Abstract
Purpose
The ketogenic diet is a low-carbohydrate, moderate protein, high-fat diet that has emerged as a potential treatment for autism spectrum disorder. Autism spectrum disorder is a neurodevelopmental disorder of social communication, and restricted, repetitive behaviors and interests in need of novel therapies. An open-label clinical trial was done in Honolulu, Hawaii to test a modified ketogenic diet for improvement of core clinical impairments in children with ASD.
Intervention
A modified ketogenic gluten-free diet regimen with supplemental MCT was completed in 15 children ages 2 to 17 years for 3 months. Clinical (ADOS-2, CARS-2) and biochemical measures were performed at baseline and 3-months on the ketogenic diet.
Main outcome
Children administered a modified ketogenic gluten-free diet with supplemental MCT significantly improved core autism features assessed from the ADOS-2 after 3 months on diet (P = 0.006). No significant difference was observed in restricted and repetitive behavior score (P = 0.125) after 3 months on the diet protocol. Substantial improvement (> 30% decrease ADOS-2 total score) was observed in six participants, moderate improvement (> 3 units) in two participants, and minor/no improvement in seven participants. Ten participants assessed at a six-month time point sustained improvement in total ADOS-2 and social affect subdomain scores comparing baseline and 6 months (P = 0.019; P = 0.023), but no significant improvement in restricted and repetitive behavior scores were noted (P = 0.197). Significant improvements in CARS-2 items after 3 months of the modified ketogenic protocol were observed in imitation, body use, and fear or nervousness (P = 0.031, P = 0.008, P = 0.039). The percent change on ADOS-2 score from baseline to 3 months was associated with baseline high-density lipoprotein levels (ρ = −0.67, P = 0.007) and albumin levels (ρ = −0.60, P = 0.019). Moreover, the percent change from baseline to 3 months in ADOS-2 scores was significantly associated with percent change in high-density lipoprotein levels (ρ = 0.54, P = 0.049) and albumin levels (ρ = 0.67, P = 0.010).
Conclusions
A modified gluten-free ketogenic diet with supplemental MCT is a potentially beneficial treatment option to improve the core features of autism spectrum disorder and warrants further investigation.
Trial registration
Trial Registry: Clinicaltrials.gov
Registration Number: NCT02477904
URL: https://www.clinicaltrials.gov/ct2/show/NCT02477904?term=ketogenic+diet&cond=Autism&rank=1
Keywords: Autism, Ketogenic diet, Neurodevelopment, Therapy, Intervention, High fat
1. Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by varying impairments in social communication, and restricted, repetitive behaviors and interests [1,2]. Symptoms of ASD also include adverse behaviors such as anxiety, focus and attention problems, impulsivity, and self-injurious behavior. Therapists attempt to modify behaviors and enhance functional communication [3]. Yet, limited improvements occur with behavioral therapy and therapy must be implemented early and intensively. There are several pharmacologic options for behavior problems in patients with autism, leading among them are FDA approved atypical antipsychotic medications such as Risperidone and Aripiprazole [4]. However, these pharmacologic treatments carry risk of adverse effects [5]. Due to the paucity of effective and safe treatments for autism, there is an increasing need to study novel interventions.
Dietary interventions have been used as an effective approach to treat neurodevelopmental disorders [6,7]. Most notably, the ketogenic diet (KD), a very low carbohydrate, moderate protein, high fat diet has been shown to significantly reduce seizure frequency in patients with epilepsy [8,9]. The KD has been proposed as an intervention to treat other neurodevelopmental disorders [10]. Studies support that the ketogenic diet alters neural cellular metabolism through utilization of ketone bodies as an alternative fuel for the brain [10]. Initial case reports and a prospective pilot study of 30 individuals with autistic behavior suggests the KD may be effective as a treatment strategy for ASD [11–13]. Follow up clinical studies of 187 Greek and 45 Egyptian children have provided additional support that the KD may safely improve symptoms of certain individuals with ASD [14,15]. In addition, research using animal mouse models of ASD have demonstrated improvements in behavior on the KD [16–19]. Yet, there remains limited evidence to support recommendations for clinical use of the KD in children with ASD.
To evaluate the effects of a modified KD in children with ASD, Shriners Hospitals for Children - Honolulu designed an open-label, observer-blinded clinical trial in a cohort of children with ASD. This pilot trial intended to test both feasibility and efficacy of a modified KD in improving ASD symptoms. We hypothesized that 3 months of a modified KD would improve the core clinical impairments in children with ASD.
2. Materials and methods
2.1. Study design and participants
We conducted an open-label, observer-blinded clinical trial to test the effects of a modified KD regimen (described in Section 2.2, hence will be referred to as modified KD/GF/MCT) on ASD behavior in children between the ages of 2 and 21 years old diagnosed with ASD at the Shriners Hospitals for Children –Honolulu. Our clinical study team included a pediatric neurologist, a registered dietitian/nutritionist, two pediatric nurse practitioners, a speech-language pathologist, and behavioral neuroscientist.
The inclusion criteria for participants were: (1) primary diagnosis of autism spectrum disorder by pediatric neurologist, (2) male or female, (3) age between 2 years to 21 years; and exclusion criteria were: (4) known cardiac disorder including arrhythmias or hypertension, (5) Body Mass Index (BMI) < 3rd percentile, (6) carnitine deficiency, (7) beta-oxidation defects, (8) inability to maintain adequate nutrition. Patients with seizures or a history of seizures were excluded from the study. The University of Hawaii Committee on Human Studies (IRB) approved this study and participant's guardians provided written informed consent.
2.2. Dietary intervention
Each participant had screening bloodwork to assess baseline status and to rule out contraindications for initiating the modified KD. These tests included a complete blood count, electrolytes, zinc, selenium, magnesium, phosphate, liver and kidney functions (total protein, albumin, AST, ALT, blood urea nitrogen, creatinine, bicarbonate, calcium), fasting lipid profile [cholesterol, triglycerides, high density lipoprotein (HDL), and Low-density lipoprotein (LDL) cholesterol], serum acylcarnitine profile, urine organic acids, serum amino acids and beta-hydroxybutyrate (BHB). Genetic testing to identify an etiology of ASD was not performed.
A Registered Dietitian/Nutritionist provided 2 h of caregiver training on the modified KD/GF/MCT protocol. We chose not to follow a standard epilepsy ketogenic diet in considering the unique metabolic considerations for children with autism. Medium-chain triglycerides (MCT) oil was incorporated into the modified KD diet profile because of primary and secondary mitochondrial dysfunction in the ASD population [20,21] and to allow for potential improvement in fatty acid utilization and ketone production. Gluten restriction was applied to all participants given the prevalence of children in our population already on a gluten restriction.
The modified KD/GF/MCT protocol limited total net carbohydrates daily intake to 20–25 g. Protein needs were calculated based on the child's age and weight, and allowed up to twice the RDA requirements. The remainder of energy needs comprised of various types of fats. Guidelines were provided on administering MCT, either from coconut or pure MCT oil to comprise 20% of energy needs. The MCT or coconut oil dose was included in the daily meal plan. Gluten restriction was maintained. Caregivers checked urine ketones twice daily for the first month, and once daily thereafter. The goal was to maintain a consistent state of ketosis, but no defined level of ketosis was targeted. Caregivers were instructed to record food intake and ketone readings electronically or in a notebook, which was reviewed by the study team at each visit. Follow up visits were scheduled at 1 month, 3 months, and 6 months after diet initiation. In addition, caregivers were able to receive additional guidance from the dietitian through phone calls and a digital online support group. Participants were given the option to continue the diet after 3 months.
2.3. Autism baseline assessment and diet outcome measures
The Autism Diagnostic Observation Schedule, 2nd edition (ADOS-2) [22] and the Childhood Autism Rating Scale-Second Edition (CARS-2) [23] were used to assess participants at baseline and 3 months. Behavior testing was completed in children that continued the diet beyond the 3-month intervention as well as those that discontinued the diet at 3 months.
ADOS-2: In research and clinical practice the ADOS-2 is frequently used to diagnose and describe ASD symptoms. The ADOS-2 is a semi-structured, standardized assessment of communication, social interaction, play/imaginative use of materials, and restricted and repetitive behaviors. Ratings assigned on the basis of observations during the administration of the ADOS-2 are converted to diagnostic algorithms which yield an ADOS-2 classification used in conjunction with other information to formulate a clinical diagnosis. Ratings also yield a Comparison Score to estimate severity of ASD symptoms relative to others with ASD of the same age and language level; and calibrated severity scores for Social Affect and Restricted, Repetitive Behavior domains to provide a clearer picture of ASD symptoms and severity. The test-retest correlations for the ADOS-2 range from 0.68 to 0.92 and indicate good stability for the Social Affect and Restricted and Repetitive Behavior domains, and excellent stability for the Overall Total [24]. Core behavioral symptoms of ASD were assessed using the ADOS-2, and administered by an examiner who met standard requirements for research reliability.
CARS-2: This questionnaire is a clinician-rated scale for key behaviors related to autism diagnosis. The CARS-2 includes 15 items rated on a 7-point scale with higher scores indicating greater severity. Interrater reliability for the CARS 2-HF has a median correlation of 73. This is consistent with inter-rater reliability results for the original CARS, which had a median correlation of 0.71 and indicates good agreement between rater [23]. The CARS-2 was completed using both direct observations of the individual's behavior and information obtained through interview of a caregiver that had knowledge of the individual's behavior across different environments. The CARS-2 questionnaire was administered by either a pediatric nurse practitioner or speech-language pathologist who were part of the study team.
2.4. Statistical analyses
The baseline study participants' demographics were summarized by descriptive statistics including means and standard deviations for continuous variables, and frequencies and proportions for categorical variables. We used an on-treatment analysis that restricted our analysis to the fifteen participants that adhered to the clinical trial for 3 months. Comparisons between baseline and 3-month diet repeated measures were conducted using Wilcoxon matched-pairs signed rank test or paired t-test, as appropriate. Comparisons between baseline, 3-month, and 6-month time points were analyzed using the Friedman non-parametric test. Post hoc multiple comparison analyses used FDR method to correct. The association between clinical measures and the changes in ASD behavior from baseline to 3-month assessment were evaluated using Spearman rank-order correlation. A P-value < 0.05 was considered statistically significant.
3. Results
3.1. Characteristics of cohort
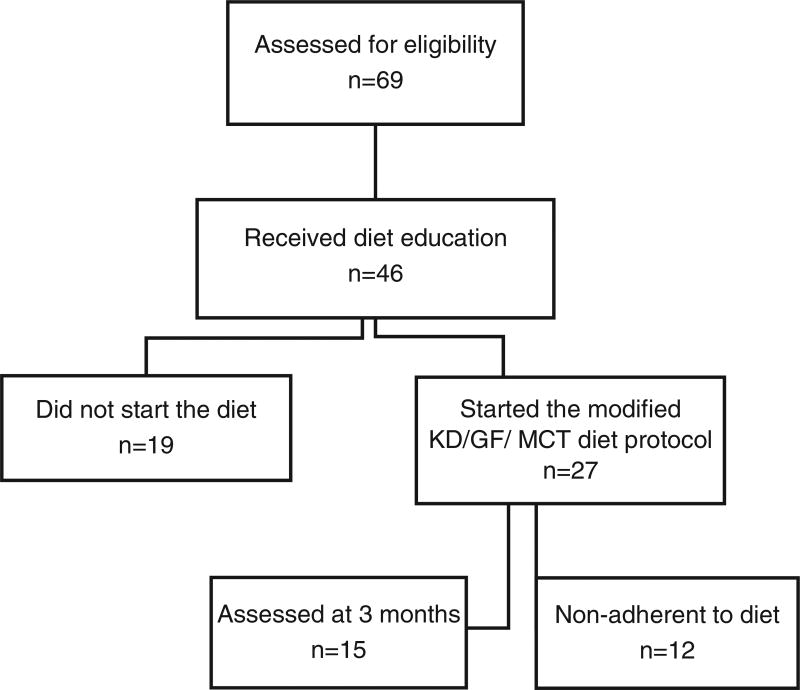
We recruited and assessed 69 pediatric subjects for eligibility in the study and 46 subjects received initial dietary education. Of these 46 subjects, 19 did not start the diet (Fig. 1). In addition, 12 subjects were excluded from analysis due to difficulties with diet compliance. A total of 15 subjects completed 3 months on the diet and 10 subjects completed 6 months. The sample size for analysis was comprised of 15 pediatric subjects (13 males, 2 females; ages 3–13 years; mean age 7.9 years, ± 3.3 years) diagnosed with ASD by clinical examination, DSM-IV-TR, DSM-V, and ADOS-2 criteria. Cohort characteristics are shown in Table 1. Of the cohort, 46.6% (7 of 15) participants were classified high level and 53.3% (8 of 15) moderate level of ASD related symptoms compared to children with ASD of the same age and similar language skills from the ADOS-2. Comorbidity of ADHD was present in 66.7% (10 of 15) participants. Some of the participants were on particular diets at the time of study initiation. These diets included gluten-free (n = 3), gluten-free/casein free (n = 1), low carbohydrate (n = 1; approximately 85 g of net carbohydrate intake daily), specific carbohydrate diet and gluten-free (n = 1), and vegan (n = 1). These restrictions were continued by the participants and tailored to meet the modified KD/GF/MCT regimen.
Fig. 1.
Diagram of ASD ketogenic diet study participation.
Table 1.
Baseline characteristics of participants.
| PID | Sex | Age | ADOS-2 Level ASD |
Comorbidities | Medication |
|---|---|---|---|---|---|
| KETOA-01 | F | 8 | High | Hypoglycemia | Trazodone, Diazepam |
| KETOA-02 | M | 11 | High | ADHD, DCD | Dexmethylphenidate |
| KETOA-03 | M | 10 | Moderate | ADHD, DCD | Focalin, Melatonin |
| KETOA-04 | M | 13 | Moderate | ADHD, ID, DCD | |
| KETOA-05 | M | 10 | High | ID, eosinophilic esophagitis | |
| KETOA-06 | M | 6 | High | ADHD, ID | Clonidine, Melatonin |
| KETOA-07 | M | 4 | High | ADHD, LD | Zyrtec, Hydrazine, Methylphenidate |
| KETOA-08 | M | 3 | Moderate | ADHD, LD | |
| KETOA-09 | M | 6 | Moderate | DCD | |
| KETOA-10 | M | 7 | Moderate | ADHD, ID, DCD | Focalin |
| KETOA-11 | M | 5 | Moderate | ID, DCD, Reactive attachment disorder | Lamotrigine |
| KETOA-12 | M | 12 | High | ADHD, DCD, ID | Advair, Flonase |
| KETOA-13 | F | 3 | Moderate | ADHD, DCD | Probiotics |
| KETOA-14 | M | 10 | Moderate | ||
| KETOA-15 | M | 11 | High | ADHD, DCD, ID | Methylphenidate |
3.2. Outcome measures after 3 months on modified KD/GF/MCT diet
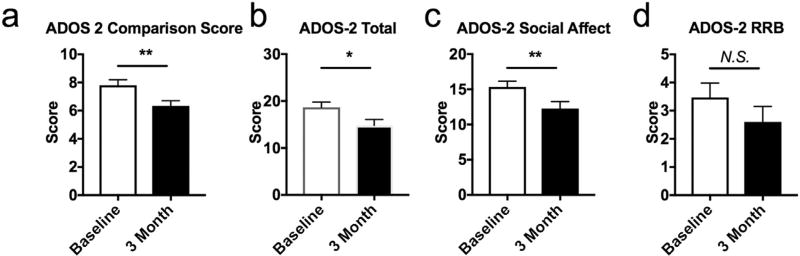
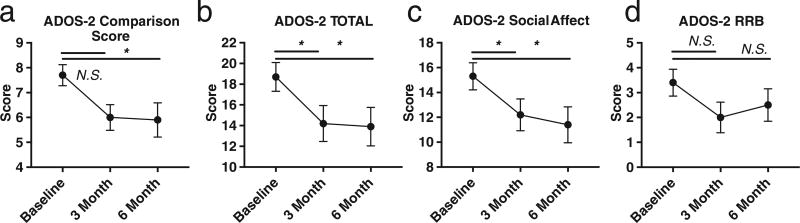
Comparison scores (P = 0.006), overall total scores (P = 0.020), and social affect scores (P = 0.006) significantly improved comparing baseline scores to 3 months on the modified KD/GF/MCT (Fig. 2a, b,c). At 3 months there was a 19.9% mean improvement in social affect score and 20.7% improvement in overall total score. Substantial improvement (> 7 units decrease of the ADOS-2 Total score) was observed in six participants, moderate improvement (> 3 units) in two participants, and minor/no improvement in seven participants (Supplementary Table 1). Approximately half (8 of 15) of participants that responded to the modified KD/GF/MCT improved their overall total ADOS-2 score by at least 4 points. Of these participants, two were “super responders” and improved social affect score by approximately 50% (8–9 points) and one of these participants improved his restricted and repetitive behavior score to zero at 3 months post diet. No significant difference was observed in restricted and repetitive behavior score (P = 0.125) (Fig. 2d). Ten of the fifteen participants were assessed by the ADOS-2 at a 6-month time point and maintained improvement in ADOS-2 Comparison Score, Total score and Social Affect sub-domain scores comparing baseline and 6 months (P = 0.033, P = 0.019, P = 0.019) (Fig. 3a, b, c), but no improvement in restricted and repetitive behavior scores were noted (P = 0.218) (Fig. 3d). Parents or caregivers self-reported improvements in eye contact, interest in other people, meaningful language, schedule transitions, focus and hyperactivity after modified KD/GF/MCT treatment.
Fig. 2.
ADOS-2 scores at baseline and 3 months on a modified KD/GF/MCT diet. a. ADOS-2 Comparison score significantly improved at 3 months on the modified KD/GF/MCT diet (Wilcoxon matched-pairs signed rank test, P = .006). b. ADOS-2 Total score significantly improved at 3 months on the modified KD/GF/MCT diet (Wilcoxon matched-pairs signed rank test, P = .020). c. ADOS-2 Social affect score significantly improved at 3 months on the modified KD/GF/MCT diet (Wilcoxon matched-pairs signed rank test, P = .006). d. ADOS-2 restricted repetitive behavior score was not significantly improved at 3 months on the modified KD/GF/MCT diet (Wilcoxon matched-pairs signed rank test, P = .125). **, P < .01; *, P < .05; N.S., not significant.
Fig. 3.
ADOS-2 scores at baseline, 3 months, and 6 months on a modified KD/GF/MCT diet. a. ADOS-2 Comparison Score significantly improved at 6 months on the modified KD/GF/MCT diet (Friedman Test, P = .057, P = .034). b. ADOS-2 Total score significantly improved at 3 and 6 months on the modified KD/GF/MCT diet (Friedman Test, P = .044, P = .019). c. ADOS-2 Social affect score significantly improved at 3 months on the modified KD/GF/MCT diet (Friedman Test, P = .044, P = .019). ADOS-2 Restricted Repetitive Behavior score did not significantly improve at 3 months or 6 months on the modified KD/GF/MCT diet (Friedman Test, P = .0736, P = .218). *, P < .05; N.S., not significant.
The total CARS-2 score significantly decreased following 3 months on the modified KD/GF/MCT (baseline, 34.96 ± 2.06; 3 months, 30.71 ± 1.6; P = 0.003, mean;SEM). Table 2 shows CARS-2 items at baseline and after 3 months. The CARS-2 items that changed significantly after 3 months on the modified KD/GF/MCT were: imitation, body use, and fear or nervousness (P = 0.031, P = 0.008, P = 0.039). The CARS-2 item relating to people showed a trend of improving after 3 months of the modified KD/GF/MCT (P = 0.093).
Table 2.
ADOS-2 and CARS-2 scores at baseline and 3 months on the modified KD/GF/MCT diet.
| Baseline M (SD) |
3 month M (SD) |
Group differences (p-value)a |
|
|---|---|---|---|
| ADOS-2 | |||
| Social Affect | 15.33(3.20) | 12.27(3.83) | 0.006 |
| Restricted & Repetitive Behavior | 3.47(1.99) | 2.60(2.13) | 0.125 |
| Overall Total | 18.67(4.25) | 14.80(5.00) | 0.017 |
| Comparison Score | 7.80(1.52) | 6.33(1.45) | 0.006 |
| CARS-2 | |||
| Relating to people | 2.35(0.72) | 2.00(0.29) | 0.094 |
| Imitation | 2.23(0.95) | 2.00(0.71) | 0.031 |
| Emotional response | 2.46(0.85) | 2.23(0.75) | 0.424 |
| Body use | 2.42(1.08) | 2.04(0.90) | 0.008 |
| Object use | 2.12(0.92) | 2.19(0.75) | 0.999 |
| Adaptation to change | 2.46(0.97) | 2.27(0.88) | 0.236 |
| Visual response | 2.23(0.75) | 2.00(0.65) | 0.406 |
| Listening response | 2.31(0.69) | 2.04(0.43) | 0.375 |
| Taste, smell, and touch response | 2.35(1.01) | 1.96(0.90) | 0.125 |
| Fear or nervousness | 2.81(0.95) | 2.35(1.01) | 0.039 |
| Verbal communication | 3.00(0.96) | 3.00(0.91) | 0.999 |
| Nonverbal communication | 2.12(0.82) | 1.81(0.60) | 0.203 |
| Activity level | 2.35(0.66) | 2.38(0.68) | 0.906 |
| Level of consistency of intellectual response | 2.50(0.95) | 2.25(0.88) | 0.929 |
| General impression | 2.83(0.58) | 2.71(0.54) | 0.265 |
Note. M = mean; SD = standard deviation.
Wilcoxon matched-pairs signed rank test.
Clinical measures at baseline and 3 months on the modified KD/GF/MCT are displayed in Table 3. As expected, levels of serum BHB were significantly elevated 3 months after initiating the modified KD/GF/MCT (P = 0.025). Mean levels were 1.351 mmol/L for the group after 3 months on the modified KD/GF/MCT. BMI significantly reduced after 3 months on the modified KD/GF/MCT (P = 0.009). However, weight did not significantly change (P = 0.916). High-density lipoprotein (HDL), Low-density lipoprotein (LDL), and cholesterol significantly increased after 3 months of the modified KD/GF/MCT (P = 0.018; P = 0.032; P = 0.003). Notably, eosinophil blood cell percent on the diet significantly decreased (P = 0.044) and there was a trend toward decreased white blood cell count (P = 0.087).
Table 3.
Clinical laboratory results at baseline and 3 months on the modified KD/GF/MCT diet.
| Baseline mean (SD) |
3 month mean (SD) |
P Value |
|
|---|---|---|---|
| Weight (kg) | 33.37(14.93) | 33.28(13.29) | 0.916 |
| BMI (kg/m2) | 18.67(3.73) | 17.67(2.78) | *0.009 |
| Beta Hydroxybutyrate (0.02–0.27 mmol/L) | 0.196(0.114) | 1.351(1.74) | *0.025 |
| Sodium (136–145 mmol/L) | 136.7(1.839) | 137.9(1.981) | 0.186 |
| Potassium (3.3–5.1 mmol/L) | 4.26(0.377) | 4.38(0.517) | 0.473 |
| Creatinine (0.20–0.70 mg/dL) | 0.47(0.082) | 0.46(0.087) | 0.868 |
| Glucose (70–99 mg/dL) | 94.5(8.00) | 89.7(8.31) | 0.178 |
| HDL (≥ 60 mg/dL) | 54.79(13.79) | 63.86(14.9) | *0.018 |
| LDL (< 100 mg/dL) | 76.7 (16.79) | 94.7(28.98) | *0.032 |
| Triglycerides (< 150 mg/dL) | 89.69 (57.8) | 71.23(22.26) | 0.283 |
| Cholesterol (< 200 mg/dL) | 151.4(24.56) | 176.7(34.05) | *0.003 |
| White Blood Cell (6.0–16.0 10(9)/L) | 8.07(2.38) | 7.10(2.49) | 0.087 |
| Neutrophils% [10–40] | 44.63(19.97) | 44.99(10.11) | 0.938 |
| Monocytes% [4–10] | 6.8(1.66) | 6.64(1.41) | 0.686 |
| Eosinophils% (0–6) | 5.83(3.89) | 4.526(3.00) | *0.044 |
3.3. Biochemical measures and the modified KD/GF/MCT diet
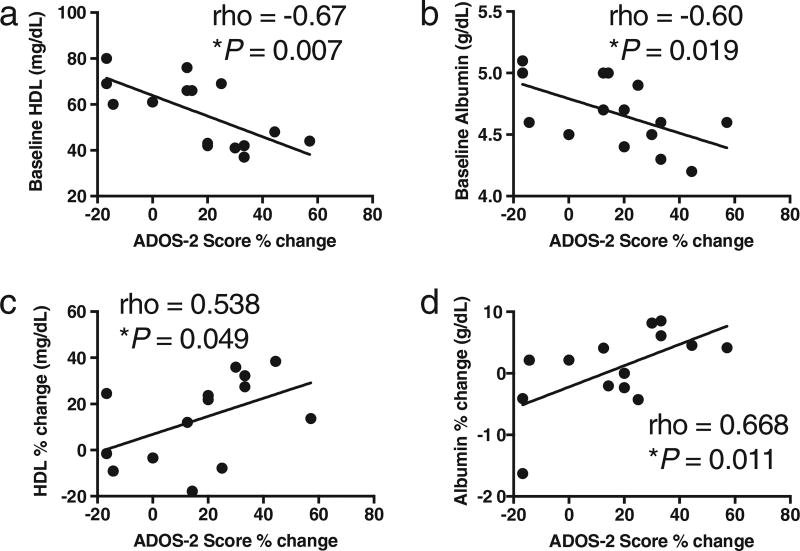
We examined associations between baseline biochemical measures and improvement in ASD behaviors as measured by ADOS-2% change from baseline to 3 months. The percent change on ADOS-2 scores from baseline to 3 months was associated with baseline HDL levels (ρ = −0.67, P = 0.007) and albumin levels (ρ = −0.60, P = 0.019) (Fig. 4a, b). No significant associations were observed for other baseline biochemical measurements. Next, we examined whether the percent change in HDL and albumin levels associated with ADOS-2% change. We found the percent change in ADOS-2 scores was significantly associated percent change on HDL (ρ = 0.538, P = 0.049) and albumin levels (ρ = 0.668, P = 0.010) from baseline to 3 months on modified KD/GF/MCT (Fig. 4c, d).
Fig. 4.
Association between baseline HDL and albumin and ADOS-2 score % change. a. Baseline HDL significantly associates with ADOS-2 score percent change. b. Baseline albumin significantly associates with ADOS-2 score percent change. c. HDL percent change significantly associates with ADOS-2 score percent change. d. Albumin percent change significantly associates with ADOS-2 score percent change. Spearman correlation.
3.4. Side effects on the modified KD/GF/MCT diet
Common side effects included diarrhea (18.8%), vomiting (18.8%), fatigue (18.8%), constipation (12.5%), dehydration (12.5%), weight loss (12.5%), acidosis (6.3%), and hypoglycemia (6.3%). Hypoglycemia was defined as a blood sugar level < 50 mg/dL. We observed weight loss as a positive side effect for 2 participants. All of the side effects occurred within the first 2–4 weeks of diet initiation.
4. Discussion
This study utilizes an on-treatment analysis to examine the efficacy of a modified KD/GF/MCT in children with ASD after 3 months. The adverse effects of the KD were minimal in our study and occurred early during initiation of the diet. Approximately 55% of patients were adherent to the diet at 3 months. After 3 months on the modified KD/GF/MCT, approximately 50% showed moderate to substantial improvement in ADOS-2 scores. In agreement with previous studies, we did not observe significant relationships between BHB levels and the degree of improvement in ASD symptoms [14,15]. The degree of improvement (20%) represented moderate changes in ADOS-2 scores. The findings were driven by improvements in the social affect domain. Moreover, 50% percent of subjects showed improvement on CARS-2 scores in the areas of imitation, body use, and fear or nervousness. These results suggest components of the KD may offer an effective and safe treatment that should be considered for the treatment of social affective impairments in children with ASD.
Our findings of ASD related improvements from the KD support prior animal and human studies investigating the relationship between ketogenic therapies and autism [11,12,14,15,17–19]. In the present study, we obtained data from 10 participants that maintained the diet past the 3-month time point and found the improvements in social behavior are maintained. Moreover, our findings of significant improvement in CARS-2 scores after 3 months on our modified KD are in agreement with a recent case control study in 45 children that found significant improvement in CARS scores after 6 months on the modified Atkins diet [15]. Taking into account this case control study and our study, both studies suggest that a specific component of a KD may mediate the beneficial effects for ASD behavior. However, uncertainty exists about the long-term effects of the KD on behavior in ASD. Thus, future research will need to examine the effects of the KD at a longer follow up period and whether termination of the diet would elicit regression of symptoms.
Emerging evidence is revealing that ASD is associated with dysregulated metabolism and altered immune inflammatory processes [25,26]. Notably, a previous matched case control study of 29 boys with ASD reported that HDL levels were significantly lower in cases as compared to controls [27]. This finding suggests that optimizing HDL levels in children with ASD may provide therapeutic benefits. Our findings are the first to support this hypothesis by showing that the percent change in HDL levels after 3 months on the KD significantly associated with percent change in ADOS-2 score. In our group, subjects with HDL levels < 45 mg/dL had the greatest improvement. High-density lipoproteins exert a role in host defense and influence immune cell response by modulation of cholesterol availability in plasma membrane lipid rafts [28]. Low HDL levels are inversely correlated with severity of sepsis and increased inflammatory response [29]. Similarly, we found that the percent change in albumin levels after 3 months on modified KD significantly associated with percent change in ADOS-2 score. Subjects with lower baseline albumin levels had higher percent change in ADOS-2 scores. Albumin is a globular protein found in human plasma that binds a variety of compounds including fatty acids. Previous studies have shown that serum levels of albumin associate with HDL levels [30,31]. We also observed this association of lower levels of albumin associating with lower levels of HDL. Croonenberghs et al. found significantly increased concentrations of total serum protein in individuals with ASD [32]. The increased levels of proteins were mainly accounted for by serum concentrations of albumin and gamma globulin, and presented in a setting of increased levels of IgG, IgG2 and IgG4. The authors hypothesized that this increased concentration of albumin is reflective of an activation of the immune response system [32].
The field of ASD research has proposed that altered immune inflammatory processes are involved in the etiology of ASD [33]. Our findings support this theory as previous work has shown that lowered levels of serum albumin and HDL are associated with children with a recent mild infection [31]. Together these findings suggest a limited biochemical profile for ASD responders of baseline HDL < 45 mg/dL and serum albumin < 4.5 g/dL. These findings also highlight the need to investigate the link to components of the ketogenic diet such as ketones that have been shown to block inflammatory related components of the immune system [34]. However, further immunological investigation is needed with a larger sample size and with children of different ages undergoing the KD. Future work should focus on identifying children with lower albumin and HDL levels, attempting interventions that target altering blood levels of HDL with the KD, and examining whether ASD behavior improves.
The neurobiological underpinnings of the KD improving ASD symptoms are unknown. While all subjects on the KD had increased BHB, only 50% of subjects demonstrated significant improvements in ASD behavior defined by 4 points or greater improvement in ADOS-2 total score. This suggests factors that convey a responder status. Furthermore, “super-responders”, as defined by total ADOS-2 score improvement of > 7 points (raw score), represented 40% of the cohort. We identified potential biochemical effects of the modified KD such as increased HDL, increased eosinophil percent, and lower white blood cell count that may play a role in predicting a responder versus non-responder. While our sample size is limited, our findings suggest that a super-responder profile may exist and relate to a pro-inflammatory condition in ASD children at baseline, and immunogenic response to the KD involving eosinophils that may mediate ASD behaviors. Among the entire cohort, there was a significant difference in pre-and post-KD eosinophil percent differential, suggesting a reduction in eosinophils following the KD. Eosinophils have classically been associated with allergy and parasitic infection [35]. We did not study these co-morbidities in our cohort. Either of these etiologies could be triggered in ASD. A reduction in eosinophils may suggest pathoetiology of allergen-specific-inflammation [36]. Several studies support a process of microglial inflammatory dysregulation resulting in the neuropathology in ASD [37–39]. There are few studies that apply anti-inflammatory therapy to rescue ASD-behavior in animal models [40]. Some groups have suggested a stratified ASD group based on inflammatory profiles [41]; however, more studies are needed that measure inflammatory profiles of children following the KD.
The KD is proposed to decrease seizure frequency by several mechanisms including reducing inflammation, and altering mitochondrial function [42]. Animal studies of the KD report direct anti-inflammatory effects of the KD [43,44]. Considering the metabolic changes that govern seizure reduction, it is plausible to hypothesize that behavioral changes in children with ASD are explained by similar mechanisms. In a survey of 733 parents of children with seizures, the KD was perceived as very effective for improving seizures as compared to other AED and non-AED treatments and was perceived as having favorable effects on sleep, communication, behavior, attention and mood [45]. Other notable areas of investigation that may provide insights into the mechanism of action of the KD involve epigenetics and the gut microbiome. Epigenetics is the study of changes to cellular function by modification to gene expression rather than altering the genetic code. Epigenetic changes occur with dietary changes and BHB has been shown to function as an epigenetic modifier of gene expression [46]. Future research will need to examine whether the KD alters the epigenetic regulation of brain cells. The gut microbiome may serve as another worthwhile area of investigation in light of recent studies showing the importance of commensal bacteria in the gut in association with healthy brain function [47]. Animal studies of ASD have supported links between the KD and gut microbiome and mitochondrial changes [48,49]. Future research will need to identify the key bacteria and cellular mechanisms that are modified by the KD and that are associated with improvements in ASD behavior in humans.
4.1. Study limitations
Several limitations exist for our study that limit the generalizability of the results to other ASD cohorts. First, we did not report ASD subjects on a standard (non-KD) diet for comparison nor did we randomize subjects to diet groups. Likewise, no data on healthy control subjects was available to provide information about the effect of the KD in typically developing individuals. The ASD cohort had 56% of subjects remain on the modified KD/GF/MCT at 3 months, and 48% at 6 months. This is lower when compared to epilepsy patients as reported by Vining et al. [9] in a multi-center study suggesting 88% retention at 3 months and 69% at 6 months. This difference may be attributed to the higher prevalence of problem feeders in this population [50,51]. Furthermore, of those recruited to start the modified KD/GF/MCT, only 55% initiated the diet after education. Providers planning to initiate the KD in patients with ASD should be aware of these challenges.
4.2. Conclusions
Components of the KD are possibly beneficial in improving social affect in children with ASD. Additional studies are needed to understand how the KD improves behavior. We propose a hypothesis similar to others that changes in carbohydrate and fat composition of the diet, cellular metabolism, inflammatory processes, and gut microbiome are responsible for the improved behaviors in children with ASD on the KD.
Supplementary Material
Acknowledgments
We would like to acknowledge the HMSA Foundation for their support in funding the Ketogenic Therapies Program at Shriners Hospitals for Children – Honolulu. We also would like to recognize the John A. Burns School of Medicine and NIH (P20GM113134) for their support. Most importantly, we thank the children and their families for their strong dedication to advancing therapies in neuroscience.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.physbeh.2018.02.006.
Authors' contributions
RWYL and MW conceived of idea, designed study, implemented study, performed data analysis, and wrote manuscript. MJC designed study, implemented study, performed data analysis, and wrote manuscript. AKM designed study. AP, RM, SY implemented study and performed data analysis. GA implemented study and wrote manuscript. LA, MN, EL, AJ implemented study.
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2(3):217–250. [PubMed] [Google Scholar]
- 2.Association D-AP. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing; Arlington: 2013. [Google Scholar]
- 3.Howlin P, Magiati I, Charman T. Systematic review of early intensive behavioral interventions for children with autism. Am. J. Int. Develop. Disabil. 2009;114(1):23–41. doi: 10.1352/2009.114:23;nd41. [DOI] [PubMed] [Google Scholar]
- 4.Ghanizadeh A, Sahraeizadeh A, Berk M. A head-to-head comparison of aripiprazole and risperidone for safety and treating autistic disorders, a randomized double blind clinical trial. Child Psychiatry Hum. Dev. 2014;45(2):185–192. doi: 10.1007/s10578-013-0390-x. [DOI] [PubMed] [Google Scholar]
- 5.LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. Pharmacy Therapeut. 2015;40(6):389. [PMC free article] [PubMed] [Google Scholar]
- 6.Millward C, Ferriter M, Calver SJ, Connell-Jones GG. Gluten-and casein-free diets for autistic spectrum disorder. Cochrane Database Syst. Rev. 2008;(2) doi: 10.1002/14651858.CD003498.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Page T. Metabolic approaches to the treatment of autism spectrum disorders. J. Autism Dev. Disord. 2000;30(5):463–469. doi: 10.1023/a:1005563926383. [DOI] [PubMed] [Google Scholar]
- 8.Wilder R. The effects of ketonemia on the course of epilepsy. Paper presented at: Mayo Clin Proc. 1921 [Google Scholar]
- 9.Vining EP, Freeman JM, Ballaban-Gil K, et al. A multicenter study of the efficacy of the ketogenic diet. Arch. Neurol. 1998;55(11):1433–1437. doi: 10.1001/archneur.55.11.1433. [DOI] [PubMed] [Google Scholar]
- 10.Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front. Pharmacol. 2012;3:59. doi: 10.3389/fphar.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evangeliou A, Vlachonikolis I, Mihailidou H, et al. Application of a ketogenic diet in children with autistic behavior: pilot study. J. Child Neurol. 2003 Feb;18(2):113–118. doi: 10.1177/08830738030180020501. [DOI] [PubMed] [Google Scholar]
- 12.Arvio M, Kuisma L, Pöntinen M. Modified Atkins diet brought back the joy of life to a developmentally severely disabled youth. Duodecim. 2010;126(5):557–560. [PubMed] [Google Scholar]
- 13.Herbert MR, Buckley JA. Autism and dietary therapy: case report and review of the literature. J. Child Neurol. 2013 Aug;28(8):975–982. doi: 10.1177/0883073813488668. [DOI] [PubMed] [Google Scholar]
- 14.Spilioti M, Evangeliou AE, Tramma D, et al. Evidence for treatable inborn errors of metabolism in a cohort of 187 Greek patients with autism spectrum disorder (ASD) Front. Hum. Neurosci. 2013;7:858. doi: 10.3389/fnhum.2013.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Rashidy O, El-Baz F, El-Gendy Y, Khalaf R, Reda D, Saad K. Ketogenic diet versus gluten free casein free diet in autistic children: a case-control study. Metab. Brain Dis. 2017:1–7. doi: 10.1007/s11011-017-0088-z. [DOI] [PubMed] [Google Scholar]
- 16.Ruskin DN, Svedova J, Cote JL, et al. Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS One. 2013;8(6):e65021. doi: 10.1371/journal.pone.0065021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruskin DN, Fortin JA, Bisnauth SN, Masino SA. Ketogenic diets improve behaviors associated with autism spectrum disorder in a sex-specific manner in the EL mouse. Physiol. Behav. 2017;168:138–145. doi: 10.1016/j.physbeh.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruskin DN, Murphy MI, Slade SL, Masino SA. Ketogenic diet improves behaviors in a maternal immune activation model of autism spectrum disorder. PLoS One. 2017;12(2):e0171643. doi: 10.1371/journal.pone.0171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro K, Baronio D, Perry IS, Riesgo RdS, Gottfried C. The effect of ketogenic diet in an animal model of autism induced by prenatal exposure to valproic acid. Nutr. Neurosci. 2017;20(6):343–350. doi: 10.1080/1028415X.2015.1133029. [DOI] [PubMed] [Google Scholar]
- 20.Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol. Psychiatry. 2012 Mar;17(3):290–314. doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weissman JR, Kelley RI, Bauman ML, et al. Mitochondrial disease in autism spectrum disorder patients: a cohort analysis. PLoS One. 2008;3(11):e3815. doi: 10.1371/journal.pone.0003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule: ADOS-2. Western Psychological Services; Los Angeles, CA: 2012. [Google Scholar]
- 23.Schopler E, Van Bourgondien ME, Wellman GJ, Love SR. The Childhood Autism Rating Scale, (CARS2) WPS; Los Angeles: 2010. [Google Scholar]
- 24.Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule–2nd Edition (ADOS-2) Western Psychological Corporation; Los Angeles, CA: 2012. [Google Scholar]
- 25.James SJ, Melnyk S, Jernigan S, Hubanks A, Rose S, Gaylor DW. Abnormal transmethylation/transsulfuration metabolism and DNA hypomethylation among parents of children with autism. J. Autism Dev. Disord. 2008;38(10):1966–1975. doi: 10.1007/s10803-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011;25(1):40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim E-K, Neggers YH, Shin C-S, Kim E, Kim EM. Alterations in lipid profile of autistic boys: a case control study. Nutr. Res. 2010;30(4):255–260. doi: 10.1016/j.nutres.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Norata GD, Pirillo A, Ammirati E, Catapano AL. Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis. 2012;220(1):11–21. doi: 10.1016/j.atherosclerosis.2011.06.045. 2012/01/01/ [DOI] [PubMed] [Google Scholar]
- 29.Catapano AL, Pirillo A, Bonacina F, Norata GD. HDL in innate and adaptive immunity. Cardiovasc. Res. 2014;103(3):372–383. doi: 10.1093/cvr/cvu150. [DOI] [PubMed] [Google Scholar]
- 30.González-Pacheco H, Amezcua-Guerra LM, Vazquez-Rangel A, et al. Levels of high-density lipoprotein cholesterol are associated with biomarkers of inflammation in patients with acute coronary syndrome. Am. J. Cardiol. 2015;116(11):1651–1657. doi: 10.1016/j.amjcard.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Aburawi EH, Grubb A, Raitakari OT, Viikari J, Pesonen EJ. Lowered levels of serum albumin and HDL-cholesterol in children with a recent mild infection. Ann. Med. 2006;38(2):154–160. doi: 10.1080/07853890500358343. [DOI] [PubMed] [Google Scholar]
- 32.Croonenberghs J, Wauters A, Devreese K, et al. Increased serum albumin, γ globulin, immunoglobulin IgG, and IgG2 and IgG4 in autism. Psychol. Med. 2002;32(8):1457–1463. doi: 10.1017/s0033291702006037. [DOI] [PubMed] [Google Scholar]
- 33.Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J. Leukoc. Biol. 2006;80(1):1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- 34.Youm Y-H, Nguyen KY, Grant RW, et al. The ketone metabolite [beta]-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015;21(3):263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambrecht BN, Hammad H. The immunology of asthma. Nat. Immunol. 2015;16(1):45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 36.Theoharides TC. Is a subtype of autism an allergy of the brain? Clin. Ther. 2013;35(5):584–591. doi: 10.1016/j.clinthera.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005;57(1):67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 38.Gupta S, Ellis SE, Ashar FN, et al. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat. Commun. 2014;5:5748. doi: 10.1038/ncomms6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown AS, Sourander A, Hinkka-Yli-Salomäki S, McKeague IW, Sundvall J, Surcel H-M. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol. Psychiatry. 2014;19(2):259–264. doi: 10.1038/mp.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naviaux J, Schuchbauer M, Li K, et al. Reversal of autism-like behaviors and metabolism in adult mice with single-dose antipurinergic therapy. Transl. Psychiatry. 2014;4(6):e400. doi: 10.1038/tp.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDougle CJ, Landino SM, Vahabzadeh A, et al. Toward an immune-mediated subtype of autism spectrum disorder. Brain Res. 2015;1617:72–92. doi: 10.1016/j.brainres.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 42.Masino SA, Rho JM. Mechanisms of Ketogenic Diet Action. 2012 [PubMed] [Google Scholar]
- 43.Dupuis N, Curatolo N, Benoist JF, Auvin S. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia. 2015;56(7) doi: 10.1111/epi.13038. [DOI] [PubMed] [Google Scholar]
- 44.Ruskin DN, Kawamura M, Jr, Masino SA. Reduced pain and inflammation in juvenile and adult rats fed a ketogenic diet. PLoS One. 2009;4(12):e8349. doi: 10.1371/journal.pone.0008349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frye RE, Sreenivasula S, Adams JB. Traditional and non-traditional treatments for autism spectrum disorder with seizures: an on-line survey. BMC Pediatr. 2011 May 18;11:37. doi: 10.1186/1471-2431-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014;25(1):42–52. doi: 10.1016/j.tem.2013.09.002. 2014/01/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dinan TG, Cryan JF. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017;46(1):77–89. doi: 10.1016/j.gtc.2016.09.007. 2017/03/01/ [DOI] [PubMed] [Google Scholar]
- 48.Newell C, Bomhof MR, Reimer RA, Hittel DS, Rho JM, Shearer J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol. Autism. 2016 Sep 01;7(1):37. doi: 10.1186/s13229-016-0099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newell C, Shutt TE, Ahn Y, et al. Tissue specific impacts of a ketogenic diet on mitochondrial dynamics in the BTBR(T+tf/j) mouse. Front. Physiol. 2016;7 doi: 10.3389/fphys.2016.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buie T, Campbell DB, Fuchs GJ, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125(Supplement 1):S1–S18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.