Abstract
Smoking is an important contributor to cardiovascular disease risk and is highly prevalent in the HIV population. In the Stopping Atherosclerosis and Treating Unhealthy Bone with Rosuvastatin in HIV trial (SATURN-HIV), a 96-week, randomized placebo-controlled study testing the effect of rosuvastatin on subclinical vascular disease and immune activation in HIV-infected adults, rosuvastatin improved immune activation and arrested common carotid artery intima media thickness (CCA IMT) progression. In this exploratory analysis, ANOVA was used to test for effect modification by smoking. One-hundred forty-seven adults were included (72 in rosuvastatin group; 75 in placebo group). Groups were similar at baseline. Overall, mean ± SD age was 45.4 ± 9.9 years, 115 (78%) were men and 100 (68%) were African American. Ninety-three (63%) were current smokers (mean ± SD 0.6 ± 0.44 packs/day) and another 24 (16%) were smokers in the past. There were statistically significant randomization group by smoking status interactions for 0–24 (p = .01) and 0–48 (p < .01) week changes in proportion of activated CD4+ T cells and for 0–48 (p < .01) and 0–96 (trend only; p = .06) week changes in CCA IMT. No effect modification by smoking was detected for changes in markers of inflammation or monocyte activation. The beneficial effect of rosuvastatin on CCA IMT was not apparent in smokers although T cell activation improved to a greater degree in this subgroup.
Keywords: : smoking, inflammation, subclinical vascular disease, HIV, antiretroviral therapy
Introduction
People living with HIV are at increased risk of cardiovascular disease (CVD) events.1 We and others have shown that 3-hydroxy-3 methylglutaryl coenzyme A reductase inhibitors or statins improve subclinical vascular disease in HIV-infected adults on antiretroviral therapy (ART).2,3 Further, in the Stopping Atherosclerosis and Treating Unhealthy Bone with Rosuvastatin in HIV (SATURN-HIV) trial, rosuvastatin improved markers of inflammation including interferon γ inducible protein-10 (IP-10) and lipoprotein-associated phospholipase A2 (Lp-PLA2), immune activation including soluble CD14 (sCD14), proportion of tissue factor expressing patrolling monocytes and proportion of activated CD4+ and CD8+ T cells4–6 and oxidized low density lipoprotein (oxLDL).7
Smoking is highly prevalent in people living with HIV8 and has long been recognized as an important contributor to CVD.9 Understanding the effect of smoking on CVD risk reduction interventions such as statins is relevant to HIV care providers because it will help to prioritize which patients should be targeted for the intervention. The aim of this exploratory analysis was to assess whether smoking status modifies the effect of rosuvastatin on select outcome measures that differed between groups over the SATURN-HIV study.
The SATURN-HIV study is a 96-week, single site, randomized, placebo-controlled trial to evaluate the effect of rosuvastatin on immune activation and subclinical vascular disease in HIV-infected adults on ART. The primary results of this trial have been published2 and full eligibility criteria may be found on clinicaltrials.gov (NCT01218802). In brief, participants were ≥18 years old with HIV-1 infection on stable ART for at least 3 months with HIV-1 RNA <1,000 copies/mL and fasting LDL ≤130 mg/dl. Additional entry criteria included proportion of CD8+ T cells that express CD38 and human leukocyte antigen (HLA)-DR ≥19% or high sensitivity C-reactive protein (hsCRP) ≥2 mg/liter. Known coronary artery disease or diabetes or inflammatory conditions were exclusionary. Randomization was to rosuvastatin 10 mg daily or matching placebo. In this exploratory, post hoc analysis, we assessed whether smoking status modified the effect of rosuvastatin on changes in markers of subclinical vascular disease, immune activation, and inflammation that differed between groups over the study. The study was approved by the University Hospitals Cleveland Medical Center Institutional Review Board.
Study Evaluations
All participants underwent carotid intima media thickness (IMT) measurement by high resolution ultrasound at 0, 48, and 96 weeks as previously described.10 Additionally, a 12-h fasting blood draw was performed at 0, 24, 48, and 96 weeks. Samples were cryopreserved at −80°C until analyzed in batches. Cellular markers of immune activation were phenotyped from peripheral blood mononuclear cells by flow cytometry as previously described.4 CD4+ and CD8+ T cells expressing CD38 and HLA-DR (activated) were quantified as a percentage of the CD4+ and CD8+ lymphocyte population, respectively. Monocyte subsets including CD14+CD16+ (inflammatory) and CD14dimCD16+ (patrolling) were quantified as a percentage of the monocyte population. Monocyte subset expression of tissue factor was also quantified. Soluble markers of monocyte activation (sCD14), systemic and vascular inflammation (IP-10 and Lp-PLA2), and oxLDL were measured by ELISA (sCD14 and IP-10; R&D Systems, Minneapolis, MN; Lp-PLA2; diaDexus, Inc., CA; oxLDL; Mercodia, Uppsala, Sweden).
Statistical Analysis
ANOVA was used to model each outcome. Outcomes evaluated were those that differed statistically between groups in the SATURN-HIV study, that is, absolute changes in log-transformed common carotid artery (CCA) IMT, IP-10, Lp-PLA2, sCD14, proportion of tissue factor expressing patrolling monocytes, proportion of activated CD4+ and CD8+ T cells and oxLDL over 48 and 96 weeks. Independent variables included in each model were baseline value for the outcome measure, group, smoking status, and a group by smoking status interaction. All statistical tests were two-sided and considered statistically significant if p < .05 in this hypothesis-generating study. Analyses were performed using SAS v. 9.4 (The SAS Institute, Cary, NC).
Results
One hundred forty-seven adults were randomized (72 to rosuvastatin and 75 to placebo). The randomization groups were similar at baseline. Overall, mean ± SD age was 45.4 ± 9.9 years, 78% were men and 68% were African American. Sixty-three percent were current smokers (mean of 0.6 ± 0.44 packs/day) and another 16% were past smokers. Mean known duration of HIV infection was 12 ± 7 years and current and nadir CD4+ T cell counts were 640 ± 300 and 200 ± 146 cell/mm,3 respectively. Most (78%) had HIV-1 RNA <50 copies/ml. At baseline, current smokers were similar to nonsmokers based on each of these factors as well.
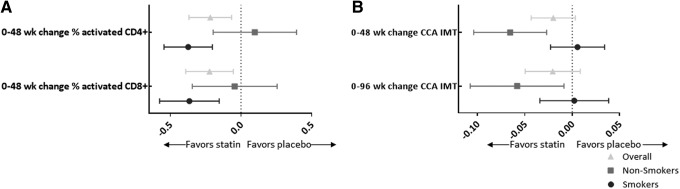
There were statistically significant randomization group by smoking status interaction terms for 0–24 (p = .01) and 0–48 (p < .01) week changes in proportion of activated CD4+ T cells and a trend toward significance for 0–48 week change in activated CD8+ T cells (p = .07). In addition, the interaction was significant for 0–48 week change in CCA IMT (p < .01) and trended toward significance for 0–96 week change in CCA IMT (p = .06). The stratum-specific estimates reveal that the improvement in CD4+ and CD8+ T cell activation with rosuvastatin is statistically significant for the smoking group only. In contrast, the beneficial effect of rosuvastatin on CCA IMT was significant for the nonsmoking group only. For smokers, rosuvastatin did improve T cell activation; however, the beneficial effect of rosuvastatin on CCA IMT was not apparent in this group (Fig. 1). No effect modification by smoking was detected for changes in other markers tested.
FIG. 1.
(A) The symbols represent the mean difference in 0–48 week change in log-transformed proportion of activated CD8+ and CD4+ T cells between rosuvastatin and placebo groups. The error bars show the 95% confidence interval around the means. (B) The symbols represent the mean difference in 0–48 and 0–96 week change in log-transformed common carotid artery intima media thickness between rosuvastatin and placebo groups. The error bars again show the 95% confidence interval around the means. For both figures, a mean difference less than 0 favors the rosuvastatin group.
Discussion
In this analysis, we have shown that the beneficial effects of rosuvastatin on T cell activation and subclinical vascular disease differ between HIV-infected smokers and nonsmokers. Interestingly, the improvement in T cell activation with rosuvastatin was only apparent in HIV-infected smokers; whereas, the beneficial effect of rosuvastatin on CCA IMT was not present in this group. At a first glance, the effect of smoking status on CCA IMT and T cell activation may seem contradictory; however, we have previously shown that the changes in T cell activation did not predict changes in CCA IMT2 and so these effects are likely independent of one another.
Although the mechanisms linking smoking to CVD are not fully known, a major contributor is smoking-induced oxidative stress that leads to endothelial dysfunction, lipid peroxidation, and platelet activation.11–13 Further, in the Women's Health Study, smoking was associated with higher levels of hsCRP, interleukin-6, soluble intercellular adhesion molecule type 1, and E-selectin—four out of the five inflammatory markers tested.14 In HIV, smokers had higher CD4+ and CD8+ T cell activation and exhaustion, measures of monocyte activation (sCD14), and microbial translocation (bacterial lipopolysaccharide).15 Atherosclerosis is known to be an inflammatory disease. T cells, macrophages, and mast cells dominate early atherosclerotic lesions and cytokines accelerate progression of the lesions, which may ultimately result in plaque rupture.16 In HIV-related CVD, there have been many links to systemic17 and vascular inflammation,18 and monocyte activation.19,20 Given this, it stands to reason that smoking may increase the risk of CVD in HIV even more than in the general population, which has been suggested by Freiberg et al. who showed a significant interaction between HIV and smoking status in risk of prevalent CVD.21
Whether smoking modifies the effect of statins on immune activation and CVD risk in the general population is largely unknown. However, in the Jupiter Trial, the hazard ratio for the combined primary endpoint of myocardial infarction, stroke, revascularization, unstable angina, or death from CVD was similar for smokers and nonsmokers and the interaction term was not statistically significant (p = .63).22 Even though this is counter to what we have shown with CCA IMT, it is possible that with the heightened immune activation that characterizes chronic HIV infection, the additional effect of smoking may be too much to correct with statins.
Because SATURN-HIV was not designed to test effect modification, we may not have detected all relevant interactions due to limited power. As only participants with fasting LDL ≤130 mg/dl and heightened hsCRP or evidence of increased T cell activation were included in this study, results may not be generalizable to all HIV-infected individuals. Last, smoking status was not a stratification variable during randomization. As such, while baseline factors were similar between randomization groups and by smoking status, all factors may not have been evenly distributed across interaction subgroups.
In conclusion, current smoking modifies the effect of rosuvastatin on CCA IMT and T cell activation. The beneficial effect of rosuvastatin on CCA IMT was not apparent in smokers even though T cell activation appears to improve more in this group. Targeting HIV-infected smokers with low LDL for primary CVD prevention utilizing rosuvastatin may not be appropriate unless smoking cessation is achieved; however, further studies are needed to confirm these findings.
Acknowledgments
This study was supported by the National Institutes of Health (grant numbers K23HL116209 to C.O.H., HD070490 and NR012642 to G.A.M.). Study drugs were provided by AstraZeneca. Technical assistance was provided by the Center for AIDS Research, Case Western Reserve University (P30 AI36219). Results were presented at the Conference on Retroviruses and Opportunistic Infections in Boston, Massachusetts, February 22–25, 2016.
Disclosure Statement
C.O.H. has served on a medical advisory board for Gilead Sciences. G.A.M. has received research grants from BMS, Gilead Sciences, Merck, and GSK and has served as a consultant to BMS, Viiv/GSK, ICON, and Gilead.
References
- 1.Petoumenos K, Reiss P, Ryom L, et al. : Increased risk of cardiovascular disease (CVD) with age in HIV-positive men: A comparison of the D:A:D CVD risk equation and general population CVD risk equations. HIV Med 2014;15:595–603 [DOI] [PubMed] [Google Scholar]
- 2.Longenecker CT, Sattar A, Gilkeson R, McComsey GA: Rosuvastatin slows progression of subclinical atherosclerosis in patients with treated HIV infection. AIDS 2016;30:2195–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo J, Lu MT, Ihenachor EJ, et al. : Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: A randomised, double-blind, placebo-controlled trial. Lancet HIV 2015;2:e52–e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funderburg NT, Jiang Y, Debanne SM, et al. : Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis 2014;58:588–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA: Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis 2014;209:1156–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funderburg NT, Jiang Y, Debanne SM, et al. : Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr 2015;68:396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hileman CO, Turner R, Funderburg NT, Semba RD, McComsey GA: Changes in oxidized lipids drive the improvement in monocyte activation and vascular disease after statin therapy in HIV. AIDS 2016;30:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mdodo R, Frazier EL, Dube SR, et al. : Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: Cross-sectional surveys. Ann Intern Med 2015;162:335–344 [DOI] [PubMed] [Google Scholar]
- 9.Doyle JT, Dawber TR, Kannel WB, Kinch SH, Kahn HA: The relationship of cigarette smoking to coronary heart disease; the second report of the combined experience of the Albany, Ny. And Framingham, Mass. Studies. JAMA 1964;190:886–890 [PubMed] [Google Scholar]
- 10.Longenecker CT, Hileman CO, Funderburg NT, McComsey GA: Rosuvastatin preserves renal function and lowers cystatin C in HIV-infected subjects on antiretroviral therapy: The SATURN-HIV trial. Clin Infect Dis 2014;59:1148–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celermajer DS, Sorensen KE, Georgakopoulos D, et al. : Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 1993;88(5 Pt 1):2149–2155 [DOI] [PubMed] [Google Scholar]
- 12.Barua RS, Ambrose JA, Srivastava S, DeVoe MC, Eales-Reynolds LJ: Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: An in vitro demonstration in human coronary artery endothelial cells. Circulation 2003;107:2342–2347 [DOI] [PubMed] [Google Scholar]
- 13.Ambrose JA, Barua RS: The pathophysiology of cigarette smoking and cardiovascular disease: An update. J Am Coll Cardiol 2004;43:1731–1737 [DOI] [PubMed] [Google Scholar]
- 14.Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM: Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol 2002;89:1117–1119 [DOI] [PubMed] [Google Scholar]
- 15.Valiathan R, Miguez MJ, Patel B, Arheart KL, Asthana D: Tobacco smoking increases immune activation and impairs T-cell function in HIV infected patients on antiretrovirals: A cross-sectional pilot study. PLoS One 2014;9:e97698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansson GK: Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–1695 [DOI] [PubMed] [Google Scholar]
- 17.Ross AC, Rizk N, O'Riordan MA, et al. : Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis 2009;49:1119–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subramanian S, Tawakol A, Burdo TH, et al. : Arterial inflammation in patients with HIV. JAMA 2012;308:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burdo TH, Lo J, Abbara S, et al. : Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011;204:1227–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker JV, Hullsiek KH, Singh A, et al. : Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS 2014;28:831–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freiberg MS, McGinnis KA, Kraemer K, et al. : The association between alcohol consumption and prevalent cardiovascular diseases among HIV-infected and HIV-uninfected men. J Acquir Immune Defic Syndr 2010;53:247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridker PM, Danielson E, Fonseca FA, et al. : Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–2207 [DOI] [PubMed] [Google Scholar]