Abstract
Congenital left ventricular aneurysm or diverticulum are rare cardiac malformations described in 809 cases since the first description in 1816, being associated with other cardiac, vascular or thoraco-abdominal abnormalities in about 70%. It appears to be a developmental anomaly, starting in the 4th embryonic week. In an experimental study, targeted knockdown of cardiac troponin T in the chick was performed at day 3, after the heart tube has formed. Morpholino treatment of gene TNNT2 at this stage led to the development of left ventricular diverticula (LVD) in the primitive left ventricular wall. Diagnosis of left ventricular aneurysms (LVA)/LVD can be made after exclusion of coronary artery disease, local or systemic inflammation or traumatic causes as well as cardiomyopathies. Clinically, most of LVA and LVD are asymptomatic or may cause systemic embolization, congestive heart failure, valvular regurgitation, ventricular wall rupture, ventricular tachycardia or sudden cardiac death. Diagnosis is established by imaging studies (echocardiography, magnetic resonance imaging or left ventricular angiography) visualizing the structural changes and accompanying abnormalities. Mode of treatment has to be individually tailored and depends on clinical presentation, accompanying abnormalities and possible complications, options include surgical resection (especially in symptomatic patients), anticoagulation after systemic embolization, radiofrequency ablation or implantation of an implantable cardioverter defibrillator (ICD) in case of symptomatic ventricular tachycardias, and occasionally combined with class I- or III-antiarrhythmic drugs. Cardiac death occurs usually in childhood, is significantly more frequent in LVA patients and caused by congestive heart failure in most of the cases, whereas patients diagnosed with LVD died more frequently from rupture of the LVD.
Keywords: Aneurysm, Congenital, Diverticulum, Left ventricular, Prognosis, Therapy
1. Introduction
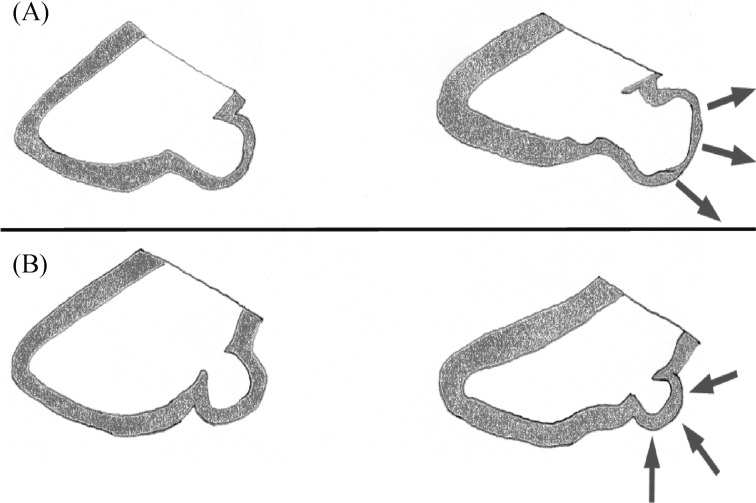
Congenital left ventricular aneurysms (LVA) and diverticula (LVD) are rare findings particularly when first diagnosed in the adulthood. They can be seen in all chambers of the heart, most frequently in the left ventricle and only occasionally in the right ventricle or in both ventricles (numerical ratio approximately 8:1:1).[1] LVA look very similar to acquired aneurysms with a wide connection to the left ventricle (ratio of the connection to the body of the anomaly > 1).[2],[3] In contrast LVD is characterized by a finger or hook like appendix emerging from the wall of the left ventricle, contracting in synchrony with the corresponding chamber.[2],[3] The ratio of the connection to the left ventricular cavity compared with the maximum diameter of the body of the anomaly is < 1 (Figure 1).
Figure 1. Schematic depicting.
Schematic depicting left ventricular aneurysm (A) and left ventricular diverticulum (B) in diastole (left panel) and corresponding structural deformation during systole (right panel).
Depending on the amount of myocardial fibers involved, aneurysms can be a- or dyskinetic or show an almost normal contractility with complete emptying in the systole with all ranges of contractility in between.[2],[3] The dimensions of the described diverticula or aneurysms ranges from 0.5 cm of diameter up to a size of 8 x 9 cm.[2],[4] Most frequently, location of LVA is the LV-apex (28%) and the perivalvular area (close to the mitral valve [= sub-mitral]; 49%) and LVD are found at the LV-apex (57%).[5],[6] Other locations include the infero-septal, antero-lateral, postero-lateral and postero-basal wall or multiple locations.[5],[6]
2. Epidemiology and classification
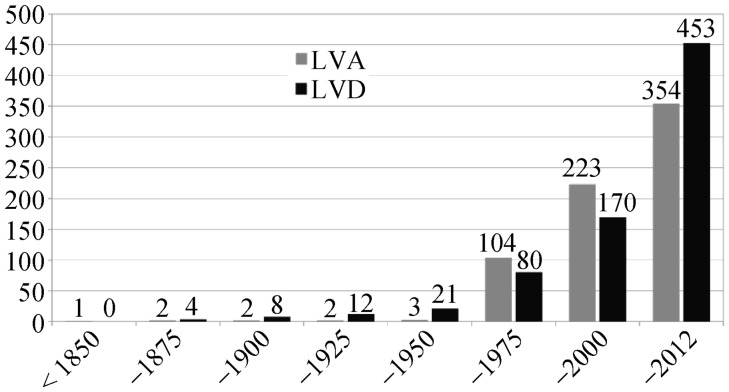
Reliable data from prospective studies regarding the epidemiology of LVA/LVD are not available. A retrospective echocardiographic study described 16 LVA/LVD out of 43,000 consecutive adult patients, resulting in a prevalence of 0.04%.[7] However, this rather low prevalence might be attributed to the fact that echocardiography underestimate the true number of LVA/LVD due to difficult visualization of the left ventricular apex, the location of almost 60% of all LVD. Another study on 12,924 consecutive autopsies in children found 750 cases with congenital cardiac defects (5.8%), and the diagnosis of a LVD was made in 3 cases (prevalence of 0.02%).[8] A more recent publication demonstrated a prevalence of 0.76% in 12,271 consecutive adult patients undergoing coronary angiography.[5] 809 cases of congenital left ventricular aneurysm or diverticulum are reported in the literature until the year 2012 (354 LVA, 453 LVD),[6] which were mostly diagnosed in childhood. These malformations were initially described in 1816 (Germany) and 1837 (England).[9],[10] First reports of an operative resection of a left ventricular diverticulum date from the year 1912.[11] The first successful resection of an apical left ventricular diverticulum in a newborn child[12] was documented in 1944.
A uniform classification system to compare the clinical presentation, morphology, treatment and prognosis would be desirable, but has not been established yet.
Logen, et al.[13] suggested two groups: Group 1 describes cases, in which the diverticulum reaches below the diaphragm and is palpable as a pulsating mass in the area of the anterior abdominal wall (usually diagnosed in childhood); Group 2 includes forms which remain above the diaphragm.
Another classification system[14] contains four groups: left ventricular aneurysm; left ventricular diverticulum; intra-abdominal; apical diverticula with “midline defects” (see below); intra-thoracal; diverticula without midline defects.
A more recent publication proposed a new classification system, integrating many types of left ventricular outpouchings (LVO) [LVO: accessory LV, LVA, LVD, left ventricular accessory chamber, and double-chambered LV (DCLV)]. Depending on the normal elliptical shape of the LV, the wall thickness, and the regional wall motion of the LVO the lesions are classified into DCLV, LVO Type I and Type II. Type II is further sub-classified into Type IIa, IIb, and IIc.[15]
3. Embryogenic and genetic hypotheses
The development of LVA/LVD begins as early as the 4th embryonic week.[6],[16] They form within the endocardial tube and develop initially at the expense of the cardiac jelly. Later they increase the ventricular volume by penetrating the myocardium and inserting additional intertrabecular spaces.[6],[16] The development of a congenital diverticulum can be explained by a partial stop in the development of the embryonic ventricle at this stage.[4],[16] Between the 14th and 18th day of the embryonic life, failure of the differentiation of the primitive intra-embryonic mesoderm into its splanchnic and somatic layers occurs.[17],[18],[19] This explains the frequent location of the LVD at the left ventricular apex and some of the accompanying abdominal wall defects.[20],[21]
More recently England, et al.[22] demonstrated that morpholino treatment of TNNT2 [a gene encoding cardiac troponin T (cTNT)] in chicken embryos resulted in development of LVD and abnormal atrial and ventricular septal growth.[22] The effect of cTNT knockdown on nitric oxide synthase 3 (NOS3, the shear stress responsive gene) and T-box transcription factor 3 (TBX3, the conduction gene) was also demonstrated in this study.[22] These findings suggests a novel role for mutations of structural sarcomeric proteins in the development of LVD and can explain the frequent association with atrial septal defect (ASD), ventricular septal defect (VSD), and ECG-abnormalities.[6],[23],[24]
4. Association with other abnormalities
Congenital left ventricular aneurysms and diverticula were associated with numerous other congenital anomalies, including those of the heart itself, or those of vascular or extra-cardiac structures.[2],[3],[6] Interestingly, the prevalence of associated cardiac and/or vascular (34% versus 11%) and extra-cardiac (33% versus 3%) anomalies was significantly higher in LVD patients. These findings suggests a different etiology and pathogenesis for LVA or LVD during the embryonic development.[6]
The most frequent associated cardiac abnormalities were in descending order ventricular septal defect, coronary anomalies, and atrial septal defect.[6]
Case reports point out numerous further associations of malformations with congenital left ventricular aneurysm and diverticulum (Table 1). Due to a change in the geometry of the aortic or mitral annulus, left ventricular aneurysms or diverticula of the sub-valvular area are very frequently associated with aortic/mitral regurgitation, occasionally in combination with mitral valve prolapse (frequently of the posterior mitral leaflet).
Table 1. Associated cardiac and vascular anomalies in patients with LVA/LVD.
| LVA | LVD | |
| Cardiac anomalies | 87 (100%) | 308 (100%) |
| Aortic valve | 9 (10.3%) | 12 (3.9%) |
| Bicuspid valve | 5 (5.8%) | 6 (2%) |
| Sub-valvular stenosis | 2 (2.3%) | 3 (1%) |
| Valvular stenosis | 2 (2.3%) | 3 (1%) |
| Mitral valve | 12 (13.8%) | 9 (2.9%) |
| Aberrant/missing papillary muscles | 5 (5.8%) | 3 (1%) |
| Mitral valve prolapse | 2 (2.3%) | 2 (< 1%) |
| Mitral cleft | 4 (4.6%) | 0 (0%) |
| Mitral stenosis | 0 (0%) | 3 (1%) |
| Dysplastic mitral valve | 1 (1.2%) | 0 (0%) |
| Double-orifice mitral valve | 0 (0%) | 1 (< 1%) |
| Pulmonary valve | 3 (3.5%) | 27 (8.8%) |
| Valvular pulmonary stenosis | 3 (3.5%) | 23 (7.5%) |
| Sub-valvular pulmonary stenosis | 0 (0%) | 3 (1%) |
| Bicuspid pulmonary valve | 0 (0%) | 1 (< 1%) |
| Tricuspid valve | 2 (2.3%) | 6 (2%) |
| Tricuspid atresia | 0 (0%) | 5 (1.6%) |
| Tricuspid dysplasia | 2 (2.3%) | 0 (0%) |
| Tricuspid stenosis | 0 (0%) | 1 (< 1%) |
| Anomalies with intra-cardiac shunting | 26 (29.9%) | 127 (41.2%) |
| Ventricular septal defect | 10 (11.5%) | 68 (22.1%) |
| Atrial septal defect | 8 (9.2%) | 25 (8.1%) |
| Tetralogy of Fallot | 3 (3.5%) | 15 (4.9%) |
| Patent ductus arteriosus | 4 (4.6%) | 11 (3.6%) |
| Pentalogy of Fallot | 0 (0%) | 4 (1.3%) |
| Persistent foramen ovale | 1 (1.2%) | 3 (1%) |
| Unroofed coronary sinus | 0 (0%) | 1 (< 1%) |
| Miscellaneous cardiac anomalies | 35 (40.2%) | 127 (41.2%) |
| Coronary anomalies | 31 (35.6%) | 39 (12.7%) |
| Cardiac dextrorotation/-position | 1 (1.2%) | 51 (16.6%) |
| Pericardial defect | 0 (0%) | 21 (6.8%) |
| Hypoplasia of the right ventricle | 2 (2.3%) | 3 (1%) |
| Divided atrium | 0 (0%) | 3 (1%) |
| Double-outlet right ventricle | 0 (0%) | 3 (1%) |
| Mesocardium | 0 (0%) | 2 (< 1%) |
| Ectopia cordis | 0 (0%) | 2 (< 1%) |
| Intra-pericardial cyst | 0 (0%) | 1 (< 1%) |
| Atrioventricular canal | 1 (1.2%) | 0 (0%) |
| Endocardial cushion defect | 0 (0%) | 1 (< 1%) |
| Sinusoidal channels between LV and coronary sinus | 0 (0%) | 1 (< 1%) |
| Vascular anomalies | 10 (100%) | 39 (100%) |
| Coarctation of the aorta | 4 (40%) | 7 (17.9%) |
| Persistent left superior vena cava | 1 (10%) | 8 (20.5%) |
| Hypoplasia of pulmonary artery | 1 (10%) | 5 (12.8%) |
| Anomalous course of the ascending aorta | 0 (0%) | 4 (10.3%) |
| Partial anomalous pulmonary venous return | 0 (0%) | 2 (5.1%) |
| Hypoplasia of the aorta | 0 (0%) | 2 (5.1%) |
| Transposition of the great arteries | 0 (0%) | 2 (5.1%) |
| Absence of the brachiocephalic trunk | 0 (0%) | 2 (5.1%) |
| Atresia of pulmonary artery | 2 (20%) | 0 (0%) |
| Occlusion of the left superior vena cava | 0 (0%) | 2 (5.1%) |
| Atresia of the internal carotid artery | 2 (20%) | 0 (0%) |
| Single pulmonary artery | 0 (0%) | 1 (2.6%) |
| Left carotid artery from the brachiocephalic trunk | 0 (0%) | 1 (2.6%) |
| Right subclavian artery from the descending aorta | 0 (0%) | 1 (2.6%) |
| Absence of the right subclavian artery | 0 (0%) | 1 (2.6%) |
| Sinus of Valsalva aneurysm | 0 (0%) | 1 (2.6%) |
LVA: left ventricular aneurysms; LVD: left ventricular diverticula.
Frequent vascular abnormalities included aortic coarctation, persistent left superior vena cava, and hypoplasia of the pulmonary artery.[6] (Table 1)
Extra-cardiac anomalies were frequently encountered in LVD patients and are only occasionally observed in LVA patients.[6] The most frequent extra-cardiac anomalies in LVD patients included alterations of the thoraco-abdominal wall (summarized as “midline defects”) such as diaphragmatic defects, umbilical hernias or omphalocele, and abnormalities of the caudal sternum. (Table 2)
Table 2. Associated non-cardiac/non-vascular anomalies in patients with LVA/LVD.
| LVA | LVD | |
| Non-cardiac/non-vascular anomalies | 21 (100%) | 251 (100%) |
| Cranial anomalies | 3 (14.3%) | 15 (6%) |
| Cranio-facial dysmorphism | 0 (0%) | 2 (< 1%) |
| Cleft palate | 0 (0%) | 2 (< 1%) |
| Aplasia of the os frontale and parietale | 0 (0%) | 1 (< 1%) |
| Agenesis of the eyes and nose | 0 (0%) | 1 (< 1%) |
| Transverse facial cleft | 0 (0%) | 1 (< 1%) |
| Hypoplasia of the tongue and mandibular arch | 0 (0%) | 1 (< 1%) |
| Mono-ventricular brain | 0 (0%) | 1 (< 1%) |
| Hypoplasia of the cerebellum | 0 (0%) | 1 (< 1%) |
| Agenesis of the corpus callosum | 0 (0%) | 1 (< 1%) |
| Tuberous sclerosis | 0 (0%) | 1 (< 1%) |
| High arched palate | 0 (0%) | 1 (< 1%) |
| Anencephaly | 0 (0%) | 1 (< 1%) |
| Cleft lip | 0 (0%) | 1 (< 1%) |
| Encephalocele | 1 (4.8%) | 0 (0%) |
| Hydrocephalus | 1 (4.8%) | 0 (0%) |
| Malformation of the external ear | 1 (4.8%) | 0 (0%) |
| Thoracic anomalies | 2 (9.5%) | 47 (18.7%) |
| Sternal defect | 2 (9.5%) | 38 (15.1%) |
| Chest deformity | 0 (0%) | 5 (2%) |
| Thoracic wall defect | 0 (0%) | 2 (< 1%) |
| Dysplastic nipple | 0 (0%) | 1 (< 1%) |
| Lung dysplasia | 0 (0%) | 1 (< 1%) |
| Abdominal anomalies | 10 (47.6%) | 182 (72.5%) |
| Diaphragmatic defect | 4 (19.1%) | 58 (23.1%) |
| Omphalocele | 0 (0%) | 39 (15.5%) |
| Abdominal defect | 2 (9.5%) | 35 (13.9%) |
| Umbilical hernia | 1 (4.8%) | 28 (11.2%) |
| Separation of the straight abdominal muscles | 1 (4.8%) | 14 (5.6%) |
| Pyloric stenosis | 1 (4.8%) | 2 (< 1%) |
| Obstruction of the utero-pelvic junction | 1 (4.8%) | 1 (< 1%) |
| Ectopic umbilicus | 0 (0%) | 1 (< 1%) |
| Fibrolipoma of the kidney | 0 (0%) | 1 (< 1%) |
| Transposition of the colon | 0 (0%) | 1 (< 1%) |
| Ectopic cardiac tissue in the abdominal wall | 0 (0%) | 1 (< 1%) |
| Diaphragmatic diverticulum | 0 (0%) | 1 (< 1%) |
| Miscellaneous anomalies | 6 (28.6%) | 7 (2.8%) |
| Scoliosis | 2 (9.5%) | 1 (< 1%) |
| Polydaktylie | 0 (0%) | 2 (< 1%) |
| Marfan syndrome | 2 (9.5%) | 0 (0%) |
| Syndactylia | 0 (0%) | 1 (< 1%) |
| Tibial aplasia | 0 (0%) | 1 (< 1%) |
| Hip dysplasia | 0 (0%) | 1 (< 1%) |
| Skeletal malformations | 0 (0%) | 1 (< 1%) |
| Niemann-Pick disease Type B | 1 (4.8%) | 0 (0%) |
| Neurofibromatosis | 1 (4.8%) | 0 (0%) |
LVA: left ventricular aneurysms; LVD: left ventricular diverticula.
5. Clinical presentation
LVA were more frequently reported from Africa and America, whereas LVD were more frequently reported from Asia and Europe.[6] Until 1950 only 24 patients with LVA/LVD were published, in the year 2000 the literature comprised already of 393 patients. Since then there were approximately 25 new LVA/LVD patients published per year (Figure 2).[6] No gender predominance was described in the literature (male gender: LVA 51.7%; LVD 51.4).[6]
Figure 2. Cumulative number of publications on patients with LVA/LVD over a period of two centuries.
LVA: left ventricular aneurysms; LVD: left ventricular diverticula.
Congenital left ventricular aneurysms or diverticula are often asymptomatic and usually found coincidentally during diagnostic procedures performed for other reasons. This may explain the late diagnosis of, on average, 31.5 years (LVA) and 29.7 (LVD) years.[6] The most frequent clinical presentation in symptomatic patients include arrhythmias, embolic events, rupture of the LVA/LVD, and congestive heart failure.[6]
5.1. Arrhythmias
The spectrum of ventricular arrhythmias is ranging from occasional ventricular premature beats to sudden cardiac death.[2] The incidence of ventricular tachyarrhythmic events at presentation was significantly higher in LVA patients compared to LVD patients (18.4% versus 9.9%).[6] However, the incidence of syncope did not differ between both groups.[6] The association of LVA/LVD and ventricular arrhythmias was reported in several publications.[25]–[28] The initial case reported in 1971[25] was a 26-year-old female with an apical LVA who had recurrent ventricular tachycardias (VT). Recently, a series of 32 patients with LVA or LVD with a history of VT, survived sudden cardiac death (SCD), and/or syncope including patients undergoing implantable cardioverter defibrillator (ICD) implantation or ablation of the clinical ventricular arrhythmia.[28] The majority of these patients had VT morphologies corresponding to the anatomical location of the LVA/LVD. Reproducible inducibility of the monomorphic VT during electrophysiologic testing suggests re-entry as the underlying mechanism of these arrhythmias.[28] Among those patients with syncopal spells or palpitations more than 90% of the documented arrhythmias were ventricular in nature.[28]
Frequent causes of complaints are supra-ventricular arrhythmias such as paroxysmal and persistent atrial fibrillation as well as atrio-ventricular (AV)-node-reentrant- and atrial tachycardia. However, it is uncertain as to whether or not these atrial arrhythmias are directly associated with congenital left ventricular aneurysm or diverticulum.[2] Symptomatic bradycardia has not been reported in the literature available.[2]
5.2. Embolic events and rupture
Only few patients in both groups presented with a history of cardio-embolic events (LVA: 5.4%; LVD: 2.9%).[6] However, thrombotic material inside of the anomaly was significantly more frequently described in the LVA group and might be related to the usually akinetic wall of an LVA.[6]
Incidence of rupture as the cause of presentation did not differ between LVA and LVD patients (4% and 4.2%).[6] Rupture in general appears to be a problem of the younger age groups as the median age at the time of rupture was in the perinatal period (LVA) and in the first 2 years of life (LVD), respectively.[6],[29] The underlying mechanism of LVD rupture might be an excessive increase in systolic pressure within the diverticulum leading to rupture of the wall. Lowe, et al.[30] recorded in simultaneous measurements a systolic pressure in a LVD up to twice as high as compared to the systemic circulation. This might be a result of a slight delay between begin of contraction of the left ventricle and of the LVD.[30]
5.3. Congestive heart failure and various symptoms
A history of congestive heart failure (CHF) was noted in 21.5% of the LVA patients compared to only 6.8% of the LVD patients (P < 0.001).[6] In children this is usually the result of volume overload in large LVA.[6],[29] In older age groups CHF was frequently associated with incompetence of the aortic or mitral valve secondary to distortion of the valve annulus by a sub-aortic or sub-mitral LVA or LVD.[5],[6],[31],[32],[33] Complaints of typical angina or atypical chest pain were described more frequently in the LVD group (16.6%).[6] Direct compression of a coronary artery causing angina pectoris was exclusively described in LVA patients.[6],[34],[35] Endocarditis at the neck of a LVD was reported in only 0.9% with no such case among LVA patients.[6]
6. Differential diagnoses
Most of the left ventricular aneurysms are acquired aneurysms forming after an acute myocardial infarction with systolic bulging of the scarred myocardium; false aneurysms (or pseudoaneurysms) resulting from healed myocardial free-wall rupture after myocardial infarction are rare. Acquired aneurysms are very difficult to distinguish from congenital left ventricular aneurysms without knowledge of the past history and the coronary angiogram. Non-cardiac or systemic diseases include sarcoidosis,[36] connective tissue disease,[37] Chagas disease,[38] tuberculosis,[39] Kawasaki disease,[40] Becet's disease,[41] human immunodefiency virus infection,[42] or traumatic causes.[43] LV aneurysms may also result from idiopathic or viral myocarditis. Among 353 patients with a histological diagnosis of myocarditis, 12 patients (3%) had single and multiple left ventricular aneurysms.[44] Cardiomyopathies also have to be excluded, since a arrhythmogenic right ventricular dysplasia can occasionally spread to the left ventricle,[45] and apical left ventricular aneurysms have also been described in hypertrophic obstructive cardiomyopathy.[46] Rarely, left ventricular aneurysms were associated with mucopolysaccharidosis Type VI (Maroteux-Lamy-Syndrom),[47] alpha-1-antitrypsin defiency,[48] glycogen storage diseases,[49] or hyperimmunoglobulin E syndrome.[50] Myocardial clefts are congenital abnormalities related to myocardial fiber or fascicle disarray and have been described in healthy volunteers.[51] Off note, Dor reported about his experimental studies in the chicken embryo where LVD could be induced by an amniotic band or pericardial hole,[52] and partial pericardial defects might result in LVA/LVD formation.[53]
6.1. Diagnostic studies
6.1.1. Laboratory tests and genetics
There are no specific laboratory tests available for the diagnosis of congenital left ventricular aneurysms and diverticula.[2],[6] Genetic testing was performed only in a minority of 15 individuals and revealed Trisomy 13 in one LVA patient,[54] clinical presentation of two other LVA patients and their relatives suggested an autosomal-dominant inheritance pattern.[55] Trisomy 18 was described in one LVD patient.[56] The remaining 10 genetic analyses were unremarkable.[6]
6.2. Imaging
6.2.1. Electrocardiogram
Typical or even pathognomonic ECG alterations are not known.[2] The prevalence of 1st degree atrio-ventricular-block, bundle branch block, and intraventricular conduction delay is high.[23],[28] However, England, et al.[22] demonstrated that TNNT2 gene knockout during heart development alters TBX3 gene expression, a marker of the developing conduction system. This might be an explanation for the high prevalence of ECG alterations in LVA/LVD patients.[23],[28] Several ECG-abnormalities were more frequently encountered in patients diagnosed with LVA or LVD.[23] These “specific” ECG-abnormalities included (1) repolarization pattern with inverted T wave > 2 mm in > 2 leads, (2) repolarization patterns with either flat, minimally inverted, or particularly tall (> 15 mm) T waves in > 2 leads, (3) Q waves 2 to 3 mm in depth and present in > 2 leads, (4) abnormal R progression in the anterior pre-cordial leads, (5) atrial fibrillation, (6) right bundle branch block (RSR pattern, QRS > 120 ms in V1 and V2), (7) early repolarization pattern (ST-elevation > 2 mm in > 2 leads), and (8) increased PR interval duration (> 0.20 s). In patients with LVA or LVD these ECG alterations are of prognostic importance, as the incidence of clinical events during a median follow-up period of 50 months was significantly higher compared to individuals with diagnosis of LVA/LVD and “non-specific” ECG patterns.[24] Recently, fetal magneto-cardiography has been proposed as an replacement for fetal ECG, providing better signal quality and information about ventricular ectopy, arrhythmic response to fetal movement, presence of ST/T-wave abnormalities, and atrial amplitude increase in fetal LVA/LVD patients.[57]
6.2.2. Chest Radiography
Depending on the size of the LVA/LVD alterations of the cardiac silhouette can occasionally be observed on chest x-ray.[6] These changes include deviation of the heart to the right (exclusively described in LVD patients),[6] cardiomegaly or localized opacifications/calcifications which was more frequently observed in LVA patients.[6],[35] Due to the relative size of the aneurysm/diverticulum compared to the left ventricle, unusual cardiac silhouettes are more frequent in described children.[2]
6.2.3. Echocardiography
Transthoracic and transesophageal echocardiography, more recently also 3-dimensional echocardiography[58] are important tools for the diagnosis of LVA and LVD as a universally available non-invasive method. Accompanying malformations can be assessed in the same setting and quantified by (colour-) Doppler flow measuring. Real-time 3-D reconstruction allows calculation of the LVA/LVD volumes.[58] Contrast harmonic power Doppler imaging provides information about contractility and myocardial perfusion of LVA or LVD.[59]–[61] Tissue-Doppler, strain and strain rate echocardiography may help distinguish contractile from akinetic lesions.[62] However, especially in adults, small apical lesions can be missed due to difficult visualization of the left ventricular apex. (Figure 3)
Figure 3. Transthoracic echocardiography.
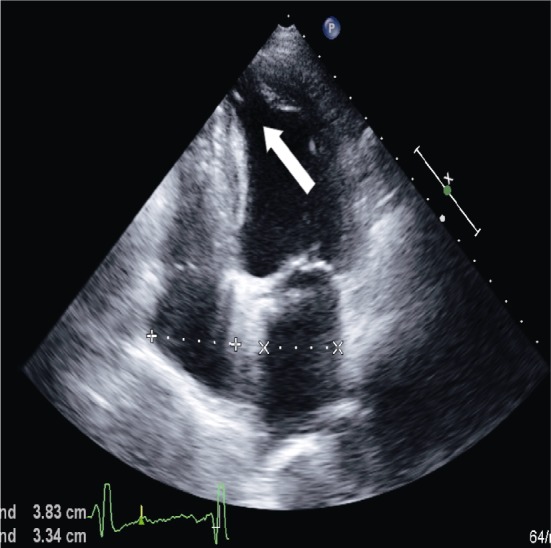
White arrow indicates apical diverticulum.
6.2.4. Radionuclide Studies
Radionuclide studies using perfusion markers like technetium (99mTc) -tetrofosmine and iodine (123I) -ß-methyl-iodophenyl-pentadecanoid (BMIPP) show the amount of active myocardium depending on the level of uptake in the wall of the LVA/LVD.[63] In some cases, this might be helpful to assess the risk of rupture of the aneurysm or diverticulum; however this method has not been established as a routine procedure yet. 123I-Metaiodbenzylguanidin - single photon emission computed tomography (MIBG-SPECT) was used in selected cases to assess the pre-synaptic cardiac noradrenaline re-uptake and the extent of distal denervation to distinguish between congenital and acquired aneurysms.[64] However, 18F-Fluorodeoxyglucose (FDG) - positron emission tomography (PET) is still the gold standard to diagnose viable myocardium.[2],[64]
6.2.5. Computertomography (CT)
There are only few data systematically analyzing the relevance of computed tomography in the diagnosis of congenital left ventricular aneurysms and diverticula.[65] In several case reports, CT could demonstrate relevant anatomical details in LVA/LVD patients, using contrast enhanced multi-detector row spiral thoracic computed tomography-scans in most cases.[4],[66],[67] (Figure 4)
Figure 4. Computed tomography: apical diverticulum.
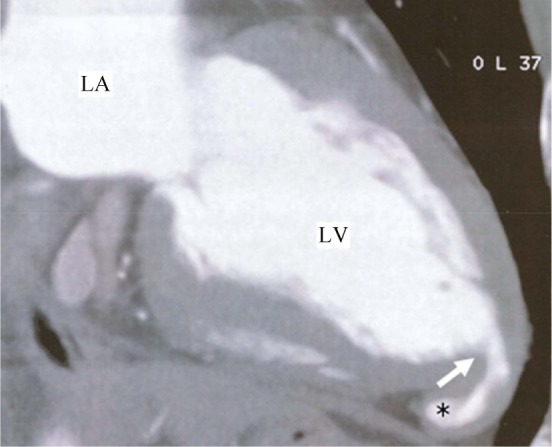
*left ventricular diverticulum. LA: left atrium; LV: left ventricle.
6.2.6. Magnet Resonance Imaging (MRI)
The relevance of MRI for diagnosing LVA or LVD still needs to be exactly defined. In selected cases, it might be important to differentiate this disorder from arrhythmogenic right ventricular dysplasia with spreading to the left ventricle due to the good visualization of fatty or fibrous infiltration of the heart muscle.[6],[7] Associated complex congenital cardiac anomalies can be visualized in an excellent fashion making MRI an adequate tool for preoperative evaluation[6] (Figure 5). MR-angiography can define the extent of bidirectional flow to and from the aneurysm or diverticulum. Technical advancements like three-dimensional (3-D) whole heart sequences allow 3-D reconstruction.[68] Time-resolved angiography with interleaved stochastic trajectories allow for dynamic acquisition and combines physiology and morphology.[69] Because of its limited availability, MRI has not been established yet as a routine.[6]
Figure 5. Magnet resonance imaging.
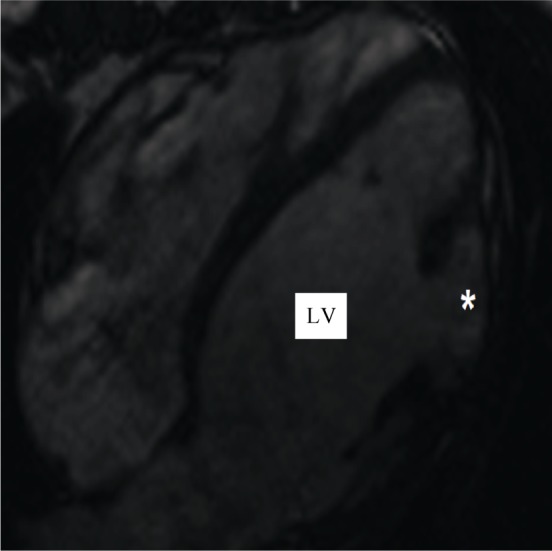
*: left ventricular diverticulum. LV: left ventricle.
6.2.7. Left ventricular angiography
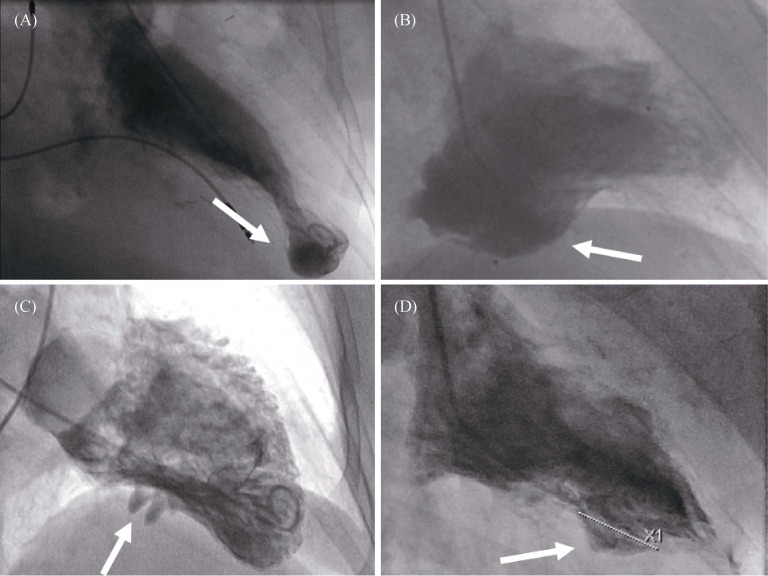
Depending on the location, the pathology can be seen in right-anterior-oblique (RAO) or left-anterior-oblique (LAO) projections. Contrast left ventriculography discloses the typical changes of a more or less contracting aneurysm or diverticulum with a small or wide communication with the left ventricle (Figure 6).[2] Due to contrast enhancement, a diverticulum can be clearly distinguished from pericardial tumors. Using standard projections, small LVA/LVD can be missed since location might be atypical or diverticula can be partially or completely filled with thrombus.[2],[6] Direct puncture and injection of contrast media into a diverticulum pulsating through the abdominal wall for simultaneous pressure measurements has been described in the literature,[30],[70] but should be clearly avoided.
Figure 6. Left ventricular angiography.
(A): apical diverticulum; (B): postero-basal aneurysm; (C): postero-basal diverticulum; (D): diaphragmatic aneurysm.
6.2.8. Electrophysiological studies
Electrophysiological studies (EPS) were performed only in a minority of all published LVA/LVD patients (10.5% and 5.7%, respectively).[6],[28] A series of 32 LVA/LVD patients with a history of VT, survived SCD, and/or syncope of whom 12 underwent EPS demonstrated VT morphologies corresponding to the anatomical location of the LVA/ LVD in 77.8%.[28] Endocardial activation sequence mapping demonstrated the origin of the VT at the LVA/LVD in 66.7%.[28] Ten patients with preoperative inducible VT underwent after aneurysmectomy or resection of the LVD a second EPS demonstrating no inducible VT in all cases (Figure 7).[71]–[73]
Figure 7. Electrophysiological study.
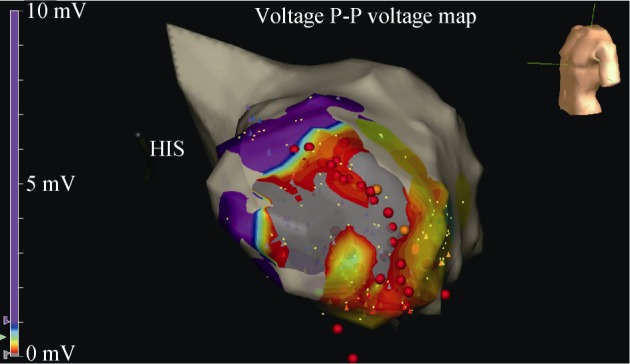
Voltage map during an ablation (red dots) of an apical aneurysm (gray area).
6.3. Histology
Histological examinations of resected LVD demonstrate the presence of all layers of the ventricular wall in 87.5% of the cases.[6] Coronary artery migration and preservation of myocardial architecture was described in almost all LVD cases, although in several cases the myofibres were partly replaced by spindle and stellate cells.[6],[74],[75] In 12.5% of LVD patients a thin walled LVD with fibrous tissue and a thick/fibrotic pericardium was described.[6] Depending on its location, blood supply of a diverticulum is derived from the right or left coronary artery or both, with a normal venous return[74],[76]–[78]. However, atypical blood supply is also mentioned in the literature (e.g. a LVD with blood supply by left internal mammary artery and inferior epigastric artery).[79] The wall of LVA mostly (–90%) show connective tissue with reticulin fibers (Masson staining) and only few disorganized muscular fibers with various degrees of vacuolization.[2],[6],[80] In the remaining LVA patients the wall of the lesion showed no microscopic features distinct from the left ventricular myocardium.[6] Vacuolization was described in 3.6%, calcifications in 8.9%, and giant cells in 3.6% of the LVA patients.[6] The internal surface is covered by simple endothelium (CD 31- and CD 34-staining) (Figure 8).[2],[74],[81],[82]
Figure 8. Histological section of a congenital left ventricular diverticulum.
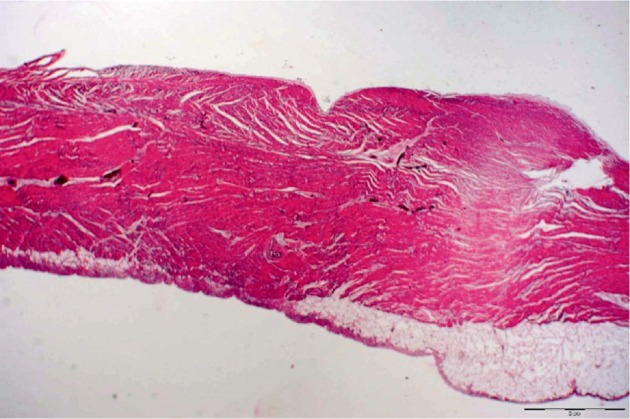
7. Management
There are no specific guidelines for management of LVA/LVD. Therefore, treatment modalities are determined by the clinical presentation and findings of the individual patient. The surgical technique depends on the type and extension of the diverticulum/aneurysm and is determined by a resection of the diverticulum or aneurysm usually under cardio-pulmonary bypass, although approximately 30% can be resected without extra-corporal circulation.[6],[83] Surgical treatment of LVA patients included in approximately three quarters aneurysmectomy alone[6] or combined with correction of accompanying congenital heart defects in 18.1%; only few LVA patients underwent ASD or VSD closure, or repair of coarctation without resection of the LVA.[6]
Isolated repair of LVD was less frequently performed (51.9%), mainly due to the higher prevalence of accompanying congenital defects in this group.[6] In the majority (–70%) isolated repair was performed by direct suturing of the LVD orifice, usually when the connection to the LV was small;[6] in the remaining LVD patients undergoing isolated repair the connection to the LV was larger than ≥ 2 cm and resection with patch closure was performed (pericardial, Dacron or polytetrafluoroethylene patches were used).[2],[6] LVD repair plus simultaneous correction of accompanying congenital heart defects was performed in 45.3% individuals.[6] Apical LVD was used in a case report as an entry for transapical aortic valve replacement and was closed by direct suture at the end of the procedure.[84] Only few patients underwent repair of their congenital cardiac defect only without resection of the LVD (one mitral valve reconstruction, one correction of a sub-aortic stenosis, and one VSD closure).[6] One patient with a LVD localized in the left ventricular outflow tract underwent transcatheter closure with an Amplatzer duct occluder.[85]
The perioperative risk under extra-corporal circulation is low for patients without other associated cardiac defects and might be less than 2%.[2],[83] Simultaneous correction of coexisting congenital or acquired cardiac anomalies increases the risk of perioperative morbidity and mortality.[2] Emergency operations with complex cardiac anatomy in the neonatal period can have mortality up to 50%.[2],[86] The perioperative mortality in 231 operated LVA and LVD cases was 15.3% and 7%, respectively.[6]
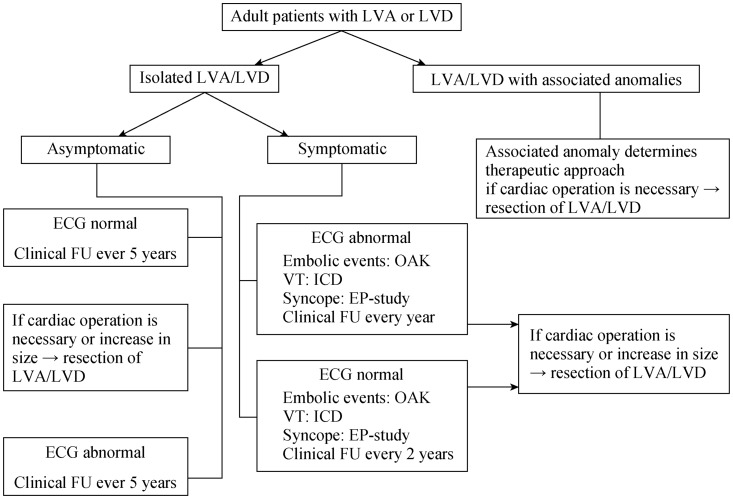
A non-surgical strategy with careful follow-up has been chosen in many case reports[56],[60],[87]–[96] and is supported by a series of 16 patients.[7] In this series, an uneventful course could be demonstrated in 94% of the patients over a period up to 127 months (mean 61 months), with an event rate of approximately 1.2% per year. No cardiac death was seen during the follow-up. In a series of 12 neonatal/juvenile patients[56] with a substantially shorter period of follow-up (mean 16 months), an event rate of 6% per year was documented, with one cardiac death due to a rupture of a diverticulum. Therefore, in this sub-population an operative therapy might be the best treatment. In patients with large hypo- or akinetic aneurysms or after systemic embolization therapeutic oral anticoagulation should be administered. Sustained VT, some of them induced by exercise,[24] are usually based on re-entry circuits originating from the congenital left ventricular diverticulum/aneurysm.[28] According to the current American College of Cardiology (ACC)/ American Heart Association (AHA) guidelines for the management of adults with congenital heart disease[97] affected patients with VT should preferably undergo definitive interventions (ICD implantation, VT ablation, or arrhythmia surgery) which have replaced in most centers class I- or III-antiarrhythmic drug treatment.[97] Radiofrequency catheter ablation alone using electro-anatomical mapping systems[98] should be reserved for patients slow, solitary VTs. In case of a sub-epicardial arrhythmogenic substrate, radiofrequency ablation can be performed via a non-surgical transthoracic epicardial approach.[98],[99] Occasionally, catheter-ablation or antiarrhythmic drug therapy may be a valuable adjunctive therapy in patients with high burden of ICD shocks.[97] Some of the patients with an implantable cardioverter defibrillator have received multiple, successful, adequate anti-tachycardia pacing and shocks during follow up.[6],[24],[27] Proposal of a therapeutic algorithm for adult patients with LVA/LVD is shown in Figure 9.
Figure 9. Therapeutic algorithm for adult patients with LVA or LVD.
Abnormal ECG: strongly suggestive of cardiovascular disease, detailed criteria in.[24] EP: electrophysiologic; FU: follow-up; ICD: implantable cardioverter-defibrillator; LVA: left ventricular aneurysm; LVD: left ventricular diverticulum; OAK: oral anticoagulation; VT: ventricular tachycardia.
8. Prognosis
Currently available data do not allow valid conclusions on morbidity and mortality of patients with congenital left ventricular aneurysm or diverticulum.[2] On the one hand, the numbers of patients in the literature included in studies are too low; on the other hand, the time period of follow-up varies.[2] The most substantial information's about outcome of affected individuals can be derived from a systematic analysis of more than 800 LVA/LVD published during two centuries.[6] Follow up duration was mean 56 months (up to 18 years) resulting in a cumulative follow-up of 1732 patient-years.[6] The cardiac event rate (occurrence of at least one of the following: arrhythmic events, rupture, sudden cardiac death, congestive heart failure, embolic events, syncope, and increase in size of LVA/LVD) differed with respect to the type of anomaly (LVA patients had significantly more adverse events during follow-up) and was the highest in the younger age groups.[6] Symptoms at the time of diagnosis and presence of several distinct ECG abnormalities (section 7.2.1) increases the incidence of adverse events during follow-up.[6],[24]
8.1. Cardiac death
Cardiac death was significantly more frequent in the LVA group (12.7% versus 3.8%; P = 0.02)[6] and occurred mostly in the age group below 18 years. Median age at the time of cardiac death was 0.8 years in LVA patients versus 2.5 years in LVD patients.[6]
8.2. Congestive heart failure
CHF as the cause of death was also significantly more frequent in LVA patients (50% versus 0.0%; P = 0.01) and occurred at a median age of 6 years.[6],[29] CHF occurred usually when the LVA was already large at baseline (≥ 400 mm2),[6],[29] most probably due to the volume overload caused by the LVA. The load placed upon the LV by a large aneurysm can be poorly tolerated by the fetal or neonatal heart because it is characterized by a low compliance and little functional reserve compared to the adult heart.[100],[101] Furthermore, the mediastinal shift of the heart caused by a large aneurysm together with pericardial effusion in some of the cases can result in a compression of the right ventricle and affect ventricular interaction.[100],[101]
8.3. Rupture
A higher incidence of rupture caused by the thin fibrous wall could be expected in LVAs,[71],[102],[103] but this is not supported by clinical data in the literature.[6] Rupture was significantly more frequently encountered in LVD patients and occurred only in patients younger than 8 years of age.[6],[29] The underlying mechanism of LVD rupture seems to be an excessive increase in systolic pressure within the diverticulum leading to rupture of the wall. This might be a result of a slight delay between begin of contraction of the left ventricle and of the LVD.[30]
8.4. Sudden cardiac death
The incidence of sudden cardiac death was similar in LVA/LVD patients.[6] The risk of SCD seems not to attenuate over time as some LVA/LVD patients died between the 25th and 62nd year of live.[6] However, the mean age at the time of SCD differed significantly between LVA and LVD patients (14.5 ± 8.1 years versus 50.5 ± 16.3 years.[6]
Changes in size of congenital left ventricular diverticula and aneurysms over time are documented primarily in pediatric cardiology.[29],[73] In a series of 16 neonatal patients,[56] there was a follow-up of size in nine cases (56%). The size increased progressively in two patients (13%) who had LVA with decreased contractility. However, the size decreased in two cases (13%) and did not change in five cases (31%).
References
- 1.Vazquez-Perez J, Gautier M, Mercier N, et al. Diverticulum of the left ventricle. Arch Mal Cœur et Vaiss. 1969;62:922. [PubMed] [Google Scholar]
- 2.Ohlow MA. Congenital left ventricular aneurysms and diverticula––Definition, pathophysiology, clinical relevance and treatment. Cardiology. 2006;106:63–72. doi: 10.1159/000092634. [DOI] [PubMed] [Google Scholar]
- 3.Ohlow MA, Secknus MA, Geller JC, et al. Kongenitale linksventrikuläre Aneurysmata und Divertikel des Erwachsenen: Pathophysiologie, klinische Präsentation und Therapieoptionen. Med Klin. 2007;102:358–365. doi: 10.1007/s00063-007-1034-3. [DOI] [PubMed] [Google Scholar]
- 4.Paz Y, Fridman E, Shakalia F, et al. Repair of a isolated huge congenital left ventricular diverticulum. J Thorac Cardiovasc Surg. 2004;128:313–314. doi: 10.1016/j.jtcvs.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 5.Ohlow MA, Secknus MA, Geller JC, et al. Prevalence and outcome of congenital left ventricular aneurysms and diverticula in an adult population. Cardiology. 2009;112:287–293. doi: 10.1159/000159122. [DOI] [PubMed] [Google Scholar]
- 6.Ohlow MA, von Korn H, Lauer B, et al. Characteristics and outcome of congenital left ventricular aneurysms and diverticula: analysis of 809 cases published since 1816. Int J Cardiol. 2015;185:34–45. doi: 10.1016/j.ijcard.2015.03.050. [DOI] [PubMed] [Google Scholar]
- 7.Mayer K, Candinas R, Radounlis C, et al. Kongenitale linksventrikuläre aneurysmen und divertikel: klinik, diagnostik und verlauf. Schweiz Med Wochenschr. 1999;129:1249–1256. [PubMed] [Google Scholar]
- 8.Albrecht G. Beitrag zur morphologie und formalen genese kongenitaler divertikel. Zentralblatt Allg Pathol. 1972;116:42–47. [PubMed] [Google Scholar]
- 9.Kreysig F. Über die Zufälle und Unterscheidungsmerkmale der Verdickung, Verdünnung und Mürbheit des Herzens. In: Kreysig F, editor. Die Krankheiten des Herzens. Zweiter Theil, zweite Abtheilung. Berlin: Maurer'sche Buchhandlung; 1816. p. 464. [Google Scholar]
- 10.O'Bryan Congenital diverticulum of the left ventricle. Prov Med Surgical Trans. 1837;6:374. [Google Scholar]
- 11.Wieting H. Eine operativ behandelte Herzmissbildung. Z Chir. 1912;144:293. [Google Scholar]
- 12.Roessler W. Erfolgreiche operative Entfernung eine ektopischen Herzdivertikel an einem Neugeborenen. Dtsch Z Chir. 1944;258:561. [Google Scholar]
- 13.Loogen F, Rippert R, Vieten H, et al. Zur Klinik des angeborenen Herzwanddivertikels. Z Kreislaufforschung. 1961;50:580. [Google Scholar]
- 14.Suilen C, Friedli B, Rutishauser W, et al. Congenital intrathoracic left ventricular diverticulum in an adult. Chest. 1990;98:750–751. doi: 10.1378/chest.98.3.750. [DOI] [PubMed] [Google Scholar]
- 15.Rad E, Awad S, Hijaz Z, et al. Congenital left ventricular outpouchings: a systematic review of 839 cases and introduction of a novel classification after two centuries. Congenit Heart Dis. 2014;9:498–511. doi: 10.1111/chd.12214. [DOI] [PubMed] [Google Scholar]
- 16.van Mierop L, Kutschke L. Embryology of the heart. Hurst's the heart. 8th ed. McGraw-Hill; 1994. [Google Scholar]
- 17.Albrecht G. Beitrag zur Morphologie und formalen Genese kongenitaler Divertikel. Zentralblatt Allg Pathol. 1972;116:42–47. [PubMed] [Google Scholar]
- 18.Duncan A, Mawson J, Duncan W, et al. Left ventricular diverticulum in an infant with pentalogy of Cantrell. Cardiol Young. 2008;18:355. doi: 10.1017/S1047951108002199. [DOI] [PubMed] [Google Scholar]
- 19.Ohlow MA, Fuhrmann JT, Lauer B, et al. Prevalence and spectrum of coronary anomalies in patients with an isolated congenital left ventricular aneurysm or diverticulum. Clin Cardiol. 2011;34:226–232. doi: 10.1002/clc.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carles D, Maugey-Lalom B, Habboud H, et al. Early prenatal diagnosis of ventricular diverticulum complicated by serous pericardial effusion. Prenat Diagn. 1995;15:778–780. doi: 10.1002/pd.1970150817. [DOI] [PubMed] [Google Scholar]
- 21.Drennan MR, van de Vijar G. Diverticulum of the human heart. J Med Ass S Afr. 1928;2:58–60. [Google Scholar]
- 22.England J, Pang KL, Parnall M, et al. Cardiac troponin T is necessary for normal development in the embryogenic chick heart. J Anat. 2016;229:436–449. doi: 10.1111/joa.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohlow MA, Lauer B, Geller JC, et al. Prevalence and spectrum of abnormal electrocardiograms in patients with an isolated congenital left ventricular aneurysm or diverticulum. Europace. 2009;11:1689–1695. doi: 10.1093/europace/eup323. [DOI] [PubMed] [Google Scholar]
- 24.Ohlow MA, Lauer B, Lotze U, et al. Long-term prognosis of adult patients with isolated congenital left ventricular aneurysm or diverticulum and abnormal ECG patterns. Circulation J. 2012;76:2465–2470. doi: 10.1253/circj.cj-12-0193. [DOI] [PubMed] [Google Scholar]
- 25.Maloy WE, Arrants JE, Sowell BF, et al. Left ventricular aneurysm of uncertain etiology with recurrent ventricular arrhythmias. N EngI J Med. 1971;285:662–663. doi: 10.1056/NEJM197109162851205. [DOI] [PubMed] [Google Scholar]
- 26.Fellows CL, Bardy GH, Ivey T, et al. Ventricular dysrhythmias associated with congenital left ventricular aneurysms. Am J Cardiol. 1986;57:997–999. doi: 10.1016/0002-9149(86)90747-2. [DOI] [PubMed] [Google Scholar]
- 27.Santamaria M, Cireddu M, Riva S, et al. Radiofrequency catheter ablation guided by non-contact mapping of ventricular tachycardia originating from an idiopathic left ventricular aneurysm. J Interv Cardiol Electrophysiol. 2007;19:49–53. doi: 10.1007/s10840-007-9132-y. [DOI] [PubMed] [Google Scholar]
- 28.Haegeli LM, Ercin E, Wolber T, et al. Arrhythmic manifestations in patients with congenital left ventricular aneurysm and diverticula. Am J Cardiol. 2011;108:1826–1830. doi: 10.1016/j.amjcard.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 29.Ohlow MA, Brunelli M, Lauer B, et al. Characteristics and outcome of primary congenital left ventricular aneurysm and diverticulum: Analysis of cases from the literature. Prenat Diagn. 2014;34:893–899. doi: 10.1002/pd.4389. [DOI] [PubMed] [Google Scholar]
- 30.Lowe J, Williams J, Robb D, et al. Congenital diverticulum of the left ventricle. Br Heart J. 1959;21:101. doi: 10.1136/hrt.21.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chesler E, Joffe N, Schamroth I, et al. Annular subvalvular left ventricular aneurysms in the South African Bantu. Circulation. 1965;32:43–51. doi: 10.1161/01.cir.32.1.43. [DOI] [PubMed] [Google Scholar]
- 32.Gueron M, Hirsch M, Opschitzer I, et al. Left ventricular diverticulum and mitral incompetence in asymptomatic children. Circulation. 1976;53:181–186. doi: 10.1161/01.cir.53.1.181. [DOI] [PubMed] [Google Scholar]
- 33.Mohanty A, Saxena A. Submitral aneurysm: unusual echocardiographic features. Heart. 2003;89:552. doi: 10.1136/heart.89.5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt H, Strauß H, Schreier R, et al. Divertikel des linken ventrikels – ein Beitrag zur differential diagnose der angina pectoris. Dt Gesundh.-Wesen. 1979;27:1249–1252. [Google Scholar]
- 35.Strauß H, Schmidt H, Gos A, et al. Kompression der linken Koronararterie durch ein subaortales linksventrikuläres diverticula. Fortschr Röntgenstr. 1985;142:694–695. doi: 10.1055/s-2008-1052741. [DOI] [PubMed] [Google Scholar]
- 36.Sekhri V, Sanal S, DeLorenzo L, et al. Cardiac sarcoidosis: a comprehensive review. Arch Med Sci. 2011;7:546–554. doi: 10.5114/aoms.2011.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frustaci A, Gentilioni N, Caldarulo M, et al. Acute myocarditis and left ventricular aneurysm as presentation of systemic lupus erythmatosus. Chest. 1996;109:282–284. doi: 10.1378/chest.109.1.282. [DOI] [PubMed] [Google Scholar]
- 38.Oliveira JA, Mello de Oliveira JS, Frederigue U, Jr, et al. Apical aneurysms of Chagas heart disease. Br Heart J. 1981;46:432–437. doi: 10.1136/hrt.46.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones KP, Tilden K. Tuberculous myocardial aneurysm with rupture and sudden cardiac death from tamponade: review of the literature and report of a case. Hawaii Med J. 1942;1:295–297. [Google Scholar]
- 40.Dimitriades VR, Brown AG, Gedalia A, et al. Kawasaki disease: pathophysiology, clinical manifestations, and management. Curr Rheumatol Rep. 2014;16:423. doi: 10.1007/s11926-014-0423-x. [DOI] [PubMed] [Google Scholar]
- 41.Han K, Siegel R, Pantuck AJ, et al. Behcet's syndrome with left ventricular aneurysm and ruptured renal artery pseudoaneurysm. Urology. 1999;54:162. doi: 10.1016/s0090-4295(98)00558-5. [DOI] [PubMed] [Google Scholar]
- 42.Kane A, Hane L, Dangou JM, et al. Left ventricular aneurysm in human immunodefiency virus infection: a case report. Arch Mal Coeur Vaiss. 1998;91:419–423. [PubMed] [Google Scholar]
- 43.Lai WT, Lin SM, Wu SJ, et al. Post-traumatic left ventricular aneurysm with massive hemopericardium in a child presenting 3 years after a fall. Pediatr Neonatol. 2013;54:406–408. doi: 10.1016/j.pedneo.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Frustaci A, Chimenetti C, Pieroni M, et al. Prognostic significance of left ventricular aneurysms with normal global function caused by myocarditis. Chest. 2000;118:1696–1702. doi: 10.1378/chest.118.6.1696. [DOI] [PubMed] [Google Scholar]
- 45.Horimoto M, Akino M, Takenaka T, et al. Evolution of left ventricular involvement in arrhythmogenic right ventricular cardiomyopathy. Cardiology. 2000;93:197–200. doi: 10.1159/000007026. [DOI] [PubMed] [Google Scholar]
- 46.Maron B, Hauser R, Roberts W, et al. Hypertophic cardiomyopathy with left ventricular apical diverticulum. Am J Cardiol. 1996;77:1263–1265. doi: 10.1016/s0002-9149(96)00180-4. [DOI] [PubMed] [Google Scholar]
- 47.Oudit G, Butany J, Williams W, et al. Left ventricular aneurysm associated with mucopolysaccharidosis type VI. Circulation. 2007;115:e60–e62. doi: 10.1161/CIRCULATIONAHA.106.656231. [DOI] [PubMed] [Google Scholar]
- 48.Corda L, Vizzardi E, De Cicco G, et al. Left ventricular pseudoaneurysm and alpha 1-antitrypsin enzyme defiency: Another pathological correlation. Int J Cardiol. 2010;4:384–386. doi: 10.1016/j.ijcard.2010.02.055. [DOI] [PubMed] [Google Scholar]
- 49.Toda G, Yoshimuta T, Kawano H, et al. Glycogen storage disease associated with left ventricular aneurysm in the elderly patient. Circ J. 2001;65:462–464. doi: 10.1253/jcj.65.462. [DOI] [PubMed] [Google Scholar]
- 50.El Noor IB, Venugopalan P, Johnston WJ, et al. Ventricular aneurysm and myocarditis in a child with hyperimmunoglobulin E syndrome. Eur Heart J. 1995;16:714–715. doi: 10.1093/oxfordjournals.eurheartj.a060980. [DOI] [PubMed] [Google Scholar]
- 51.Afonso L. Myocardial cleft, crypt, diverticulum or aneurysm? Does it really matter? Clin Cardiol. 2009;32:E48–E51. doi: 10.1002/clc.20466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dor X. Embryologie experimentale et malformations cardiaques. Encyclopedie medico-chirurgicale, Coeur Vaiss. 1981;76:513–523. [Google Scholar]
- 53.Auch-Schwelk W, Bonzel T, Krause T, et al. Differential diagnosis of chest pain and diagnostic findings in pericardial defects combined with coronary artery disease. Clin Cardiol. 1988;11:650–657. doi: 10.1002/clc.4960110912. [DOI] [PubMed] [Google Scholar]
- 54.Papagiannis J, Van Praagh R, Schwindt O, et al. Congenital left ventricular aneurysm: Clinical, imaging, pathologic and surgical findings in seven new cases. Am Heart J. 2001;141:491–499. doi: 10.1067/mhj.2001.113076. [DOI] [PubMed] [Google Scholar]
- 55.Eriksson H, Cooper S, Rosenbaum K, et al. Familial occurrence of congenital aneurysms of the muscular interventricular septum. Pediatr Cardiol. 1998;19:249–252. doi: 10.1007/s002469900297. [DOI] [PubMed] [Google Scholar]
- 56.Cavalle-Garrido T, Cloutier A, Harder J, et al. Evolution of fetal aneurysms and diverticula of the heart: an echocardiographic study. Am J Perinatol. 1997;14:393–400. doi: 10.1055/s-2007-994167. [DOI] [PubMed] [Google Scholar]
- 57.Peters C, Wacker-Gussmann A, Strasburger J, et al. Electrophysiologic features of fetal ventricular aneurysms and diverticula. Prenat Diagn. 2015;35:129–136. doi: 10.1002/pd.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruiz-Esparza E, Roldan FJ, Vazquez-Antona C, et al. 2D and 3D echocardiography of a left ventricular diverticulum. Echocardiography. 2009;26:1087–1088. doi: 10.1111/j.1540-8175.2009.00958.x. [DOI] [PubMed] [Google Scholar]
- 59.Ueda T, Mizushige K, Yukiiri K, et al. Contrast harmonic power Doppler imaging of congenital ventricular diverticulum – a case report. Angiology. 2001;52:357–359. doi: 10.1177/000331970105200510. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi M, Nishikimi T, Tamano K, et al. Multiple left ventricular diverticula detected by second harmonic imaging: a case report. Circ J. 2003;67:972–974. doi: 10.1253/circj.67.972. [DOI] [PubMed] [Google Scholar]
- 61.Estevez CM, Weyman AE, Feigenbaum H, et al. Detection of left ventricular diverticulum by cross-sectional echocardiography. Chest. 1976;69:544–546. doi: 10.1378/chest.69.4.544. [DOI] [PubMed] [Google Scholar]
- 62.Hajsadeghi S, Pazoki M, Talebitaher M, et al. Giant congenital left ventricular diverticulum associated with endocarditis: a diagnosis made by tissue doppler echocardiography. J Cardiol Cases. 2016;14 doi: 10.1016/j.jccase.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshida T, Niwano S, Izumi T, et al. Arrhythmogenic giant sub-mitral left ventricular diverticulum. Heart. 2002;88:52. doi: 10.1136/heart.88.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paul M, Schäfers M, Grude M, et al. Idiopathic left ventricular aneurysm and sudden cardiac death in young adults. Europace. 2006;8:607–612. doi: 10.1093/europace/eul074. [DOI] [PubMed] [Google Scholar]
- 65.Srichai M, Hecht E, Kim D, et al. Ventricular diverticula on cardiac CT: more common than previously thought. An J Roengenol. 2007;189:204–208. doi: 10.2214/AJR.06.1223. [DOI] [PubMed] [Google Scholar]
- 66.Di Bernardo S, Sekarski N, Meijboom E, et al. Left ventricular diverticulum in a neonate with Cantrell syndrome. Heart. 2004;90:1320. doi: 10.1136/hrt.2004.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beregi J, Aumegeat V, Coullet J, et al. Case report. Congenital left ventricular aneurysm diagnosed by spiral CT angiography. J Comput Assist Tomogr. 1996;20:484–486. doi: 10.1097/00004728-199605000-00033. [DOI] [PubMed] [Google Scholar]
- 68.Uribe S, Muthurangu V, Boubertakh R, et al. Whole heart cine MRI using real-time respiratory self gating. Magn Reson Med. 2007;57:606–613. doi: 10.1002/mrm.21156. [DOI] [PubMed] [Google Scholar]
- 69.Sharma A, Kumar S. Overview of left ventricular outpouchings on cardiac magnetic resonance imaging. Cardiovasc Diagn Ther. 2015;5:464–470. doi: 10.3978/j.issn.2223-3652.2015.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skapinker S. Diverticulum of the left ventricle of the heart. Arch Surg. 1951;63:629–634. doi: 10.1001/archsurg.1951.01250040643009. [DOI] [PubMed] [Google Scholar]
- 71.Yamashiro S, Kuniyoshi Y, Miyagi K, et al. Two cases of ventricular tachycardia with congenital left ventricular malformation in an adult. Ann Thorasc Cardiovasc Surg. 2004;10:42–46. [PubMed] [Google Scholar]
- 72.Shen E, Fukuyama O, Herre J, et al. Ventricular tachycardia with congenital ventricular diverticulum. Chest. 1991;100:283–285. doi: 10.1378/chest.100.1.283. [DOI] [PubMed] [Google Scholar]
- 73.Rajasinghe H, Lorenz H, Longaker M, et al. Arrhythmogenic ventricular aneurysms unrelated to coronary artery disease. Ann Thorac Surg. 1995;59:1079–1084. doi: 10.1016/0003-4975(95)00121-z. [DOI] [PubMed] [Google Scholar]
- 74.Adler E, El Fiky M. Zur Chirurgie der Herzwandivertikel. Zbl Chirurgie. 1967;35:2471–2483. [PubMed] [Google Scholar]
- 75.Mady C. Left ventricular diverticulum: analysis of two operated cases and review of literature. Angiology. 1982;33:280–286. doi: 10.1177/000331978203300409. [DOI] [PubMed] [Google Scholar]
- 76.Pitsis A, Visouli A, Kelpis T, et al. Diverticulum of the left ventricle: etiology and surgical treatment. Heart Surg Forum. 2008;11:e75–e77. doi: 10.1532/HSF98.20071205. [DOI] [PubMed] [Google Scholar]
- 77.Korver A, Haas F, Freund M, et al. Pentalogy of Cantrell. Pediatr Cardiol. 2008;29:146–149. doi: 10.1007/s00246-007-9032-z. [DOI] [PubMed] [Google Scholar]
- 78.Wennevold A, Andersen E, Efsen F, et al. Congenital apical aneurysm of the left ventricle: surgical removal in two infants. Eur J Cardiol. 1978;7:411–419. [PubMed] [Google Scholar]
- 79.Murphy D, Aberdeen E, Dobbs R, et al. Surgical treatment of syndrome consisting of thoraco-abdominal wall, diaphragmatic, pericardial, and ventricular septal defects; and left ventricular diverticulum. Ann Thorac Surg. 1968;6:528–534. doi: 10.1016/s0003-4975(10)66119-x. [DOI] [PubMed] [Google Scholar]
- 80.Pitol R, Cardoso C, Cardoso RC, et al. Congenital ventricular diverticulum associated with ventricular tachycardia. Arq Bras Cardiol. 2005;84:81–87. doi: 10.1590/s0066-782x2005000200016. [DOI] [PubMed] [Google Scholar]
- 81.Astley R, Parsons C. Diverticulum of left ventricle. Report of case. Br Heart J. 1953;15:289. [Google Scholar]
- 82.Cantrell J, Haller J, Ravitch M, et al. A syndrome of congenital defects involving the abdominal wall, sternum, diaphragm, pericardium, and heart. Surg Gynaecol Obstet. 1958;107:602–614. [PubMed] [Google Scholar]
- 83.Okereke O, Cooley D, Frazier O, et al. Congenital diverticulum of the ventricle. J Thorac Cardiovasc Surg. 1986;91:208–214. [PubMed] [Google Scholar]
- 84.Ferrati E, van Steenberghe M, Namasivayam J, et al. Feasibility of transapical aortic valve replacement through a left ventricular apical diverticulum. J Cardiothorasc Surg. 2013;8:3. doi: 10.1186/1749-8090-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jain S, Mahajan R, Rohin M, et al. Percutaneous transcatheter device closure of an isolated congenital LV diverticulum: first case report. Pediatr Cardiol. 2011;32:1219–1222. doi: 10.1007/s00246-011-9998-4. [DOI] [PubMed] [Google Scholar]
- 86.Gruberg L, Goldstein S, Pfister A, et al. Cantrell's-Syndrome: Left ventricular diverticulum in an adult patient. Circulation. 2000;101:109–110. doi: 10.1161/01.cir.101.1.109. [DOI] [PubMed] [Google Scholar]
- 87.Parthenakis F, Kochiadakis G, Patrianakos A, et al. Peripheral arterial embolism due to a left ventricular diverticulum in a young adult. Chest. 2005;127:1452–1454. doi: 10.1378/chest.127.4.1452. [DOI] [PubMed] [Google Scholar]
- 88.Huang G, Pavan D, Antonini-Canterin F, et al. Asymptomatic isolated congenital left ventricular muscular diverticulum in an adult: a case report. Echocardiography. 2003;20:191–195. doi: 10.1046/j.1540-8175.2003.03004.x. [DOI] [PubMed] [Google Scholar]
- 89.Brachlow A, Sable C, Smith S, et al. Fetal diagnosis and postnatal follow up of an asymptomatic congenital left ventricular diverticulum. Pediatr Cardiol. 2002;23:658–660. doi: 10.1007/s00246-002-9002-4. [DOI] [PubMed] [Google Scholar]
- 90.Ghannem M, Lozinguez O, Godard S, et al. Isolated congenital diverticulum of the left ventricle disclosed by ventricular tachycardia in a 72-year-old woman. Ann Cardiol Angeiol. 1997;46:663–666. [PubMed] [Google Scholar]
- 91.Wu J, Yu C. Isolated congenital left ventricular diverticulum. Pediatr Cardiol. 1996;17:254–256. doi: 10.1007/BF02524804. [DOI] [PubMed] [Google Scholar]
- 92.Roche R, Long J, Bitar G, et al. Myocardial diverticulum of the left ventricle. Review of the literature apropos of a case. Ann Cardiol Angeiol. 1996;45:68–70. [PubMed] [Google Scholar]
- 93.Speechly-Dick M, Oliver R, Slapak G, et al. Congenital left ventricular diverticula: a rare cause of sudden cardiac death. Postgrad Med J. 1992;68:378–380. doi: 10.1136/pgmj.68.799.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Treisman B, Cooley D, Luftschanowski R, et al. Diverticulum or aneurysm of the left ventricle. Am J Cardiol. 1973;32:119–123. doi: 10.1016/s0002-9149(73)80097-9. [DOI] [PubMed] [Google Scholar]
- 95.Ehlers K, Engle M, Levin A, et al. Left ventricular abnormality with late mitral insufficiency and abnormal electrocardiogram. Am J Card. 1970;26:333–340. doi: 10.1016/0002-9149(70)90726-5. [DOI] [PubMed] [Google Scholar]
- 96.Gembruch U, Steil E, Redel D, et al. Prenatal diagnosis of left ventricular aneurysm. Prenat Diagn. 1990;10:203–209. doi: 10.1002/pd.1970100312. [DOI] [PubMed] [Google Scholar]
- 97.Bhatt A, Foster E, Kuehl K, et al. Congenital heart disease in older adults – AHA scientific statement. Circulation. 2015;131:1–48. doi: 10.1161/CIR.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 98.Ouyang F, Antz M, Deger F, et al. An unrecognized sub-epicardial re-entrant tachycardia attributable to left ventricular aneurysm in patients with normal coronary arteriograms. Circulation. 2003;107:2702–2709. doi: 10.1161/01.CIR.0000068343.69532.B6. [DOI] [PubMed] [Google Scholar]
- 99.Ogawa M, Mioshi K, Morito N, et al. Successful catheter ablation of ventricular tachycardia originating from the idiopathic saccular apical left ventricular aneurysm. Int J Cardiol. 2004;93:343–346. doi: 10.1016/S0167-5273(03)00222-5. [DOI] [PubMed] [Google Scholar]
- 100.Mathias A, Fredouille C, Nesmann C, et al. Prenatal diagnosis of left ventricular aneurysm: a report of three cases and a review. Cardiol Young. 1999;9:175–184. doi: 10.1017/s1047951100008404. [DOI] [PubMed] [Google Scholar]
- 101.Anderson PA. Myocardial development. In: Long WA, editor. Fetal and neonatal cardiology. Philadelphia, USA: W.B. Saunders; 1990. pp. 17–38. [Google Scholar]
- 102.Abrahams D, Barton C, Cockshott W, et al. Annular subvalvular left ventricular aneurysms. Quart J Med. 1962;31:345–360. [PubMed] [Google Scholar]
- 103.Rimailho A, Cabrol C, Soyer R. Aneurisme idiopathique du ventricule gauche. A prospos de 4 cas. Arch Mal Coer. 1981;84:443–451. [PubMed] [Google Scholar]