Abstract
Ulcus vulvae acutum is a rare clinical condition characterized by the presence of multiple acute painful genital ulcers of non-venereal origin associated with systemic symptoms in young women. The aetiopathogenesis of the disease is not fully understood, although recent reports have associated it with the Epstein–Barr virus. Diagnosis is difficult and generally made by exclusion after venereal diseases, and autoimmune, inflammatory, traumatic, and neoplastic causes. We describe a case of adolescent female with an episode of ulcus vulvae acutum associated with infectious mononucleosis. The diagnosis was supported by the clinical symptoms, elevated circulating levels of liver enzymes, positive EBV serology, cervical and inguinal lymphadenomegaly, and hepatosplenomegaly. The patient presented a history of aphthous stomatitis. Negative Pathergy test and the absence of any other related symptoms allowed us to exclude the Behçhet syndrome. Lesions healed with no sequelae or recurrences.
Keywords: Ulcus vulvae acutum, Genital ulcers, Non-venereal origin
Highlights
-
•
Ulcus vulvae acutum is a rare clinical condition characterized by the presence of multiple acute painful genital ulcers.
-
•
Diagnosis is difficult and made by exclusion after venereal diseases, autoimmune, inflammatory, traumatic, neoplastic causes.
-
•
Lesions healed with no sequelae or recurrences.
1. Introduction
Ulcus vulvae acutum is a rare clinical condition characterized by the presence of multiple acute genital ulcers of non-venereal origin associated with malaise, fever and other systemic symptoms in young women. These ulcers usually heal spontaneously but tend to persist for some weeks and leave some scarring (1). The cause of this distressing syndrome ulcer is still unknown, although several infectious agents have been associated with the disease. Diagnosis is difficult and generally made by exclusion. We describe a case of adolescent female with an episode of ulcus vulvae acutum associated with infectious mononucleosis.
Case report: a 16-year-old woman was admitted to our clinic with painful vulvar ulcers suddenly onset a couple of days before. The patient reported high fever over the past days (up to 38 °C), malaise, mild headache, together with mild cough and oral aphthae. She presented history of few episodes of aphthous stomatitis during the year, spontaneously resolved. Genital examination revealed vulvar edema, two ulcerated lesions two-three centimeters of labia majora bilaterally with fibrin debris at the bottom and hyperemic edges as well as other ulcers smaller than one centimeter diameter at the level of labial commissure. The patient reported protected sexual contacts. Moreover, it was described a one millimeter oral aphthae, cervical and inguinal lymphadenomegaly (Fig. 1, Fig. 2). Complete blood count (CBC) and serological tests for a wide spectrum of infectious diseases were performed. Serology was negative for HIV, hepatitis B and C, HSV, TPHA, and CMV. CBC showed low leucocytes and platelet levels (GB 3.79 × 10 ^ 3 μL, PLT 106.000 × 10 ^ 3 μL) and augmentation of C-reactive protein (CPR 17.28 mg/L). The dosage of serum IgG, IgA and IgM resulted normal. During hospitalization the patient presented severe asthenia, high fever and sore throat. On her third day of hospitalization, it was registered an increase of liver enzymes (GPT/GOT 114/184 UI/L, LDH 629 UI/L). Vaginal smears for HSV and Trichomonas vaginalis came out negative as well as smears of the lesions for HSV. A biopsy for histological examination was not performed because the patient refused. Pathergy test was performed in suspicion of Behçet syndrome, and resulted negative. Of the exams executed, just EBV serology showed the presence of high levels of VCA IgM and EA, compatible with acute EBV infection. Complete abdomen ultrasound revealed hepatosplenomegaly. No antibiotic therapy was administered but a local treatment with emollients and analgesics was performed. Spontaneous resolution of the local disease occurred after 20 days. After discharge form our Unit, the patient subsequently was admitted to ENT department for the treatment of a persistent sore throat in necrotic tonsillitis with dysphagia as consequences of mononucleosis. Woman received corticosteroid and antibiotic therapy.
Fig. 1.

Vulvar edema and ulcer in a 16 years old patient.
Fig. 2.
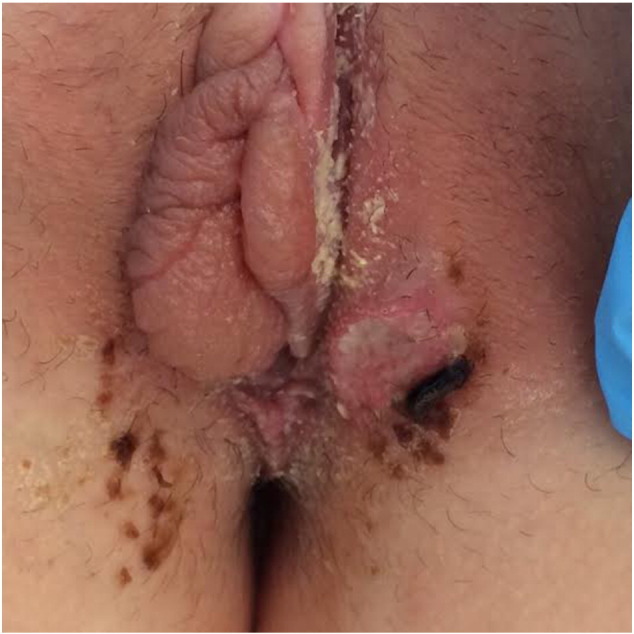
Vulvar edema and ulcer in a 16 years old patient.
2. Discussion
To make a right diagnosis in young patients with vulvar ulcers it is fundamental to perform an adequate anamnesis, starting from the history of sexual contacts (including abuse) in order to hypothesize possible sexually transmitted infections or other infective and non-infective diseases as origin (Table 1) [1], [2], [3]. Infectious causes like HSV, Syphilis, lymphogranuloma venereum, chancroid and HIV should be considered in young patients who are sexually active, being HSV etiology the most common and potentially also non sexually-transmitted. HSV-PCR assay is thus the first step in the management of vulvar ulcers [[4], [5] JEADV 2004, Mayo 2010]. Instead, when a documented sexually transmitted infectious etiology lacks or in the presence of a virgin female, other infective agents are to be considered besides inflammatory, autoimmune, traumatic and neoplastic causes [6]. The occurrence of acute disease comprising fever, genital ulceration and lymphadenopathy was a phenomenon first described in 1913 by Lipschütz in an adolescent girl without a history of sexual contact and thus formerly termed “ulcus vulvae acutum” or “Lipschütz ulcers” [7]. This entity is also recently known as “Reactive nonsexually related acute genital ulcers” (RNSRAGU) referring to genital ulceration that appears in response to an acute illness rather than as a manifestation of an underlying chronic systemic disease [5]. Numerous etiologies have been proposed such as Mycoplasma infection, paratyphoid fever, influenza A infection, cytomegalovirus (CMV) associated acute mononucleosis and mostly EBV infection, as in the present case, but pathogenesis is still unknown [1]. If the hypothesis of an interplay between a direct cytolytic effect of EBV replication in the vulvar epithelium and the associated inflammatory reaction is true, whether the infectious agent reaches the genital mucosa directly, via hematogenous transport by circulating infected T lymphocytes or through autoinoculation of oral secretion it is similarly debated [5], [8]. Moreover Farhi et al. [9] found negative results of in situ hybridization for EBV in three of four samples studied suggesting that ulcus vulvae acutum may be more likely to result from an indirect immune reaction than from a direct epithelial cytopathogenic effect of EBV [10]. The disease could be considered also a reactive process typically triggered by a distant infection, whose association with acute genital ulcers may be temporal, but not causal. In fact, the systemic infective illness leads the recruitment of cytotoxic T-lymphocytes, found in the infiltrate, that are responsible of marked inflammation and consequently genital ulceration [5], [10]. Moreover, a new recent report has emphasized the possible role of local immunological mechanisms in the development of the disease presenting two cases of ulcus vulvae acutum in young females with partial IgA deficiency [8]. They supposed that, in order to compensate the decreased levels of IgA, it will produce a pronounced T-helper 1 reaction, resulting in a stronger local cytotoxic immune response of the mucosa. As suggested by the Authors we evaluated IgA levels in our patient that resulted normal. At last, among the etiopathogenetic hypothesis of ulcus vulvae acutum, there is a type of aphthosis, whose spectrum ranges from aphthous minor to complex aphthosis (referred to recurrent, severe oral and genital ulcerations without other systemic manifestations of Behçet's disease) to full-blown Behçhet's disease [10]. In fact, as for oral aphthae, the stress caused by an infection could be a risk factor for aphtosis. In our case the patient presented genital lesions and a history of aphthous stomatitis but not any other symptoms thus the Behçhet syndrome has been excluded. Hupper et al., described a case series in which most were consistent with aphthous major and one third of patients with complex aphthosis [6].
Table 1.
Differential diagnosis of vulvar ulcers in adolescent.
| Girls [1–3] |
|---|
| Infectious |
| Venereal |
| Herpes simplex virus |
| Syphilis |
| Lymphogranuloma venereum |
| Chancroid |
| Granuloma inguinale |
| Human immunodeficiency virus (HIV) |
| Nonvenereal |
| Epstein–Barr virus |
| Cytomegalovirus |
| Mycobacteria |
| Candida |
| Parasite |
| Paratyphoid fever |
| Inflammatory |
| Lichen planus |
| Lichen sclerosus et atrophicans |
| Inflammatory bowel disease |
| Pyoderma gangrenosum |
| Aphthae (idiopathic) |
| Complex aphthosis |
| Behcet disease |
| Sweet syndrome |
| Drug reaction |
| Pemphigus vulgaris |
| Bullous pemphigoid |
| Paraneoplastic pemphigoid |
| Reiter syndrome |
| Systemic lupus erythematosus |
| Malignancy |
| Hemophagocytic syndrome |
| Langerhans cell histiocytosis |
| Lymphoma/leukemia |
| Trauma |
| Caustic burns |
| Foreign body |
| Sexual injury |
| Factitial |
Although Lipschütz ulcers are quite rare, this clinical condition should always be considered in differential diagnosis of genital ulcers in young women and further studies are required to better understand what the real etiopathogenesis of the disease is.
References
- 1.Brinca A., Carvalho M.J., Figueiredo A., Canelas M.M., Vieira R. Lipchütz ulcer (ulcus vulvae acutum) — a rare cause of genital lesion. An. Bras. Dermatol. 2012;87:622–624. doi: 10.1590/s0365-05962012000400018. [DOI] [PubMed] [Google Scholar]
- 2.Barnes C.J., Alio A.B., Cuningham B.B., Friedlander S.F. Epstein-Barr virus-associated genital ulcers: an under-recognized disorder. Pediatr Dermatol. 2007;24:130–134. doi: 10.1111/j.1525-1470.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- 3.Simpson M.C., Cooper A.S., Boardman L.A. Vulvar aphthous ulcers in adolescent and young women. J. Pediatr. Adolesc. Gynecol. 2008;21:81–82. [Google Scholar]
- 4.Cheng S.X., Chapman M.S., Margesson L.J., Birenbaum D. Genital ulcers caused by Epstein–Barr virus. J. Am. Acad. Dermatol. 2004;51:824–826. doi: 10.1016/j.jaad.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Lehman J.S., Bruce A.J., Wetter D.A., Ferguson S.B., Rogers R.S., 3rd. Reactive nonsexually related acute genital ulcers: review of cases evaluated at Mayo Clinic. J. Am. Acad. Dermatol. 2010;63:44–51. doi: 10.1016/j.jaad.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 6.Huppert J.S., Gerber M.A., Deitch H.R., Mortensen J.E., Staat M.A., Adams Hillard P.J. Vulvar ulcers in young females: a manifestation of aphthosis. J. Pediatr. Adolesc. Gynecol. 2006;19:195–204. doi: 10.1016/j.jpag.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Lipchütz B. Über eine eigenartige geschwürsform des weiblichen genitales (ulcus vulvae acutum) Arch. Dermatol. Syphilol. (Berlin) 1913;114:363–395. [Google Scholar]
- 8.Kinyó Á., Nagy N., Oláh J., Kemény L., Bata-Csörgő Z. Ulcus vulvae acutum Lipschütz in two young female patients. Eur. J. Dermatol. 2014;24:361–364. doi: 10.1684/ejd.2014.2311. [DOI] [PubMed] [Google Scholar]
- 9.Farhi D., Wendling J., Molinari E., Raynal J., Carcelain G., Morand P. Non-sexually related acute genital ulcers in 13 pubertal girls: a clinical and microbiological study. Arch. Dermatol. 2009;145:38–45. doi: 10.1001/archdermatol.2008.519. [DOI] [PubMed] [Google Scholar]
- 10.Sárdy M., Wollenberg A., Niedermeier A., Flaig M.J. Genital ulcers associated with Epstein–Barr virus infection (ulcus vulvae acutum) Acta Derm. Venereol. 2011;91:55–59. doi: 10.2340/00015555-0979. [DOI] [PubMed] [Google Scholar]


