Abstract
Background
Ventricular premature complexes (VPCs) with a burden higher than 10% to 20% of total daily heart beats can cause VPC-induced cardiomyopathy. The systolic blood pressure response (SBPR) is the difference between the SBP during maximal exercise and rest. A low SBPR was recently identified to be a marker of cardiomyopathy. The aim of this manuscript was to clarify the association between VPC burden and SBPR.
Methods
From January to December 2015, all patients with a VPC burden larger than 240 beats/day on Holter recordings and treadmill exercise tests were enrolled. The patients with a heart rhythm other than sinus rhythm, coronary artery disease, and severe cardiomyopathy were excluded. The SBPR was measured during a treadmill test. The basic characteristics and echocardiographic findings were collected.
Results
All patients were classified into three groups: Group 1; 240-1,000 VPCs/day (n = 78), Group 2; 1,000-10,000 VPCs/day (n = 54), and Group 3; > 10,000 VPCs/day (n = 21). Group 1 had a higher SBPR than the other groups. Multivariate analysis revealed that only VPC burden was associated with SBPR. Receiver operating characteristic curve analysis showed that a VPC burden > 1,055 beats/day predicted a SBPR < 40 mmHg. The results were consistent in all subgroups. There were no significant differences in echocardiographic findings among the groups.
Conclusions
AVPC burden higher than 1,055 beats/day was associated with a reduced SBPR.
Keywords: Cardiomyopathy, Systolic blood pressure response, Ventricular premature complex
INTRODUCTION
Ventricular premature complexes (VPCs) were once thought to be a benign rhythm in patients without structural heart disease.1 However, it is now known that VPCs can cause impaired ventricular contractility and enlarged ventricular size, known as VPC-induced cardiomyopathy.2,3 Previous studies have suggested a correlation between VPC burden and left ventricular function, and that a higher VPC burden is associated with a lower left ventricular (LV) ejection fraction (EF), a larger LV end diastolic diameter (EDD), and a larger LV end systolic diameter (ESD).4,5 Other studies have revealed improved LV systolic function after the suppression of VPCs in patients with dilated cardiomyopathy.6-8 However, the cutoff value of VPC burden causing cardiomyopathy has yet to be clearly defined. Using the LVEF and LV size as parameters for VPC-induced cardiomyopathy, several studies have reported that a VPC burden higher than 10% to 20% of total daily heart beats contributes to VPC-induced cardiomyopathy.2,9
Blood pressure is a simple routine measurement in most clinical departments. A systolic blood pressure response (SBPR) is defined as the difference between systolic blood pressure during maximal exercise and rest. It has been reported that the SBPR during exercise has an inverse relationship with cardiac mortality in patients with chronic heart failure.10-12 In patients with heart failure, a low cardiac output causes low blood pressure. Therefore, a higher SBPR indicates a more preserved cardiac function and higher inotropic reserve.10-12 Further, in patients with chronic heart failure and dilated cardiomyopathy, a low SBPR has also been shown to result in a poorer prognosis.13 In addition to patients with heart failure and dilated cardiomyopathy, O'Neal et al. showed a similar prognostic role of SBPR in the general population.14 Their study included a large number of cases with along follow-up duration. A low SBPR has also been associated with all-cause mortality and myocardial infarction, and the cutoff value of SBPR for predicting a poorer prognosis has been reported to range from 38 to 42 mmHg.10-14
Conventionally, LVEF and LV size have been used to evaluate VPC-induced cardiomyopathy. However, SBPR has been shown to be even more sensitive than conventional parameters in disclosing cardiomyopathy.13 Considering the poor prognosis of cardiomyopathy and heart failure,15-17 the relationship between SBPR and cardiomyopathy should be more clearly investigated. To the best of our knowledge, no previous studies have addressed the relationship between VPC burden and SBPR. Therefore, the aim of this study was to (1) evaluate the relationship between VPC burden and SBPR, (2) determine the best cutoff value of VPC burden for predicting a low SBPR, and (3) compare the sensitivity of SBPR and conventional markers for predicting VPC-induced cardiomyopathy.
MATERIAL AND METHODS
Patient enrollment
To determine the distribution of SBPR in patients with frequent VPCs, we enrolled all patients with a VPC burden exceeding 240 beats on 24-hour Holter electrocardiographic monitoring and treadmill exercise stress tests at our hospital from January 2015 to December 2015. Patients with a duration between Holter monitoring and treadmill exercise tests over 3 months and those with a heart rhythm other than a sinus rhythm were excluded. To avoid confounding from ischemic heart disease, the patients with a positive treadmill exercise test result or with other evidence of marked coronary artery disease were also excluded. During the treadmill test, the patients with ventricular tachycardia were excluded, but the patients with isolated VPCs or VPC couplets were enrolled. Patients with a LVEF less than 35%, marked concentric left ventricular hypertrophy with a ventricular wall thicker than 16 mm, dilated cardiomyopathy, hypertrophic cardiomyopathy with a septal wall thicker than 20 mm or severe valvular heart disease were also excluded.
VPC burden and systolic blood pressure response
VPC burden was assessed by 24-hour Holter electrocardiographic monitoring. Heart rhythm was also assessed using the same procedure. According to previous studies,18,19 the patients were classified into three groups based on VPC burden: Group 1; 240-1,000 beats/day, Group 2; 1,000-10,000 beats/day, and Group 3, > 10,000 beats/day.
SBPR was calculated from the recorded blood pressure during the treadmill exercise test. The difference in the SBP during maximal exercise and rest was recorded as the SBPR.
Treadmill exercise test
The treadmill exercise stress test (ML4500, Fukuda Denshi, Tokyo) was performed using the Bruce protocol. Electrocardiography and blood pressure were recorded before exercise, every 3 minutes during exercise, and after exercise. The initial systolic blood pressure (SBP) and maximum SBP were recorded, and the SBPR was calculated accordingly.
Data collection
The basic characteristics (age, gender, hemoglobin, serum level of glutamate-pyruvate transaminase, creatinine, uric acid, total cholesterol, and low-density lipoprotein), disease history (hypertension and diabetes mellitus), medical history (angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, beta blockers, diuretics, dihydropyridine calcium channel blockers, non-dihydropyridine calcium channel blockers, and statins), echocardiographic findings (LVEF, LVEDD, and LVESD) were collected. After data collection, the identification numbers were deleted to protect the privacy of the enrolled patients. This study was approved by the Institutional Review Board of Taipei Medical University.
Statistical analysis
All quantitative data were expressed as the mean ± standard deviation. One-way ANOVA with the Bonferroni post-hoc test was used to compare differences among the three groups. Categorical variables were compared using the chi-square test and Fisher’s exact test. All variables with a p-value less than 0.2 in univariate linear regression analysis were included in multivariate linear regression analysis. The VPC burden cut-off value was determined based on receiver operating characteristic (ROC) curve analysis. On the SBPR-VPC distribution figures, a fit line was added with the Loess method. The results with a p-value less than 0.05 were considered to be statistically significant.
RESULTS
Table 1 shows the basic characteristics of the enrolled patients (n = 153). There were 78 patients in Group 1, 54 in Group 2, and 21 in Group 3. Group 2 had the lowest diabetes mellitus rate and Group 3 had the highest diabetes mellitus rate. Group 2 had a significantly lower rate of diabetes mellitus than the other two groups. Group 1 had the highest prescription rate of statins, and the rate was significantly higher than in the other two groups. There were no significant differences in the other basic characteristics among the three groups.
Table 1. Basic characteristics.
| Group 1 | Group 2 | Group 3 | p value | |
| Case number, n | 78 | 54 | 21 | - |
| Age, mean ± SD, y | 63.1 ± 11.5 | 59.5 ± 12.2 | 60.6 ± 12.2 | 0.22 |
| Hemoglobin, mean ± SD, g/dl | 13.9 ± 1.3 | 13.6 ± 1.8 | 13.6 ± 1.0 | 0.49 |
| GPT, mean ± SD, U/l | 25.9 ± 10.8 | 22.5 ± 9.8 | 25.8 ± 11.6 | 0.21 |
| Creatinine, mean ± SD, mg/dl | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.3 | 0.07 |
| Uric acid, mean ± SD, mg/dl | 5.8 ± 2.4 | 5.6 ± 1.5 | 5.4 ± 1.4 | 0.7 |
| Total cholesterol, mean ± SD, mg/dl | 186.3 ± 40.1 | 183.3 ± 36.8 | 179.8 ± 29.7 | 0.78 |
| LDL, mean ± SD, mg/dl | 108.7 ± 39.4 | 109.3 ± 29.5 | 100.9 ± 35.2 | 0.64 |
| LV ejection fraction, mean ± SD, % | 71.1 ± 6.9 | 70.2 ± 9.3 | 68.3 ± 7.8 | 0.36 |
| LV EDD, mean ± SD, mm | 44.9 ± 5.7 | 45.2 ± 5.1 | 45.9 ± 4.1 | 0.73 |
| LVESD, mean ± SD, mm | 26.3 ± 4.8 | 26.9 ± 5.4 | 28.2 ± 4.5 | 0.3 |
| Female gender, % | 0.436 | 0.426 | 0.571 | 0.29 |
| Hypertension, % | 0.577 | 0.5 | 0.714 | 0.24 |
| Diabetes, % | 0.208 | 9.6%* | 0.333 | 0.05 |
| ACEI/ARB, % | 0.39 | 0.308 | 0.524 | 0.22 |
| Beta blocker, % | 0.506 | 0.673 | 0.714 | 0.08 |
| Diuretics, % | 0.078 | 0.096 | 0.048 | 0.78 |
| Dihydropyridine CCB, % | 0.351 | 0.212 | 0.381 | 0.18 |
| Non-dihydropyridine CCB, % | 0.104 | 0.038 | 0 | 0.14 |
| Statin, % | 55.8%* | 0.308 | 0.381 | 0.02 |
ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; EDD, end diastolic diameter; ESD, end systolic diameter; GPT, glutamic-pyruvic transaminase; LDL, low-density lipoprotein; LV, left ventricle; SD, standard deviation.
Figure 1A shows that the initial blood pressure was the same in all three groups. However, Group 1 had a higher maximal blood pressure and SBPR. Figure 1B shows the distribution of the SBPR according to VPC burden. As the VPC burden increased, the SBPR decreased rapidly in the beginning and then remained at a low level.
Figure 1.
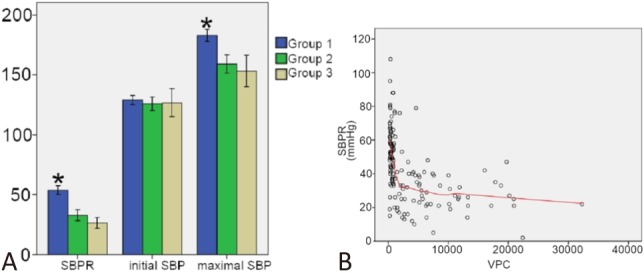
(A) Systolic blood pressure response (SBPR), initial systolic blood pressure (SBP), and maximal SBP of each group. (B) Distribution map of the SBPR to the ventricular premature complex (VPC) burden. * p < 0.05.
Table 2 shows the univariate and multivariate analyses for SBPR. The univariate analysis showed that age, gender, and VPC burden were associated with SBPR. We included the parameters with a p-value less than 0.2 in the univariate analysis into the multivariate analysis. The included parameters included age, uric acid level, gender, statin usage, and VPC burden. The multivariate analysis revealed that only VPC burden was associated with SBPR.
Table 2. Univariable and multivariable analyses of a systolic blood pressure response.
| 95% confidence interval | p value | ||
| Lower bound | Upper bound | ||
| Univariable analysis | |||
| Age | 0.01 | 0.59 | 0.05 |
| Hemoglobin | -1.27 | 4.14 | 0.30 |
| GPT | -0.22 | 0.41 | 0.56 |
| Creatinine | -18.5 | 11.9 | 0.67 |
| Uric acid | -0.34 | 3.08 | 0.11 |
| Total cholesterol | -0.13 | 0.04 | 0.31 |
| LDL | -0.14 | 0.06 | 0.43 |
| Ejection fraction | -0.15 | 0.67 | 0.21 |
| LV EDD | -0.67 | 0.56 | 0.86 |
| LV ESD | -1.02 | 0.27 | 0.25 |
| Gender | -13.3 | -0.92 | 0.03 |
| Hypertension | -4.7 | 8.12 | 0.60 |
| Diabetes | -3.02 | 13.3 | 0.22 |
| ACEI/ARB | -7.98 | 5.19 | 0.68 |
| Beta blocker | -10.1 | 2.91 | 0.28 |
| Diuretics | -8.82 | 14.7 | 0.62 |
| Dihydropyridine CCB | -6.40 | 7.47 | 0.88 |
| Non-dihydropyridine CCB | -6.11 | 19.4 | 0.30 |
| Statin | -0.59 | 12.1 | 0.08 |
| VPC burden | -0.002 | -0.001 | < 0.005 |
| Multivariable analysis | |||
| Age | -0.03 | 0.52 | 0.08 |
| Uric acid | -1.09 | 1.99 | 0.56 |
| Gender | -10.6 | 2.11 | 0.19 |
| Statin | -3.15 | 9.30 | 0.33 |
| VPC burden | -0.002 | -0.001 | < 0.005 |
ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; EDD, end diastolic diameter; ESD, end systolic diameter; GPT, glutamic-pyruvic transaminase; LDL, low-density lipoprotein; LV, left ventricle; VPC, ventricular premature complex.
Previous studies have reported a cut-off value of SBPR for predicting a poorer outcome of 40 mmHg. Figure 2 shows the ROC curve of VPC burden and a SBPR of < 40 mmHg. The area under the ROC curve was 0.85, and the best cut-off value of VPC burden for predicting a SBPR < 40 mmHg was 1,055 beats/day. The sensitivity was 79.2% and the specificity was 82.9%.
Figure 2.
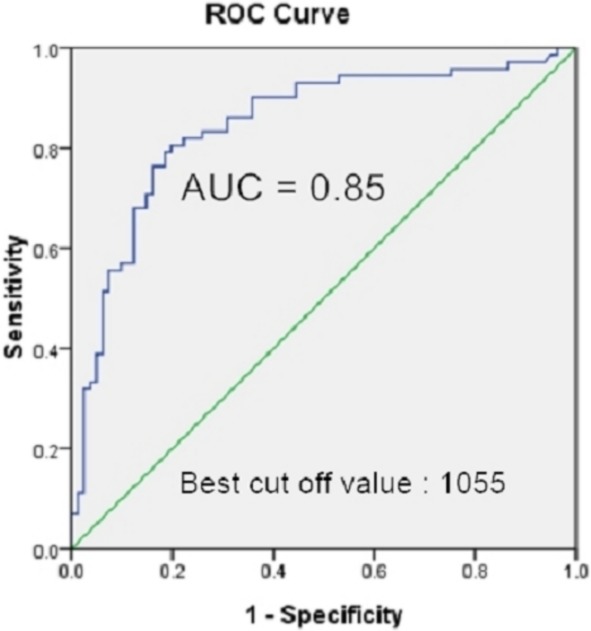
Receiver operating characteristic (ROC) curve for the ventricular premature complex (VPC) burden to predict a systolic blood pressure response less than 40 mmHg. The best cut-off value of the VPC burden was 1055 beats /day. The area under the curve (AUC) was 0.85.
Figure 3 shows the results of subgroup analysis. In all subgroups, a VPC burden < 1,055 beats/day predicted a SBPR of < 40 mmHg. In the subgroup with a LVEDD of > 52 mm, the result was marginal (95% confidence interval 0.03-0.99). There were only 11 cases in that subgroup.
Figure 3.
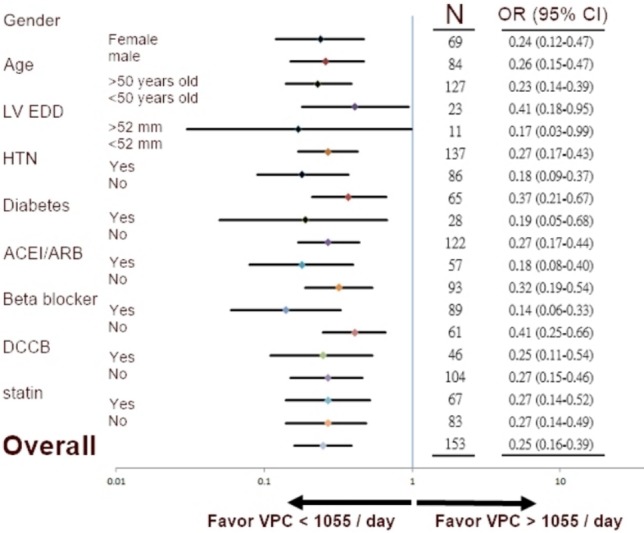
Subgroup analysis of a ventricular premature complex (VPC) burden of 1055 beats/day and systolic blood pressure response of < 40 mmHg.
Figure 4 shows the echocardiographic findings. Figure 4A shows the LVEF, LVEDD, and LVESD in each group. There were no significant differences among the three groups. Figure 4B shows the distribution of the LVEF, LVEDD, and LVESD according to VPC burden. Although Figure 4A shows no statistical differences, Figure 4B shows the trend of a decreasing LVEF and increasing left ventricular diameter as the VPC burden increased.
Figure 4.
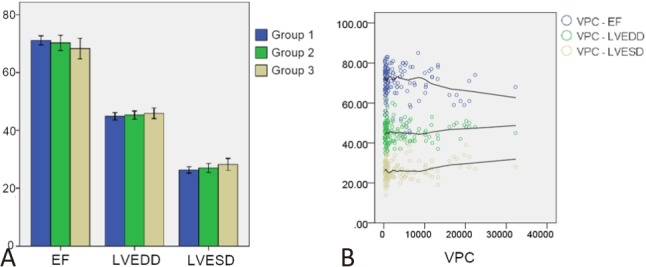
(A) Left ventricular ejection fraction (EF), left ventricular end diastolic diameter (LVEDD), and left ventricular end systolic diameter (LVESD) of each group. (B) Distribution map of the EF, LVEDD, and LVESD according to the ventricular premature complex (VPC) burden.
DISCUSSION
Association between VPC burden and SBPR
Evidence has shown that a VPC burden as high as 10,000 to 20,000 beats/day is associated with cardiomyopathy. The diagnosis of cardiomyopathy relies on echocardiographic measurements including LVEF and left ventricular diameter. In addition to echocardiographic findings, SBPR has recently been identified as a parameter for predicting impaired heart function.14,20 For the first time, we proved that VPC burden was associated with SBPR. In the distribution map and multivariate analysis, a lower VPC burden was associated with a higher SBPR.
Previous studies have reported that SBPR may serve as a more sensitive parameter for cardiomyopathy.10-14 Even with similar initial echocardiographic findings, a lower SBPR can predict a poor outcome in 5 to 10 years.14 In the present study, the SBPR was significantly lower in the groups with a VPC burden of 1,000 to 10,000 beats/day and more than 10,000 beats/day. The distribution map also showed that the SBPR decreased rapidly as the VPC burden increased. Because SBPR is more sensitive than conventional parameters for cardiomyopathy, the association between VPC burden and SBPR may potentially reflect VPC-induced cardiomyopathy.
Previous studies have reported a cut-off value of SBPR of about 40 mmHg, regardless of whether or not the patients had heart failure. Therefore, in this study, we set the SBPR cut-off value at 40 mmHg. With this definition, a VPC burden as low as 1,055 beats/day predicted a lower SBPR (less than 40 mmHg).
VPC burden and echocardiographic findings
In this study, the echocardiographic measurements did not differ significantly among the three groups with different VPC burdens. Even in the group with a VPC burden of larger than 10,000 beats/day, the LVEF and LV diameters were similar to those in the other groups. However, previous studies have reported an association between a high VPC burden and a low LVEF and large LV diameters.4,21-23 This discrepancy may due to the small number of cases and short follow-up period in the present study. Although there was no statistical difference in the distribution map (Figure 4B), there was a trend toward a decreasing LVEF and increasing LVEDD and LVESD as the VPC burden increased. To a certain degree, our findings are comparable to previous studies, even with different statistical results.
Different rates of diabetes and prescriptions of statins
Diabetes mellitus is an independent risk factor for cardiovascular disease. Because of autonomic neuropathy, patients with diabetes mellitus have a higher risk of arrhythmias.24,25 Hyperglycemia can induce VPCs by affecting calcium balance.26 In the present study, the prevalence rate of diabetes mellitus was statistically different among the three groups. Group 3 had the highest prevalence of diabetes mellitus, which was compatible with hyperglycemia-induced VPCs. This finding may mean that diabetes mellitus is a contributing factor to a reduced SBPR. However, diabetes mellitus was not an independent factor in the multivariate analysis. In addition, the prevalence of diabetes mellitus was lower in Group 2 than in Group 1. This further suggests that diabetes mellitus is less likely to be associated with SBPR. To clearly illustrate the relationship between diabetes mellitus and SBPR, further studies with a larger number of cases and longer follow-up period are necessary.
The rate of statin prescriptions was also significantly higher in Group 1. Statin agents have pleiotropic effects such as lowering cholesterol, anti-inflammation, and anti-oxidation. Several studies have shown that statin usage is associated with a reduced VPC burden due to their cholesterol-lowering activity.27-29 However, other studies have been unable to show similar preventative effects of ventricular arrhythmic events with statin treatment.30-32 Consequently, the relationship between statins and VPCs is still uncertain. In our study, Group 1 had a higher statin prescription rate and lower VPC burden. However, the use of statins was not associated with SBPR in the multivariate analysis, which is consistent with a previous study.33 In summary, although we showed that the group with a lower VPC burden was associated with a higher statin prescription rate, there was no association between statin usage and SBPR.
Association between age and gender and SBPR
In the univariate analysis, there were correlations between SBPR and age and gender. However, in the multivariate analysis, neither age nor gender was associated with SBPR. Further, in the subgroup analysis, we found that regardless of whether the age was older or younger than 50 years and regardless of whether the gender was male or female, < 1,055 VPCs/day could predict a SBPR > 40 mmHg. Accordingly, age and gender had no significant impact on our main findings.
Study limitations
This was a retrospective observational study. Therefore, we could not clarify if the relationship between VPC burden and SBPR was a causal relationship or not. Without any intervention and a prospective design, it was not possible to illustrate the direct effect of VPC burden on SBPR. In addition, the number of cases was small and the follow-up period was short. Some meaningful parameters such as echocardiographic measurements for cardiomyopathy were also lacking. SBPR alone has not been widely accepted as a cardiac outcome endpoint, however there were no significant differences in other well-known cardiac outcome endpoints, which may be due to the small scale of this study. A further large-scale study is necessary to address the definite relationship between VPC burden and SBPR.
This study was also limited by its short follow-up period, which may have resulted in a lack of significant differences inclinical outcomes. Although SBPR has been shown to be related to clinical outcomes, the direct relationship between VPC burden, SBPR and clinical outcome should be evaluated in other studies with a longer follow-up period and even a prospective study design.
Although catheter-based ablation has been proven to be an effective strategy to eliminate VPCs,34,35 none of the enrolled patient in this study received such treatment, making comparisons of before and after treatment impossible. Further extensive studies should be undertaken to evaluate changes in SBPR after VPC elimination. Furthermore, in this study, we did not evaluate VPC burden during the treadmill test. In clinical practice, VPC burden may be affected by exercise. Further studies are needed to investigate the associations between VPC burden during exercise, SBPR and clinical prognosis.
CONCLUSIONS
A VPC burden of over 1055 beats/day was associated with a low SBPR. Based on the prognostic role of low SBPR, more attention should be paid to VPCs in real word practice.
Acknowledgments
This study was approved by Taipei Medical University – Joint Institutional Review Board. The approved number is N201610043.
REFERENCES
- 1.Kennedy HL, Whitlock JA, Sprague MK, et al. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N Engl J Med. 1985;312:193–197. doi: 10.1056/NEJM198501243120401. [DOI] [PubMed] [Google Scholar]
- 2.Latchamsetty R, Bogun F. Premature ventricular complexes and premature ventricular complex induced cardiomyopathy. Curr Probl Cardiol. 2015;40:379–422. doi: 10.1016/j.cpcardiol.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Sarrazin JF, Labounty T, Kuhne M, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm. 2009;6:1543–1549. doi: 10.1016/j.hrthm.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–869. doi: 10.1016/j.hrthm.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 5.Del Carpio Munoz F, Syed FF, Noheria A, et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J Cardiovasc Electrophysiol. 2011;22:791–798. doi: 10.1111/j.1540-8167.2011.02021.x. [DOI] [PubMed] [Google Scholar]
- 6.Chugh SS, Shen WK, Luria DM, Smith HC. First evidence of premature ventricular complex-induced cardiomyopathy: a potentially reversible cause of heart failure. J Cardiovasc Electrophysiol. 2000;11:328–329. doi: 10.1111/j.1540-8167.2000.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 7.Yokokawa M, Good E, Crawford T, et al. Recovery from left ventricular dysfunction after ablation of frequent premature ventricular complexes. Heart Rhythm. 2013;10:172–175. doi: 10.1016/j.hrthm.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 8.El Kadri M, Yokokawa M, Labounty T, et al. Effect of ablation of frequent premature ventricular complexes on left ventricular function in patients with nonischemic cardiomyopathy. Heart Rhythm. 2015;12:706–713. doi: 10.1016/j.hrthm.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Hasdemir C, Ulucan C, Yavuzgil O, et al. Tachycardia-induced cardiomyopathy in patients with idiopathic ventricular arrhythmias: the incidence, clinical and electrophysiologic characteristics, and the predictors. J Cardiovasc Electrophysiol. 2011;22:663–668. doi: 10.1111/j.1540-8167.2010.01986.x. [DOI] [PubMed] [Google Scholar]
- 10.Kallistratos MS, Poulimenos LE, Pavlidis AN, et al. Prognostic significance of blood pressure response to exercise in patients with systolic heart failure. Heart Vessels. 2012;27:46–52. doi: 10.1007/s00380-010-0115-z. [DOI] [PubMed] [Google Scholar]
- 11.Raphael CE, Whinnett ZI, Davies JE, et al. Quantifying the paradoxical effect of higher systolic blood pressure on mortality in chronic heart failure. Heart. 2009;95:56–62. doi: 10.1136/hrt.2007.134973. [DOI] [PubMed] [Google Scholar]
- 12.Nishiyama Y, Morita H, Harada H, et al. Systolic blood pressure response to exercise as a predictor of mortality in patients with chronic heart failure. Int Heart J. 2010;51:111–115. doi: 10.1536/ihj.51.111. [DOI] [PubMed] [Google Scholar]
- 13.Tateishi E, Noguchi T, Goto Y, et al. Prognostic impact of blood pressure response plus gadolinium enhancement in dilated cardiomyopathy. Heart. 2015;101:774–780. doi: 10.1136/heartjnl-2014-307007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Neal WT, Qureshi WT, Blaha MJ, et al. Systolic blood pressure response during exercise stress testing: The Henry Ford ExercIse Testing (FIT) Project. J Am Heart Assoc. 2015;4:e002050. doi: 10.1161/JAHA.115.002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tung YC, Chou SH, Liu KL, et al. Worse prognosis in heart failure patients with 30-day readmission. Acta Cardiol Sin. 2016;32:698–707. doi: 10.6515/ACS20151113A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang HY, Wang CC, Wu YW, et al. One-year outcomes of acute decompensated systolic heart failure in Taiwan: Lessons from TSOC-HFrEF Registry. Acta Cardiol Sin. 2017;33:127–138. doi: 10.6515/ACS20170202A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang CC, Chang HY, Yin WH, et al. TSOC-HFrEF Registry: a registry of hospitalized patients with decompensated systolic heart failure: description of population and management. Acta Cardiol Sin. 2016;32:400–411. doi: 10.6515/ACS20160704A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanei Y, Friedman M, Ogawa N, et al. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol. 2008;13:81–85. doi: 10.1111/j.1542-474X.2007.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YH, Zhong L, Roger VL, et al. Frequency, origin, and outcome of ventricular premature complexes in patients with or without heart diseases. Am J Cardiol. 2014;114:1373–1378. doi: 10.1016/j.amjcard.2014.07.072. [DOI] [PubMed] [Google Scholar]
- 20.Gupta MP, Polena S, Coplan N, et al. Prognostic significance of systolic blood pressure increases in men during exercise stress testing. Am J Cardiol. 2007;100:1609–1613. doi: 10.1016/j.amjcard.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 21.Lee AK, Deyell MW. Premature ventricular contraction-induced cardiomyopathy. Curr Opin Cardiol. 2016;31:1–10. doi: 10.1097/HCO.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 22.Niwano S, Wakisaka Y, Niwano H, et al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart. 2009;95:1230–1237. doi: 10.1136/hrt.2008.159558. [DOI] [PubMed] [Google Scholar]
- 23.Zhong L, Lee YH, Huang XM, et al. Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: a single-center retrospective study. Heart Rhythm. 2014;11:187–193. doi: 10.1016/j.hrthm.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 24.Movahed MR, Hashemzadeh M, Jamal M. Increased prevalence of ventricular fibrillation in patients with type 2 diabetes mellitus. Heart Vessels. 2007;22:251–253. doi: 10.1007/s00380-006-0962-9. [DOI] [PubMed] [Google Scholar]
- 25.Nakou ES, Mavrakis H, Vardas PE. Are diabetic patients at increased risk of arrhythmias? Hellenic J Cardiol. 2012;53:335–339. [PubMed] [Google Scholar]
- 26.Erickson JR, Pereira L, Wang L, et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–376. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dewey FE, Perez M, Hadley D, et al. Statin use and ventricular arrhythmias during clinical treadmill testing. J Cardiovasc Electrophysiol. 2009;20:193–199. doi: 10.1111/j.1540-8167.2008.01284.x. [DOI] [PubMed] [Google Scholar]
- 28.Kishi T, Yamada A, Okamatsu S, et al. Atorvastatin might improve ventricular electrostability and decelerate the deterioration of renal function in patients with heart failure and diabetes mellitus. J Cardiol. 2009;53:341–348. doi: 10.1016/j.jjcc.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Goonasekara CL, Balse E, Hatem S, et al. Cholesterol and cardiac arrhythmias. Expert Rev Cardiovasc Ther. 2010;8:965–979. doi: 10.1586/erc.10.79. [DOI] [PubMed] [Google Scholar]
- 30.Rahimi K, Mcgale P, Majoni W, et al. Effect of statins on ventricular arrhythmic events: a collaborative meta-analysis of randomised controlled trials. European Heart Journal. 2009;30(suppl 1):591. [Google Scholar]
- 31.Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Drugs. 2004;64 Suppl 2:43–60. doi: 10.2165/00003495-200464002-00005. [DOI] [PubMed] [Google Scholar]
- 32.Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 33.Zaleski AL, Mentch ML, Pescatello LS, et al. Effects of atorvastatin on resting and peak exercise blood pressure among normotensive men and women. Cholesterol. 2014;2014:720507. doi: 10.1155/2014/720507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung FP, Lin YJ, Chang SL, et al. Long-term follow-up of catheter ablation of ventricular arrhythmias: experiences from a tertiary referral center in Taiwan. Acta Cardiol Sin. 2015;31:8–17. doi: 10.6515/ACS20140721A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao JN, Hu YF, Lin WS, et al. Gender difference in idiopathic right ventricular outflow tract-ventricular tachycardia. Acta Cardiol Sin. 2013;29:304–310. [PMC free article] [PubMed] [Google Scholar]


