Abstract
Colistin represents one of the few available drugs for treating infections caused by carbapenem-resistant Enterobacteriaceae. As such, the recent plasmid-mediated spread of the colistin resistance gene mcr-1 poses a significant public health threat, requiring global monitoring and surveillance. Here, we characterize the global distribution of mcr-1 using a data set of 457 mcr-1-positive sequenced isolates. We find mcr-1 in various plasmid types but identify an immediate background common to all mcr-1 sequences. Our analyses establish that all mcr-1 elements in circulation descend from the same initial mobilization of mcr-1 by an ISApl1 transposon in the mid 2000s (2002–2008; 95% highest posterior density), followed by a marked demographic expansion, which led to its current global distribution. Our results provide the first systematic phylogenetic analysis of the origin and spread of mcr-1, and emphasize the importance of understanding the movement of antibiotic resistance genes across multiple levels of genomic organization.
The recent plasmid-mediated spread of the mobilized colistin resistance gene mcr-1 poses a significant public health threat, requiring worldwide monitoring and surveillance. Here, Wang et al. compile and analyze a data set of 457 mcr-1-positive sequenced isolates to investigate the origin and global distribution of mcr-1.
Introduction
Colistin was largely abandoned as a treatment for bacterial infections in the 1970s owing to its toxicity and low renal clearance, but has been reintroduced in recent years as an antibiotic of ‘last resort’ against multi-drug-resistant infections1. It is therefore alarming that the prevalence of resistance to colistin has become a significant concern, following the identification of plasmid-mediated colistin resistance conferred by the mcr-1 gene in late 20152. Colistin resistance is emblematic of the growing problems of antimicrobial resistance worldwide.
Up until 2015, resistance to colistin had only been linked to mutational and regulatory changes mediated by chromosomal genes3,4. The mobilized colistin gene mcr-1 was first described in a plasmid carried by an Escherichia coli isolated in China in April 20112. The presence of colistin resistance on mobile genetic elements poses a significant public health risk, as these can spread rapidly by horizontal transfer, and may entail a lower fitness cost5. At the time of writing, mcr-1 has been identified in numerous countries across five continents. Significantly, mcr-1 has also been observed on plasmids containing other antimicrobial resistance genes such as carbapenemases6–8 and extended-spectrum β-lactamases9–11.
The mcr-1 element has been characterized in a variety of genomic backgrounds, consistent with the gene being mobilized by a transposon12–16. To date, mcr-1 has been observed on a wide variety of plasmid types, including IncI2, IncHI2, and IncX417. Intensive screening efforts for mcr-1 have found it to be highly prevalent in a number of environmental settings, including the Haihe River in China18, recreational water at public urban beaches in Brazil19, and fecal samples from otherwise healthy individuals20,21. Although both Brazil and China have now banned the use of colistin in agriculture, the evidence that mcr-1 can spread within hospital environments even in the absence of colistin use22 as well as in the community21 raises the possibility that the spread of mcr-1 will not be contained by these bans.
The global distribution of mcr-1 over at least five continents is well documented, but little is known about its origin, acquisition, emergence, and spread. In this study, we aim to shed light on these fundamental issues using whole-genome sequencing (WGS) data from 110 novel mcr-1-positive isolates from China in conjunction with an extensive collection of publicly available sequence data sourced from the NCBI repository as well as the Short Read Archive (SRA).
Our data and analyses support an initial single mobilization event of mcr-1 by an ISApl1-mcr-1-orf-ISApl1 transposon around 2006. The transposon was immobilized on several plasmid backgrounds following the loss of the flanking ISApl1 elements, and spread through plasmid transfer. The current distribution of mcr-1 points to a possible origin in Chinese livestock. Our results illustrate the complex dynamics of antibiotic resistance genes across multiple embedded genetic levels (transposons, plasmids, bacterial lineages and bacterial species), previously described as a nested ‘Russian doll’ model of genetic mobility23.
Results
Data set
We compiled a global data set of 457 mcr-1-positive isolates (Fig. 1a), including 110 new WGS from China, of which 107 were sequenced with Illumina short reads and three with PacBio long-read technology. One hundred and ninety-five isolates were sourced from publicly available assemblies in the NCBI GenBank repository (73 completed plasmids, 1 complete chromosome, 121 assemblies). A further 152 sequences were sourced from the NCBI SRA, after being identified as mcr-1-positive using a k-mer index of a snapshot of the SRA as of December 2016 (see Methods). The whole data set consists of 256 short-read data sets, 6 long-read PacBio WGS, 121 draft assemblies, and 74 completed assemblies. Accession numbers and metadata for the 457 isolates are provided in supplementary data 1.
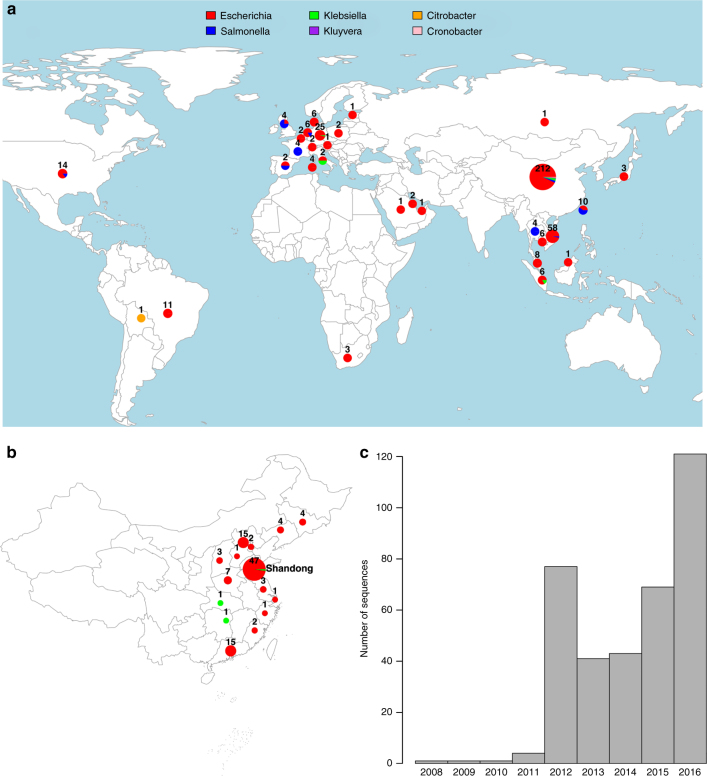
Fig. 1.
Overview of the mcr-1-positive isolates included. a Global map of mcr-1-positive isolates included colored by genus with the number and size of pies providing the sample size per location; b Map of novel Chinese isolates sequenced for this study; c Histogram of sampling dates (years) of the isolates. Maps were created using the R package rworldmap using the public domain Natural Earth data set
Isolates carrying mcr-1 were identified from 31 countries (Fig. 1a). The countries with the largest numbers of mcr-1-positive samples are China (212), Vietnam (58) and Germany (25). Within China, nearly half (45%) of positive isolates stem from the Shandong province (Fig. 1b). The vast majority of mcr-1-positive isolates belong to E. coli (411), but the data set also comprises mcr-1-positive isolates from another seven bacterial species: Salmonella enterica (29), Klebsiella pneumoniae (8), Escherichia fergusonii (2), Kluyvera ascorbata (2), Citrobacter braakii (2), Cronobacter sakazakii (1), and Klebsiella aerogenes (1) (Fig. 1a). The majority of isolates for which sampling dates were available (80%), were collected between 2012 and 2016, with the oldest available isolates dating back to 2008 (Fig. 1c). Isolates with metadata on the sample source (n = 360) came from a range of animal (n = 222), human (n = 108) and environmental (n = 30) hosts.
The large number of mcr-1-positive isolates from China, and the high incidence in the Shandong province can be largely ascribed to the inclusion of our 110 newly sequenced isolates including 49 from Shandong and to another 37 isolates from a previous large sequencing effort13. However, even after discounting the isolates from these two sources, China remains, together with Vietnam one of the two countries with the highest number of sequenced mcr-1-positive isolates.
Evolutionary model
It has been proposed that mcr-1 is mobilized by a composite transposon formed of a ~2600 bp region containing mcr-1 (1626 bp) and a putative open reading frame encoding a PAP2 superfamily protein (765 bp), flanked by two ISApl1 insertion sequences12. ISApl1 is a member of the IS30 family of insertion sequences, which utilize a ‘copy-out, paste-in’ mechanism with a targeted transposition pathway requiring the formation of a synaptic complex between an inverted repeat (IR) in the transposon circle and an IR-like sequence in the target. Snesrud and colleagues12 hypothesized that after the initial formation of such a composite transposon, these insertion sequences would have been lost over time, leading to the stabilization of mcr-1 in a diverse range of plasmid backgrounds (Fig. 2). In the following, we sought to test this model by performing an explicit phylogenetic analysis of the region surrounding mcr-1 using our comprehensive global data set.
Fig. 2.
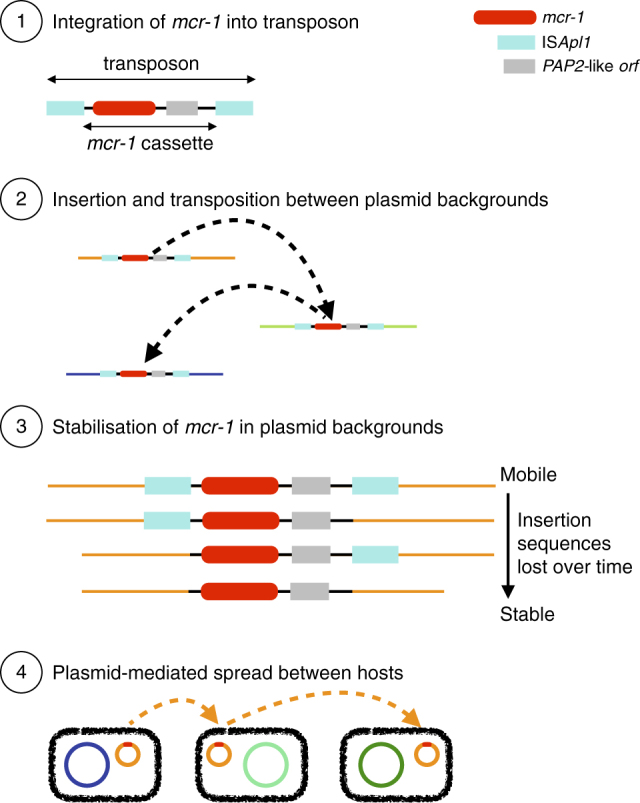
Schematic representation of the evolutionary model for the steps in the spread of the mcr-1 gene. (1) The formation of the original composite transposon, followed by (2) transposition between plasmid backgrounds and (3) stabilization via loss of ISApl1 elements before (4) plasmid-mediated spread
Immediate genomic background of mcr-1
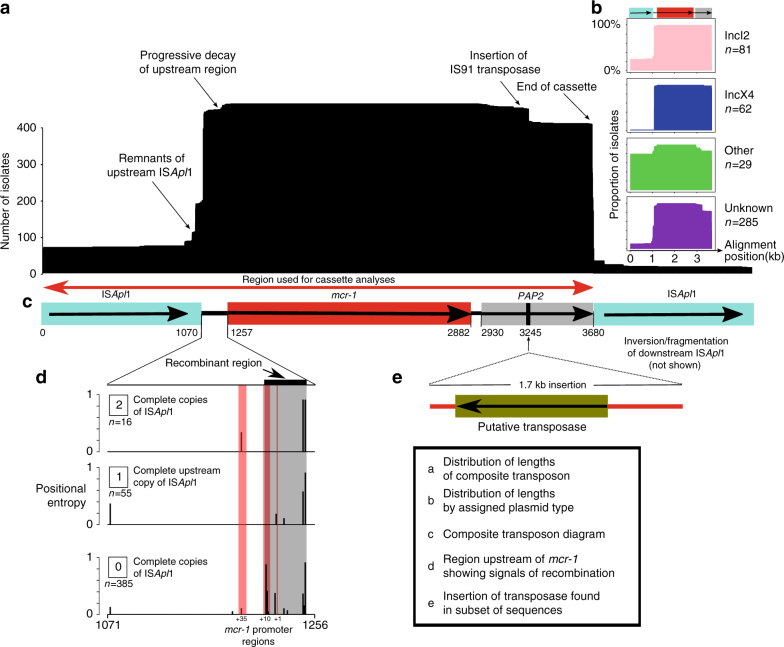
If there had been a unique formation event for the composite transposon, followed by progressive transposition and loss of insertion sequences, we would expect to be able to identify a common immediate background region for mcr-1 in all samples. Indeed, we were able to identify and align a shared region or remnants of it in all 457 sequences surrounding mcr-1 (see Methods), supporting a single common origin for all mcr-1 elements sequenced to date (Fig. 3a). The majority of the sequences contained no trace of ISApl1 (n = 260) indicating that the mcr-1 transposon had been completely stabilized in their genomic background. Forty-two sequences contained indication of the presence of ISApl1 both upstream and downstream, either in full copies (n = 16), a full copy upstream and a partial copy downstream (n = 7), a partial copy upstream and a full copy downstream (n = 1), or partial copies upstream and downstream (n = 18). Some sequences only had ISApl1 present upstream as a complete (n = 55) or partial (n = 99) sequence, and one sequence had only a partial downstream ISApl1 element. The downstream copy of ISApl1 was inverted in some sequences (n = 3) and some sequences had full copies of ISApl1 present elsewhere on the same contig (n = 7), consistent with its high observed activity in transposition24.
Fig. 3.
The genetic element carrying mcr-1 is a composite transposon and is alignable across our global data set. a Length distribution of the alignment across sequences. b Length distribution subset by plasmid type. c The composite transposon, consisting of ISApl1, mcr-1, a PAP2 orf, and ISApl1. The region indicated by the red arrow was used in phylogenetic analyses, after the removal of recombination. d The 186 bp region upstream of mcr-1 showed strong signals of recombination (gray box) that coincided with the promotor regions of mcr-1 (red box), and this diverse region was removed from the subsequent alignment. e Twenty-eight sequences from Vietnam had a 1.7 kb insertion containing a putative transpose, suggesting subsequent rearrangement after initial mobilization
Further inspection of the transposon alignment revealed that the 186 bp region between the 3’-end of the upstream ISApl1 and mcr-1 contained IR-like sequences similar to the IRR and IRL of ISApl1 (respectively: 93–142 bp, 23/50 identity; and 125–175 bp, 21/50 identity). The most variable positions in this 186 bp region were at 177 bp and 142 bp, approximately coinciding with the end of the alignment with the IRs and were more variable in sequences lacking ISApl1, suggesting possible loss of function of the transposition pathway associated with ISApl1 (Fig. 3d). Some of these SNPs occurred in a stretch previously identified as the promoter region for mcr-125, and this region showed strong signals of recombination. A small number of sequences (3%) had SNPs present in mcr-1 itself. These tended to be at the upstream/5’-end of the sequence, particularly in the first three positions. A subset of the sequences from Vietnam (n = 28) included a secondary 1.7 kb insertion downstream of mcr-1 containing a putative transposase, indicating subsequent rearrangements involving this region after initial mobilization of the transposon (Fig. 3e).
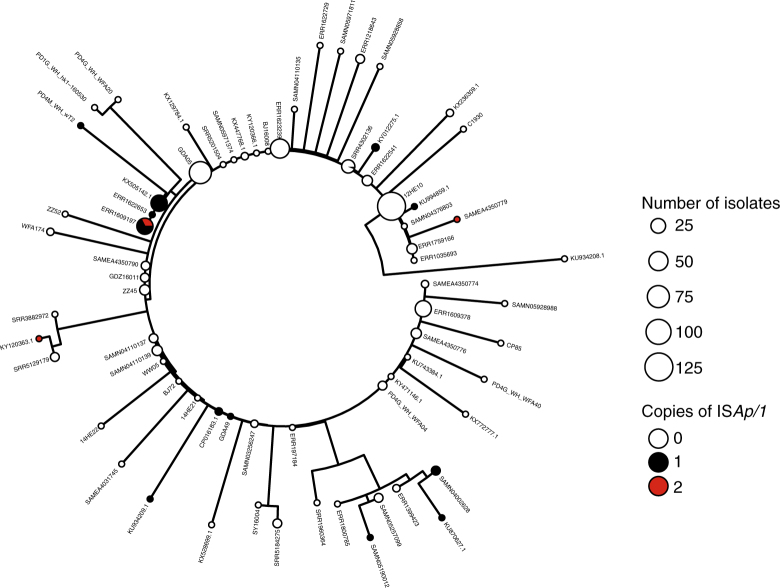
To reconstruct the phylogenetic history of the composite mcr-1 transposon, we created a sequence alignment for 457 sequences (Fig. 3c) after removal of recombinant regions identified with ClonalFrameML, including the region immediately upstream of mcr-1 between positions 1212–1247 (Fig. 3d). A midpoint-rooted maximum parsimony phylogeny showed that there was a dominant sequence type with subsequent diversification, likely indicating the ancestral form of the composite transposon (Fig. 4). There was no discernible clustering of isolates by bacterial species (Supplementary Fig. 1) or sample source (Supplementary Fig. 2), suggesting the composite transposon does not evolve differently in these different backgrounds.
Fig. 4.
Phylogeny of the mcr-1 composite transposon indicates a dominant sequence type with subsequent diversification. Midpoint-rooted maximum parsimony phylogeny based on the 3522 bp alignment of 457 sequences (recombinant regions removed). Size of points indicates the number of identical sequences, with a representative sequence for each shown next to each tip
A Bayesian dating approach (BEAST) was applied to infer a timed phylogeny of the maximal alignable region of the mcr-1 carrying transposon (see Methods). Based on this 3522 site alignment we infer a common ancestor for 364 dated isolates in 2006 (Supplementary Fig. 3; 2002–2008 95% highest poster density (HPD) with a strict clock and coalescent model) with a mutation rate around 7.51 × 10−5 substitutions per site per year (Supplementary Table 1). There was no clear overall geographic clustering in the Maximum Clade Credibility (MCC) tree (Supplementary Figure 4).
Wider genomic background of mcr-1
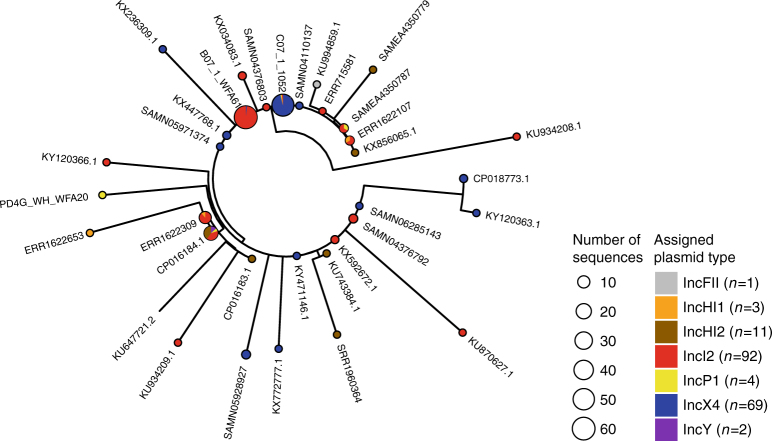
Next, we explored the wider genomic background upstream and downstream of the conserved transposon sequences. We had sufficiently long assembled contigs for 182 isolates to identify plasmid types based on co-occurrence with plasmid replicons (see Methods) and identified mcr-1 in 13 different plasmid backgrounds. IncI2 and IncX4 were the dominant plasmid types, accounting for 51 and 38% of the isolates, respectively (Fig. 5) similar to the proportions observed by Matamoros et al.15. One isolate in our data set was definitively located on a complete chromosome. Though, we cannot rule out the presence of a few other chromosomal copies of mcr-1 located on short contigs.
Fig. 5.
The distribution of plasmid types shown on the transposon phylogeny. Maximum parsimony tree (homoplastic sites removed, midpoint rooted, as in Fig. 4) based on the composite transposon alignment for 172 sequences containing a plasmid replicon on the same contig i.e. those with an assigned plasmid type (color). IncI2 and IncX4 are the most common plasmid types. An example sequence ID is shown for each unique sequence
The distribution of transposons carrying one or two copies of ISApl1 was highly heterogeneous across these plasmid types. For example, sequences with one or two copies of ISApl1 were found on six and four types, respectively, which supports their mobility compared with those without ISApl1, which were found in five plasmid types. Of the contigs carrying one copy of ISApl1, 61% were found in IncI2 plasmids, and 50% of contigs carrying two copies of ISApl1 belonged to IncHI2 plasmids. Conversely, the common IncX4 plasmids carried only two transposons with two copies of ISApl1 and none with a single copy of the element.
We identified two extended plasmid backbone sequences that could be aligned. The first such alignment encompasses a shared sequence of 7161 bp between 108 plasmid backgrounds and has been previously referred to as ‘Type A’13. These sequences contain 54 sequences co-occurring with an IncI2 replicon, with 54 of unknown plasmid type, and encompass a large fraction of the genetic diversity found in the mcr-1 transposon, although a large proportion (9/108) belonged to the dominant sequence type (i.e., B07_1_WFA61 in Fig. 4). The second alignment is 34,761 bp long and is common to nine IncX4 plasmids and partly overlaps with a background previously defined as ‘Type D’13.
We applied BEAST to infer a timed phylogeny for each of these alignable regions after removal of SNPs showing evidence of recombination. For the IncI2 background we infer that a common ancestor to all 108 isolates existed in 2006 (1998–2010 95% CI relaxed exponential clock model) assuming a constant population size model (Supplementary Fig. 5). For the IncX4 backgrounds we dated the common ancestor of the eight isolates to 2011 (2010–2013 95% CI relaxed exponential clock model) assuming a constant population size model (Supplementary Fig. 6). Posterior density distributions of root dating under different population models are shown in Supplementary Figs. 7–8. The difference in dating inferred for these two plasmid backgrounds and the recent date obtained for IncX4 highlight the dynamic nature of the integration of the mcr-1 carrying transposon, even if in the IncX4 phylogeny isolates from East Asia and Europe and the Americas cluster together. The inferred mutation rates obtained for the IncI2 and IncX4 backgrounds consistently lie around 5–10 × 10−5 substitutions per site per year (Supplementary Table 1).
Environmental distribution of the composite mcr-1 transposon
It has been suggested that agricultural use of colistin, as has been widespread in China since the early 1980s21, caused the initial emergence and spread of mcr-126,27. According to the evolutionary model in Fig. 2, the ancestral mobilizable state is represented by the transposon carrying both its ISApl1 elements. The transposon is thought to lose its capability for mobilization after the loss of both ISApl1 elements, although a single copy is reportedly sufficient to keep some ability to mobilize, with the upstream copy being functionally more important12. Comparing human (n = 108) and non-human (n = 252) isolates, there were significantly more sequences with some trace of the insertion sequence ISApl1 both upstream and downstream in non-human isolates (32/220 vs. 5/108, χ2-test, p = 0.033). This comparison held when only comparing agricultural isolates to human isolates (n = 213) (28/213 vs. 5/108, χ2-test, p = 0.029). Furthermore, of the 42 isolates that had ISApl1 fragments both upstream and downstream, the majority were from Asia (n = 30) with only a quarter from Europe (n = 10) (χ2 test, p = 0.12). This result is not driven by an over representation of agricultural isolates from Asia in our data set (χ2 test, p = 0.38).
Discussion
We assembled a global data set of 457 mcr-1-positive sequenced isolates and could show that there was a single integration event of mcr-1 into an ISApl1 composite transposon, followed by its subsequent spread between multiple genomic backgrounds. Our phylogenetic analyses point to a date for the insertion of mcr-1 into the gene transposon shared across our isolates in the mid 2000s (2002–2008 95% HPD). We could identify the likely sequence of the ancestral transposon type and show the pattern of diversity supports a single mobilization with subsequent diversification during global spread.
Despite the limited number of WGSs for samples before 2012, with the oldest sequence available from 2008 (Fig. 1c), our estimate is consistent with the majority of available evidence from retrospective surveillance data,26 which has found the presence of mcr-1 in samples dating back to 2005 in Europe28. One retrospective study of Chinese isolates from 1970–2014 reported three mcr-1-positive E. coli dating from the 1980s29, although mcr-1 then did not reappear until 2004. This observation seems surprising in light of our results, which clearly exclude such an early spread of mcr-1 at least on this ISApl1 transposon background.
Our estimates of the age of spread of the representative IncI2 and IncX4 plasmid backgrounds are more recent, dating to ~ 2008 and 2013, respectively, but are both consistent with the age of the transposon mobilization event. We did not constrain the evolutionary rates in any of our phylogenetic analyses. It is thus encouraging that the different rates are highly consistent between the mcr-1 transposon and the two plasmid backgrounds. Although this points to high internal consistency between our estimates, we were surprisingly unable to find any previously published estimates for the evolutionary rate of bacterial plasmids.
The current distribution and observed genetic patterns are in line with a center of origin in China. This is the place where we observe the highest proportion of isolates carrying intact or partial copies of the ISApl1 flanking elements. Transposon sequences carrying ISApl1 elements were also overrepresented in environmental and agriculture isolates, relative to those collected from humans. This pattern is in line with agricultural settings acting as the source of mcr-1 within bacteria isolated from humans21. The current global distribution has been achieved through multiple translocations, and is illustrated by the interspersed geographic origins in our phylogenetic reconstructions. A likely driver for the global spread is trade, in particular food animals30 and meat, although direct global movement by colonized or infected humans20 is also likely to have played a role in the current distribution.
The origin of mcr-1 prior to its mobilization remains elusive. Despite an exhaustive search of sequence repositories, including the short-read archive, we found not a single mcr-1 sequence outside the ISApl1 transposon background. ISApl1 was first identified in the pig pathogen Actinobacillus pleuropneumoniae31 suggesting that it may also have been an ancestral host for mcr-1, although to our knowledge no mcr-1-positive A. pleuropneumoniae isolates have been described. The phosphoethanolamine transferase from Paenibacillus sophorae has also been proposed as a possible candidate32. However, this seems most unlikely as Paenibacili are Gram-positive and are thus intrinsically resistant to polymixins33. Moreover, although the two sequences share functional similarities, this should be interpreted as a case of possible parallel evolution rather than direct filiation33. Moraxella has also been suggested as being the source of mcr-134, following the identification of genes in Moraxella with limited homology to mcr-1 (~60% nucleotide sequence identity). However, this sequence identity is too low for Moraxella to be considered as viable candidates for the origin of mcr-1. The search for the initial source of mcr-1 remains open until a mcr-1 sequence is identified outside of the ISApl1 sequence background.
We note that there are an increasing number of mobilized genes that can confer colistin resistance, with mcr-2 reported less than a year after mcr-1 was initially described35 and more recent descriptions of the phylogenetically distant mcr-3, mcr-4, and mcr-536–38. There appear to be commonalities between the mechanisms of the mcr genes, despite their different sequences and locations near to different insertion sequences. For example, mcr-2 has 76.7% nucleotide identity to mcr-1 and was found in colistin-resistant isolates that did not contain mcr-1, and appeared to be mobilized on an IS1595 transposon35. Despite the different insertion sequences, intriguingly, this mobile element also contained a similar protein downstream of the mcr gene. Indeed, in mcr-1, -2, and -3, the mcr gene has a downstream open reading frame encoding, respectively, a putative PAP2 protein12, a PAP2 membrane-associated lipid phosphatase35, and a diacylglycerol kinase36, all of which have transmembrane domains and are involved in the phosphatidic acid pathway39,40. Although the PAP2-like orf in mcr-1 has been shown not to be required for colistin resistance41, the presence of similar sequences downstream of other mcr genes implies some functional role, either in the formation of the mobile element and/or in its continued mobilization.
In summary, we assembled the largest data set to date of mcr-1-positive sequenced isolates through our own sequencing efforts combined with an exhaustive search of publicly available sequence databases including unassembled data sets from the SRA. Although this allowed us to obtain a truly global data set of 457 mcr-1-positive isolates covering 31 countries and five continents, we appreciate that the data is likely affected by complex sampling biases, with an over representation of samples from places with active surveillance and well-funded research communities. Equally, although we took advantage of the most sophisticated bioinformatics and phylogenetic tools currently available, the complex ‘Russian doll’ dynamics of the transposon, plasmids, and bacterial host limits our ability to reach strong inferences on some important aspects of the spread of mcr-1. Nevertheless, we believe our results highlight the potential for phylogenetic reconstruction of antimicrobial resistance elements at a global scale. We hope that future efforts relying on more sophisticated computational tools and even more extensive genetic sequence data will become part of the routine toolbox in infectious disease surveillance.
Methods
Compilation of genomic data set
We blasted for mcr-1 in all NCBI GenBank assemblies (as of 16th March 2017, n = 90,759) using a 98% identity cutoff. 195 records (0.21%; 121 assemblies, 73 complete plasmids, one complete chromosome) contained at least one contig with a full-length hit to mcr-1 (1626 bases). We only included samples with a single copy of mcr-1. The only isolate with multiple copies was a previously published isolate with three chromosomal copies of mcr-1 and seven copies of ISApl142.
We searched a snapshot of all WGS bacterial raw read data sets in the SRA (December 2016), looking for samples containing mcr-1 by using a k-mer index (k = 31), which we had previously constructed43; software available at: https://github.com/phelimb/bigsi. A total of 184 data sets were found to contain at least 70% of the 31-mers in mcr-1. After removing duplicates (i.e., those with a draft assembly available) we could assemble contigs with mcr-1 for 152 of these.
Our final data set comprised 457 isolates from six genera across 31 different countries, ranging in date from 2008 to 2017. Where only a year was provided as the date of isolate collection the date was set to the midpoint of that year.
Whenever identified isolates did not comprise previously assembled genomes or complete plasmids, the raw fastq files were first inspected using FastQC and trimmed and filtered on a case-by-case basis. De novo assembly was then conducted using Plasmid SPADES 3.10.0 using the–careful switch and otherwise default parameters44. For those isolates sequenced using PacBio a different pipeline was employed. Correction, trimming, and assembly of raw reads was performed using Canu45 and assembled reads were corrected and trimmed using the tool Circlator46. The quality of resultant assemblies was assessed using infoseq. In both cases the mcr-1 carrying contigs in this final data set were identified using blastn v2.2.3147.
We ran Plasmid Finder 1.348 with 95% identity to identify plasmid replicons on the mcr-1 carrying contigs. In total, 182 unique contigs could be assigned a plasmid type using this method.
Novel samples from China
We selected 110 mcr-1-positive isolates from China for WGS from a larger survey effort of both clinical and livestock isolates. A total of 2824 non-repetitive clinical isolates, including 1637 E. coli and 1187 K. pneumoniae were collected from 15 provinces of mainland China from 2011 to 2016. Seventy-two isolates were resistant to polymyxin B, comprising 40 E. coli and four K. pneumoniae carrying mcr-1. Livestock samples were collected from four provinces of China in 2013 and 2016. One broiler farm of the Shandong province provided chicken anal swabs, liver, heart, and wastewater isolated in 2013. In 2016, samples including feces, wastewater, anal swabs, and internal organs of sick livestock were collected from swine farms, cattle farms, and broiler farms in four provinces (Jilin, Shandong, Henan, and Guangdong). A total of 601 E. coli and 126 K. pneumoniae were isolated, of which 167 (137 E. coli and 30 K. pneumoniae) were resistant to polymyxin B. We detected mcr-1 in 135 E. coli and two K. pneumoniae, as well as in eight E. coli isolated from environmental samples, which were collected from influents and effluents of four tertiary care teaching hospitals.
All of the isolates were sent to the microbiology laboratory of Peking University People’s Hospital and confirmed by routine biochemical tests, the Vitek system (bioMérieux, Hazelwood, MO, USA) and/or MALDI-TOF (Bruker Daltonics, Bremen, Germany). The minimal inhibitory concentrations (MICs) of polymyxin B was determined using the broth dilution method. The breakpoints of polymyxin B for Enterobacteriaceae were interpreted with the European Committee on Antimicrobial Susceptibility Testing (EUCAST, http://www.eucast.org/clinical_breakpoints) guidelines. Colistin-resistant isolates (MIC of ≥2 μg/ml) were screened for mcr-1 by PCR and sequencing as described previously49.
Identification and alignment of mcr-1 transposon
We searched for the mcr-1 carrying transposon region across isolates by blasting for its major components: ISApl1 (Actinobacillus pleuropneumoniae reference sequence: EF407820), mcr-1 (from E. coli plasmid pHNSHP45: KP347127.1), and short sequences representing the sequences immediately upstream and downstream of mcr-1 (from KP347127.1) using blastn-short. Contiguous sequences containing mcr-1 were aligned using Clustal Omega50 and then manually curated and amended using jalview51, resulting in a 3679 bp alignment containing the common ~2600 bp identified by Snesrud and colleagues12. The downstream copy of ISApl1 was more often fragmented or inverted. Twenty-eight isolates, which were all assemblies from the same study in Vietnam had a ~1.7 kb insertion downstream of mcr-1 (Fig. 3e) before the downstream ISApl1 element.
Phylogenetic analyses
For constructing the transposon phylogeny, we excluded the downstream ISApl1 and the insertion sequence observed in a small number of samples, as well as regions identified as having signals of recombination by ClonalFrameML52, resulting in a 3522 bp alignment. We removed two homoplastic sites (requiring >1 change on the phylogeny), before constructing a maximum parsimony neighbor-joining tree based on the Hamming distance between sequences. We calculated branch lengths using non-negative least squares with nnls.phylo in phangorn v2.2.053. Phylogenies were visualized with ggtree v1.8.154.
Phylogenetic dating
Given recombination can conceal the clonal phylogenetic signal we also applied ClonalFrameML52 to identify regions of high recombination in a subset of IncI2 and IncX4 plasmid background alignments. Where recombination hotspots were identified, they were removed from the alignment. In the IncI2 alignment, this resulted in removing 1281 positions. No regions of high recombination were detected in the IncX4 alignment. We applied root-to-tip correlations to test for a temporal signal in the data using TempEST55. There was a significantly positive slope for all three alignments (Supplementary Fig. 9–11).
We applied BEAUTi and BEAST v2.4.756,57 to estimate a timed phylogeny from an alignment of IncI2 plasmids (7161 sites, 110 isolates) and IncX4 plasmids (34,761 sites, 8 isolates). Sequences were annotated using their known sampling times expressed in years. For both plasmid alignments, the HKY substitution model was selected based on evaluation of all possible substitution models in bModelTest. BEAST analyses were then applied under both a coalescent population model (the coalescent Bayesian skyline implementation) and an exponential growth model (Coalescent Exponential population implementation). In addition, a strict clock, with a lognormal prior, and a relaxed clock (both lognormal and exponential) were tested. MCMC was run for 50,000,000 iterations sampling every 2000 steps and convergence was checked by inspecting the effective sample sizes and parameter value traces in the software Tracer v1.6.0. Analyses were repeated three times to ensure consistency between the obtained posterior distributions. Posterior trees for the best fitting model were combined in TreeAnnotator after a 10% burn-in to provide an annotated MCC tree. MCC trees were plotted using ggtree54 for both backgrounds: IncI2 (Supplementary Fig. 12) and IncX4 (Supplementary Fig. 13). The model fit across analyses was compared using the Akaike’s information criteria model through 100 bootstrap resamples as described in Baele and colleagues58 and implemented in Tracer v1.6 (Supplementary Table 2).
Phylogenetic dating on the transposon was performed using an alignment of 364 isolates, which included only those with information on isolation date, across 3522 sites. As before BEAST analyses were applied under both a coalescent population model (coalescent Bayesian skyline implementation) and an exponential growth model (coalescent exponential population implementation). In addition, a strict clock, with a lognormal prior, and a relaxed clock (both lognormal and exponential) were tested. Analyses were run under a HKY substitution model for 600 million iterations sampling every 5000 steps. Only analyses using a strict clock model reached convergence after 600 million iterations. The resultant set of trees were thinned by sampling every 10 trees and excluding a 10% burn-in and combined using TreeAnnotator to produce a MCC tree. MCC trees were plotted using ggTree48. As before the model fit was evaluated using AICM’s implemented in Tracer v1.6.
Environmental distribution
For the purpose of testing the distribution of sequences containing some trace of ISApl1, we classed isolates into broad categories as either environmental (n = 39; bird, cat, dog, fly, food, penguin, reptile, vegetables), agricultural (n = 213; chicken, cow, pig, poultry feed, sheep, turkey), or human (n = 108). We did not correct for study site with subsampling as we found great diversity within sites, consistent with a recent study showing multiple diverse mcr-1-positive strains within a single hospital sewage sample59.
Data access
All the data generated or analyzed in this study are available within the paper and its supplementary information files. Accession numbers and metadata for all 457 isolates is provided in supplementary data 1. The newly sequenced 110 mcr-1-positive genomes have been submitted to the Short Read Archive under Bioproject: PRJNA408214, Accession: SRP118547.
Electronic supplementary material
Description of Additional Supplementary File
Acknowledgements
L.v.D., X.D., H.W., T.S., L.A.W., and FB acknowledge financial support from the Newton Trust UK-China NSFC initiative (grants MR/P007597/1 and 81661138006). F.B. additionally acknowledges support from the BBSRC GCRF scheme. L.P.S. was supported by a PhD scholarship from EPSRC (EP/F500351/1). P.B. is funded by Wellcome Trust on a “Genomic Medicine and Statistics DPhil” grant. A.R. was co-funded by the European Union: European regional development fund (ERDF), by the Conseil Régional de La Réunion and by the Centre de Coopération internationale en Recherche agronomique pour le Développement (CIRAD). H.W. was supported by National Natural Science Foundation of China (81625014). L.A.W. is supported by a Dorothy Hodgkin Fellowship funded by the Royal Society (Grant Number DH140195) and a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant Number 109385/Z/15/Z). Z.I. was funded by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (Grant Number 102541/A/13/Z). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author contributions
H.W. and F.B. conceived the project and designed the experiments. R.W., Q.W., X.W., L.J. Q.Z., Y.L., and H.W. collected samples. R.W., Q.W., X.W., L.J., Q.Z., and Y.L. performed microbial identification, antimicrobial susceptibility testing, screening for mcr-1, and DNA extraction for WGS. R.W. L.v.D., L.P.S. assembled the new sequence data. L.v.D. and L.P.S. curated the global data set and performed the computational analyses. T.D.-S. advised on functional aspects of colistin resistance. A.R., L.A.W., and X.D. helped with the phylogenetic reconstructions. P.B. and Z.I. performed the search for mcr-1-positive samples on the Short Read Archive. H.W. takes responsibility for the accuracy and availability of the epidemiological and raw sequence data, and F.B. for all bioinformatics and computational methods and results. L.v.D., L.P.S., and F.B. wrote the paper with contributions from X.D. and H.W. All authors read and commented on successive drafts and all approved the content of the final version.
Competing interests
The authors declare no competing financial interests.
Footnotes
These authors contributed equally: Ruobing Wang, Lucy van Dorp, Liam P. Shaw.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-03205-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hui Wang, Email: wanghui@pkuph.edu.cn.
Francois Balloux, Email: f.balloux@ucl.ac.uk.
References
- 1.Grégoire, N., Aranzana-Climent, V., Magréault, S., Marchand, S. & Couet, W. Clinical pharmacokinetics and pharmacodynamics of colistin. Clin. Pharmacokinet.56 1441–1460 (2017). [DOI] [PubMed]
- 2.Liu YY, et al. Emergence of plasmid-mediated colistin resistance mechanism mcr-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 3.Olaitan AO, Morand S, Rolain JM. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014;5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JY, Choi MJ, Choi HJ, Ko KS. Preservation of acquired colistin resistance in Gram-negative bacteria. Antimicrob. Agents Chemother. 2016;60:609–612. doi: 10.1128/AAC.01574-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carattoli A. Plasmids and the spread of resistance. Int. J. Med. Microbiol. 2013;303:298–304. doi: 10.1016/j.ijmm.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Poirel L, Kieffer N, Liassine N, Thanh D, Nordmann P. Plasmid-mediated carbapenem and colistin resistance in a clinical isolate of Escherichia coli. Lancet Infect. Dis. 2016;16:281. doi: 10.1016/S1473-3099(16)00006-2. [DOI] [PubMed] [Google Scholar]
- 7.Du H, Chen L, Tang YW, Kreiswirth BN, Rolain JM. Emergence of the mcr-1 colistin resistance gene in carbapenem-resistant Enterobacteriaceae. Lancet Infect. Dis. 2016;16:287–288. doi: 10.1016/S1473-3099(16)00056-6. [DOI] [PubMed] [Google Scholar]
- 8.Yao X, Doi Y, Zeng L, Lv L, Liu JH. Carbapenem-resistant and colistin-resistant Escherichia coli co-producing NDM-9 and MCR-1. Lancet Infect. Dis. 2016;16:288–289. doi: 10.1016/S1473-3099(16)00057-8. [DOI] [PubMed] [Google Scholar]
- 9.Zhang XF, et al. Possible transmission of mcr-1 –harboring Escherichia coli between aompanion animals and human. Emerg. Infect. Dis. 2016;22:1679–1681. doi: 10.3201/eid2209.160464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falgenhauer L, et al. Colistin resistance gene mcr-1 in extended-spectrum β-lactamase-producing and carbapenemase-producing Gram-negative bacteria in Germany. Lancet Infect. Dis. 2016;16:282–283. doi: 10.1016/S1473-3099(16)00009-8. [DOI] [PubMed] [Google Scholar]
- 11.Haenni M, et al. Co-occurrence of extended spectrum β lactamase and MCR-1 encoding genes on plasmids. Lancet Infect. Dis. 2016;16:281–282. doi: 10.1016/S1473-3099(16)00007-4. [DOI] [PubMed] [Google Scholar]
- 12.Snesrud E, et al. A model for transposition of the colistin resistance gene mcr-1 by ISApl1. Antimicrob. Agents Chemother. 2016;60:6973–6976. doi: 10.1128/AAC.01457-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, et al. Comprehensive resistome analysis reveals the prevalence of NDM and MCR-1 in Chinese poultry production. Nat. Microbiol. 2017;2:16260. doi: 10.1038/nmicrobiol.2016.260. [DOI] [PubMed] [Google Scholar]
- 14.Li R, et al. Genetic characterization of mcr-1 -bearing plasmids to depict molecular mechanisms underlying dissemination of the colistin resistance determinant. J. Antimicrob. Chemother. 2017;72:393–401. doi: 10.1093/jac/dkw411. [DOI] [PubMed] [Google Scholar]
- 15.Matamoros S, et al. Global phylogenetic analysis of Escherichia coli and plasmids carrying the mcr-1 gene indicates bacterial diversity but plasmid restriction. Sci. Rep. 2017;7:15364. doi: 10.1038/s41598-017-15539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou HW, et al. Occurrence of plasmid- and chromosome-carried mcr-1 in waterborne Enterobacteriaceae in China. Antimicrob. Agents Chemother. 2017;61:e00017–e00017. doi: 10.1128/AAC.00017-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matamoros, S. et al. Global phylogenetic analysis of Escherichia coli and plasmids carrying the mcr-1 gene indicates bacterial diversity but plasmid restriction. Sci. Rep.7, 15364 (2017). [DOI] [PMC free article] [PubMed]
- 18.Yang D, et al. The occurrence of the colistin resistance gene mcr-1 in the Haihe River (China) Int. J. Environ. Res. Public Health. 2017;14:576. doi: 10.3390/ijerph14060576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes MR, et al. Colistin-resistant mcr-1 -positive escherichia coli on public beaches, an infectious threat emerging in recreational waters. Antimicrob. Agents Chemother. 2017;61:e00234–17. doi: 10.1128/AAC.00234-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Wintersdorff CJH, et al. Detection of the plasmid-mediated colistin-resistance gene mcr-1 in faecal metagenomes of Dutch travellers. J. Antimicrob. Chemother. 2016;71:3416–3419. doi: 10.1093/jac/dkw328. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, et al. Prevalence, risk factors, outcomes, and molecular epidemiology of mcr-1-positive Enterobacteriaceae in patients and healthy adults from China: an epidemiological and clinical study. Lancet Infect. Dis. 2017;17:390–399. doi: 10.1016/S1473-3099(16)30527-8. [DOI] [PubMed] [Google Scholar]
- 22.Tian GB, et al. MCR-1-producing Klebsiella pneumoniae outbreak in China. Lancet Infect. Dis. 2017;17:577. doi: 10.1016/S1473-3099(17)30266-9. [DOI] [PubMed] [Google Scholar]
- 23.Sheppard AE, et al. Nested Russian doll-like genetic mobility drives rapid dissemination of the carbapenem resistance gene blaKPC. Antimicrob. Agents Chemother. 2016;60:3767–3778. doi: 10.1128/AAC.00464-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snesrud E, et al. Analysis of serial isolates ofmcr-1-positive Escherichia coli reveals a highly active ISApl1 transposon. Antimicrob. Agents Chemother. 2017;61:e00056–17. doi: 10.1128/AAC.00056-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poirel L, et al. Genetic features of mcr-1-producing colistin-resistant Escherichia coli isolates in South Africa. Antimicrob. Agents Chemother. 2016;60:4394–4397. doi: 10.1128/AAC.00444-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poirel L, Nordmann P. Emerging plasmid-encoded colistin resistance: the animal world as the culprit? J. Antimicrob. Chemother. 2016;71:2326–2327. doi: 10.1093/jac/dkw074. [DOI] [PubMed] [Google Scholar]
- 27.Schwarz S, Johnson AP. Transferable resistance to colistin: a new but old threat. J. Antimicrob. Chemother. 2016;71:2066–2070. doi: 10.1093/jac/dkw274. [DOI] [PubMed] [Google Scholar]
- 28.Haenni M, et al. Co-occurrence of extended spectrum β-lactamase and mcr-1 encoding genes on plasmids. Lancet Infect. Dis. 2016;16:281–282. doi: 10.1016/S1473-3099(16)00007-4. [DOI] [PubMed] [Google Scholar]
- 29.Shen Z, Wang Y, Shen Y, Shen J, Wu C. Early emergence of mcr-1 in Escherichia coli from food-producing animals. Lancet Infect. Dis. 2016;16:293. doi: 10.1016/S1473-3099(16)00061-X. [DOI] [PubMed] [Google Scholar]
- 30.Grami R, et al. Impact of food animal trade on the spread of mcr-1 -mediated colistin resistance, Tunisia, July 2015. Eurosurveillance. 2016;21:30144. doi: 10.2807/1560-7917.ES.2016.21.8.30144. [DOI] [PubMed] [Google Scholar]
- 31.Tegetmeyer HE, Jones SCP, Langford PR, Baltes N. ISApl1, a novel insertion element of Actinobacillus pleuropneumoniae, prevents ApxIV-based serological detection of serotype 7 strain AP76. Vet. Microbiol. 2008;128:342–353. doi: 10.1016/j.vetmic.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 32.Gao R, et al. Dissemination and Mechanism for the MCR-1 Colistin Resistance. PLOS Pathog. 2016;12:e1005957. doi: 10.1371/journal.ppat.1005957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Conza JA, Radice MA, Gutkind G. O. mcr-1: rethinking the origin. Int. J. Antimicrob. Agents. 2017;50:737. doi: 10.1016/j.ijantimicag.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Kieffer N, Nordmann P, Poirel L. Moraxella species as potential sources of mcr-like polymyxin resistance determinants. Antimicrob. Agents Chemother. 2017;61:e00129–17. doi: 10.1128/AAC.00129-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xavier BB, et al. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Eurosurveillance. 2016;21:30280. doi: 10.2807/1560-7917.ES.2016.21.27.30280. [DOI] [PubMed] [Google Scholar]
- 36.Yin W, et al. Novel plasmid-mediated colistin resistance gene mcr-3 Escherichia coli. MBio. 2017;8:e00543–17. doi: 10.1128/mBio.00543-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carattoli A, et al. Novel plasmid-mediated colistin resistance mcr-4 g ene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Eurosurveillance. 2017;22:30589. doi: 10.2807/1560-7917.ES.2017.22.31.30589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borowiak M, et al. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J. Antimicrob. Chemother. 2017;72:3317–3324. doi: 10.1093/jac/dkx327. [DOI] [PubMed] [Google Scholar]
- 39.Athenstaedt K, Daum G. Phosphatidic acid, a key intermediate in lipid metabolism. Eur. J. Biochem. 1999;266:1–16. doi: 10.1046/j.1432-1327.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- 40.Epand RM, Walker C, Epand RF, Magarvey NA. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta - Biomembr. 2016;1858:980–987. doi: 10.1016/j.bbamem.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Zurfluh K, Kieffer N, Poirel L, Nordmann P, Stephan R. Features of the mcr-1 cassette related to colistin resistance. Antimicrob. Agents Chemother. 2016;60:6438–6439. doi: 10.1128/AAC.01519-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu CY, et al. Complete genome sequencing revealed novel genetic contexts of the mcr-1 gene in Escherichia coli strains. J. Antimicrob. Chemother. 2016;72:1253–1255. doi: 10.1093/jac/dkw541. [DOI] [PubMed] [Google Scholar]
- 43.Bradley, P., Bakker, H. den, Rocha, E., McVean, G. & Iqbal, Z. Real-time search of all bacterial and viral genomic data. bioRxiv 234955 10.1101/234955 (2017).
- 44.Antipov D, et al. plasmidSPAdes: assembling plasmids from 555whole-genome sequencing data. Bioinformatics. 2016;32:3380–3387. doi: 10.1093/bioinformatics/btv688. [DOI] [PubMed] [Google Scholar]
- 45.Koren, S. et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. gr.215087.116 10.1101/gr.215087.116 (2017). [DOI] [PMC free article] [PubMed]
- 46.Hunt M, et al. Circlator: automated circularization of genome assemblies using long sequencing reads. Genome Biol. 2015;16:294. doi: 10.1186/s13059-015-0849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Camacho C, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carattoli A, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014;58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, et al. Molecular epidemiology of colistin-resistant Enterobacteriaceae in inpatient and avian isolates from China: high prevalence of mcr-negative Klebsiella pneumoniae. Int. J. Antimicrob. Agents. 2017;50:536–541. doi: 10.1016/j.ijantimicag.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Sievers F, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview Version 2 - a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Didelot X, Wilson DJ. ClonalFrameML: efficient inference of recombination in whole bacterial genomes. PLOS Comput. Biol. 2015;11:e1004041. doi: 10.1371/journal.pcbi.1004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schliep KP. phangorn: phylogenetic analysis in R. Bioinformatics. 2011;27:592–593. doi: 10.1093/bioinformatics/btq706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu G, Smith DK, Zhu H, Guan Y, Lam TTY. ggtree: an r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017;8:28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- 55.Rambaut A, Lam TT, Max Carvalho L, Pybus OG. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen) Virus Evol. 2016;2:vew007. doi: 10.1093/ve/vew007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bouckaert R, et al. BEAST 2: a software platform for Bayesian evolutionary analysis. PLOS Comput. Biol. 2014;10:e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baele G, et al. Improving the accuracy of demographic and molecular clock model comparison while accommodating phylogenetic uncertainty. Mol. Biol. Evol. 2012;29:2157–2167. doi: 10.1093/molbev/mss084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao F, Feng Y, Lü X, McNally A, Zong Z. Remarkable diversity of Escherichia coli carrying mcr-1 from hospital sewage with the identification of two new mcr-1 variants. Front. Microbiol. 2017;8:2094. doi: 10.3389/fmicb.2017.02094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary File