Abstract
Background
Elevated homocysteine (Hcy) has been reported to be associated with cardiovascular events in atrial fibrillation (AF) patients, while the age-related expression pattern of plasma Hcy in AF remains unknown. The study was aimed to investigate the effect of advanced age on plasma Hcy levels and its association with ischemic stroke in non-valvular AF patients.
Methods
A total of 2562 consecutive patients with non-valvular AF and 535 controls were enrolled and divided into six age groups. Plasma Hcy levels were analyzed among different age groups, and the effect of advanced age on Hcy was investigated.
Results
Plasma Hcy levels did not show any difference among groups aged below 65 years, while it increased sharply in patients aged 65–74 years and aged over 75 years (15.7 ± 4.6 µmol/L, 17.1 ± 4.9 µmol/L, both P < 0.01 compared with the first four age groups). Hcy was much higher in AF patients than in controls at the same age group (all P < 0.05). The proportion of patients with hyperhomocysteinemia increased gradually with age from 32.3%, 29.2%, 31.2%, 32.4%, 45.9%, to 51.4% in six age groups. The concentration of Hcy in AF patients with ischemic stroke increased progressively with age, and was higher than those without stroke at the same age. Logistic regression analysis demonstrated that age 65–74 years [odds ratios (OR): 1.742, 95% confidence interval (CI): 1.223–2.482, P = 0.002] and age ≥ 75 years (OR: 2.637, 95% CI: 1.605–4.335, P < 0.001) were significantly independent predictors of elevated plasma Hcy levels.
Conclusions
Advanced age was significantly associated with elevated Hcy levels, which may provide a possible explanation for the progressive increase in ischemic stroke especially in elderly AF patients.
Keywords: Advanced age, Atrial fibrillation, Homocysteine, Ischemic stroke
1. Introduction
It is well-established in many epidemiological studies that the prevalence and incidence of atrial fibrillation (AF) increase with age. With the progressive aging of the population, it affects about 4% of those aged 65 years and more than 10% of those aged 80 years.[1] In addition, advanced age is an independent risk factor for AF-related ischemic stroke.[2] The importance of age has been highlighted in the CHA2DS2-VASc scoring system in the guidelines.[3] So aging has a great impact on the development and the prognosis of AF patients. Improved understanding of age-related changes will help to the management of AF patients especially for elderly patients.
Homocysteine (Hcy), a sulfur-containing amino acid, is an intermediate by-product during the metabolism of dietary methionine. Recently, elevated plasma Hcy has gained a great deal of interest because of its high prevalence in the general population and close relationship with a number of vascular diseases, including hypertension, coronary artery disease, stroke, venous thromboembolism, etc.[4] And hyperhomocysteinemia is an independent risk factor for ischemic stroke.[5],[6]
In our previous studies, we have found that high Hcy is associated with early recurrence of atrial tachyarrhythmia after catheter ablation in persistent AF patients.[7] In addition, we have reported elevated Hcy increases the risk of left atrial/left atrial appendage thrombus in non-valvular AF patients with low CHA2DS2-VASc score of 0–1.[8] Despite Hcy has an important role in the cardiovascular events in AF patients, the expression pattern of plasma Hcy in AF patients remains unknown. We have a hypothesis that the expression of Hcy may demonstrate an age-related manner in AF patients, and elevated Hcy may have some relationship with previous ischemic stroke in AF patients. Therefore, the present study was to investigate the effect of advanced age on plasma Hcy levels and its association with ischemic stroke in non-valvular AF patients.
2. Methods
2.1. Study population
A total of 2562 patients with non-valvular AF were consecutively enrolled in Beijing Anzhen Hospital between January 2015 and December 2016, the study populations were described before.[9] The definition and classification of AF were according to the published guideline.[3] Paroxysmal AF was defined as recurrent AF that terminates spontaneously within seven days, and persistent AF including persistent AF and long-standing persistent AF was defined as any AF episode lasting longer than seven days or requiring termination by cardioversion. The study complied with the Declaration of Helsinki. The protocol was approved by the institutional ethics review committee, and all patients provided written informed consent.
The exclusion criteria included: secondary AF, organic valvular heart diseases, congenital heart diseases, ischemic stroke within 12 months, significant stenosis (> 70%) of the carotid arteries, deep venous thrombosis, pulmonary embolism, left ventricular thrombus, hyperthyroidism, malignant tumor, pregnant status, severe liver/renal dysfunction and taking drugs that significantly affect Hcy metabolism (ie, VitB6, VitB12, folic acid).
All patients were divided into six age groups, aged below 35 years, aged 35–44 years, aged 45–54 years, aged 55–64 years, aged 65–74 years, and aged over 75 years. These age groups included 105, 198, 555, 849, 612, and 243 AF patients respectively, respectively. The control population comprised 535 subjects from the same geographic area without structural cardiovascular diseases, none of them had abnormal liver or renal function. AF patients and controls were entirely unrelated, and none of the study populations were being treated with folid acid/vitamin B supplementation at the moment of enrollment.
2.2. Blood sampling and assays
Venous blood samples were obtained from the basilic vein the morning after admission (after overnight fast). Blood samples were collected in tubes containing ethylene diamine tetraacetic acid (EDTA) as the anticoagulant, placed in a refrigerator (4–5 °C) within 15–30 min and centrifuged for 10 min within 60 min. The plasma samples were transported at room temperature to our laboratory. All samples were assayed in the laboratory of our hospital, and laboratory personnel were blinded to the clinical status. Laboratory data including Hcy and creatinine levels were collected and analyzed. Plasma Hcy levels were quantitatively determined by cycling enzymatic method (Beckman Coulter AU5400 automatic biochemical analyzer, CA, USA). The baseline glomerular filtration rate (GFR) was evaluated using the Cockcroft-Gault formula. The CHA2DS2-VASc score was calculated for each patient as follows: two points were assigned for a history of stroke or transient ischemic attack, or age ≥ 75 years; and one point was assigned for age 65–74 years, history of hypertension, diabetes mellitus, heart failure, vascular disease, and female sex.
2.3. Ultrasound evaluation
All patients routinely underwent transthoracic echocardiography after admission. A Vivid 9 cardiovascular ultrasound system (GE Healthcare, PA, USA) with a 5 MHz omniplane probe was used to acquire transthoracic echocardiogram images. The left atrial diameter (LAD), left ventricular ejection fraction (LVEF) and other parameters were measured from the parasternal M-mode or 2D images. All measurements were performed and interpreted by experienced physicians who were blind to the study.
2.4. Statistical analysis
All statistical analysis was performed with SPSS19.0 (Chicago, IL, USA). Values were presented as mean ± SD for normally distributed continuous variables, and proportions for categorical variables. The differences between continuous values were assessed using an unpaired 2-tailed t-test for normally distributed continuous variables, a Mann-Whitney test for skewed variables. Categorical variables were compared using χ2 test or Fisher exact test if necessary. Multivariate logistic regression analysis, which included variables with a P value < 0.05 found on univariate analysis, was performed to identify the predictors of Hcy levels. All odds ratios (ORs) were given with the 95% confidence interval (CI). All probability values were 2-sided and a P value < 0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics of patients
A total of 2562 patients with non-valvular AF were included in this study. The baseline clinical and laboratory characteristics of AF patients and controls are showed in Table 1. It was more likely for AF patients to have hypertension, diabetes mellitus, coronary artery disease, chronic heart failure, ischemic stroke, and to take oral anticoagulation drug and antiplatelet drug.
Table 1. Baseline characteristics of the study population.
| Clinical variable | AF patients (n = 2562) | Controls (n = 535) |
| Age, yrs | 59 ± 10 | 58 ± 8 |
| Male sex | 1683 (65.7%) | 355 (66.4%) |
| BMI, kg/m2 | 25 ± 6 | 24 ± 5 |
| Persistent AF | 875 (34.2%) | - |
| Hypertension | 948 (37.0%) | 138 (25.8%)* |
| Diabetes mellitus | 317 (12.4%) | 4 (0.7%)* |
| Dyslipidemia | 605 (23.6%) | 114 (21.3%) |
| Coronary artery disease | 402 (15.7%) | 22 (4.1%)* |
| Chronic heart failure | 79 (3.1%) | 0 (0)* |
| Ischemic stroke | 255 (9.9%) | 0 (0)* |
| Creatinine, µmol/L | 78 ± 12 | 75 ± 10 |
| GFR, mL/min | 86 ± 15 | 92 ± 13 |
| LAD, mm | 39 ± 7 | 36 ± 6 |
| LVEF, % | 56 ± 9 | 58 ± 10 |
| Oral anticoagulation drug | 487 (19.0%) | 0 (0)* |
| Oral antiplatelet drug | 1096 (42.8%) | 86 (16.0%)* |
* P < 0.01. AF: atrial fibrillation; BMI: body mass index; CAD: coronary artery disease; GFR: glomerular filtration rate; LAD: left atrial dimension; LVEF: left ventricular ejection fraction.
3.2. Plasma Hcy levels in AF patients
As showed in Table 2, when classifying AF patients into six groups according to age, it can be seen that plasma Hcy levels did not show any difference among groups aged below 35 years, aged 35–44 years, aged 45–54 years, and aged 55–64 years (13.4 ± 3.5 µmol/L, 13.8 ± 3.2 µmol/L, 13.8 ± 3.3 µmol/L, 13.9 ± 3.3 µmol/L, all P > 0.05), while it increased sharply in those patients aged 65–74 years and aged over 75 years (15.7 ± 4.6 µmol/L, 17.1 ± 4.9 µmol/L, all P < 0.01 when compared with the first four groups). The similar age-related pattern of Hcy levels was observed in controls. Notably, plasma Hcy was much higher in AF patients than in controls at the same age group (Table 2, all P < 0.05). And Hcy was significantly higher in men than in women at the same age group (Table 3, P < 0.05 for aged 45–54 group, P < 0.01 for other five age groups).
Table 2. Plasma Hcy levels stratified by age.
| Age groups, yrs | AF patients |
Controls |
||
| n | Hcy, µmol/L | n | Hcy, µmol/L | |
| < 35 | 105 | 13.4 ± 3.5 | 30 | 12.6 ± 3.3※ |
| 35–44 | 198 | 13.8 ± 3.2 | 52 | 13.0 ± 3.1※ |
| 45–54 | 555 | 13.8 ± 3.3 | 108 | 13.1 ± 3.3※ |
| 55–64 | 849 | 13.9 ± 3.3 | 156 | 13.1 ± 3.4※ |
| 65–74 | 612 | 15.7 ± 4.6* | 121 | 14.8 ± 4.2*※ |
| ≥ 75 | 243 | 17.1 ± 4.9# | 68 | 16.4 ± 4.5#※ |
| All | 2562 | 14.6 ± 3.8 | 535 | 13.9 ± 3.6 |
*P < 0.01, group 65–74 vs. group < 35, 35–44, 45–54, 55–64; #P < 0.01, group ≥ 75 vs. group < 35, 35–44, 45–54, 55–64. ※P < 0.05, AF vs. control at the same age group. AF: atrial fibrillation; Hcy: homocysteine.
Table 3. Plasma Hcy levels stratified by age and sex in AF patients.
| Age groups, yrs | Men |
Women |
||
| n | Hcy, µmol/L | n | Hcy, µmol/L | |
| < 35 | 90 (85.7%) | 13.7 ± 3.6 | 15 (14.3%) | 11.9 ± 2.7* |
| 35–44 | 162 (81.8%) | 14.1 ± 3.2 | 36 (18.2%) | 12.5 ± 2.9* |
| 45–54 | 477 (85.9%) | 13.9 ± 3.3 | 78 (14.1%) | 13.0 ± 3.0※ |
| 55–64 | 522 (61.5%) | 14.4 ± 3.4 | 327 (38.5%) | 13.1 ± 3.1* |
| 65–74 | 300 (49.0%) | 17.3 ± 4.8 | 312 (51.0%) | 14.2 ± 3.7* |
| ≥ 75 | 132 (54.3%) | 18.6 ± 5.2 | 111 (45.7%) | 15.3 ± 4.5* |
| All | 1683 (65.7%) | 15.1 ± 4.1 | 879 (34.3%) | 13.7 ± 3.6 |
*P < 0.01, women vs. men at the same age group; ※P < 0.05, women vs. men at the same age group. AF: atrial fibrillation; Hcy: homocysteine.
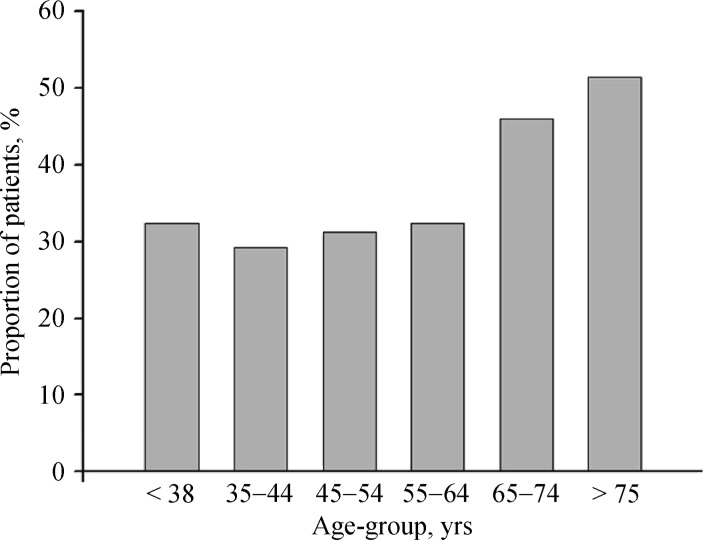
Hyperhomocysteinemia was always defined as the concentration of plasma Hcy above 15 µmol/L. In our study population of non-valvular AF patients, the proportion with hyperhomocysteinemia increased gradually with age from 32.3%, 29.2%, 31.2%, 32.4%, 45.9%, to 51.4% in six age groups (Figure 1), demonstrating a much higher proportion in elderly persons over 65 years.
Figure 1. Age distribution of increased plasma Hcy (≥15 µmol/L) among AF patients.
AF: atrial fibrillation; Hcy: homocysteine.
3.3. Association of Hcy with stroke in AF patients
Table 4 shows plasma Hcy levels in AF patients according to stroke and age. The concentration of Hcy in AF patients with ischemic stroke increased progressively with age (-, 15.1 ± 4.3 µmol/L, 15.7 ± 4.9 µmol/L, 16.3 ± 4.1 µmol/L, 16.8 ± 5.5 µmol/L, 17.6 ± 5.7 µmol/L), while in patients without stroke, it remained the same level in patients aged below 65 years, and then increased sharply in those aged 65–74 years and aged over 75 years (15.5 ± 4.2 µmol/L, 17.0 ± 4.8 µmol/L, both P < 0.01 when compared with the first four groups).
Table 4. Plasma Hcy levels according to stroke and age catagories.
| < 35 |
35–44 |
45–54 |
55–64 |
65–74 |
≥ 75 |
|||||||
| n | Hcy, µmol/L | n | Hcy, µmol/L | n | Hcy, µmol/L | n | Hcy, µmol/L | n | Hcy, µmol/L | n | Hcy, µmol/L | |
| Stroke (+) | 0 (0) | - | 6 (3.03%) | 15.1 ± 4.3 | 36 (6.49%) | 15.7 ± 4.9 | 90 (10.6%) | 16.3 ± 4.1 | 72 (11.8%) | 16.8 ± 5.5 | 51 (20.9%) | 17.6 ± 5.7 |
| Stroke (−) | 105 (100%) | 13.4 ± 3.5 | 192 (96.97%) | 13.8 ± 3.4* | 519 (93.51%) | 13.7 ± 3.3* | 759 (89.4%) | 13.6 ± 3.3* | 540 (88.2%) | 15.5 ± 4.2* | 192 (79.1%) | 17.0 ± 4.8 |
*P < 0.01, group stroke (+) vs. group stroke (−) at the same age. Hcy: homocysteine.
As showed in Table 4, compared with those without stroke at the same age (except group aged < 35 years without stroke), patients with the history of ischemic stroke had higher levels of Hcy (for age groups 35–44, 45–54, 55–64, 65–74, all P < 0.01, for age group > 75, P = 0.359). What's more, there was a prominent increase with age in the prevalence of previous ischemic stroke, from 0, 3.03%, 6.49%, 10.6%, 11.8%, to 20.9% in these six age groups (Table 4).
3.4. Association of Hcy with CHA2DS2-VASc score
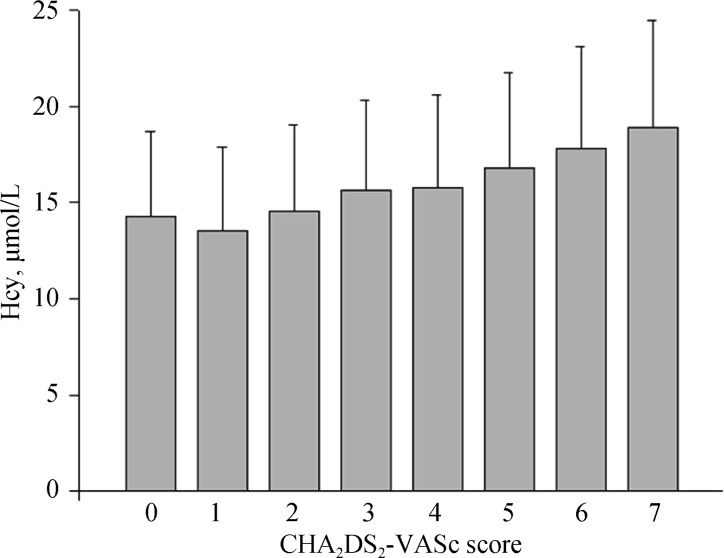
Figure 2 shows the association of Hcy with CHA2DS2-VASc score. There was a gradual increase of Hcy levels among the patients with CHA2DS2-VASc scores of 0–7, with the value of 14.3 ± 4.4 µmol/L, 13.6 ± 4.3 µmol/L, 14.6 ± 4.5 µmol/L, 15.6 ± 4.7 µmol/L, 15.8 ± 4.8 µmol/L, 16.8 ± 5.0 µmol/L, 17.8 ± 5.3 µmol/L, 18.9 ± 5.6 µmol/L, respectively.
Figure 2. Hcy levels in different CHA2DS2-VASc score categories.
Hcy: homocysteine.
3.5. Clinical factors associated with elevated Hcy levels
Several clinical factors were tested to predict plasma Hcy levels (≥ 15 µmol/L) using the logistic regression univariate and multivariate analysis (Table 5). Univariate analysis demonstrated that male sex (P = 0.000), age 65–74 years (P = 0.008), age ≥ 75 years (P = 0.001), and coronary artery disease (P = 0.001) were associated with elevated plasma Hcy levels. Hypertension, diabetes mellitus, stroke, and chronic heart failure had no correlation with elevated Hcy. In multivariate analysis, male sex (OR: 0.464, 95% CI: 0.334–0.645, P = 0.000), age 65–74 years (OR: 1.742, 95% CI: 1.223–2.482, P = 0.002), age ≥ 75 years (OR: 2.637, 95% CI: 1.605–4.335, P < 0.001), and coronary artery disease (OR: 1.655, 95% CI: 1.064–2.574, P = 0.026) remained independent predictors of elevated Hcy levels (Table 5). Consequently, advanced age was significantly associated with elevated Hcy levels in AF patients.
Table 5. Clinical factors associated with Hcy levels (≥15 µmol/L) by logistic regression analysis.
| Variable | Univariate analysis |
Multivariate analysis |
||||
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Sex | 0.544 | 0.399–0.742 | 0.000 | 0.464 | 0.334–0.645 | 0.000 |
| Age 65–74 yrs | 1.451 | 1.070–1.954 | 0.008 | 1.742 | 1.223–2.482 | 0.002 |
| Age ≥ 75 yrs | 2.162 | 1.364–3.428 | 0.001 | 2.637 | 1.605–4.335 | 0.000 |
| Hypertension | 1.258 | 0.949–1.668 | 0.110 | |||
| Diabetes mellitus | 0.762 | 0.515–1.128 | 0.175 | |||
| Coronary artery disease | 2.074 | 1.359–3.163 | 0.001 | 1.655 | 1.064–2.574 | 0.026 |
| Stroke | 1.492 | 0.947–2.349 | 0.084 | |||
| Chronic heart failure | 1.668 | 0.637–4.369 | 0.298 | |||
CI: confidence interval; Hcy: homocysteine; OR: odds ratios.
4. Discussion
In this study, we for the first time found that plasma Hcy levels increased sharply in elderly AF patients, which was parallel with the increased prevalence of ischemic stroke in patients over 65 years. And logistic regression analysis demonstrated that advanced age was significantly associated with elevated Hcy levels in AF patients.
As the most common sustained cardiac arrhythmia in clinical practice, AF has become an emerging socioeconomic burden globally. Advanced age is a most important unmodifiable risk factor for AF and AF-related adverse comorbidities including ischemic stroke.[10],[11] Aging profoundly influences the development and prognosis of AF patients.[12] Improved understanding of age-related changes will help to the management of AF patients especially for elderly patients. Identification of novel modifiable risk factors will be essential to reduce morbidity and result in more effective stroke prevention in AF patients.
Moderately elevated plasma Hcy levels are widely prevalent in the general population and are associated with an increased risk of many vascular diseases, independent of the conventional cardiovascular risk factors.[13] The possible mechanisms involving in biological damage to vascular endothelial cells include by generating oxidative stress, decreasing NO bioavailability and inducing inflammatory response.[14],[15] Studies also have reported that hyperhomocysteinemia can activate matrix metalloproteinase, disrupts connexin-43 and increase collagen/elastin ratio, leading to a substrate for arrhythmogenesis and sudden cardiac death.[16],[17] Furthermore, elevated Hcy confers an independent risk for the thromboembolic and cardiovascular events in AF patients in our recent studies and other researchers' findings.[7],[8],[18]–[20] A high level of Hcy is pathophysiologically linked with left atrial enlargement and ion channel remodeling, demonstrating the pro-arrhythmic effects on developing AF.[21]–[23]
It is now highly recognized that supplementation of diet with folic acid and vitamin B can lower Hcy levels. Several studies and meta-analysis, including the HOPE-2 trial and the VITATOPS trial, have showed a reduced risk of stroke by means of vitamin supplementation.[24] Therefore, plasma Hcy may represent a potentially modifiable risk factor for AF and AF-related ischemic stroke.
The relationship between advanced age and Hcy in non-valvular AF patients remained unknown. In the present study, we found that Hcy levels did not show any difference among four groups aged below 65 years, then increased significantly in those patients aged 65–74 years and aged over 75 years. Interestingly, the expression pattern of plasma Hcy showed a sharp increase in patients aged over 65 years, not increasing linearly with age. And plasma Hcy was much higher in men than in women at the same age group. It may be attributable to differences in muscle mass and sex hormone status.[25]–[27] We found the proportion with hyperhomocysteinemia in AF patients demonstrated an age-dependent manner and a much higher proportion in elderly persons, similar to Spence's findings on age distribution of increased plasma Hcy among patients referred to vascular prevention clinics.[28]
As expected, we found the concentration of Hcy in AF patients who had ischemic stroke increased progressively with age, and was higher than those who had not stroke at the same age. It was in line with Friedman's observations,[29] however, they did not examine age-related changes of Hcy among different age groups in detail. And the prevalence of ischemic stroke gradually increased from 3.03% in AF patients aged 35–44 years to 20.9% in those aged > 75 years, which were consistent with the findings in Framingham study,[30] indicating more particular medical intervention should be paid to elderly AF patients to avoid the high risks for stroke. The prevalence of stroke in AF patients paralleled with the steep increase in Hcy levels especially in those aged over 65 years. Taken together, these results suggested that elevated Hcy may provide a possible explanation for the progressive increase in ischemic stroke especially in elderly AF patients.
We used variables from the components of the CHA2DS2-VASc score to enter the logistic regression models to predict the hyperhomocysteinemia. It demonstrated that age 65–74 years and age ≥ 75 years were significantly associated with elevated Hcy, indicating advanced age was a strong independent predictor of high Hcy levels in AF patients.
4.1. Clinical implications and limitations
Elevated Hcy, as an independent risk factor even a possible causal metabolic biomarker for AF and AF-related ischemic stroke, may have profound clinical implications, as plasma Hcy levels can be modified simply by supplementation of diet with folic acid and vitamin B. Until now, several clinical trials have got promising achievements as significantly reduced risk of stroke.[31],[32] Unfortunately, some efforts to treat hyperhomocysteinaemia using vitamin supplementation have yielded little clinical benefit. It may lie in a number of complex reasons in the real world, including dietary factors, renal function, the baseline levels of folic acid, vitamin B6 and B12, the dose and duration of B vitamins, etc.[24],[33]
In the present study, these age-related changes in Hcy levels and the the prevalence of stroke in AF patients provided therapeutic evidence that particular care should be given to those elderly patients for its high stroke risk overall. Surely, further randomized clinical trials are needed to clarify whether Hcy-lowering treatments result in reduced stroke in non-valvular AF patients.[34]
Our study presents several limitations. Firstly, the results of this observational study were a single-center experience and should be cautiously interpreted. The conclusion may be more precise if it is prospectively validated in an external AF patient population. Secondly, the concentration of folic acid and vitamin B12 were not measured in this study, which may influence plasma Hcy levels. Finally, MTHFR C677T polymorphism was not assessed in the present study.
4.2. Conclusions
In conclusion, this study provided important insights into the correlation between advanced age, plasma Hcy levels and ischemic stroke in AF patients. Advanced age was significantly associated with elevated Hcy levels, which may provide a possible explanation for the progressive increase in ischemic stroke especially in elderly AF patients. Particular care should be given to those elderly patients for its high Hcy levels and high stroke risk overall.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81670294, 81200141); Beijing Novel Program (No. 2011081, Z131103000413116).
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee: Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 4.Han L, Wu Q, Wang C, et al. Homocysteine, ischemic stroke, and coronary heart disease in hypertensive patients: a population-based, prospective cohort study. Stroke. 2015;46:1777–1786. doi: 10.1161/STROKEAHA.115.009111. [DOI] [PubMed] [Google Scholar]
- 5.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke. JAMA. 2002;288:2015–2222. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 6.Shi Z, Guan Y, Huo YR, et al. Elevated total homocysteine levels in acute ischemic stroke are associated with long-term mortality. Stroke. 2015;46:2419–2425. doi: 10.1161/STROKEAHA.115.009136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao Y, Yao W, Bai R, et al. Plasma homocysteine levels predict early recurrence after catheter ablation of persistent atrial fibrillation. Europace. 2017;19:66–71. doi: 10.1093/europace/euw081. [DOI] [PubMed] [Google Scholar]
- 8.Yao Y, Shang MS, Gao LJ, et al. Elevated homocysteine increases the risk of left atrial/left atrial appendage thrombus in non-valvular atrial fibrillation with low CHA2DS2-VASc score. Europace. doi: 10.1093/europace/eux189. Published Online First. 16 June 2017. [DOI] [PubMed] [Google Scholar]
- 9.Du X, Ma C, Wu J, et al. Rationale and design of the Chinese Atrial Fibrillation Registry Study. BMC Cardiovasc Disord. 2016;16:130. doi: 10.1186/s12872-016-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marinigh R, Lip GYH, Fiotti N, et al. Age as a risk factor for stroke in atrial fibrillation patients. J Am Coll Cardiol. 2010;56:827–837. doi: 10.1016/j.jacc.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 11.van Walraven C, Hart RG, Connolly S, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation: the stroke prevenion investgators. Stroke. 2009;40:1410–1416. doi: 10.1161/STROKEAHA.108.526988. [DOI] [PubMed] [Google Scholar]
- 12.Wolff A, Shantsila E, Lip GY, et al. Impact of advanced age on management and prognosis in atrial fibrillation: insights from a population-based study in general practice. Age Ageing. 2015;44:874–878. doi: 10.1093/ageing/afv071. [DOI] [PubMed] [Google Scholar]
- 13.Wang TJ, Gona P, Larson MG, et al. Multiple Biomarkers for the Prediction of First Major Cardiovascular Events and Death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 14.Steed MM, Tyagi SC. Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxid Redox Signal. 2011;15:1927–1943. doi: 10.1089/ars.2010.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lentz SR. Mechanisms of homocysteine-induced atherothrombosis. J Thromb Haemost. 2005;3:1646–1654. doi: 10.1111/j.1538-7836.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- 16.Maldonado C, Soni CV, Todnem ND, et al. Hyperhomocysteinemia and sudden cardiac death: potential arrhythmogenic mechanisms. Curr Vasc Pharmacol. 2010;8:64–74. doi: 10.2174/157016110790226552. [DOI] [PubMed] [Google Scholar]
- 17.Moshal KS, Metreveli N, Frank I, et al. Mitochondrial MMP activation, dysfunction and arrhythmogenesis in hyperhomocysteinemia. Curr Vasc Pharmacol. 2008;6:84–92. doi: 10.2174/157016108783955301. [DOI] [PubMed] [Google Scholar]
- 18.Marcucci R, Betti I, Cecchi E, et al. Hyperhomocysteinemia and vitamin B6 deficiency: new risk markers for nonvalvular atrial fibrillation? Am Heart J. 2004;148:456–461. doi: 10.1016/j.ahj.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Naji F, Suran D, Kanic V, et al. High homocysteine levels predict the recurrence of atrial fibrillation after successful electrical cardioversion. Int Heart J. 2010;51:30–33. doi: 10.1536/ihj.51.30. [DOI] [PubMed] [Google Scholar]
- 20.Ay H, Arsava EM, Tokgozoglu SL, et al. Hyperhomocysteinemia is associated with the presence of left atrial thrombus in stroke patients with nonvalvular atrial fibrillation. Stroke. 2003;34:909–912. doi: 10.1161/01.STR.0000060202.63475.BA. [DOI] [PubMed] [Google Scholar]
- 21.Cai B, Shan L, Gong D, et al. Homocysteine modulates sodium channel currents in human atrial myocytes. Toxicology. 2009;256:201–206. doi: 10.1016/j.tox.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Law Phillip, Kharche Sanjay, Stott Jonathan, et al. Effects of elevated homocysteine hormone on electrical activity in the human atrium: a simulation study. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:3936–3939. doi: 10.1109/IEMBS.2009.5333530. [DOI] [PubMed] [Google Scholar]
- 23.Shimano M, Inden Y, Tsuji Y, et al. Circulating homocysteine levels in patients with radiofrequency catheter ablation for atrial fibrillation. Europace. 2008;10:961–966. doi: 10.1093/europace/eun140. [DOI] [PubMed] [Google Scholar]
- 24.Spence JD, Stampfer MJ. Understanding the complexity of homocysteine lowering with vitamins: the potential role of subgroup analyses. JAMA. 2011;306:2610–2611. doi: 10.1001/jama.2011.1834. [DOI] [PubMed] [Google Scholar]
- 25.Nygård O, Refsum H, Ueland PM, et al. Major lifestyle determinants of plasma total homocysteine distribution: the Hordaland Homocysteine Study. Am J Clin Nutr. 1998;67:263–270. doi: 10.1093/ajcn/67.2.263. [DOI] [PubMed] [Google Scholar]
- 26.Andersson A, Brattstrom L, Israelsson B, et al. Plasma homocysteine before and after methionine loading with regard to age, gender and menopausal status. Eur J Clin Invest. 1992;22:79–87. doi: 10.1111/j.1365-2362.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 27.Verhoef P, Meleady R, Daly LE, et al. Homocysteine, vitamin status and risk of vascular disease; effects of gender and menopausalstatus. European COMAC Group. Eur Heart J. 1999;20:1234–1244. doi: 10.1053/euhj.1999.1522. [DOI] [PubMed] [Google Scholar]
- 28.Spence D. Mechanisms of thrombogenesis in atrial fibrillation. Lancet. 2009;373:1006. doi: 10.1016/S0140-6736(09)60604-8. [DOI] [PubMed] [Google Scholar]
- 29.Friedman HS. Serum homocysteine and stroke in atrial fibrillation. Ann Intern Med. 2001;134:253–254. doi: 10.7326/0003-4819-134-3-200102060-00027. [DOI] [PubMed] [Google Scholar]
- 30.Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–1764. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 31.Antoniades C, Antonopoulos AS, Tousoulis D, et al. Homocysteine and coronary atherosclerosis: from folate fortification to the recent clinical trials. Eur Heart J. 2009;30:6–15. doi: 10.1093/eurheartj/ehn515. [DOI] [PubMed] [Google Scholar]
- 32.Spence JD. Homocysteine-lowering therapy: a role in stroke prevention? Lancet Neurol. 2007;6:830–838. doi: 10.1016/S1474-4422(07)70219-3. [DOI] [PubMed] [Google Scholar]
- 33.Rimm EB, Stampfer MJ. Folate and cardiovascular disease: one size does not fit all. Lancet. 2011;378:544–546. doi: 10.1016/S0140-6736(11)61057-X. [DOI] [PubMed] [Google Scholar]
- 34.Spence JD. Homocysteine lowering for stroke prevention: unravelling the complexity of the evidence. Int J Stroke. 2016;11:744–747. doi: 10.1177/1747493016662038. [DOI] [PubMed] [Google Scholar]