtii ST4SA and Lactobacillus plantarum 423 in mice. Appl. Environ. Microbiol. 2015;81:5993–6002. doi: 10.1128/AEM.01247-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shaner NC, Campbell RE, Steinbach PA, Giepmans BNG, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 95.Chapagain PP, Regmi CK, Castillo W. Fluorescent protein barrel fluctuations and oxygen diffusion pathways in mCherry. J. Chem. Phys. 2011;135:235101. doi: 10.1063/1.3660197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Merzlyak EM, Goedhart J, Shcherbo D, Bulina ME, Shcheglov AS, Fradkov AF, Gaintzeva A, Lukyanov KA, Lukyanov S, Gadella TWJ, Chudakov DM. Bright monomeric red fluorescent protein with an extended fluorescence lifetime. Nat. Methods. 2007;4:555–557. doi: 10.1038/nmeth1062. [DOI] [PubMed] [Google Scholar]
- 97.Subach OM, Malashkevich VN, Zencheck WD, Morozova KS, Piatkevich KD, Almo SC, Verkhusha VV. Structural characterization of acylimine-containing blue and red chromophores in mTagBFP and TagRFP fluorescent proteins. Chem. Biol. 2010;17:333–341. doi: 10.1016/j.chembiol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Flors C, Fryer MJ, Waring J, Reeder B, Bechtold U, Mullineaux PM, Nonell S, Wilson MT, Baker NR. Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, Singlet Oxygen Sensor Green®. J. Exper. Botany. 2006;57:1725–1734. doi: 10.1093/jxb/erj181. [DOI] [PubMed] [Google Scholar]
- 99.Ragas X, Jimenez-Banzo A, Sanchez-Garcia D, Batllori X, Nonell S. Singlet oxygen photosensitisation by the fluorescent probe Singlet Oxygen Sensor Green®. Chem. Commun. 2009;20:2920–2922. doi: 10.1039/b822776d. [DOI] [PubMed] [Google Scholar]
- 100.Shcherbo D, Murphy CS, Ermakova GV, Solovieva EA, Chepurnykh TV, Shcheglov AS, Verkhusha VV, Pletnev VZ, Hazelwood KL, Roche PM, Lukyanov S, Zaraisky AG, Davidson MW, Chudakov DM. Far-red fluorescent tags for protein imaging in living tissues. Biochem. J. 2009;418:567–574. doi: 10.1042/BJ20081949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Strack RL, Hein B, Bhattacharyya D, Hell SW, Keenan RJ, Glick BS. A rapidly maturing far-red derivative of DsRed-Express2 for whole-cell labeling. Biochemistry. 2009;48:8179–8281. doi: 10.1021/bi900572b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pletnev S, Shcherbo D, Chudakov DM, Pletneva N, Merzlyak EM, Wlodawer A, Dauter Z, Pletnev V. A crystallographic study of bright far-red fluorescent protein mKate reveals pH-induced cis-trans isomerization of the chromophore. J. Biol. Chem. 2008;283:28980–28987. doi: 10.1074/jbc.M800599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leem JW, Choi SH, Kim SR, Kim SW, Choi KH, Kim YL. Scalable and continuous nanomaterial integration with transgenic fibers for enhanced photoluminescence. Mater. Horiz. 2017;4:281–289. doi: 10.1039/C6MH00423G. [DOI] [Google Scholar]
- 104.Zeng LY, Luo LJ, Pan YW, Luo S, Lu GM, Wu AG. In vivo targeted magnetic resonance imaging and visualized photodynamic therapy in deep-tissue cancers using folic acid-functionalized superparamagnetic-upconversion nanocomposites. Nanoscale. 2015;7:8946–8954. doi: 10.1039/C5NR01932J. [DOI] [PubMed] [Google Scholar]
- 105.Fang CH, Jia HL, Chang S, Ruan QF, Wang P, Chen T, Wang JF. (Gold core)/(titania shell) nanostructures for plasmon-enhanced photon harvesting and generation of reactive oxygen species. Energy Environ. Sci. 2014;7:3431–3438. doi: 10.1039/C4EE01787K. [DOI] [Google Scholar]
- 106.Stiel AC, Trowitzsch S, Weber G, Andresen M, Eggeling C, Hell SW, Jakobs S, Wahl MC. 1.8 angstrom bright-state structure of the reversibly switchable fluorescent protein Dronpa guides the generation of fast switching variants. Biochem. J. 2007;402:35–42. doi: 10.1042/BJ20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Evdokimov AG, Pokross ME, Egorov NS, Zaraisky AG, Yampolsky IV, Merzlyak EM, Shkoporov AN, Sander I, Lukyanov KA, Chudakov DM. Structural basis for the fast maturation of Arthropoda green fluorescent protein. EMBO Rep. 2006;7:1006–1012. doi: 10.1038/sj.embor.7400787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pletneva NV, Pletnev VZ, Sarkisyan KS, Gorbachev DA, Egorov ES, Mishin AS, Lukyanov KA, Dauter Z, Pletnev S. Crystal structure of phototoxic orange fluorescent proteins with a tryptophan-based chromophore. PLoS ONE. 2015;10:e0145740. doi: 10.1371/journal.pone.0145740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pletneva N, Pletnev V, Tikhonova T, Pakhomov AA, Popov V, Martynov VI, Wlodawer A, Dauter Z, Pletnev S. Refined crystal structures of red and green fluorescent proteins from the button polyp Zoanthus. Acta Crystallogr. D. 2007;63:1082–1093. doi: 10.1107/S0907444907042461. [DOI] [PubMed] [Google Scholar]
- 110.Sarkisyan KS, Zlobovskaya OA, Gorbachev DA, Bozhanova NG, Sharonov GV, Staroverov DB, Egorov ES, Ryabova AV, Solntsev KM, Mishin AS, Lukyanov KA. KillerOrange, a genetically encoded photosensitizer activated by blue and green light. PLoS ONE. 2015;10:e0145287. doi: 10.1371/journal.pone.0145287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wardlaw JL, Sullivan TJ, Lux CN, Austin FW. Photodynamic therapy against common bacteria causing wound and skin infections. Vet. J. 2012;192:374–377. doi: 10.1016/j.tvjl.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 112.Yin R, Dai TH, Avci P, Jorge AES, de Melo WCMA, Vecchio D, Huang YY, Gupta A, Hamblin MR. Light based anti-infectives: ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr. Opin. Pharmacol. 2013;13:731–762. doi: 10.1016/j.coph.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fagan R, McCormack DE, Dionysiou DD, Pillai SC. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicon. Proc. 2016;42:2–14. doi: 10.1016/j.mssp.2015.07.052. [DOI] [Google Scholar]
- 114.Wigginton KR, Pecson BM, Sigstam T, Bosshard F, Kohn T. Virus inactivation mechanisms: impact of disinfectants on virus function and structural integrity. Environ. Sci. Technol. 2012;46:12069–12078. doi: 10.1021/es3029473. [DOI] [PubMed] [Google Scholar]
- 115.Nakano R, Hara M, Ishiguro H, Yao YY, Ochiai T, Nakata K, Murakami T, Kajioka J, Sunada K, Hashimoto K, Fujishima A, Kubota Y. Broad spectrum microbicidal activity of photocatalysis by TiO2. Catalysts. 2013;3:310–323. doi: 10.3390/catal3010310. [DOI] [Google Scholar]
- 116.Hajkova P, Spatenka P, Horsky J, Horska I, Kolouch A. Photocatalytic effect of TiO2 films on viruses and bacteria. Plasma Process. Polym. 2007;4:S397–S401. doi: 10.1002/ppap.200731007. [DOI] [Google Scholar]
- 117.Liga MV, Maguire-Boyle SJ, Jafry HR, Barron AR, Li QL. Silica decorated TiO2 for virus inactivation in drinking water—Simple synthesis method and mechanisms of enhanced inactivation kinetics. Environ. Sci. Technol. 2013;47:6463–6470. doi: 10.1021/es400196p. [DOI] [PubMed] [Google Scholar]
- 118.Alves E, Faustino MAF, Neves MGPMS, Cunha A, Nadais H, Almeida A. Potential applications of porphyrins in photodynamic inactivation beyond the medical scope. J. Photochem. Photobiol. C. 2015;22:34–57. doi: 10.1016/j.jphotochemrev.2014.09.003. [DOI] [Google Scholar]
- 119.Ben Amor T, Jori G. Sunlight-activated insecticides: historical background and mechanisms of phototoxic activity. Insect Biochem. Mol. Biol. 2000;30:915–925. doi: 10.1016/S0965-1748(00)00072-2. [DOI] [PubMed] [Google Scholar]
- 120.Bonnett R, Krysteva MA, Lalov IG, Artarsky SV. Water disinfection using photosensitizers immobilized on chitosan. Water Res. 2006;40:1269–1275. doi: 10.1016/j.watres.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 121.Jori G, Magaraggia M, Fabris C, Soncin M, Camerin M, Tallandini L, Coppellotti O, Guidolin L. Photodynamic inactivation of microbial pathogens: disinfection of water and prevention of water-borne diseases. J. Environ. Pathol. Toxicol. Oncol. 2011;30:261–271. doi: 10.1615/JEnvironPatholToxicolOncol.v30.i3.90. [DOI] [PubMed] [Google Scholar]
- 122.Kim DW, Lee OJ, Kim SW, Ki CS, Chao JR, Yoo H, Yoon SI, Lee JE, Park YR, Kweon H, Lee KG, Kaplan DL, Park CH. Novel fabrication of fluorescent silk utilized in biotechnological and medical applications. Biomaterials. 2015;70:48–56. doi: 10.1016/j.biomaterials.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 123.Iizuka T, Sezutsu H, Tatematsu K, Kobayashi I, Yonemura N, Uchino K, Nakajima K, Kojima K, Takabayashi C, Machii H, Yamada K, Kurihara H, Asakura T, Nakazawa Y, Miyawaki A, Karasawa S, Kobayashi H, Yamaguchi J, Kuwabara N, Nakamura T, Yoshii K, Tamura T. Colored fluorescent silk made by transgenic silkworms. Adv. Funct. Mater. 2013;23:5232–5239. doi: 10.1002/adfm.201300365. [DOI] [Google Scholar]
- 124.Teule F, Miao YG, Sohn BH, Kim YS, Hull JJ, Fraser MJ, Lewis RV, Jarvis DL. Silkworms transformed with chimeric silkworm/spider silk genes spin composite silk fibers with improved mechanical properties. Proc. Nat. Acad. Sci. USA. 2012;109:923–928. doi: 10.1073/pnas.1109420109. [DOI] [PMC free article] [PubMed] [Google Scholar]


