Abstract
Object We aimed to identify the β-adrenoceptor (β-AR) subtypes involved in isoprenaline-induced relaxation of guinea pig colonic longitudinal smooth muscle using pharmacological and biochemical approaches. Methods Longitudinal smooth muscle was prepared from the male guinea pig ascending colon and contracted with histamine prior to comparing the relaxant responses to three catecholamines (isoprenaline, adrenaline, and noradrenaline). The inhibitory effects of subtype-selective β-AR antagonists on isoprenaline-induced relaxation were then investigated. Results The relaxant potencies of the catecholamines were ranked as: isoprenaline > noradrenaline ≈ adrenaline, whereas the rank order was isoprenaline > noradrenaline > adrenaline in the presence of propranolol (a non-selective β-AR antagonist; 3 × 10−7 M). Atenolol (a selective β1-AR antagonist; 3 × 10−7–10−6 M) acted as a competitive antagonist of isoprenaline-induced relaxation, and the pA2 value was calculated to be 6.49 (95% confidence interval: 6.34–6.83). The relaxation to isoprenaline was not affected by ICI-118,551 (a selective β2-AR antagonist) at 10−9–10−8 M, but was competitively antagonized by 10−7–3 × 10−7 M, with a pA2 value of 7.41 (95% confidence interval: 7.18–8.02). In the presence of propranolol (3 × 10−7 M), the relaxant effect of isoprenaline was competitively antagonized by bupranolol (a non-selective β-AR antagonist), with a pA2 value of 5.90 (95% confidence interval: 5.73–6.35). Conclusion These findings indicated that the β-AR subtypes involved in isoprenaline-induced relaxation of colonic longitudinal guinea pig muscles are β1-AR and β3-AR.
Keywords: β-adrenoceptor, smooth muscle relaxation, guinea pig colonic longitudinal smooth muscle, isoprenaline
Introduction
The β-adrenoceptor (β-AR) belongs to the family of G protein-coupled receptors and is currently classified into three subtypes (β1, β2, and β3) (1,2,3). Although the β-AR is distributed throughout the body, it exists at a particularly high density in smooth muscle and plays an important role in its physiological relaxant responses. The expression of each subtype shows some degree of tissue specificity, and also varies greatly between species. For example, the β2-AR is the major subtype in human (2, 4) and guinea pig (5,6,7) bronchial smooth muscle, while the β1-AR is the major subtype in mouse bronchial smooth muscle (8). Therefore, although animal studies of the β-AR provide important information, care is required when extrapolating these results to humans.
The major β-AR in gastrointestinal smooth muscle was classified as β1-AR by Lands et al. (9, 10). However, Koike et al. identified the β2-AR subtype in the guinea pig tenia cecum, indicating that gastrointestinal smooth muscle expressed other β-AR subtypes, in addition to β1-AR (11). Furthermore, Koike et al. reported the possibility that guinea pig tenia cecum expressed the β3-AR subtype, which is insensitive to propranolol at concentrations up to ≈10−6 M and is involved in the relaxant responses to isoprenaline, noradrenaline, and adrenaline (12,13,14). Horinouchi et al. subsequently reported that the β3-AR was also expressed in the guinea pig stomach, duodenum, and the longitudinal muscle of the ileum (15, 16). Therefore, although the physiological roles of the β3-AR are still unclear, the presence of this receptor should be taken into account when considering the β-AR subtypes in gastrointestinal smooth muscle. These previous reports have investigated the β-AR subtypes expressed in gastrointestinal smooth muscle from the stomach to the small intestine, but few studies have examined the smooth muscle of the large intestine.
The function of the smooth muscle of the large intestine is controlled by excitatory parasympathetic nerves and inhibitory sympathetic nerves, and abnormalities of these autonomic nerves lead to dysfunction of the large intestine (17, 18). Indeed, anticholinergic drugs that inhibit parasympathetic nerve activation-associated enhancement of gastrointestinal motility are used to treat conditions associated with an overactive bowel, such as irritable bowel syndrome. In addition, parasympathomimetic drugs such as synthetic choline esters and cholinesterase inhibitors are used to treat conditions associated with an underactive bowel, such as post-surgical intestinal paralysis. Agonists or antagonists of intestinal smooth muscle β-ARs could provide alternatives to parasympathomimetic or anticholinergic drug therapies for large intestine dysfunction.
The present study aimed to clarify the functional β-AR subtypes in the smooth muscle of the large intestine using pharmacological and biochemical approaches. We compared the rank order of efficacy of three catecholamines (isoprenaline, noradrenaline, and adrenaline) in longitudinal muscle preparations of the guinea pig colon, and examined the effects of selective β-AR antagonists on the isoprenaline-induced relaxation response. In addition, we examined the mRNA expression of β-AR subtypes within the colonic longitudinal muscle.
Materials and methods
Animals
Male Hartley guinea pigs (5–8 weeks old, weighing 340–490 g; Sankyo Labo Service, Tokyo, Japan) were housed under controlled conditions (temperature 20–22°C, relative air humidity 50 ± 5%, fixed 12-h light [08:00 to 20:00]/12-h dark cycle). Food and water were available to all animals ad libitum, and only healthy animals were used for the experiments. This study was approved by the Toho University Animal Care and User Committee (approval number: 15–51-294, accredited on May 22, 2015; approval number: 16–52-294, accredited on May 16, 2016; approval number: 17–53-294, accredited on March 17, 2017) and conducted in accordance with the User Guidelines of the Laboratory Animal Center of the Faculty of Pharmaceutical Sciences, Toho University.
Preparation of longitudinal smooth muscle preparations of the guinea pig colon
The guinea pigs were euthanized by cervical dislocation and exsanguinated from a carotid artery; the ascending colon (length: about 80 mm) was isolated immediately after the dissection of the abdomen, washed well with Locke–Ringer solution containing 154 mM NaCl, 5.6 mM KCl, 2.2 mM CaCl2, 2.1 mM MgCl2, 5.9 mM NaHCO3, and 2.8 mM d-(+)-glucose, and then divided into two parts in the circular muscle direction. The longitudinal smooth muscle bundles were then isolated using a glass rod, swab, and tweezers, and divided in two in the longitudinal direction. Four preparations were made from each guinea pig using this procedure.
Recording of isotonic tension changes
The longitudinal smooth muscle preparations were suspended in an organ bath filled with 20 mL Locke–Ringer solution, which was aerated with mixed gas (95% O2 and 5% CO2) and kept at 32°C. Tension changes were recorded isotonically via a kymograph and lever. The initial tension load was 0.5 g, and each preparation was initially incubated for 15 min prior to inducing contraction by adding histamine.
Examination of sustained responses of the colonic longitudinal smooth muscle to
contractile agents
First of all, we examined the sustainability and stability of colonic longitudinal smooth muscle contractile responses to five drugs in order to determine the most suitable constrictor of this muscle for detection of β-AR-mediated relaxation. The contractile drugs tested were serotonin, prostaglandin F2α (PGF2α), carbachol, histamine, and bethanechol.
After the initial 15-min incubation, the colonic preparation was contracted by adding histamine (10−6 M) at least twice at 10-min intervals. After an equilibration period of 10 min, histamine (10−8–3 × 10−5 M) was cumulatively applied to the bath medium until a maximum response was obtained, which was subsequently washed out. Following this procedure, concentration-response curves for the test drugs were obtained by their cumulative application using the following concentration ranges: serotonin (10−8–3 × 10−6 M); PGF2α (10−8–10−4 M); carbachol (10−8–10−4 M); histamine (10−8–3 × 10−5 M); and bethanechol (10−8–3 × 10−4 M). From these concentration-response curves, we determined the concentrations that caused sub-maximal contractile responses. These concentrations, which were then used to examine the sustainability and stability of the contractile responses, were 10−6 M for serotonin, 3 × 10−5 M for PGF2α, 3 × 10−5 M for carbachol, 10−5 M for histamine, and 10−4 M for bethanechol. After these procedures, the contractile response to each test drug was recorded for 3 h.
Comparison of the relaxant potencies of isoprenaline, adrenaline, and noradrenaline
This series of experiments was carried out in the presence of normetanephrine (3 × 10−7 M) and desipramine (10−6 M) in order to rule out the contribution of amine transporters to the relaxant effects of catecholamines. Normetanephrine is an uptake 1 inhibitor and desipramine is an uptake 2 inhibitor.
After the initial 15-min incubation, the colonic preparation was contracted by histamine (10−5 M). When the contraction reached a steady-state, the preparation was relaxed by the application of isoprenaline. Before starting the control experiment, this procedure was repeated again. After the second relaxant response to isoprenaline (preliminary experiment), the preparation was incubated for 40 min in Locke–Ringer solution. Normetanephrine (3 × 10−7 M) and desipramine (10−6 M) were then added to the bath solution, and the colonic longitudinal smooth muscle preparations were contracted by histamine (10−5 M). When the contraction reached a steady-state level, isoprenaline, adrenaline, or noradrenaline was cumulatively applied to the bath solution in order to obtain concentration-response curves in the absence of propranolol. After washing out the reagents using fresh bath solution, the colonic longitudinal smooth muscle preparations were incubated for 60 min, with the bath solution changed every 20 min. Thereafter, normetanephrine (3 × 10−7 M), desipramine (10−6 M), and propranolol (3 × 10−7 M) were added to the bath solution, and a sustained contraction was evoked by applying histamine (10−5 M). When the histamine-induced contraction reached a steady-state level, isoprenaline, adrenaline, or noradrenaline was again cumulatively applied in order to obtain concentration-response curves in the presence of propranolol (3 × 10−7 M). After cumulative application of these catecholamines, the preparation was completely relaxed by the application of papaverine (10−4 M). All experiments were carried out in the presence of indomethacin (3 × 10−6 M) and prazosin (10−5 M) to prevent any possible effects of endogenous PGs or α-ARs.
Evaluation of the effects of β-AR antagonists on the isoprenaline-induced relaxation
After conducting the preliminary procedures described in previous section, the colonic longitudinal smooth muscle preparations were contracted with histamine (10−5 M). When the contraction reached a steady-state level, isoprenaline was cumulatively applied to the bath solution in order to obtain a concentration-response curve. The reagents were then washed out using fresh bath solution and the colonic longitudinal smooth muscle preparations were incubated for 60 min, with the bath solution changed every 20 min. Thereafter, the preparations were contracted with histamine (10−5 M) in the presence of the indicated β-AR antagonists; when the histamine-induced contraction reached a steady-state level, isoprenaline was cumulatively applied in order to obtain a concentration-response curve. The β-AR antagonists used were propranolol (3 × 10−9–10−6 M), atenolol (3 × 10−7–10−6 M), ICI-118,551 (10−9–3 × 10−7 M), or bupranolol (10−6–3 × 10−6 M) plus propranolol (3 × 10−7 M). Following the cumulative application of isoprenaline, the preparation was completely relaxed by applying papaverine (10−4 M). This series of experiments was also carried out in the presence of indomethacin (3 × 10−6 M) and prazosin (10−5 M).
Reverse transcription polymerase chain reaction (RT-PCR) analysis of β-AR subtype
mRNA expression
Guinea pigs were euthanized by cervical dislocation and exsanguinated from a carotid artery; the ascending colon (length: about 80 mm), right atria, and ileum (length: about 80 mm) were isolated immediately after the dissection of the abdomen and washed thoroughly with Locke–Ringer solution. Colonic and ileal longitudinal muscle preparations were prepared as described earlier. The smooth muscle and atrial preparations were frozen in liquid nitrogen and used for extraction of total RNA using RNAiso PlusTM (TAKARA BIO Inc., Shiga, Japan), according to the manufacturer’s instructions. The first-strand cDNA (10 µL) was synthesized from total RNA (0.5 µg) using ReverTra Ace® qPCR RT Master Mix with gDNA Remover (TOYOBO Co., Ltd. Osaka, Japan), according to the manufacturer’s instructions. Prior to the start of the reverse transcription reaction, the total RNA solution was incubated at 65°C for 5 min in order to denature the RNA, and then cooled on ice. To check for genomic DNA contamination, the same procedure was also applied to a solution containing no reverse transcriptase. cDNA (0.5 µL) was amplified using Go Taq® Green Master Mix (Promega Corp., Madison, Wis., USA) with the specific forward and reverse primer sets shown in Table 1 (Eurofins Genomics, Tokyo, Japan), according to the manufacturer’s instructions. The PCR conditions for β-ARs were 95°C for 2 min followed by 35 cycles of 98°C for 30 sec, 55°C for 2 sec, 72°C for 30 sec, and finally 72°C for 5 min. The PCR for β-actin was 95°C for 2 min followed by 30 cycles of 98°C for 30 sec, 55°C for 2 sec, 72°C for 30 sec, and finally 72°C for 5 min. The PCR products were electrophoresed in TAE buffer using a 1.5% agarose gel containing ethidium bromide (0.5 µg/mL) to confirm target gene amplification.
Table 1. Primer sequences used for RT-PCR.
| Code | Primer sequence (5′–3′) | Gene bankaccession no. | PCRproduct size |
| β1-AR | Forward:CCGCTGCTACAACGATCCCAAG | EU332753 | 444 bp |
| Reverse:AGCCAGTTGAAGAAGACGAAGAGGCG | |||
| β2-AR | Forward:CTGGTCATCACAGCCATTGCC | AJ459814 | 434 bp |
| Reverse:TGGTTCGTGAAGAAGTCACA | |||
| β3-AR | Forward:GTGGGAGGCAACCTGCTGGT | U51098 | 383 bp |
| Reverse:CGCCACCACTGGCTCAT | |||
| β-actin | Forward:ATCCTGCGTCTGGACCTGGCTG | AF508792 | 559 bp |
| Reverse:CCTGCTTGCTGATCCACATCTGCTG |
Drugs
Atenolol, bethanechol chloride, carbamylcholine chloride (carbachol), desipramine hydrochloride, (–)-epinephrine (+)-bitartrate salt, ethidium bromide, histamine dihydrochloride, ICI-118,551 hydrochloride, indomethacin, (–)-isoproterenol hydrochloride, dl-normetanephrine hydrochloride, dl-propranolol hydrochloride, and prazosin hydrochloride were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA); (–)-(R)-norepinephrine hydrogen tartrate monohydrate, serotonin creatinine sulfate, and papaverine hydrochloride were obtained from Wako Pure Chemical Industries (Osaka, Japan); and prostaglandin F2α was obtained from Fuji Pharma Co., Ltd. (Tokyo, Japan). (±)-Bupranolol hydrochloride was kindly donated by Kaken Pharmaceutical Co., Ltd. (Tokyo, Japan).
Atenolol was dissolved in 0.1 N HCl to produce a stock solution of 10−2 M. Indomethacin was dissolved in 100% ethanol to produce a stock solution of 10−2 M. All other drugs were prepared as aqueous solutions and diluted with distilled water. Drugs were added directly to the organ bath and expressed as the molar concentration (M) in the bath medium.
Data analysis
The extent of relaxation induced by the β-AR agonists (isoprenaline, adrenaline, and noradrenaline) was calculated with respect to the tone level following the application of 10−4 M papaverine (100% relaxation), and to the steady-state tone level prior to the application of each relaxant (0% relaxation).
The potencies of β-AR agonists were expressed as pD2 (pEC50) values (the negative logarithm of the effective agonist concentration producing a response that is 50% of the maximum response). Data were plotted as a function of the catecholamine concentration and fitted to the equation:E = Emax × Anh / (EC50nh + Anh)where E is the % relaxation at a given concentration, Emax is the maximum response, A is the agonist (relaxant) concentration, nH is the slope function, and EC50 is the agonist concentration producing a 50% response. Curve-fitting was carried out using GraphPad PrismTM (Version 4.00; GraphPad Software, Inc., San Diego, CA, USA).
The β-AR antagonist potencies are expressed as the pA2 value, which was calculated according to the method originally reported by Arunlakshana and Schild (19).
Data are presented as the mean ± S.E.M. or the mean and the 95% confidence interval (C.I.), and n refers to the number of preparations. Significant differences between the means were evaluated by GraphPad PrismTM (Version 4.00) using one-way analysis of variance followed by Tukey’s multiple comparison test. A P-value of less than 0.05 was considered to be statistically significant.
Results
Sustained contractile responses of colonic longitudinal smooth muscle segments to
contractile agents
Serotonin (10−6 M) produced a very weak contraction that reached its peak level 5–10 min after application (Fig. 1A). In addition, this contraction was not sustained and declined to the level observed prior to serotonin application after 30–60 min (Figs. 1A, 2). PGF2α (3 × 10−5 M) evoked a medium contraction that reached its peak level 5–10 min after application and was sustained over 3 h (Figs. 1B, 2). However, PGF2α-induced tone was difficult to recover after washout; it took over 1 h for the tone induced by PGF2α (3 × 10−5 M) to recover to the level observed prior to its application (Fig. 1B). Therefore, PGF2α was judged to be unsuitable for repeated administrations. Carbachol (3 × 10−5 M) evoked a strong contraction that reached a peak level 5–20 min after application (Fig. 1C). However, carbachol-induced tone was not sustained and decreased gradually within the observation period of 3 h. Therefore, carbachol was also judged to be inappropriate for the evaluation of relaxation responses to β-AR agonists (Figs. 1C, 2). Both histamine (10−5 M) and bethanechol (10−4 M) produced strong contractions that were established within 60–90 min of application and were sustained for at least 90 min (Figs. 1D, 1E, 2). Based on these findings, both histamine and bethanechol were judged to be suitable for the determination of β-AR agonist-induced relaxation responses. However, we chose histamine as a contractile agonist in order to rule out any possible physiological interactions between muscarinic receptors and adrenergic receptors.
Fig. 1.
Representative traces of the contractile responses of isolated segments of the longitudinal smooth muscle of the guinea pig colon to various stimulants over a 3-h period.
Fig. 2.
Analysis of the 3-h contractile responses of isolated segments of guinea pig colonic longitudinal smooth muscle to the indicated compounds. Contractions are expressed as a percentage of the contraction induced by 10−6 M histamine (100%), which was applied before starting the 3-h recording period; n = 4 for each data point.
The relaxant responses to repeat applications of isoprenaline
Figure 3 shows the reproducibility of concentration-response curves for isoprenaline-induced relaxation, determined using the same longitudinal smooth muscle preparations of the guinea pig colon. The concentration-response curves for isoprenaline were almost identical for the first and second applications, and no significant differences were found in the pD2 values or the maximum responses (Emax); the pD2 and Emax values for the first vs. second response were 8.08 ± 0.02 vs. 8.19 ± 0.08 (n = 6) and 95.9 ± 1.9% vs. 98.5 ± 0.7% (n = 6), respectively.
Fig. 3.
Reproducibility of the concentration-response curves for isoprenaline-induced relaxation of the isolated guinea pig colonic longitudinal smooth muscle preparations. Concentration-response curves for isoprenaline were obtained in the same preparations. Relaxation is expressed as a percentage of the maximum relaxation induced by 10−4 M papaverine (100%), which was applied at the end of each experiment; n = 3 for each data point.
This finding indicated that reproducible relaxant responses were obtained at least twice by cumulative addition of isoprenaline to the same preparation of ascending colonic longitudinal muscle. We therefore regarded the first relaxant response as the control response, while the effects of β-AR antagonists were examined in the second relaxation response.
Rank order of relaxant potency of isoprenaline, adrenaline, and noradrenaline
Figure 4 shows the concentration-response curves for the three catecholamines tested (isoprenaline, adrenaline, and noradrenaline). Each catecholamine produced potent relaxant effects in both the absence and presence of propranolol (3 × 10−7 M) (Fig. 4A, B); they all relaxed the colonic longitudinal smooth muscle to the muscle tone level observed prior to the application of histamine (10−5 M).
Fig. 4.
Relaxant responses to isoprenaline, adrenaline, and noradrenaline in guinea pig colonic longitudinal smooth muscle preparations contracted with histamine (10−5 M) in the absence (A) and presence (B) of propranolol (3 × 10−7 M). Relaxation is expressed as a percentage of the maximum relaxation induced by 10−4 M papaverine (100%), which was applied at the end of each experiment; n = 3 for each data point.
In the absence of propranolol, the pD2 values were 8.17 ± 0.02 (n = 3) for isoprenaline, 6.85 ± 0.04 (n = 3) for adrenaline, and 7.05 ± 0.05 (n = 3) for noradrenaline; the rank order of relaxant effect potency was thus isoprenaline > noradrenaline ≥ adrenaline (Fig. 4A). In the presence of propranolol (3 × 10−7 M), the pD2 values were 6.67 ± 0.04 (n = 3) for isoprenaline, 5.40 ± 0.09 (n = 3) for adrenaline, and 5.94 ± 0.14 (n = 3) for noradrenaline; a rank order of isoprenaline > noradrenaline > adrenaline (Fig. 4B).
These results suggest that β-AR-mediated relaxation of the colonic longitudinal muscle is induced via β1-AR and/or β3-AR in the absence of propranolol, and by β3-AR in the presence of propranolol (3 × 10−7 M).
Effects of propranolol on the isoprenaline-induced relaxation
Figure 5 shows the effect of propranolol on the relaxant response to isoprenaline. Isoprenaline-induced relaxation was shifted to the right by propranolol at both 3 × 10−9 M (Fig. 5A) and 10−8 M (Fig. 5B). The slope of the regression line for the Schild plot of propranolol (3 × 10−9–10−8 M) vs. isoprenaline was 1.08, which was not significantly different from unity (95% C.I.: 0.38–1.79, n = 6) (Fig. 5E). These findings indicated that propranolol acted as a competitive antagonist of the relaxant response to isoprenaline, with a pA2 value of 8.56 (95% C.I.: 8.38–9.18, n = 6).
Fig. 5.
Inhibitory effects of propranolol on isoprenaline-induced relaxation of isolated guinea pig colonic longitudinal muscle preparations. A–D: Effects of the indicated concentrations of propranolol on isoprenaline-induced relaxation. A sustained contraction was generated by histamine (10−5 M) and isoprenaline was then applied to the bath solution cumulatively. Relaxation is expressed as a percentage of the maximum relaxation induced by 10−4 M papaverine (100%), which was applied at the end of each experiment; n = 3 for each data point. E: Schild plot analyses of the effects of the indicated concentrations of propranolol against isoprenaline; n = 6 for each line.
Figures 5C, D show the effects of higher concentrations of propranolol (3 × 10−7–10−6 M) on the relaxant response to isoprenaline. Isoprenaline-induced relaxation was shifted to the right by propranolol at both 3 × 10−7 M (Fig. 5C) and 10−6 M (Fig. 5D). However, the slope of the Schild plot regression line was less than unity (–0.35) (Fig. 5E). These findings indicated that isoprenaline-induced relaxation was not suppressed further in the presence of more than 3 × 10−7 M propranolol.
Effects of atenolol on the isoprenaline-induced relaxation
Figure 6 shows the effect of atenolol on the relaxant response to isoprenaline. Isoprenaline-induced relaxation was shifted to the right by atenolol at 3 × 10−7–10−6 M (Fig. 6A, B). The slope of the Schild plot regression line was 0.84, which was not significantly different from unity (95% C.I.: 0.37–1.30, n = 6) (Fig. 6C). This indicated that atenolol acted as a competitive antagonist of isoprenaline-induced relaxation, with a pA2 value of 6.49 (95% C.I.: 6.34–6.85, n = 6).
Fig. 6.
Inhibitory effects of atenolol on isoprenaline-induced relaxation of isolated guinea pig colonic longitudinal muscle preparations. A, B: Effects of the indicated concentrations of atenolol on isoprenaline-induced relaxation of colonic longitudinal muscle preparations contracted with histamine (10−5 M). Relaxation is expressed as a percentage of the maximum relaxation induced by 10−4 M papaverine (100%), which was applied at the end of each experiment; n = 3 for each data point. C: Schild plot analysis of the effects of the indicated concentrations of atenolol against isoprenaline; n = 6.
Effects of ICI-118,551 on the isoprenaline-induced relaxation
Figure 7 shows the effect of ICI-118,551 on the relaxant response to isoprenaline. Isoprenaline-induced relaxation was not substantially affected by ICI-118,551 (10−9–10−8 M) (Fig. 7A, B). Figure 7C–E shows that higher concentrations of ICI-118,551 (10−7–3 × 10−7 M) shifted isoprenaline-induced relaxation to the right. The slope of the Schild plot regression line was 0.93, which was not significantly different from unity (95% C.I.: 0.49–1.36, n = 8) (Fig. 7E). This indicated that ICI-118,551 acted as a competitive antagonist of isoprenaline-induced relaxation, with a pA2 value of 7.41 (95% C.I.: 7.18–8.02, n = 8).
Fig. 7.
Effects of ICI-118,551 on isoprenaline-induced relaxation of isolated guinea pig colonic longitudinal muscle preparations. A–D: Effects of the indicated concentrations of ICI-118,551 on isoprenaline-induced relaxation. Relaxation is expressed as a percentage of the maximum relaxation induced by 10−4 M papaverine (100%), which was applied at the end of each experiment; n = 4 for each data point. E: Schild plot analysis of the effects of the indicated concentrations of ICI-118,551 against isoprenaline; n = 8.
Effects of bupranolol on the isoprenaline-induced relaxation
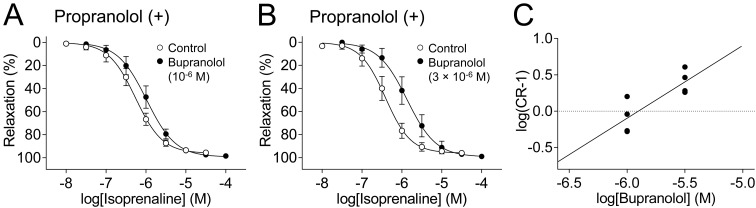
Figure 8 shows that bupranolol (10−6–3 × 10−6 M) shifted isoprenaline-induced relaxation to the right in the presence of 3 × 10−7 M propranolol (Fig. 8A, B). The slope of the Schild plot regression line was 1.00, which was not significantly different from unity (95% C.I.: 0.31–1.69, n = 8) (Fig. 8C). This indicated that bupranolol acted as a competitive antagonist of isoprenaline-induced relaxation in the presence of 3 × 10−7 M propranolol, with a pA2 value of 5.90 (95% C.I.: 5.73–6.35, n = 8).
Fig. 8.
Inhibitory effects of bupranolol on isoprenaline-induced relaxation of isolated guinea pig colonic longitudinal muscle preparations, in the presence of propranolol. The effects of the indicated concentrations of bupranolol on isoprenaline-induced relaxation were investigated in the presence of propranolol (3 × 10−7 M). Relaxation is expressed as a percentage of the maximum relaxation induced by 10−4 M papaverine (100%), which was applied at the end of each experiment; n = 4 for each data point. C: Schild plot analysis of the effects of the indicated concentrations of bupranolol against isoprenaline; n = 8.
Expression of β-AR subtype mRNAs in colonic longitudinal muscle segments
PCR products for β1-, β2-, and β3-ARs were detected in colonic longitudinal muscle and ileal smooth muscle, with the expected corresponding PCR products of 444, 434, and 383 base pairs, respectively (Fig. 9). In contrast, only β1- and β2-AR mRNAs were detected in the atrium (Fig. 9). A PCR product for β-actin, as an internal standard, was also detected in all three preparations; this had the expected size of 559 base pairs. No positive bands were observed in PCR products generated in the absence of reverse transcription.
Fig. 9.
Representative images of agarose gels showing expression of the indicated mRNAs in guinea pig colonic longitudinal smooth muscle (lane 1), atria (lane 2), and ileal longitudinal smooth muscle (lane 3). PCR products were separated by 1.5% agarose gel electrophoresis and cDNA was detected using ethidium bromide. PCR products for β1-, β2-, and β3-ARs were detected as the expected corresponding PCR products of 444, 434, and 383 base pairs, respectively. RT(+): reverse transcription, RT(–): no reverse transcription. These results were representative of four experiments.
Discussion
These pharmacological and biochemical studies were carried out to identify the β-AR subtypes that mediated the relaxant responses of guinea pig colonic longitudinal smooth muscle. In order to achieve this, we determined the rank order of relaxant potency of three catecholamines, and examined the inhibitory effects of non-selective and selective β-AR antagonists on isoprenaline-induced relaxation. Furthermore, we examined colonic longitudinal smooth muscle for the expression of mRNAs encoding β1-, β2-, and β3-ARs.
First, we examined the rank order of potency of three catecholamines (isoprenaline, adrenaline, and noradrenaline). The rank order of the pD2 values determined by this study (isoprenaline > noradrenaline ≥ adrenaline) suggested that the major β-AR subtypes involved in the relaxation of guinea pig colonic longitudinal smooth muscle were β1-AR, β3-AR, or both β1-AR and β3-AR. This series of experiments did not indicate whether this rank order reflected the involvement of β1-AR or β3-AR, since the pD2 values for noradrenaline (7.05 ± 0.05) and adrenaline (6.85 ± 0.04) were similar. On the other hand, in the presence of a high concentration of propranolol (3 × 10−7 M), the rank order of the pD2 values of these three catecholamines was isoprenaline > noradrenaline > adrenaline; this was consistent with β3-AR involvement.
Propranolol is usually recognized as a nonselective competitive antagonist of β1- and β2-ARs, with pA2 values of around 8.5 for both receptor subtypes (20, 21). However, propranolol also shows an antagonistic effect on β3-AR, although this effect requires concentrations that are more than 100-fold higher; published pA2 values of propranolol against the β3-AR are around 6.0 (22, 23). We therefore next examined the effects of propranolol on isoprenaline-induced relaxation in order to distinguish effects mediated by β3-AR from those involving β1-AR, which is inhibited by lower concentrations of propranolol. Propranolol at 3 × 10−9–10−8 M competitively antagonized the relaxant responses to isoprenaline (Fig. 5A, B, E), indicating that β1- or β2-ARs were involved in this response. Higher concentrations of propranolol (3 × 10−7–10−6 M) also inhibited isoprenaline-induced relaxation, but the isoprenaline concentration-response curve was not shifted any further to the right under these conditions (Fig. 5C, D, E). In contrast to our present findings, propranolol was previously shown to act as a competitive antagonist of isoprenaline-induced relaxation at concentrations of 10−8–3 × 10−6 M in guinea pig esophageal smooth muscle; this tissue expresses β1- and β2-ARs, but not β3-AR (20). These isoprenaline vs. propranolol results indicate that at least two β-AR subtypes mediate colonic longitudinal smooth muscle relaxation; one is sensitive to lower concentrations of propranolol (less than around 10−7–3 × 10−7 M), and the other is insensitive or poorly sensitive to even higher concentrations of propranolol (more than 3 × 10−7–10−6 M). The former involves the β1-AR and the latter involves the β3-AR.
In order to identify the β-AR subtype that is responsible for isoprenaline-induced relaxation and sensitive to propranolol at ≤ 3 × 10−7 M, we examined the effects of atenolol (a selective β1-AR antagonist) and ICI-118,551 (a selective β2-AR antagonist) on this effect. Isoprenaline-induced relaxation was competitively antagonized by atenolol, with a pA2 value of atenolol vs. isoprenaline of 6.49. This was similar to the previously reported pA2 value (6.5–7.0) of atenolol against the β1-AR subtype (5, 21, 24). Although ICI-118,551 is a selective β2-AR antagonist, it can also act as a competitive antagonist of β1-AR at higher concentrations; the pA2 values of ICI-118,551 vs. β2- and β1-AR are around 9.0 and 7.0, respectively (20, 21). In the present study, isoprenaline-induced relaxation was not affected by ICI-118,551 at 10−9–10−8 M, thus ruling out the possibility of β2-AR involvement. In contrast, isoprenaline-induced relaxation was competitively antagonized by 10−7–3 × 10−7 M ICI-118,551, and the calculated pA2 value (7.41) was consistent with the pA2 value of ICI-118,551 for the β1-AR. These findings thus indicate that the β1-AR was the main mediator of ≤ 3 × 10−7 M-propranolol sensitivity in this gastrointestinal smooth muscle preparation.
Finally, we examined the effects of bupranolol on isoprenaline-induced relaxation in the presence of propranolol (3 × 10−7 M) to identify the β-AR that was resistant to this concentration of propranolol in this preparation. We used bupranolol because this compound antagonizes β1- and β2-ARs with pA2 values of ≈9.0, and also antagonizes β3-AR with a pA2 value of 6.0 (3, 13, 25,26,27). We have been using this β-AR antagonist from the beginning of our smooth muscle β3-AR studies and have found that the β3-AR pA2 value is always within relatively narrow range of 5.5–6.0. In the presence of propranolol (3 × 10−7 M), isoprenaline-induced relaxation was competitively antagonized by bupranolol (10−6–3 × 10−6 M) with a pA2 value of 5.90 (Fig. 8); this was consistent with its pA2 value against the β3-AR. These findings indicate that the β3-AR is involved in the propranolol (3 × 10−7 M)-insensitive component of isoprenaline-induced relaxation of colonic longitudinal smooth muscle.
The motility of large intestinal smooth muscle is suppressed by sympathetic nerve activity (17, 18). The present study suggests that the activation of sympathetic nerves of the large intestine inhibits its motility through stimulating β1- and/or β3-ARs. Therefore, agonists of β1- and/or β3-AR might be effective for the treatment of diseases caused by overactivity of the colon, such as irritable bowel syndrome, while antagonists of these receptor subtypes might be effective for diseases caused by underactivity of the colon, such as intestinal palsy. However, further experiments will be required to determine whether the present guinea pig results are applicable to humans.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
References
- 1.Arch JR, Kaumann AJ. β3 and atypical β-adrenoceptors. Med Res Rev. 1993; 13(6): 663–729. doi: 10.1002/med.2610130604 [DOI] [PubMed] [Google Scholar]
- 2.Johnson M. The β-adrenoceptor. Am J Respir Crit Care Med. 1998; 158(5 Pt 3): S146–53. doi: 10.1164/ajrccm.158.supplement_2.13tac110 [DOI] [PubMed] [Google Scholar]
- 3.Koike K, Takayanagi I. β3-adrenoceptor mechanisms in guinea-pig taenia caecum. Life Sci. 1998; 62(17–18): 1503–7. doi: 10.1016/S0024-3205(98)00097-6 [DOI] [PubMed] [Google Scholar]
- 4.Nijkamp FP. β-adrenergic receptors in the lung: an introduction. Life Sci. 1993; 52(26): 2073–82. doi: 10.1016/0024-3205(93)90722-F [DOI] [PubMed] [Google Scholar]
- 5.O’Donnell SR, Wanstall JC. The importance of choice of agonist in studies designed to predict β2: β1 adrenoceptor selectivity of antagonists from pA2 values on guinea-pig trachea and atria. Naunyn Schmiedebergs Arch Pharmacol. 1979; 308(3): 183–90. doi: 10.1007/BF00501381 [DOI] [PubMed] [Google Scholar]
- 6.Tanaka Y, Yamashita Y, Yamaki F, Horinouchi T, Shigenobu K, Koike K. MaxiK channel mediates β2-adrenoceptor-activated relaxation to isoprenaline through cAMP-dependent and -independent mechanisms in guinea-pig tracheal smooth muscle. J Smooth Muscle Res. 2003; 39(6): 205–19. doi: 10.1540/jsmr.39.205 [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y, Yamashita Y, Horinouchi T, Yamaki F, Koike K. Evidence showing that β-adrenoceptor subtype responsible for the relaxation induced by isoprenaline is principally β2 but not β1 in guinea-pig tracheal smooth muscle. Auton Autacoid Pharmacol. 2004; 24(2): 37–43. doi: 10.1111/j.1474-8673.2004.00314.x [DOI] [PubMed] [Google Scholar]
- 8.Henry PJ, Goldie RG. β1-adrenoceptors mediate smooth muscle relaxation in mouse isolated trachea. Br J Pharmacol. 1990; 99(1): 131–5. doi: 10.1111/j.1476-5381.1990.tb14666.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lands AM, Arnold A, McAuliff JP, Luduena FP, Brown TG, Jr . Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967; 214(5088): 597–8. doi: 10.1038/214597a0 [DOI] [PubMed] [Google Scholar]
- 10.Lands AM, Luduena FP, Buzzo HJ. Differentiation of receptors responsive to isoproterenol. Life Sci. 1967; 6(21): 2241–9. doi: 10.1016/0024-3205(67)90031-8 [DOI] [PubMed] [Google Scholar]
- 11.Koike K, Takayanagi I, Muramatsu M, Ohki S, Horinouchi T. Involvement of β3-adrenoceptor in the relaxation response in guinea pig taenia caecum. Jpn J Pharmacol. 1994; 66(2): 213–20. doi: 10.1254/jjp.66.213 [DOI] [PubMed] [Google Scholar]
- 12.Koike K, Horinouchi T, Takayanagi I. Signal transduction pathway involved in β3-adrenoceptor-mediated relaxation in guinea pig taenia caecum. Jpn J Pharmacol. 1995; 68(1): 41–6. doi: 10.1254/jjp.68.41 [DOI] [PubMed] [Google Scholar]
- 13.Koike K, Horinouchi T, Takayanagi I. Possible mechanisms of β-adrenoceptor-mediated relaxation induced by noradrenaline in guinea pig taenia caecum. Eur J Pharmacol. 1995; 279(2–3): 159–63. doi: 10.1016/0014-2999(95)00147-D [DOI] [PubMed] [Google Scholar]
- 14.Koike K, Horinouchi T, Yamamoto Y. The β3-adrenoceptor-mediated relaxation induced by epinephrine in guinea pig taenia caecum. J Smooth Muscle Res. 2000; 36(3): 93–9. doi: 10.1540/jsmr.36.93 [DOI] [PubMed] [Google Scholar]
- 15.Horinouchi T, Koike K. Cyclic AMP-independent relaxation mediated by β3-adrenoceptors on guinea pig gastrointestine. Eur J Pharmacol. 2002; 442(1–2): 137–46. doi: 10.1016/S0014-2999(02)01505-4 [DOI] [PubMed] [Google Scholar]
- 16.Horinouchi T, Tanaka Y, Koike K. β-adrenoceptor subtypes involved in relaxations of guinea-pig gastrointestinal smooth muscles: distribution and signaling pathway of β3-adrenoceptors. Nihon Yakurigaku Zasshi. 2003; 122 Suppl: 54P–56P. [PubMed]
- 17.Ridolfi TJ, Tong WD, Kosinski L, Takahashi T, Ludwig KA. Recovery of colonic transit following extrinsic nerve damage in rats. Scand J Gastroenterol. 2011; 46(6): 678–83. doi: 10.3109/00365521.2011.560682 [DOI] [PubMed] [Google Scholar]
- 18.Ridolfi TJ, Tong WD, Takahashi T, Kosinski L, Ludwig KA. Sympathetic and parasympathetic regulation of rectal motility in rats. J Gastrointest Surg. 2009; 13(11): 2027–33. doi: 10.1007/s11605-009-0999-z [DOI] [PubMed] [Google Scholar]
- 19.Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959; 14(1): 48–58. [DOI] [PMC free article] [PubMed]
- 20.Horinouchi T, Tanaka Y, Koike K. Function of β1-adrenoceptors and mRNA expression of β1- and β2-adrenoceptors in guinea-pig esophagus. Eur J Pharmacol. 2003; 473(1): 79–82. doi: 10.1016/S0014-2999(03)01943-5 [DOI] [PubMed] [Google Scholar]
- 21.Tanaka Y, Yamashita Y, Michikawa H, Horinouchi T, Koike K. Pharmacological characterization of the β-adrenoceptor that mediates the relaxant response to noradrenaline in guinea-pig tracheal smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 2007; 375(1): 51–64. doi: 10.1007/s00210-006-0130-x [DOI] [PubMed] [Google Scholar]
- 22.Van Liefde I, Van Witzenburg A, Vauquelin G. Multiple beta adrenergic receptor subclasses mediate the l-isoproterenol-induced lipolytic response in rat adipocytes. J Pharmacol Exp Ther. 1992; 262(2): 552–8. [PubMed] [Google Scholar]
- 23.Tanaka E, Yamakawa A, Yamamura M, el Borai N, Ito K, Nakano S, Nakazawa H. Regulation of heat production of brown adipocytes via typical and atypical beta-adrenoceptors in the rat. Jpn J Physiol. 1995; 45(6): 1043–51. doi: 10.2170/jjphysiol.45.1043 [DOI] [PubMed] [Google Scholar]
- 24.Mustafa SM, Yousif M, Cherian A, Oriowo MA. β1- and β3-adrenoceptors mediate relaxation in ovine trachealis smooth muscle. J Auton Pharmacol. 1999; 19(4): 193–9. doi: 10.1046/j.1365-2680.1999.00134.x [DOI] [PubMed] [Google Scholar]
- 25.Koike K, Ichino T, Horinouchi T, Takayanagi I. The β2- and β3-adrenoceptor-mediated relaxation induced by isoprenaline and salbutamol in guinea pig taenia caecum. J Smooth Muscle Res. 1997; 33(3): 99–106. doi: 10.1540/jsmr.33.99 [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto Y, Mori A, Koike K. β-adrenoceptors in the detrusor of guinea pig bladder. J Smooth Muscle Res. 1998; 34(5–6): 233–42. doi: 10.1540/jsmr.34.233 [DOI] [PubMed] [Google Scholar]
- 27.Akimoto Y, Horinouchi T, Tanaka Y, Koike K. The β2- and β3-adrenoceptor-mediated relaxation induced by fenoterol in guinea pig taenia caecum. J Smooth Muscle Res. 2002; 38(4–5): 145–51. doi: 10.1540/jsmr.38.145 [DOI] [PubMed] [Google Scholar]