Abstract
Purpose
To evaluate osseous changes of temporomandibular joint (TMJ) in patients with rheumatoid arthritis (RA) using cone-beam computed tomography (CBCT) and to correlate the imaging findings with the severity of TMJ dysfunction, clinical findings, and laboratory findings.
Materials and Methods
This study consisted of 28 subjects, including 14 RA patients and 14 controls, who were scheduled to undergo CBCT imaging for the diagnosis of a complaint not related to or affecting the TMJ. The Fonseca's questionnaire was used to assess the severity of TMJ dysfunction. Rheumatoid factor (RF) and the erythrocyte sedimentation rate (ESR) were assessed in the RA patients. CBCT was then performed in all subjects and osseous TMJ abnormalities were assessed.
Results
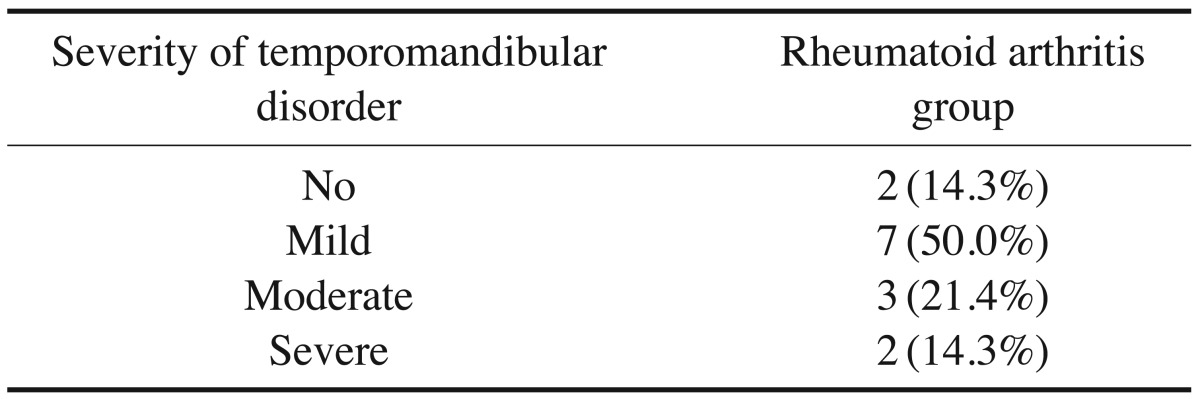
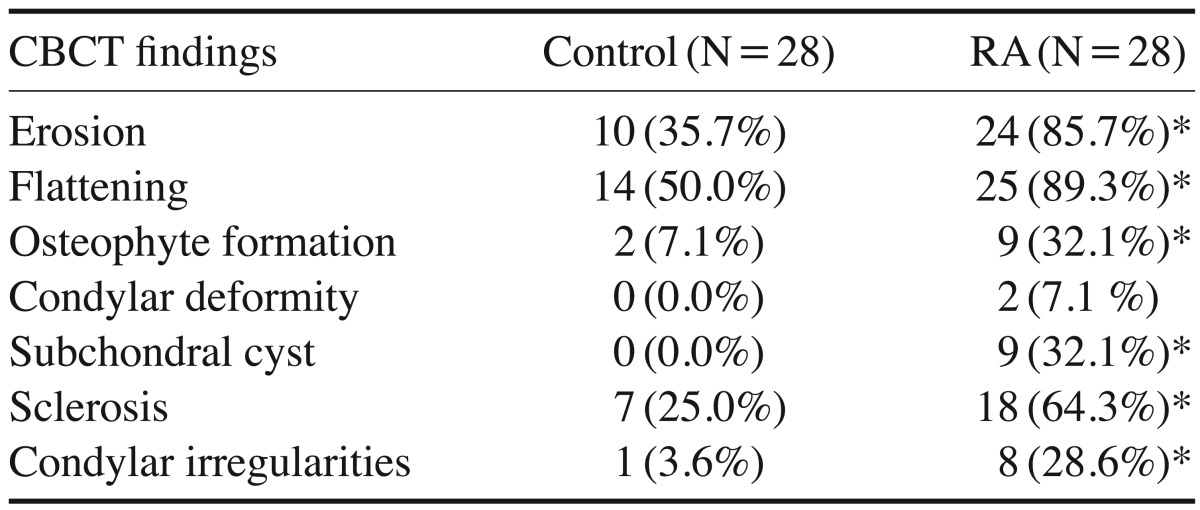
According to the Fonseca's questionnaire, 14.3% of the patients had no TMJ dysfunction, while 50%, 21.4%, and 14.3% had mild, moderate, and severe dysfunction, respectively. RF was positive in 64.3% of patients, and the ESR level was high in 100%. Imaging findings revealed a statistically significantly higher prevalence of erosion (85.7%), flattening (89.3%), osteophyte formation (32.1%), subchondral cyst (32.1%), sclerosis (64.3%), and condylar irregularities (28.6%) in the RA patients than in the controls. No correlations were found between CBCT findings and the clinical findings, the severity of TMJ dysfunction, disease duration, or laboratory results.
Conclusion
RA patients might show extensive osseous abnormalities with no/mild clinical signs or symptoms of TMJ dysfunction that necessitate TMJ imaging for these patients. CBCT is a valuable and efficient modality that can assess osseous TMJ changes in RA patients.
Keywords: Arthritis, Rheumatoid; Cone-Beam Computed Tomography; Temporomandibular Joint
Introduction
Rheumatoid arthritis (RA) is a chronic systemic disease that involves the joints in a symmetrical manner.1 It involves inflammation of the synovial tissue of the joint, leading to cartilage destruction and bone resorption, and ending in complete loss of the joint uniformity.2 It has been stated in the literature that bone destruction of the joints in RA occurs within 2–3 years after the onset of the disease, and then rapidly progresses.3
According to the American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines,4 as many as 70% of patients may test positive for rheumatoid factor (RF), an autoimmune antibody.2 Additionally, the erythrocyte sedimentation rate (ESR), which indicates inflammatory activity,5 is often elevated.
The temporomandibular joint (TMJ) is an important joint in the body that plays a crucial role in jaw movement during chewing, swallowing, and speech.6 Previous studies reported that 2%–86% of TMJs are affected in patients with RA.1,7
In RA patients, the most common clinical features of temporomandibular disorder (TMD) are joint pain, stiffness, swelling, limitation of mouth opening, mandible deviation, and joint sounds, such as clicking and crepitation.1
Various methods exist to screen for TMD, the most common of which is the Research Diagnostic Criteria for Temporomandibular Disorders.8 This method involves clinical and radiographic joint assessment, in addition to the evaluation of the patient's psychologic status and pain-related disability.9 Another method of TMD evaluation is the Fonseca's questionnaire, which is low-cost and easy to apply because it is a questionnaire.8
In RA, the radiographic features of the osseous components of the TMJ include erosion, resorption, flattening, and sclerosis of the articulating condyle and the glenoid fossa. Later, osteophyte formation can be observed in the condylar head.10 Moreover, the joint space may be reduced, subchondral cysts may be formed, and a sharp-pointed condyle (referred to as a ‘sharpened pencil’) or even complete destruction of the condylar head may occur, causing anterosuperior positioning of the condyle, which may lead to malocclusion.10,11 The imaging techniques for TMJ evaluation include conventional tomography, arthrography, computed tomography (CT), cone-beam CT (CBCT), and magnetic resonance imaging.11 CT has been considered to be the modality of choice for 3-dimensional imaging of the osseous components of the TMJ due to its high resolution and accuracy.12,13,14 A relatively recent imaging modality is CBCT, which has become widely available for dental use. The advantages of CBCT over CT include its low radiation dose and higher spatial resolution.15
Many studies have been conducted to investigate the ability of CT to detect osseous involvement of the TMJ in RA patients; these studies revealed that CT was highly accurate for detecting degenerative changes in the TMJ.14,16,17,18,19 CBCT has been used to assess TMJ condylar resorption, which occurs in early RA patients.20
Insufficient research has investigated the use of CBCT to evaluate the TMJ in patients with RA. Consequently, the aim of this study was to evaluate osseous changes in the TMJ in RA patients using CBCT and to correlate the imaging findings with the severity of TMJ dysfunction, clinical findings, and laboratory findings.
Materials and Methods
The current study was discussed and approved by the Institutional Review Board of the Faculty of Dentistry, Cairo University. During the course of the study, informed written consent was provided by all participants.
The study sample included 28 subjects between 20 and 45 years of age, with a mean age of 38 years. They were allocated into 2 groups: the RA group, which comprised 14 RA patients (1 male and 13 females), and the control group, which comprised 14 healthy subjects (2 males and 12 females). The RA group was selected from the patients receiving care at the outpatient clinic of the Rheumatology and Rehabilitation Department, Faculty of Medicine, Cairo University. The control group was selected from the patients receiving care at the outpatient clinic of the Oral and Maxillofacial Radiology Department, Faculty of Dentistry, Cairo University.
The subjects who were included in the control group had already been referred for a CBCT examination to assess complaints not related to or affecting the TMJ; these included impactions and preoperative implant site assessment of missing teeth. The selected individuals were examined for any local factors that could affect TMJ function (such as jaw-related trauma, complete or partial dentures, and bruxism or premature contact).
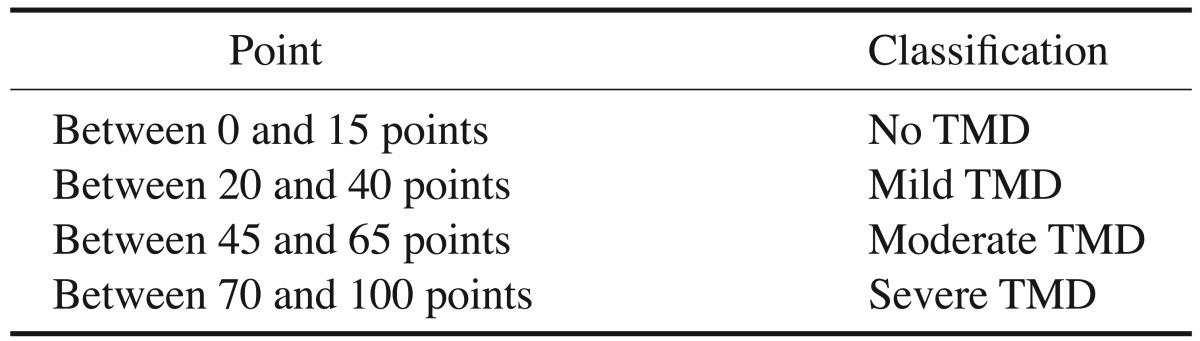
The prevalence and severity of TMD were assessed in both groups using the Fonseca's questionnaire. All subjects in the control group who showed any degree of TMD were excluded from the study. The questionnaire consisted of 10 questions and the subjects were informed that each question should be answered with “yes,” “no,” or “sometimes.” Each answer was scored as follows: “no,” 0; “sometimes,” 5; and “yes,” 10 (Table 1). The number of each reply (“no,” “sometimes,” and “yes”) was counted and the total number of each reply was multiplied by its score. The final value was then compared to the clinical index, and TMD severity was thereby determined (Table 2).21
Table 1. The questions of Fonseca's questionnaire.
Table 2. The classification of clinical index of temporomandibular disorder (TMD).
The inclusion criterion for the RA group was having had RA for more than 2 years. The exclusion criteria included the presence of any systemic disease affecting the TMJ other than RA, the presence of any local factor disturbing TMJ function, trigeminal neuralgia, facial nerve paralysis, having received a TMJ injection in the last 6 months, and pregnancy.
RA patients were assessed for the presence/absence of the clinical features of TMD, including joint pain, tenderness, morning stiffness, swelling, joint sounds, mandibular deviation, and limitation of mouth opening (a ruler was used to measure the maximum mouth opening, and a value less than 4 cm was considered to indicate limited mouth opening).1,22,23
Laboratory investigations were performed, including the assessment of RF positivity and the ESR level through a blood test. The blood tests were performed at the Cairo University Hospital Laboratories.
CBCT of the TMJ was conducted for each subject using a Planmeca® Promax3D Mid (Planmeca Oy, Helsinki, Finland) CBCT machine at the Department of Oral and Maxillofacial Radiology, Faculty of Dentistry, Cairo University. The imaging parameters were a field of view of 200×100 mm, a 500×500×500 image matrix, a voxel size of 400 µm, 90 kVp, 10 mA, and an exposure time of 18.5 s.
CBCT image analysis was performed using the Planmeca Romexis viewer software 4.2.0.R (Helsinki, Finland) specific to that CBCT machine. The TMJ was assessed in both the corrected sagittal and coronal views to guarantee an accurate visualization of the TMJ from the anteroposterior and the mesiolateral aspects. Each image was carefully inspected to spot any osseous abnormalities, such as erosion (an area of decreased density or loss of continuity of the cortical bone at the articular surface), flattening (blunting of the round shape of the condylar head), osteophyte formation (outward growth of osseous tissue), condylar deformity (major changes in the morphological shape of the condyle), subchondral cyst (a well-circumscribed radiolucency in the subcortical area with or without destruction of the cortex), sclerosis (an area of increased bone density extending to the bone marrow), and condylar irregularities (an irregular surface or resorptive changes of the condylar surface).
The images were analyzed by 3 radiologists with varying levels of experience, and a calibration session was performed to set the criteria for the TMJ assessment. Image analysis was performed by each observer on an individual basis without prior knowledge of the subject's group or the clinical or laboratory information of the patients. Whenever a disagreement existed between the observers, the final decision was reached by consensus.
Qualitative data are presented as number and percentages. Quantitative data with a parametric distribution are presented as mean, standard deviations, and ranges.
Comparisons of qualitative data between the 2 groups were made using the chi-square test and/or the Fisher exact test (if the expected count in any cell was less than 5). Comparisons of quantitative data with a parametric distribution between the 2 independent groups were made using the independent t-test. The clinical correlations were investigated using the chi-square test. All P values resulted from 2-sided statistical tests. P values <.05 were considered to indicate statistical significance.
Results
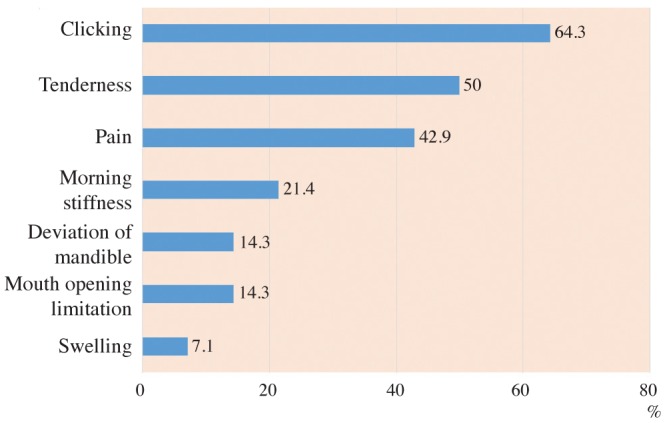
The prevalence and severity of TMD in the RA group according to the Fonseca's questionnaire are shown in Table 3. The proportions of patients with TMJ clinical findings in the RA group are presented in Figure 1. A positive RF test was found in 9 patients (64.3%) and the ESR level was high in all patients (100%).
Table 3. The frequencies and percentages of occurrence of the severity of temporomandibular disorder in the rheumatoid arthritis group.
Fig. 1. A bar chart demonstrates the proportions of rheumatoid arthritis (RA) patients showing various temporomandibular joint clinical findings.
In the CBCT images, the comparison between the right and left TMJs in the control and RA groups was not statistically significant; thus, the TMJs of both sides were combined for each group and considered as a single sample, such that each study group comprised 28 TMJs. Statistically significant differences were found between the 2 groups in the CBCT findings of osseous features of the TMJ. Condylar deformity was only detected in RA patients (Table 4). The osseous changes are illustrated in Figures 2, 3, 4, 5, 6, 7, 8. No correlations were found between CBCT findings and the clinical findings, the severity of TMJ dysfunction, disease duration, or laboratory results.
Table 4. The comparison between the computed tomographic (CBCT) findings in the control and rheumatoid arthritis (RA) groups.
*: p<0.05
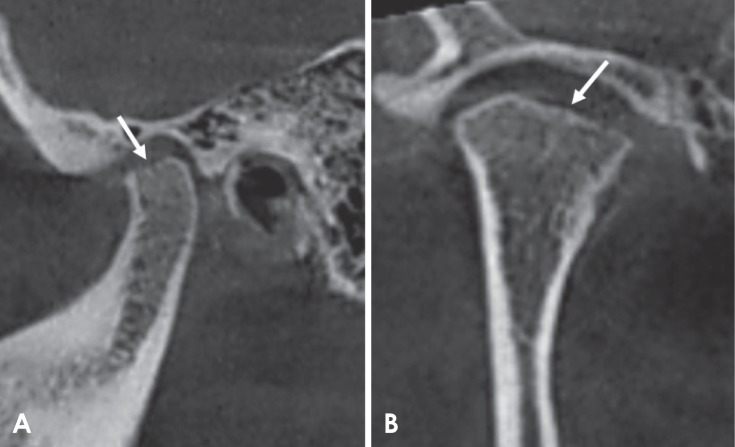
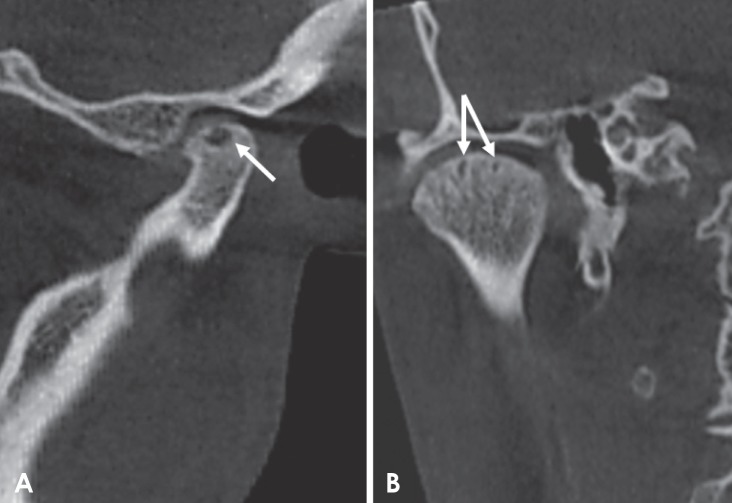
Fig. 2. Corrected and reformatted sagittal cone-beam computed tomographic images of rheumatoid arthritis patients revealing erosion as the loss of cortical continuity (arrow).
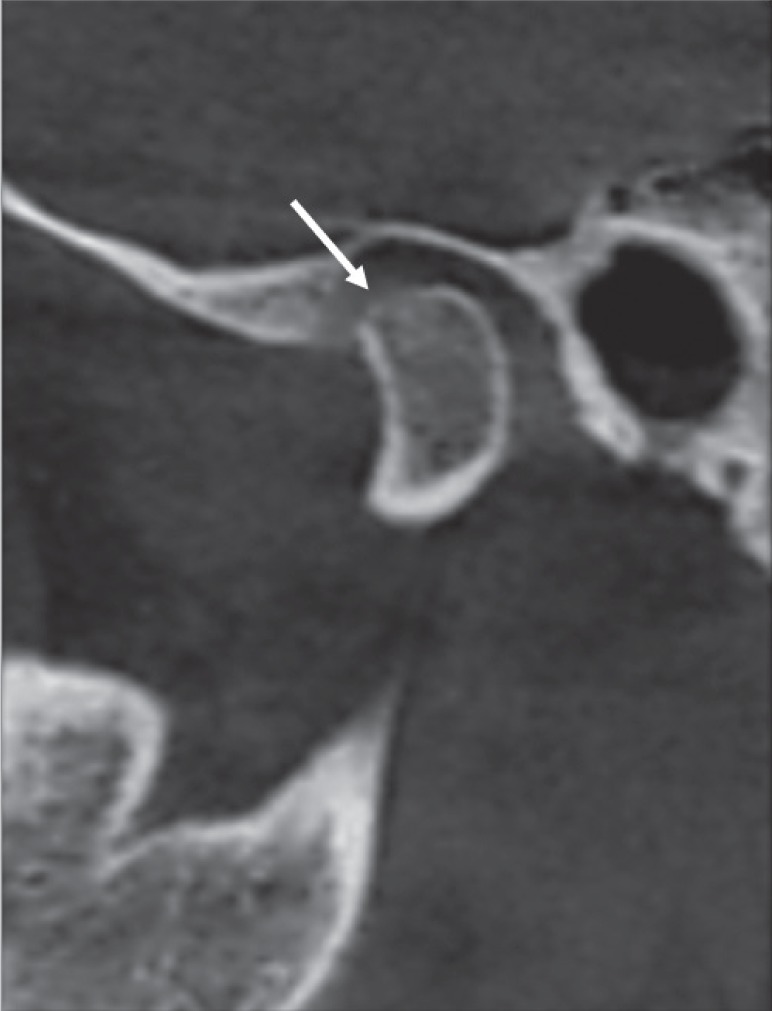
Fig. 3. Reformatted cone-beam computed tomographic images of a rheumatoid arthritis patient showing flattening of the condyle. A. Corrected sagittal view reveals flattening of the superior surface of the condyle (arrow). B. Corrected coronal view shows flattening of the medial part of the same condyle (arrow).
Fig. 4. Corrected sagittal cone-beam computed tomographic images of a rheumatoid arthritis patient showing an osteophyte (arrow).
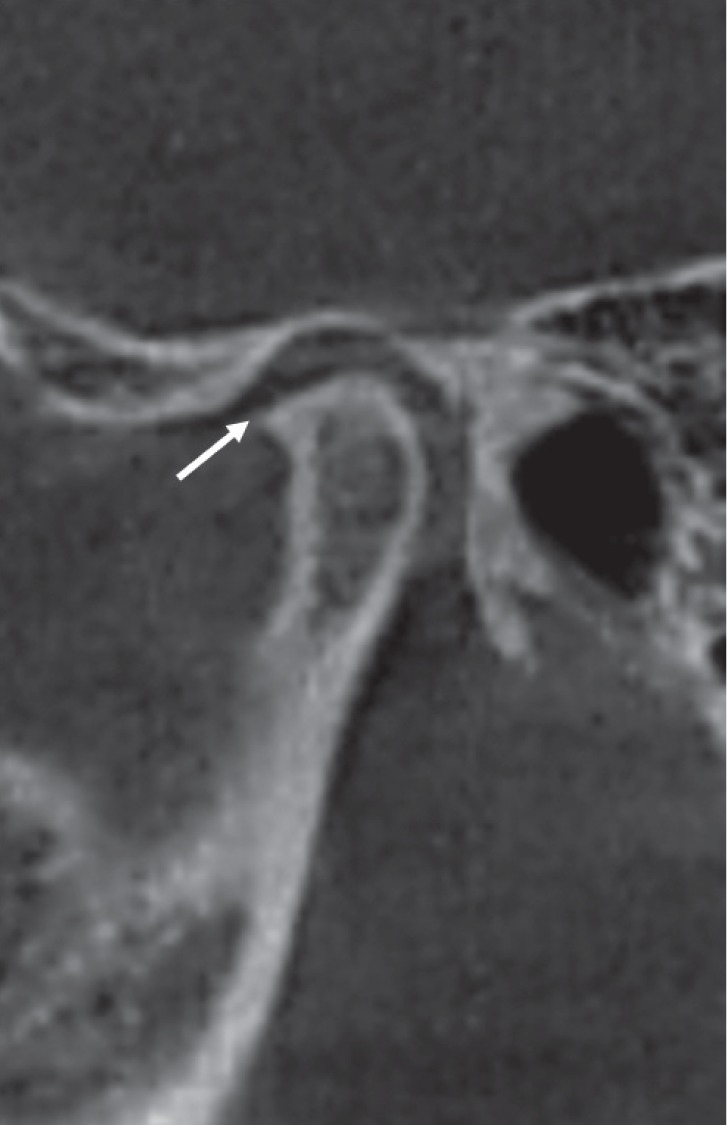
Fig. 5. Corrected sagittal cone-beam computed tomographic images of a rheumatoid arthritis patient showing condylar deformity. Note the presence of sclerosis and osteophyte formation.
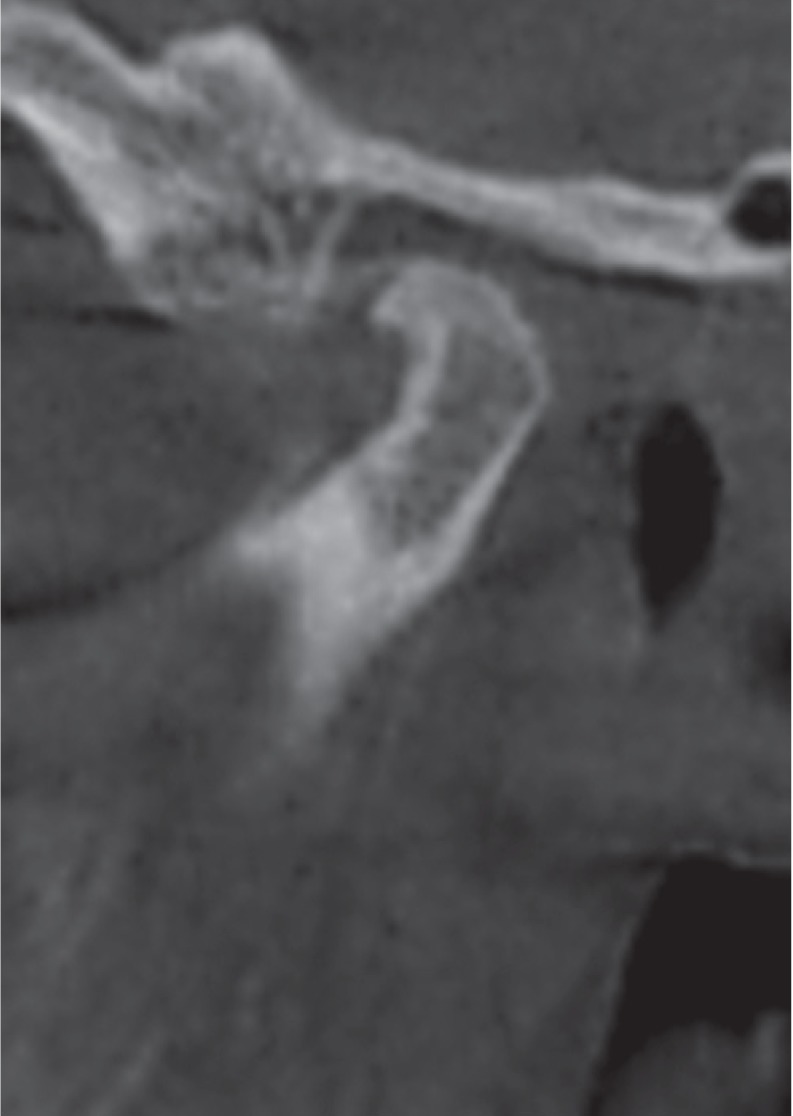
Fig. 6. Corrected reformatted conebeam computed tomographic images of a rheumatoid arthritis patient showing subchondral cysts (arrows). A. Corrected sagittal view. B. Corrected coronal view. Note the extensive subchondral sclerosis depicted in the coronal view.
Fig. 7. Corrected coronal cone-beam computed tomographic images of a rheumatoid arthritis patient showing surface irregularities in the superior aspect of the condyle. Note the extensive sclerosis and subchondral cysts.
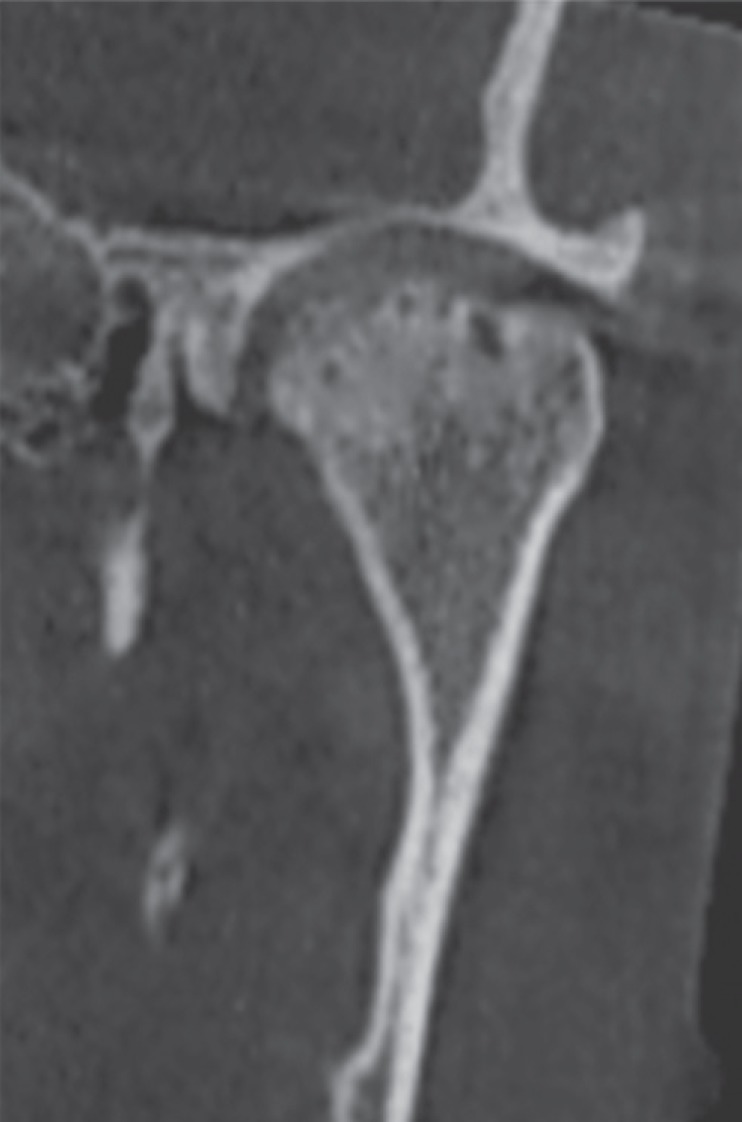
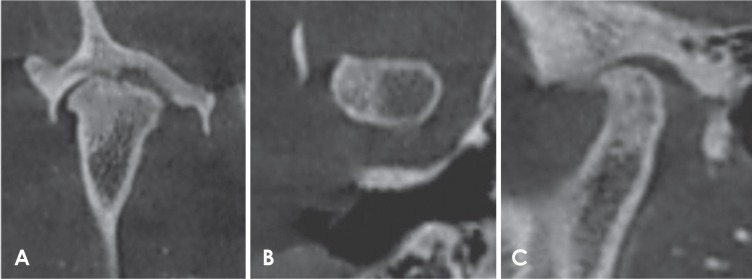
Fig. 8. Reformatted cone-beam computed tomographic images of a rheumatoid arthritis patient. A. Corrected coronal view. B. Axial view. C. Corrected sagittal view shows surface irregularities of the condyle and extensive sclerosis.
Discussion
RA is an inflammatory chronic disease characterized by uncontrolled proliferation of the synovial tissues of the joints.2 It has a 3 : 1 female-to-male predilection.24 In this study, the selected age of the studied population ranged between 20 and 45 years old; this was to ensure that all patients had undergone the complete calcification of cortical borders, which occurs by the age of 20 years old,11 and to exclude age-related degenerative arthritic changes.25
The Fonseca's questionnaire has been used to identify TMD patients and to classify them according to the severity of the disorder; this method was chosen due to its high efficiency and simplicity.21
The severity of TMJ dysfunction in the RA group was variable, with the majority of patients classified as having mild TMD (50%); however, no TMJ dysfunction was detected in 14.3% of the cases. Our results do not agree with those of Hiz et al.,26 who found that the majority of patients were classified as having moderate TMD (60%) and that no TMJ dysfunction was detected in only 3.3% of cases.
Clicking was the most frequent clinical TMJ symptom in the RA group, followed by tenderness and pain (64.3%, 50%, and 42.9%, respectively). Morning stiffness, mandibular deviation, and limitation of mouth opening occurred less frequently (21.4%, 14.3%, and 14.3%, respectively), and swelling was the least frequent clinical finding (7.1%). Our results are consistent with those of Helenius et al.27 and Ozcan et al.,28 who found that clicking was the most frequent clinical symptom; in contrast, Deoghare and Degwekar18 and Ardic et al.12 reported that the most common clinical findings were joint tenderness and pain, respectively.
RF is a non-specific antibody that may be produced in some autoimmune diseases and might be present in approximately 70% of RA patients.2,4 In this study, RF was positive in 64.3% of the patients. This agrees with the results of Gheita et al.,14 Kurup et al.,29 and Yilmaz et al.,1 who found RF positivity in 75%, 67%, and 60.71% of cases, respectively.
ESR is a diagnostic test commonly used to detect inflammation resulting from autoimmune diseases.5 Although it is a non-specific test, it is usually used to monitor the disease course.2,5 The ESR level was elevated in all of the studied patients; however, lower frequencies were detected by Kurup et al.29; Yilmaz et al.,1 and Voog et al.,16 who found that the ESR was elevated in 87%, 28.57%, and 53% of cases, respectively.
It is well known that CBCT scans with high-resolution protocols are accompanied by a high radiation dose.30 According to Zhang et al.,31 no difference was found between CBCT images scanned with high-resolution protocols and those scanned with normal-resolution protocols in the detection of condylar defects. As such, the normal-resolution protocol was used in the current study to minimize the patient radiation dose.
The comparison between the CBCT osseous findings in both groups revealed that the RA group had a statistically significant higher prevalence of erosion, flattening, osteophyte formation, subchondral cysts, sclerosis, and condylar irregularities than the control group.
CBCT showed erosion in 35.7% and 85.7% of the controls and RA patients, respectively. These results are similar to those of Goupille et al.19 who detected cortical erosion in both controls and RA patients, with frequencies of 57.6% and 88.4%, respectively. Similarly, Deoghare and Degwekar18 and Hajati et al.20 detected erosion in 85% and 72% of patients, respectively. However, lower frequencies of erosion were detected by Bayar et al.,17 Voog et al.,16 and Gheita et al.14 in their RA patients (13.3%, 50%, and 62.5%, respectively).
Flattening was detected in both groups, with frequencies of 50% and 89.3% for the control and RA groups, respectively. A lower frequency in RA patients (30%) was found by Voog et al.16
Osteophytes were detected in the RA group with a frequency of 32.1%. This is in accordance with Gheita et al.,14 who detected osteophytes in 33.33% of the patients they studied. A lower frequency (10%) was detected by Voog et al.16
Subchondral cyst was only detected in the RA group, with a frequency of 32.1%. Bayar et al.,17 Voog et al.,16 and Gheita et al.14 observed subchondral cysts in 23.3%, 30%, and 20.83% of their RA patients, respectively, while a lower frequency (10%) was reported by Deoghare and Degwekar.18
Sclerosis was detected in both the controls and the RA patients (25% and 64.3%, respectively). Diverse results were reported by Voog et al.16 and Gheita et al.14 in their RA patients (75% and 41.67%, respectively).
The frequencies of condylar irregularities and condylar deformity in the RA group were 28.6% and 7.1% respectively. A higher frequency of condylar deformity (37.5%) was reported by Gheita et al.14
In RA, infectious or autoimmune mechanisms are responsible for the proliferation of synovial fibroblasts and macrophages. This is followed by lymphocytic infiltration and endothelial proliferation. Eventually, the inflamed synovium grows, resulting in the formation of a pannus that invades the bone and cartilage and causes their destruction. Further joint destruction occurs due to the release of interleukins, cytokines, growth factors, and proteinases.2
Afterward, the body tries to repair the joint by broadening the joint surface to better withstand incoming forces, leading to osteophyte formation.32 This may explain the higher frequency of the occurrence of osteophytes in the RA group in the present study.
Two theories have been proposed regarding subchondral cysts. The first is the synovial intrusion theory, which suggests that these cysts could result from the proliferated synovium that invades the bone. This is justified by the similarity between the cystic fluid and the synovial fluid and the presence of some pieces of the surface cartilage within the cyst. The second theory is the bony contusion theory, which suggests that the loss of bone surface cartilage results in contact between the 2 opposing bony surfaces, causing bone microfractures and necrosis. Then, the bone starts the healing process by resorbing the necrotic areas, which provide a passage for the synovial fluid to intrude into the bone, resulting in subchondral cyst formation.33
Although flattening and sclerosis are pathologic processes, they may occur as a result of physiological remodeling to help the joint to withstand the additional forces applied to it.11,34 This may explain the occurrence of flattening and sclerosis in both groups in this study.
In the present study, no correlation was found between the CBCT findings and the clinical findings, the severity of TMJ dysfunction, or the laboratory results. Moreover, there was no correlation between the disease duration and the CBCT findings.
Since the inflammatory exudate in the joint is drained efficiently by special retrodiscal tissue that is rich in blood vessels, pain and other clinical symptoms may be avoided.1 This may explain the lack of a correlation between the clinical and imaging findings and may also clarify the presence of certain osseous findings in the control group.
The absence of a correlation between CBCT findings and clinical findings, TMJ dysfunction severity, or laboratory results is very alarming, because a patient may be asymptomatic but have degenerative changes that may worsen if not detected, thereby complicating the clinical situation and the final management of the case.
In conclusion, RA patients may show extensive osseous changes with no/mild clinical signs or symptoms of TMD. Therefore, TMJ imaging is mandatory for RA patients even in the absence of clinical symptoms to avoid the occurrence of severe complications. CBCT is an effective imaging modality with a relatively low dose that can be used to assess the osseous changes caused by this disease.
References
- 1.Yilmaz HH, Yildirim D, Ugan Y, Tunc SE, Yesildag A, Orhan H, et al. Clinical and magnetic resonance imaging findings of the temporomandibular joint and masticatory muscles in patients with rheumatoid arthritis. Rheumatol Int. 2012;32:1171–1178. doi: 10.1007/s00296-010-1743-4. [DOI] [PubMed] [Google Scholar]
- 2.Rindfleisch JA, Muller D. Diagnosis and management of rheumatoid arthritis. Am Fam Physician. 2005;72:1037–1047. [PubMed] [Google Scholar]
- 3.Kretapirom K, Okochi K, Nakamura S, Tetsumura A, Ohbayashi N, Yoshino N, et al. MRI characteristics of rheumatoid arthritis in the temporomandibular joint. Dentomaxillofac Radiol. 2013;42:31627230. doi: 10.1259/dmfr/31627230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 Update. Arthritis Rheum. 2002;46:328–346. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- 5.Assasi N, Blackhouse G, Campbell K, Hopkins RB, Levine M, Richter T, et al. Comparative value of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) testing in combination versus individually for the diagnosis of undifferentiated patients with suspected inflammatory disease or serious infection: a systematic review and economic analysis. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2015. [PubMed] [Google Scholar]
- 6.Campos PS, Reis FP, Aragão JA. Morphofunctional features of the temporomandibular joint. Int J Morphol. 2011;29:1394–1397. [Google Scholar]
- 7.Scrivani SJ, Keith DA, Kaban LB. Temporomandibular disorders. N Engl J Med. 2008;359:2693–2705. doi: 10.1056/NEJMra0802472. [DOI] [PubMed] [Google Scholar]
- 8.Campos JA, Carrascosa AC, Bonafé FS, Maroco J. Severity of temporomandibular disorders in women: validity and reliability of the Fonseca Anamnestic Index. Braz Oral Res. 2014;28:16–21. doi: 10.1590/s1806-83242013005000026. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad M, Hollender L, Anderson Q, Kartha K, Ohrbach R, Truelove EL, et al. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): development of image analysis criteria and examiner reliability for image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:844–860. doi: 10.1016/j.tripleo.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bag AK, Gaddikeri S, Singhal A, Hardin S, Tran BD, Medina JA, et al. Imaging of the temporomandibular joint: an update. World J Radiol. 2014;6:567–582. doi: 10.4329/wjr.v6.i8.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perschbacher S. Temporomandibular joint abnormalities. In: White SC, Pharoah MJ, editors. Oral radiology: principles and interpretation. 7th ed. St. Louis: Mosby-Year Book Inc; 2014. pp. 492–523. [Google Scholar]
- 12.Ardic F, Gokharman D, Atsu S, Guner S, Yilmaz M, Yorgancioglu R. The comprehensive evaluation of temporomandibular disorders seen in rheumatoid arthritis. Aust Dent J. 2006;51:23–28. doi: 10.1111/j.1834-7819.2006.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 13.Cara AC, Gaia BF, Perrella A, Oliveira JX, Lopes PM, Cavalcanti MG. Validity of single- and multislice CT for assessment of mandibular condyle lesions. Dentomaxillofac Radiol. 2007;36:24–27. doi: 10.1259/dmfr/54883281. [DOI] [PubMed] [Google Scholar]
- 14.Gheita T, Dahaba M, Ahmed E, Khalifa S, Basmy A. Using clinical and multislice computer tomographic features to assess temporomandibular joint osseous involvement in rheumatoid arthritis: a preliminary study. Turk J Rheumatol. 2012;27:47–55. [Google Scholar]
- 15.Alkhader M, Ohbayashi N, Tetsumura A, Nakamura S, Okochi K, Momin MA, et al. Diagnostic performance of magnetic resonance imaging for detecting osseous abnormalities of the temporomandibular joint and its correlation with cone beam computed tomography. Dentomaxillofac Radiol. 2010;39:270–276. doi: 10.1259/dmfr/25151578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voog U, Alstergren P, Eliasson S, Leibur E, Kallikorm R, Kopp S. Inflammatory mediators and radiographic changes in temporomandibular joints of patients with rheumatoid arthritis. Acta Odontol Scand. 2003;61:57–64. doi: 10.1080/ode.61.1.57.64. [DOI] [PubMed] [Google Scholar]
- 17.Bayar N, Kara SA, Keles I, Koç MC, Altinok D, Orkun S. Temporomandibular joint involvement in rheumatoid arthritis: a radiological and clinical study. Cranio. 2002;20:105–110. doi: 10.1080/08869634.2002.11746198. [DOI] [PubMed] [Google Scholar]
- 18.Deoghare A, Degwekar SS. Clinical and CT scan evaluation of temporomandibular joints with osteoarthritis and rheumatoid arthritis. J Indian Acad Oral Med Radiol. 2010;22(Suppl 1):1–5. [Google Scholar]
- 19.Goupille P, Fouquet B, Cotty P, Goga D, Valat JP. Temporomandibular joint and rheumatoid polyarthritis: correlations between clinical and tomodensitometric abnormalities. Rev Rhum Mal Osteoartic. 1992;59:213–218. [PubMed] [Google Scholar]
- 20.Hajati AK, Alstergren P, Näsström K, Bratt J, Kopp S. Endogenous glutamate in association with inflammatory and hormonal factors modulates bone tissue resorption of the temporomandibular joint in patients with early rheumatoid arthritis. J Oral Maxillofac Surg. 2009;67:1895–1903. doi: 10.1016/j.joms.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nomura K, Vitti M, Oliveira AS, Chaves TC, Semprini M, Siéssere S, et al. Use of the Fonseca's questionnaire to assess the prevalence and severity of temporomandibular disorders in Brazilian dental undergraduates. Braz Dent J. 2007;18:163–167. doi: 10.1590/s0103-64402007000200015. [DOI] [PubMed] [Google Scholar]
- 22.Sato H, Osterberg T, Ahlqwist M, Carlsson GE, Gröndahl HG, Rubinstein B. Association between radiographic findings in the mandibular condyle and temporomandibular dysfunction in an elderly population. Acta Odontol Scand. 1996;54:384–390. doi: 10.3109/00016359609003556. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher C, Gallagher V, Whelton H, Cronin M. The normal range of mouth opening in an Irish population. J Oral Rehabil. 2004;31:110–116. doi: 10.1046/j.0305-182x.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsiklakis K. Cone beam computed tomographic findings in temporomandibular joint disorders. Alpha Omegan. 2010;103:68–78. doi: 10.1016/j.aodf.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Alexiou K, Stamatakis H, Tsiklakis K. Evaluation of the severity of temporomandibular joint osteoarthritic changes related to age using cone beam computed tomography. Dentomaxillofac Radiol. 2009;38:141–147. doi: 10.1259/dmfr/59263880. [DOI] [PubMed] [Google Scholar]
- 26.Hiz O, Ediz L, Ozkan Y, Bora A. Clinical and magnetic resonance imaging findings of the temporomandibular joint in patients with rheumatoid arthritis. J Clin Med Res. 2012;4:323–331. doi: 10.4021/jocmr1084w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helenius LM, Tervahartiala P, Helenius I, Al-Sukhun J, Kivisaari L, Suuronen R, et al. Clinical, radiographic and MRI findings of the temporomandibular joint in patients with different rheumatic diseases. Int J Oral Maxillofac Surg. 2006;35:983–989. doi: 10.1016/j.ijom.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Ozcan I, Ozcan KM, Keskin D, Bahar S, Boyacigil S, Dere H. Temporomandibular joint involvement in rheumatoid arthritis: correlation of clinical, laboratory and magnetic resonance imaging findings. B-ENT. 2008;4:19–24. [PubMed] [Google Scholar]
- 29.Kurup S, Gharote H, Jose R. A radiographic evaluation of temporomandibular and hand (Metacarpophalangeal)/wrist joints of patients with adult rheumatoid arthritis. Dent Res J (Isfahan) 2012;9(Suppl 1):S32–S38. [PMC free article] [PubMed] [Google Scholar]
- 30.Scarfe WC, Li Z, Aboelmaaty W, Scott SA, Farman AG. Maxillofacial cone beam computed tomography: essence, elements and steps to interpretation. Aust Dent J. 2012;57(Suppl 1):46–60. doi: 10.1111/j.1834-7819.2011.01657.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang ZL, Shi XQ, Ma XC, Li G. Detection accuracy of condylar defects in cone beam CT images scanned with different resolutions and units. Dentomaxillofac Radiol. 2014;43:20130414. doi: 10.1259/dmfr.20130414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain AM, Packota G, Major PW, Flores-Mir C. Role of different imaging modalities in assessment of temporomandibular joint erosions and osteophytes: a systematic review. Dentomaxillofac Radiol. 2008;37:63–71. doi: 10.1259/dmfr/16932758. [DOI] [PubMed] [Google Scholar]
- 33.Audrey HX, Abd Razak HR, Andrew TH. The truth behind subchondral cysts in osteoarthritis of the knee. Open Orthop J. 2014;8:7–10. doi: 10.2174/1874325001408010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schellhas KP, Piper MA, Omlie MR. Facial skeleton remodeling due to temporomandibular joint degeneration: an imaging study of 100 patients. Cranio. 1992;10:248–259. doi: 10.1080/08869634.1992.11677918. [DOI] [PubMed] [Google Scholar]