Abstract
Soil microorganisms are key drivers of plant productivity in terrestrial ecosystems, yet controls on their diversities and abundances are not fully elucidated. Phenolic acids, released through plant root exudation and residues decomposition, are usually referred as autotoxins of several crops, including cucumber. In this study, effects of vanillic acid (VA) on cucumber rhizosphere microbial communities were investigated by treating cucumber seedlings with VA every two days for five times. Amplicon sequencing, PCR-denaturing gradient gel electrophoresis and quantitative PCR were used to analyzed the 16S rRNA genes of total bacterial, Pseudomonas and Bacillus spp. communities. Results showed that VA at 0.05 μmol g−1 soil changed total bacterial community diversity and composition. In particular, VA inhibited the relative abundances of genera with plant-beneficial potentials, such as Bacillus and Lysobacter spp. Moreover, VA changed Pseudomonas and Bacillus spp. community compositions by altering the number and/or relative abundances of their OTUs; and decreased Bacillus spp. community abundance at 0.02 to 0.2 μmol g−1 soil and Pseudomonas spp. community abundance at 0.2 μmol g−1 soil. Overall, VA changed cucumber seedling rhizosphere total bacterial, Pseudomonas and Bacillus spp. communities, which maybe be associated with the adverse effects of VA on cucumber growth under soil conditions.
Introduction
Allelopathy is the phytotoxicity of a compound or a group of compounds released from plant parts through leaching, root exudation, volatilization, or residue decomposition to susceptible plants1. Several plant species can inhibited the growth of its own kind through autotoxicity, a special kind of allelopathy2. In agricultural ecosystems, autotoxicity is implied as one of the main causing agents of soil sickness, the phenomenon of growth and yield reduction and disease increases when one crop repeatedly grown on the same land2,3. Autotoxicity can be overcame by several agricultural practices, such as crop rotation, selecting crop varieties resistant to autotoxins, and adopting a proper fallow period so that there is enough time for the decomposition of autotoxins2. Cucumber (Cucumis sativus L.), a popular vegetable with high economic importance that often continuously monocropped in the greenhouse, is vulnerable to soil sickness4,5. Previous studies demonstrated that phenolic acids (such as derivatives of cinnamic and benzoic acids) could exert detrimental effects on cucumber and were potential autotoxins of cucumber in both hydroponic solution and soil conditions3,6,7.
Soil microorganisms are responsible for the key processes associated with soil fertility and plant health, hence, greatly influence the functioning of terrestrial ecosystems8. Accumulating evidence suggests that changes in soil microbial communities may lead to alterations in the functions performed by the community, which can have a feedback effect on plant health and fitness9. Recent studies indicated that phenolic acids could act as specific substrates or signaling molecules for a large group of microbial species in the soil10. In vitro studies found that phenolic acids were able to influence the growth and physiological status of specific microorganisms, such as Pseudomonas syringae, Fusarium oxysporum and Rhizoctonia solani3,11–13. However, little information is available on how these acids can affect microbial communities in the soil14,15.
Pseudomonas and Bacillus spp. are ubiquitous in terrestrial ecosystems, and are frequently found in associations with plants, either as mutualists, saprophytes or pathogens. Particularly, some species of these two genera play major roles in nutrient mobilization, plant growth promotion and protection, and thus play an important role in agriculture16,17. For example, P. putida, B. pumilus, B. subtilis are able to promote cucumber growth, induce systemic resistance, and directly inhibit plant pathogens, such as Fusarium oxysporum f.sp. cucumerinum, a host-specific soil-borne pathogen of cucumber18,19. It has been shown that agricultural management regimes, including crop continuous monocropping, affected Pseudomonas and Bacillus spp. communities16,20,21. However, how phenolic acids affect soil Pseudomonas and Bacillus spp. communities are still not clear.
Previously, we observed that vanillic acid (4-hydroxy-3-methoxybenzoic acid, a dihydroxybenzoic acid derivative) accumulated in the soil after continuous monocropping of cucumber22. Vanillic acid also inhibited cucumber seedling growth and changed the whole bacterial community structure as evaluated by PCR-denaturing gradient gel electrophoresis (DGGE)23. However, detailed changes in rhizosphere microbial compositions are still unclear. In the present study, we further analyzed cucumber rhizosphere bacterial community with high-throughput sequencing technique, which can provide a higher resolution and a better understanding of environmental microbial communities than the PCR-based fingerprinting techniques24. Moreover, cucumber rhizosphere Pseudomonas and Bacillus spp. community structures and abundances were estimated by PCR-DGGE and quantitative PCR, respectively.
Results
Illumina Miseq sequencing data
In total, Illumina Miseq sequencing generated 21,2367 quality bacterial 16S rRNA gene sequences with an average read length of 396 bp. The Good’s coverage of each sample, which reflects the captured diversity, was larger than 98% (data not shown). Rarefaction curves of OTUs of all samples tended to approach the saturation plateau (Figure S1), which indicated that our sequencing data represented most of the total bacterial community composition.
Bacterial Community Composition
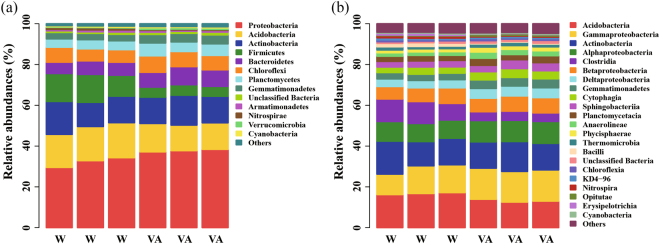
Across all samples, 32 bacterial phyla were detected and 1.06% of the bacterial sequences were unclassified at the phylum level. Proteobacteria, Acidobacteria, Actinobacteria, Firmicutes, Bacteroidetes and Chloroflexi were the dominant phyla (relative abundance > 5%), which accounted for more than 86% of the bacterial sequences (Fig. 1a). Compared with rhizosphere soils treated with water, rhizosphere soils treated with 0.05 μmol g−1 soil vanillic acid had higher relative abundances of Proteobacteria, Planctomycetes, Bacteroidetes, Gemmatimonadetes and Verrucomicrobia, but lower relative abundances of Acidobacteria, Firmicutes and Nitrospirae (P < 0.05) (Figs 1a, 2).
Figure 1.
Relative abundances of main bacterial phyla (a) and classes (b) in cucumber rhizosphere soils treated with water (W) or vanillic acid at 0.05 μmol g−1 soil (VA). Bacterial phyla and classes with average relative abundances >0.5% in at least one treatment were shown. Data are represented as the means of three independent replicates.
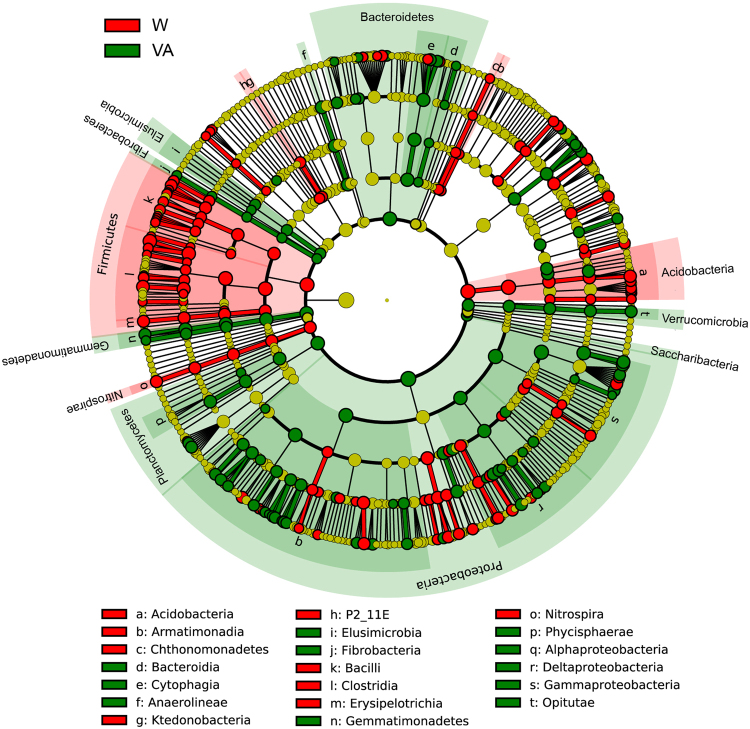
Figure 2.
Cladograms, generated from LEfSe analysis, represent the polygenetic distribution of cucumber rhizosphere soil bacterial taxa. LEfSe analysis was based on the data of three independent replicates of each treatment. Bacterial taxa that are significantly enriched in each treatment with LDA scores larger than 2.0 are shown. Significantly discriminant taxon nodes are colored: red for cucumber rhizosphere soils treated with water (W), green for vanillic acid at 0.05 μmol g−1 soil (VA). Yellow circles represent non-significant differences in abundance between treatments for that particular taxon. Each circle’s diameter is proportional to the taxon’s abundance. Labels are shown of the phylum, class and order levels. The LDA scores of each significantly discriminant taxon from the phylum to genus levels are shown in Figure S2.
At the class level, more than 70 bacterial taxa were detected. The top three classes were Acidobacteria, Gammaproteobacteria and Actinobacteria, which accounted for about 42% of the bacterial sequences (Fig. 1b). Cucumber rhizosphere soils treated with 0.05 μmol g−1 soil vanillic acid had higher relative abundances of Alphaproteobacteria, Deltaproteobacteria, Gemmatimonadetes, Cytophagia, Anaerolineae and Opitutae, but lower relative abundances of Acidobacteria, Clostridia, Bacilli, Nitrospira and Erysipelotrichia (P < 0.05) (Figs 1b, 2).
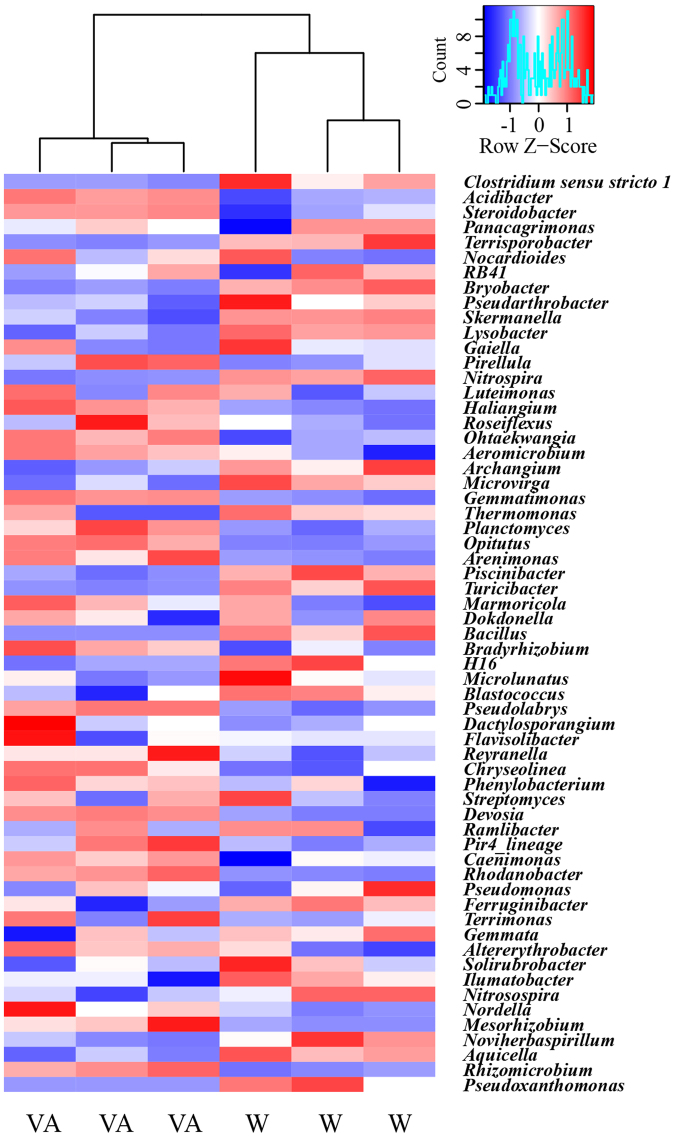
At the genus level, more than 530 bacterial taxa were detected. Gemmatimonas, RB41, Dokdonella, Bradyrhizobium, Nitrospira, Rhizomicrobium and Pseudolabrys spp. were dominant classified genera (relative abundance > 1%) (Fig. 3). Cucumber rhizosphere soils treated with 0.05 μmol g−1 soil vanillic acid had higher relative abundances of Acidibacter, Steroidobacter, Haliangium, Gemmatimonas, Opitutus, Ohtaekwangia, Pseudolabrys, Planctomyces, Arenimonas, Bradyrhizobium, Rhizomicrobium, Rhodanobacter, Devosia and Chryseolinea spp., but lower relative abundances of Terrisporobacter, Bryobacter, Turicibacter, Nitrospira, Bacillus, Skermanella, Piscinibacter, Lysobacter, Archangium, Microvirga, Noviherbaspirillum and Aquicella spp. (P < 0.05).
Figure 3.
Heat map showing the relative abundances of dominant classified bacterial genera in cucumber rhizosphere soils treated with water (W) or vanillic acid at 0.05 μmol g−1 soil (VA). Classified bacterial genera with average relative abundances >0.3% were identified in each sample by colors deduced from the raw Z-scores. Hierarchical clustering of samples was performed using the average clustering method with the Euclidean distances.
A total of 2,060 OTUs were identified at 97% similarity across all samples. Most dominated OTUs (relative abundance > 0.5%) were mainly aligned to bacterial phylum Proteobacteria, Firmicutes, Acidobacteria, Bacteroidetes and Actinobacteria (Table 1). The relative abundances of seven OTUs, aligned to Acidibacter, Steroidobacter, Nitrosomonadaceae norank, Subgroup 7 norank, BIrii41 norank and Bradyrhizobium spp., were higher in rhizosphere soils treated with 0.05 μmol g−1 soil vanillic acid; while 11 OTUs, aligned to Terrisporobacter, Clostridium sensu stricto 1, Peptostreptococcaceae unclassified, Archangium, Piscinibacter, Turicibacter, SC-I-84 norank and Bryobacter spp. were higher in rhizosphere soils treated with water (P < 0.05).
Table 1.
The most abundant bacterial OTUs in cucumber rhizosphere soils treated with water (W) or vanillic acid at 0.05 μmol g−1 soil (VA). OTUs were delineated at 97% sequence similarity. Only OTUs with average relative abundances >0.5% in at least one treatment were presented. Values were expressed as mean ± standard error (n = 3). OTU ID in bold indicates its relative abundance was significant different between treatments according to Welch’s t test (P < 0.05, Bonferroni corrected).
| OTU ID | Phylum | Class | Genus | Relative abundances (%) | |
|---|---|---|---|---|---|
| W | VA | ||||
| OTU1259 | Proteobacteria | Gammaproteobacteria | Acidibacter | 1.75 ± 0.24 | 3.72 ± 0.52 |
| OTU394 | Firmicutes | Clostridia | Clostridium sensu stricto 1 | 3.14 ± 0.47 | 1.40 ± 0.52 |
| OTU1239 | Proteobacteria | Gammaproteobacteria | Panacagrimonas | 1.99 ± 0.73 | 2.09 ± 0.11 |
| OTU1472 | Proteobacteria | Gammaproteobacteria | Steroidobacter | 1.37 ± 0.13 | 1.89 ± 0.12 |
| OTU725 | Acidobacteria | Acidobacteria | Acidobacteria norank | 2.16 ± 0.12 | 0.91 ± 0.36 |
| OTU429 | Firmicutes | Clostridia | Terrisporobacter | 2.18 ± 0.24 | 0.85 ± 0.55 |
| OTU1435 | Firmicutes | Clostridia | Clostridium sensu stricto 1 | 1.34 ± 0.13 | 0.80 ± 0.09 |
| OTU750 | Acidobacteria | Acidobacteria | Acidobacteria unclassified | 1.08 ± 0.06 | 1.05 ± 0.05 |
| OTU914 | Bacteroidetes | Cytophagia | Cytophagaceae norank | 0.99 ± 0.02 | 0.97 ± 0.01 |
| OTU1125 | Actinobacteria | Actinobacteria | Pseudarthrobacter | 1.15 ± 0.15 | 0.76 ± 0.06 |
| OTU906 | Firmicutes | Clostridia | Peptostreptococcaceae unclassified | 1.37 ± 0.20 | 0.49 ± 0.37 |
| OTU631 | Acidobacteria | Acidobacteria | Acidobacteria unclassified | 0.93 ± 0.03 | 0.79 ± 0.03 |
| OTU1545 | Firmicutes | Clostridia | Clostridium sensu stricto 1 | 1.05 ± 0.16 | 0.47 ± 0.17 |
| OTU654 | Acidobacteria | Acidobacteria | Acidobacteria norank | 0.96 ± 0.06 | 0.54 ± 0.11 |
| OTU1754 | Proteobacteria | Betaproteobacteria | Nitrosomonadaceae norank | 0.51 ± 0.04 | 0.97 ± 0.10 |
| OTU1955 | Proteobacteria | Gammaproteobacteria | Luteimonas | 0.59 ± 0.05 | 0.74 ± 0.03 |
| OTU301 | Actinobacteria | Actinobacteria | Aeromicrobium | 0.50 ± 0.09 | 0.74 ± 0.11 |
| OTU690 | Proteobacteria | Deltaproteobacteria | Archangium | 0.68 ± 0.03 | 0.56 ± 0.05 |
| OTU1324 | Acidobacteria | Acidobacteria | Subgroup 7 norank | 0.42 ± 0.08 | 0.78 ± 0.11 |
| OTU1210 | Proteobacteria | Gammaproteobacteria | Thermomonas | 0.63 ± 0.02 | 0.55 ± 0.04 |
| OTU665 | Chloroflexi | Anaerolineae | Anaerolineaceae norank | 0.56 ± 0.01 | 0.52 ± 0.01 |
| OTU1321 | Acidobacteria | Acidobacteria | Acidobacteria norank | 0.49 ± 0.04 | 0.58 ± 0.08 |
| OTU148 | Proteobacteria | Betaproteobacteria | Piscinibacter | 0.68 ± 0.04 | 0.38 ± 0.13 |
| OTU924 | Firmicutes | Erysipelotrichia | Turicibacter | 0.90 ± 0.11 | 0.17 ± 0.28 |
| OTU1691 | Proteobacteria | Betaproteobacteria | SC-I-84 norank | 0.68 ± 0.03 | 0.37 ± 0.14 |
| OTU1282 | Proteobacteria | Betaproteobacteria | Nitrosomonadaceae norank | 0.45 ± 0.03 | 0.57 ± 0.05 |
| OTU1127 | Actinobacteria | Actinobacteria | Marmoricola | 0.42 ± 0.09 | 0.59 ± 0.09 |
| OTU1074 | Bacteroidetes | Cytophagia | Cytophagaceae norank | 0.45 ± 0.04 | 0.56 ± 0.05 |
| OTU660 | Proteobacteria | Deltaproteobacteria | BIrii41 norank | 0.37 ± 0.04 | 0.60 ± 0.05 |
| OTU1983 | Acidobacteria | Acidobacteria | Acidobacteria norank | 0.57 ± 0.01 | 0.39 ± 0.05 |
| OTU156 | Proteobacteria | Alphaproteobacteria | Bradyrhizobium | 0.41 ± 0.03 | 0.53 ± 0.03 |
| OTU1649 | Actinobacteria | Actinobacteria | Microlunatus | 0.53 ± 0.07 | 0.39 ± 0.04 |
| OTU1913 | Proteobacteria | Gammaproteobacteria | Steroidobacter | 0.36 ± 0.03 | 0.53 ± 0.06 |
| OTU1140 | Actinobacteria | Actinobacteria | Blastococcus | 0.51 ± 0.03 | 0.36 ± 0.08 |
| OTU704 | Acidobacteria | Acidobacteria | Acidobacteria norank | 0.53 ± 0.03 | 0.32 ± 0.05 |
| OTU168 | Acidobacteria | Acidobacteria | Bryobacter | 0.58 ± 0.02 | 0.24 ± 0.11 |
Bacterial Community Diversity and Structure
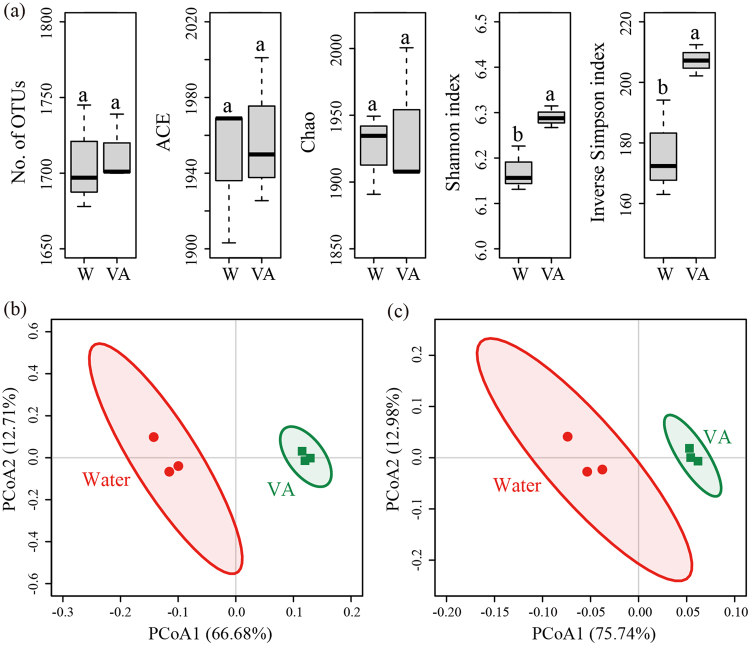
Rhizosphere soils treated with vanillic acid had higher Shannon and inverse Simpson indices than rhizosphere soils treated with water (P < 0.05) (Fig. 4a). However, number of OTUs, ACE and Chao indices were similar in rhizosphere soils treated with water and vanillic acid (Fig. 4a).
Figure 4.
Alpha diversity and beta diversity indices of bacterial communities in cucumber rhizosphere soils treated with water (W) or vanillic acid at 0.05 μmol g−1 soil (VA). For alpha diversity, number of OTUs observed (No. of OTUs), ACE, Chao, Shannon and Inverse Simpson indices were calculated using random subsamples of 24,245 16S rRNA gene sequences per sample. OTUs were delineated at 97% sequence similarity. The box plot shows median (black line), first quartile–third quartile percentiles (box range) and 1.5× the interquartile range (whiskers). There were three independent replicates of each treatment. Different letters indicate significant difference based on Welch’s t test (P < 0.05). For beta diversity, differences in Bray-Curtis (b) and UniFrac distances (c) of bacterial communities at the OTU level were visualized by principal coordinates analyses. Ellipses indicate 95% confidence interval for replicates.
Principal coordinates analysis at the OTU level, based on both Bray-Curtis and UniFrac distance dissimilarities, revealed a clear separation between rhizosphere soils treated with water and vanillic acid (Fig. 4b,c).
Bacillus and Pseudomonas spp. Community Compositions
Eight and five OTUs aligned to Bacillus and Pseudomonas spp., respectively, were detected in all treatments as determined by Illumina Miseq sequencing (Table S1). OTU275, OTU423 and OTU1458 were classified as B. luciferensis, P. flexibilis and P. resinovorans, respectively; while most of these OTUs could be aligned at the species level. For Bacillus spp., six OTUs were detected in rhizosphere soils treated with vanillic acid and eight OTUs in rhizosphere soils treated with water; the relative abundances of OTU275, OTU302 and OTU1222 were lower in rhizosphere soils treated with vanillic acid than in rhizosphere soils treated with water (P < 0.05). For Pseudomonas spp., the relative abundance of OTU1587 were higher in rhizosphere soils treated with vanillic acid than in rhizosphere soils treated with water (P < 0.05).
Bacillus and Pseudomonas spp. Community Structures
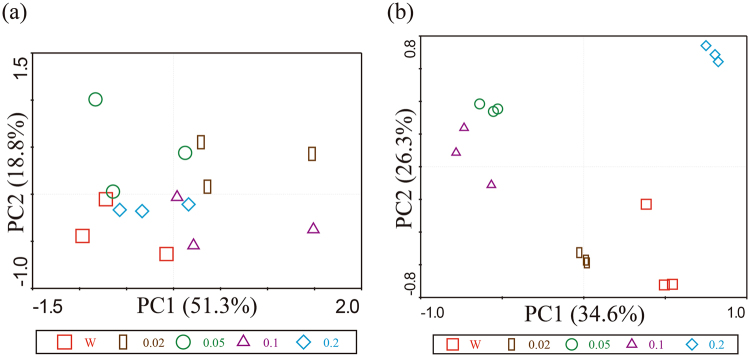
For Bacillus spp. community, PCR-DGGE analyses showed that cucumber rhizosphere soils treated with water and different concentrations of vanillic acid (0.02, 0.05, 0.1, 0.2 μmol g−1 soil) had similar DGGE banding patterns (Figure S3a). Principal component analysis also could not clearly separate the five treatments from each other (Fig. 5a). Number of bands, Shannon-Wiener index and evenness index were not affected by exogenously applied vanillic acid (Figure S4).
Figure 5.
Principal component analysis of Bacillus (a) and Pseudomonas (b) spp. communities in cucumber rhizosphere soils based on PCR-DGGE analysis. W represents cucumber rhizosphere soils treated with water. 0.02, 0.05, 0.1 and 0.2 represent cucumber rhizosphere soils treated with vanillic acid at 0.02, 0.05, 0.1, 0.2 μmol g−1 soil concentrations, respectively.
For Pseudomonas spp. community, visual inspection of the DGGE profiles revealed different banding patterns among treatments (Figure S3b). Principal component analysis clearly distinguished the five treatments from each other (Fig. 5b). Compared with rhizosphere soils treated with water, rhizosphere soils treated with 0.2 μmol g−1 soil vanillic acid had lower number of bands, and rhizosphere soils treated with 0.02 μmol g−1 soil vanillic acid had higher Shannon-Wiener index and evenness index (Figure S4).
Bacillus and Pseudomonas spp. Community Abundances
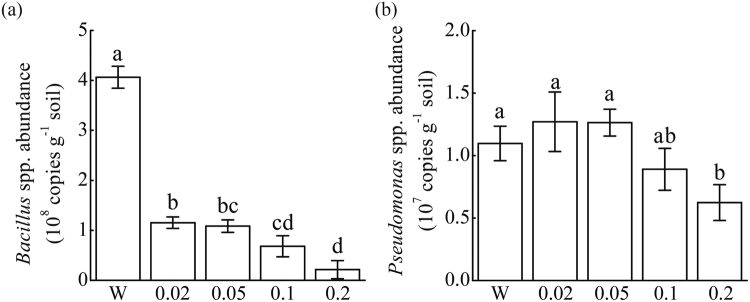
Quantitative PCR revealed that all concentrations of vanillic acid (0.02, 0.05, 0.1, 0.2 μmol g−1 soil) significantly decreased rhizosphere Bacillus spp. community abundance (P < 0.05) (Fig. 6a). The lowest Bacillus spp. community abundance was observed in rhizosphere soils treated with 0.2 μmol g−1 soil vanillic acid. Vanillic acid at 0.02, 0.05 and 0.1 μmol g−1 soil did not significantly affect cucumber rhizosphere Pseudomonas spp. community abundance (Fig. 6b). Vanillic acid at 0.2 μmol g−1 soil significantly decreased rhizosphere Pseudomonas spp. community abundance (P < 0.05). Bacillus and Pseudomonas spp. community abundances in rhizosphere soils treated with water was 19 and 1.76 times of these in rhizosphere soils treated with 0.2 μmol g−1 soil vanillic acid, respectively.
Figure 6.
Abundances of Bacillus (a) and Pseudomonas (b) spp. communities in cucumber rhizosphere soils as determined by quantitative PCR. W represents cucumber rhizosphere soils treated with water. 0.02, 0.05, 0.1 and 0.2 represent cucumber rhizosphere soils treated with vanillic acid at 0.02, 0.05, 0.1, 0.2 μmol g−1 soil concentrations, respectively. Data are represented as the means of three independent replicates with standard error bars. Different letters indicate significant difference based on Tukey’s HSD test test (P < 0.05).
Discussion
Plant-derived carbon metabolites, released through root exudation and residue composition, can be assimilated by soil microorganisms, the growths of which are usually limited by carbon resources25. It was observed that phenolic acids were rapidly decomposed by soil microorganisms after entering the soil26,27. Therefore, vanillic acid was applied into the soil periodically as described before26. Generally, the bioactivity of toxic compounds on plants and microorganisms was concentration dependent28. Previously, we found that the concentration of vanillic acid in cucumber rhizosphere was about 0.05 μmol g−1 soil in a continuous monocropping system22. In both natural and agricultural ecosystems, the concentration of soil phenolic acids was shown to range from 0.01 to 0.5 μmol g−1 soil29–31. Therefore, concentrations of vanillic acid used in this study (0.02 to 0.2 μmol g−1 soil) were within the realistic range of concentrations in the soil reported before.
Blum et al.32 found that phenolic acids inhibited cucumber seedling growth but stimulated the rhizosphere phenolic acid-utilizing bacteria. We also demonstrated that vanillic acid at concentration ≥0.05 μmol g−1 soil inhibited cucumber seedling growth and increased rhizosphere bacterial community abundance23. In this study, high-throughput amplicon sequencing of bacterial 16S rRNA gene was used to further illustrate the taxonomies of these responsive bacteria. Results showed that relative abundances of some bacteria such as Rhodanobacter, Steroidobacter and Ohtaekwangia spp., which have been reported to be involved in phenolic compounds degradation33–35, were enriched in cucumber rhizosphere soils treated with vanillic acid. Future in vitro studies should focus on validating the capabilities of these microorganisms to metabolize vanillic acid.
In continuous monocropping systems, plant usually showed reduced growth and vigor, and increased disease index5,36 For example, continuous monocropping of cucumber stimulated the abundance of Fusarium oxysporum f.sp. cucumerinum, the causing agent of cucumber Fusarium wilt disease4. Illumina MiSeq sequencing showed that, compared with rhizosphere soils treated with water, rhizosphere soils treated with vanillic acid (0.05 μmol g−1 soil) had higher relative abundances of Bacillus, Archangium and Lysobacter spp., which contained species to promote plant growth and inhibit plant pathogens19,37,38. Quantitative PCR also found that all concentrations of vanillic acid (0.02 to 0.2 μmol g−1 soil) decreased cucumber rhizosphere Bacillus spp. abundance. Some species in Bacillus spp. are able to inhibit plant soil-borne pathogens, including Fusarium oxysporum f.sp. cucumerinum, and promote cucumber growth19. Therefore, decreases in cucumber rhizosphere plant-beneficial microorganisms induced by vanillic acid may contribute to increased soil-borne diseases in the continuous monocropping system.
In this study, vanillic acid increased the relative abundances of Arenimonas39, Gemmatimonas40, Haliangium41, Opitutus42, Pseudolabrys43, Steroidobacter44 and Rhodanobacter spp.45, which contained taxa with denitrification capabilities, dissimilatory reduction of nitrate to nitrous oxide and N2. Meanwhile, vanillic acid decreased the relative abundance of Nitrospira spp.46, which had nitrification capabilities, oxidation of ammonia to nitrite. These results validated previous studies showing that phenolic acids were able to inhibit nitrification47 and some denitrifiers were able to use phenolic acids as carbon sources48. Nitrogen is one of the major limiting elements in agricultural ecosystems49. Our results indicated that there was possibility that phenolic acids could influence plant growth through regulating rhizosphere nitrogen transformations, such as inhibiting nitrification and promoting denitrification.
Illumina MiSeq sequencing and PCR-DGGE analyses showed that vanillic acid changed the structure and composition of cucumber rhizosphere Pseudomonas spp. community. Illumina MiSeq sequencing revealed that vanillic acid also changed the composition of cucumber rhizosphere Bacillus spp. community. Moreover, quantitative PCR analysis showed that vanillic acid decreased Bacillus spp. community abundance at 0.02 to 0.2 μmol g−1 soil, and decreased Pseudomonas spp. community abundance at 0.2 μmol g−1 soil. It has been shown that agricultural intensification could negatively influence soil Pseudomonas and Bacillus spp. communities16,20,21,50. For example, continuous monocropping of Radix pseudostellariae changed the community structure and decreased the abundance of soil Pseudomonas spp.20. Continuous monocropping of Helianthus tuberosus changed soil Pseudomonas and Bacillus spp. community structures51. It is well known that continuous monocropping can lead to accumulation of autotoxins, including phenolic acids, in the soil6,22,48. Thus, accumulation of phenolic autotoxins may be linked the effects of continuous monocropping on Pseudomonas and Bacillus spp. communities.
Plants can influence the diversity and composition of rhizosphere microbial communities through releasing root exudates10. Generally, rhizosphere microbial communities have higher abundances but lower diversities than those of the bulk soil8. It has been observed that artificially applied phenolic acids can alter the composition and diversity of soil microbial communities in absence of host plants10,15. Phenolic autotoxins can damage plant root and enhance ion leakage52 and these changes may also affect rhizosphere microbial communities. Therefore, besides its direct effects, vanillic acid may also indirectly changed cucumber rhizosphere microbial communities through its effects on physiological status of cucumber. These also suggest that rhizosphere and bulk microbial communities may respond differently to phenolic acids, which need to be further elucidated.
Phenolic acids are ubiquitous secondary metabolites in plants2,6. However, the composition of phenolic acids in root exudates or rhizosphere soils differed among crop species2,7,22,31. Liu et al.15 found that benzoic acid, a phenolic compound found in peanut (Arachis hypogaea) root exudates, increased the relative abundance of Burkholderia spp. in soil. However, vanillic acid did not affect the relative abundance of Burkholderia spp. in this study. These indicated that different phenolic acids may have different effects on soil microbial communities. Previous studies have also revealed the structure-function relationships of the phytotoxic and antimicrobial activities of phenolic acids6,53. For example, cinnamic acid derivatives had higher inhibitory effects than their corresponding benzoic acid derivatives on cucumber seedlings54. Phenolic acids with the hydroxyl group in the position para to the carboxyl side chain, such as benzoic acid, can be easily metabolized by the maize pathogen Cochliobolus heterostrophus; while phenolic acids lacking the para-hydroxy group or their para-methoxy substituted derivatives, such as ferulic acid, have higher antifungal activity53. These evidences may help to explain phenolic acids act as autotoxins of several crops though all plants releases phenolic acids.
Conclusion
Overall, our results revealed that vanillic acid changed cucumber rhizosphere total bacterial, Pseudomonas and Bacillus spp. community compositions, and Pseudomonas and Bacillus spp. community abundances. In particular, Illumina MiSeq sequencing showed that vanillic acid at 0.05 μmol g−1 soil inhibited the relative abundances rhizosphere microorganisms with plant-beneficial potentials. Quantitative PCR analysis showed that vanillic acid decreased Bacillus spp. community abundance at 0.02 to 0.2 μmol g−1 soil, and decreased Pseudomonas spp. community at 0.2 μmol g−1 soil. It is clear that phenolic acids are toxic to plants2,6,7,52. Results obtained from the present study suggested that phenolic acids may also inhibit plant growth through changing rhizosphere microbial communities, which need to be further explored by evaluating the effects of phenolic acids on the functions of rhizosphere microbial communities and their relationships with plant performance.
Materials and Methods
Pot Experiment
The soil used in this experiment was collected from the upper soil layer (0–15 cm) of an open field in the experimental station of Northeast Agricultural University, Harbin, China (45°41′N, 126°37′E), which was covered with grasses and undisturbed for more than 15 years. The soil has a sandy loam texture, contained organic matter, 3.67%; available N, 89.02 mg kg−1; Olsen P, 63.36 mg kg−1; available K, 119.15 mg kg−1; EC (1:2.5, w/v), 0.33 mS cm−1; and pH (1:2.5, w/v), 7.78. Cucumber seedlings (cv. Jinlv 3) with two cotyledons were transplanted into pots contained 150 g soil and maintained in a greenhouse (32 °C day/22 °C night, relative humidity of 60–80%, 16 h light/8 h dark). There was one cucumber seedling per pot. No fertilizer was added to the soil.
Cucumber seedlings at the one-leaf stage were treated with different concentrations of vanillic acid (0.02, 0.05, 0.1, 0.2 μmol g−1 soil) every two days for five times as recommended before26. The solution pH was adjusted to 7.0 with 0.1 M NaOH solution, because the soil pH is widely accepted as a dominant factor that regulates soil microbial communities55. Cucumber seedlings treated with distilled water were served as the control. Soil water content was adjusted every two days with distilled water to maintain a constant weight of pots. There were five treatments (four concentrations of vanillic acid and one control) in total. Each treatment had five seedlings and was replicated three times.
Rhizosphere Soil Sampling and DNA Extraction
Ten days after the first application of vanillic acid, cucumber rhizosphere soil samples were collected as described before5. Briefly, cucumber roots were gently removed from the pot, and soils loosely attached to cucumber roots were carefully removed by manual shaking. Then, soils tightly adhering to roots were removed from the root surface by a sterile brush and considered as rhizosphere soils. After sieving (2 mm), rhizosphere soil samples were stored at −70 °C. Samples from five plants in each replicate were combined to make a composite sample. There were three composite rhizosphere samples for each treatment.
Total soil DNA was extracted with the PowerSoil DNA Isolation Kit (MO BIO Laboratories, Carlsbad, USA) as per the manufacturer’s instructions. Each composite soil sample was extracted in triplicate and the extracted DNA solutions were pooled.
High-throughput Amplicon Sequencing and Data Processing
Total rhizosphere soil bacterial community compositions were estimated with high-throughput sequencing on an Illumina MiSeq platform. Primer set of F338/R806 was used to amplify V3-V4 regions of the bacterial 16S rRNA gene as described before4,56. Both the forward and reverse primers also had a six-bp barcode unique to each soil sample. The PCR protocol was: 95 °C for 3 min; followed by 27 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 45 s; and a final extension at 72 °C for 10 min. Each DNA sample was independently amplified in triplicate. Products of the triplicate PCR reactions were pooled and purified using an Agarose Gel DNA purification kit (TaKaRa, China). Then, purified amplicons were quantified by a TBS-380 micro fluorometer with Picogreen reagent (Invitrogen, USA), and mixed accordingly to achieve the equal concentration in the final mixture. The mixture was then paired-end sequenced (2 × 300) on an Illumina Miseq platform at Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China.
Raw sequence reads were de-multiplexed, quality-filtered, and processed using FLASH57 as described before4. Operational taxonomic units (OTUs) were delineated at 97% sequence similarity with UPARSE using an agglomerative clustering algorithm58. Then, a representative sequence of each OTU was taxonomically classified through BLAST in Ribosomal Database Project (RDP) database59. Chimeric sequences were identified and removed using USEARCH 6.1 in QIIME60. To avoid potential bias caused by sequencing depth, a random subsampling effort of 24,245 16S rRNA gene sequences per sample was performed for further analysis. The data set was deposited in the NCBI-Sequence Read Archive with the submission Accession Number SRP119631.
PCR-DGGE Analysis
Semi-nested PCR protocols were used to amplify Pseudomonas and Bacillus spp. 16S rDNA fragments. Primer sets of PsF/PsR and GC-338F/518 R were used for the first and second round of PCR amplification of Pseudomonas spp.16, respectively; while BacF/BacR and GC-338F/518 R were used for Bacillus spp.21. The PCR protocol was: 95 °C for 5 min; followed by 28 cycles of 95 °C for 30 s, 65 °C for 30 s for PsF/PsR and BacF/BacR (56 °C for 45 s for GC-338F/518 R), 72 °C for 90 s; and a final extension at 72 °C for 10 min.
For DGGE analysis, 6% (w/v) acrylamide gel with 45–65% denaturant gradient was used for Pseudomonas and Bacillus communities51. The gel was run in a 1 × TAE (Tris-acetate-EDTA) buffer for 14 h under conditions of 60 °C and 80 V with a DCode universal mutation detection system (Bio-Rad Lab, LA, USA). After the electrophoresis, the gel was stained in 1:3300 (v/v) GelRed (Biotium, USA) nucleic acid staining solution for 20 min. DGGE profiles were photographed with an AlphaImager HP imaging system (Alpha Innotech Crop., CA, USA) under UV light.
Quantitative PCR Assay
Abundances of Pseudomonas and Bacillus spp. communities were estimated by quantitative PCR assays with primer sets of PsF/PsR16 and BacF/BacR21, respectively, as described before51,61. The PCR protocol was: 95 °C for 5 min; followed by 30 cycles of 95 °C for 30 s, 65 °C for 30 s, 72 °C for 90 s; and a final extension at 72 °C for 10 min. Standard curves were made with a 10-fold dilution series (102–108) of plasmids containing 16S rRNA genes of Pseudomonas and Bacillus spp. from soil samples. Sterile water was used as a negative control to replace the template. All amplifications were done in triplicate. The specificity of the products was confirmed by melting curve analysis and agarose gel electrophoresis. The threshold cycle (Ct) values obtained for each sample were compared with the standard curve to determine the initial copy number of the target gene.
Statistical Analysis
For Illumina Miseq sequencing data, the defined OTUs were used to calculate taxon accumulation curves with the ‘vegan’ package in ‘R’62. Alpha diversity indices, Chao, ACE, Shannon index and inverse Simpson index were calculated using QIIME60. For beta diversity analysis, weighted UniFrac distances and Bray-Curtis distances were calculated using QIIME60 and ‘vegan’ package in ‘R’62, respectively. Principal coordinates analysis was conducted to visualize the community similarity with the ‘vegan’ package in ‘R’62. Linear discriminant effect size (LEfSe) analysis was used to identify microbial taxa that were significantly associated with each treatment with an alpha value of 0.05 for the Kruskal-Wallis test and a threshold of 2.0 for logarithmic linear discriminant analysis (LDA) scores63. Differences in relative abundances of microbial taxa between treatments were analyzed using Welch’s t test with Bonferroni correction in ‘STAMP’64.
The DGGE profiles banding patterns were analyzed with Quantity One V4.5 (Bio-Rad Lab, LA, USA). Principal component analysis was used to compare the band patterns between samples with Canoco for Windows 4.5 software (Plant Research International, Wageningen, the Netherlands). The microbial community diversity indices, including number of bands, Shannon-Wiener index and evenness index, were calculated as described before5.
Data were analyzed by analysis of variance (ANOVA). For alpha diversity indices from Illumina Miseq sequencing, mean comparison between treatments was performed based on the Welch’s t test at the 0.05 probability level. For diversity indices from the PCR-DGGE analysis, mean comparison between treatments was performed based on the Tukey’s honestly significant difference (HSD) test at 0.05 probability level.
Electronic supplementary material
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31772361), ‘Academic Backbone’ Project of Northeast Agricultural University (17XG05), University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT-2015002), and China Agricultural Research System (CARS-23-C-10).
Author Contributions
X.Z. and F.W. conceived and designed the study. X.Z. performed experiments, analyzed the data and wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-23406-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Inderjit CRM, Vivanco JM. Can plant biochemistry contribute to understanding of invasion ecology? Trends Plant Sci. 2006;11:574–580. doi: 10.1016/j.tplants.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Singh HP, Batish DR, Kohli RK. Autotoxicity: concept, organisms and ecological significance. Crit Rev Plant Sci. 1999;18:757–772. doi: 10.1080/07352689991309478. [DOI] [Google Scholar]
- 3.Zhou X, Wu F. p-Coumaric acid influenced cucumber rhizosphere soil microbial communities and the growth of Fusarium oxysporum f. sp. cucumerinum Owen. PloS one. 2012;7:e48288. doi: 10.1371/journal.pone.0048288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou X, Liu J, Wu F. Soil microbial communities in cucumber monoculture and rotation systems and their feedback effects on cucumber seedling growth. Plant Soil. 2017;415:507–520. doi: 10.1007/s11104-017-3181-5. [DOI] [Google Scholar]
- 5.Zhou X, Yu G, Wu F. Effects of intercropping cucumber with onion or garlic on soil enzyme activities, microbial communities and cucumber yield. Eur J Soil Biol. 2011;47:279–287. doi: 10.1016/j.ejsobi.2011.07.001. [DOI] [Google Scholar]
- 6.Blum U. Allelopathic interactions involving phenolic acids. J Nematol. 1996;28:259–267. [PMC free article] [PubMed] [Google Scholar]
- 7.Yu JQ, Matsui Y. Phytotoxic substances in the root exudates of Cucumis sativus L. J Chem Ecol. 1994;20:21–31. doi: 10.1007/BF02065988. [DOI] [PubMed] [Google Scholar]
- 8.Berendsen RL, Pieterse CM, Bakker PA. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17(478-486):2012. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 9.van der Putten WH, et al. Plant–soil feedbacks: the past, the present and future challenges. J Ecol. 2013;101:265–276. doi: 10.1111/1365-2745.12054. [DOI] [Google Scholar]
- 10.Badri DV, Chaparro JM, Zhang R, Shen Q, Vivanco JM. Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J Biol Chem. 2013;288:4502–4512. doi: 10.1074/jbc.M112.433300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bais HP, Prithiviraj B, Jha AK, Ausubel FM, Vivanco JM. Mediation of pathogen resistance by exudation of antimicrobials from roots. Nature. 2005;434:217–221. doi: 10.1038/nature03356. [DOI] [PubMed] [Google Scholar]
- 12.Lanoue A, et al. De novo biosynthesis of defense root exudates in response to Fusarium attack in barley. New Phytol. 2010;185:577–588. doi: 10.1111/j.1469-8137.2009.03066.x. [DOI] [PubMed] [Google Scholar]
- 13.Walker TS, Bais HP, Halligan KM, Stermitz FR, Vivanco JM. Metabolic profiling of root exudates of Arabidopsis thaliana. J Agr Food Chem. 2003;51:2548–2554. doi: 10.1021/jf021166h. [DOI] [PubMed] [Google Scholar]
- 14.Zhou X, Yu G, Wu F. Responses of soil microbial communities in the rhizosphere of cucumber (Cucumis sativus L.) to exogenously applied p-hydroxybenzoic acid. J Chem Ecol. 2012;38:975–983. doi: 10.1007/s10886-012-0156-0. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Li X, Jia Z, Zhang T, Wang X. Effect of benzoic acid on soil microbial communities associated with soilborne peanut diseases. Appl Soil Ecol. 2017;110:34–42. doi: 10.1016/j.apsoil.2016.11.001. [DOI] [Google Scholar]
- 16.Garbeva P, van Veen JA, van Elsas JD. Assessment of the diversity, and antagonism towards Rhizoctonia solani AG3, of Pseudomonas species in soil from different agricultural regimes. FEMS Microbiol Ecol. 2004;47:51–64. doi: 10.1016/S0168-6496(03)00234-4. [DOI] [PubMed] [Google Scholar]
- 17.Pérez-García A, Romero D, de Vicente A. Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotechnol. 2011;22:187–193. doi: 10.1016/j.copbio.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Wei G, Kloepper JW, Tuzun S. Induced systemic resistance to cucumber diseases and increased plant growth by plant growth-promoting rhizobacteria under field conditions. Phytopathology. 1996;86:221–224. doi: 10.1094/Phyto-86-221. [DOI] [Google Scholar]
- 19.Cao Y, et al. Bacillus subtilis SQR 9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol Fert Soils. 2011;47:495–506. doi: 10.1007/s00374-011-0556-2. [DOI] [Google Scholar]
- 20.Chen J, et al. Assessment of the diversity of Pseudomonas spp. and Fusarium spp. in Radix pseudostellariae rhizosphere under monoculture by combining DGGE and quantitative PCR. Front Microbiol. 2017;8:1748. doi: 10.3389/fmicb.2017.01748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garbeva P, van Veen JA, van Elsas JD. Predominant Bacillus spp. in agricultural soil under different management regimes detected via PCR-DGGE. Microbial Ecol. 2003;45:302–316. doi: 10.1007/s00248-002-2034-8. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Yu G, Wu F. Soil phenolics in a continuously mono-cropped cucumber (Cucumis sativus L.) system and their effects on cucumber seedling growth and soil microbial communities. Eur J Soil Sci. 2012;63:332–340. doi: 10.1111/j.1365-2389.2012.01442.x. [DOI] [Google Scholar]
- 23.Zhou X, Wu F. Artificially applied vanillic acid changed soil microbial communities in the rhizosphere of cucumber (Cucumis sativus L.) Can J Soil Sci. 2013;93:13–21. doi: 10.4141/cjss2012-039. [DOI] [Google Scholar]
- 24.Schöler A, Jacquiod S, Vestergaard G, Schulz S, Schloter M. Analysis of soil microbial communities based on amplicon sequencing of marker genes. Biol Fertil Soils. 2017;53:485–489. doi: 10.1007/s00374-017-1205-1. [DOI] [Google Scholar]
- 25.Hodge A, Robinson D, Fitter A. Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci. 2000;5:304–308. doi: 10.1016/S1360-1385(00)01656-3. [DOI] [PubMed] [Google Scholar]
- 26.Shafer SR, Blum U. Influence of phenolic acids on microbial populations in the rhizosphere of cucumber. J Chem Ecol. 1991;17:369–389. doi: 10.1007/BF00994339. [DOI] [PubMed] [Google Scholar]
- 27.Souto XC, Chiapusio G, Pellissier F. Relationships between phenolics and soil microorganisms in spruce forests: Significance for natural regeneration. J Chem Ecol. 2000;26:2025–2034. doi: 10.1023/A:1005504029243. [DOI] [Google Scholar]
- 28.Belz RG, Hurle K, Duke SO. Dose-Response—A Challenge for Allelopathy? Nonlinearity Biol Toxicol Med. 2005;3:173–211. doi: 10.2201/nonlin.003.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muscolo A, Sidari M. Seasonal fluctuations in soil phenolics of a coniferous forest: effects on seed germination of different coniferous species. Plant Soil. 2006;284:305–318. doi: 10.1007/s11104-006-0040-1. [DOI] [Google Scholar]
- 30.Piotrowski JS, Morford SL, Rillig MC. Inhibition of colonization by a native arbuscular mycorrhizal fungal community via Populus trichocarpa litter, litter extract, and soluble phenolic compounds. Soil Biol Biochem. 2008;40:709–717. doi: 10.1016/j.soilbio.2007.10.005. [DOI] [Google Scholar]
- 31.Li ZB, Zhou RJ, Fu JF. Allelopathic effects of phenolic acids from Ginseng rhizosphere soil on Cylindrocarpon destructans (zinss) Scholten. Allelopathy J. 2018;43:53–64. doi: 10.26651/allelo.j./2018-43-1-1129. [DOI] [Google Scholar]
- 32.Blum U, Staman KL, Flint LJ, Shaffer SR. Induction and/or selection of phenolic acid-utilizing bulk-soil and rhizosphere bacteria and their influence on phenolic acid phytotoxicity. J Chem Ecol. 2000;26:2059–2078. doi: 10.1023/A:1005560214222. [DOI] [Google Scholar]
- 33.Cébron A, et al. Root exudates modify bacterial diversity of phenanthrene degraders in PAH-polluted soil but not phenanthrene degradation rates. Environ Microbiol. 2011;13:722–736. doi: 10.1111/j.1462-2920.2010.02376.x. [DOI] [PubMed] [Google Scholar]
- 34.Hou J, et al. PGPR enhanced phytoremediation of petroleum contaminated soil and rhizosphere microbial community response. Chemosphere. 2015;138:592–598. doi: 10.1016/j.chemosphere.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 35.Bacosa HP, Suto K, Inoue C. Bacterial community dynamics during the preferential degradation of aromatic hydrocarbons by a microbial consortium. Int Biodeterior Biodegrad. 2012;74:109–115. doi: 10.1016/j.ibiod.2012.04.022. [DOI] [Google Scholar]
- 36.Cook RJ. Toward cropping systems that enhance productivity and sustainability. Proc Natl Acad Sci. 2006;103:18389–18394. doi: 10.1073/pnas.0605946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Böhlendorf B, et al. Antibiotics from gliding bacteria, LXXIII indole and ouinoline derivatives as metabolites of tryptophan in Myxobacteria. Eur J Org Chem. 1996;1:49–53. [Google Scholar]
- 38.Folman LB, Postma J, van Veen JA. Characterisation of Lysobacter enzymogenes (Christensen and Cook 1978) strain 3.1T8, a powerful antagonist of fungal diseases of cucumber. Microbiol Res. 2003;158:107–115. doi: 10.1078/0944-5013-00185. [DOI] [PubMed] [Google Scholar]
- 39.Remmas N, Melidis P, Katsioupi E, Ntougias S. Effects of high organic load on amoA and nirS gene diversity of an intermittently aerated and fed membrane bioreactor treating landfill leachate. Bioresource Technol. 2016;220:557–565. doi: 10.1016/j.biortech.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Jones CM, Graf DR, Bru D, Philippot L, Hallin S. The unaccounted yet abundant nitrous oxide-reducing microbial community: a potential nitrous oxide sink. ISME J. 2013;7:417–426. doi: 10.1038/ismej.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mcilroy SJ, et al. Identification of active denitrifiers in full-scale nutrient removal wastewater treatment systems. Environ Microbiol. 2016;18:50–64. doi: 10.1111/1462-2920.12614. [DOI] [PubMed] [Google Scholar]
- 42.Sanford RA, et al. Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proc Natl Acad Sci. 2012;109:19709–19714. doi: 10.1073/pnas.1211238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kämpfer P, et al. Pseudolabrys taiwanensis gen. nov., sp. nov., an alphaproteobacterium isolated from soil. Int J Syst Evol Microbiol. 2006;56:2469–2472. doi: 10.1099/ijs.0.64124-0. [DOI] [PubMed] [Google Scholar]
- 44.Fahrbach M, et al. Steroidobacter denitrificans gen. nov., sp. nov., a steroidal hormone-degrading gammaproteobacterium. Int J Syst Evol Microbiol. 2008;58:2215–2223. doi: 10.1099/ijs.0.65342-0. [DOI] [PubMed] [Google Scholar]
- 45.Green SJ, et al. Denitrifying bacteria from the genus Rhodanobacter dominate bacterial communities in the highly contaminated subsurface of a nuclear legacy waste site. Appl Environ Microbiol. 2012;78:1039–1047. doi: 10.1128/AEM.06435-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daims H, et al. Complete nitrification by Nitrospira bacteria. Nature. 2015;528:504–509. doi: 10.1038/nature16461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amor L, Eiroa M, Kennes C, Veiga MC. Phenol biodegradation and its effect on the nitrification process. Water Res. 2005;39:2915–2920. doi: 10.1016/j.watres.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Siqueira JO, Nair MG, Hammerschmidt R, Safir GR. Significance of phenolic compounds in plant-soil-microbial systems. Crit. Rev. Plant Sci. 1991;10:63–121. doi: 10.1080/07352689109382307. [DOI] [Google Scholar]
- 49.Canfield DE, Glazer AN, Falkowski PG. The evolution and future of Earth’s nitrogen cycle. Science. 2010;330:192–196. doi: 10.1126/science.1186120. [DOI] [PubMed] [Google Scholar]
- 50.Garbeva P, Postma J, van Veen JA, van Elsas JD. Effect of above-ground plant species on soil microbial community structure and its impact on suppression of Rhizoctonia solani AG3. Environ Microbiol. 2006;8:233–246. doi: 10.1111/j.1462-2920.2005.00888.x. [DOI] [PubMed] [Google Scholar]
- 51.Zhou X, et al. Dynamics of soil bacterial communities in Jerusalem artichoke monocropping system. Allelopathy J. 2016;39:167–178. [Google Scholar]
- 52.Yu JQ, Matsui Y. Effects of root exudates of cucumber (Cucumis sativus) and allelochemicals on ion uptake by cucumber seedlings. J Chem Ecol. 1997;23:817–827. doi: 10.1023/B:JOEC.0000006413.98507.55. [DOI] [Google Scholar]
- 53.Shalaby S, Horwitz BA, Larkov O. Structure-activity relationships delineate how the maize pathogen Cochliobolus heterostrophus uses aromatic compounds as signals and metabolites. Mol Plant Microbe In. 2012;25:931–940. doi: 10.1094/MPMI-01-12-0015-R. [DOI] [PubMed] [Google Scholar]
- 54.Gerig TM, Blum U. Effects of mixtures of four phenolic acids on leaf area expansion of cucumber seedlings grown in Portsmouth B 1 soil materials. J Chem Ecol. 1991;17:29–40. doi: 10.1007/BF00994420. [DOI] [PubMed] [Google Scholar]
- 55.Fierer N, Jackson RB. The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci. 2006;103:626–631. doi: 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Derakhshani H, Tun HM, Khafipour E. An extended single-index multiplexed 16S rRNA sequencing for microbial community analysis on MiSeq illumina platforms. J Basic Microb. 2016;56:321–326. doi: 10.1002/jobm.201500420. [DOI] [PubMed] [Google Scholar]
- 57.Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 59.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drigo B, van Veen JA, Kowalchuk GA. Specific rhizosphere bacterial and fungal groups respond differently to elevated atmospheric CO2. ISME J. 2009;3:1204–1217. doi: 10.1038/ismej.2009.65. [DOI] [PubMed] [Google Scholar]
- 62.R. Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ (2013).
- 63.Segata N, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30:3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.