Abstract
Human parainfluenza viruses (family Paramyxoviridae), human metapneumovirus, and respiratory syncytial virus (family Pneumoviridae) infect most infants and children within the first few years of life and are the etiologic agents for many serious acute respiratory illnesses. These virus infections are also associated with long-term diseases that impact quality of life, including asthma. Despite over a half-century of vaccine research, development, and clinical trials, no vaccine has been licensed to date for the paramyxoviruses or pneumoviruses for the youngest infants. In this study, we describe the recent reclassification of paramyxoviruses and pneumoviruses into distinct families by the International Committee on the Taxonomy of Viruses. We also discuss some past unsuccessful vaccine trials and some currently preferred vaccine strategies. Finally, we discuss hurdles that must be overcome to support successful respiratory virus vaccine development for the youngest children.
Keywords: : respiratory syncytial virus, measles virus, human metapneumovirus, parainfluenza virus, Sendai virus
Reclassification and Comparison of Paramyxoviridae and Pneumoviridae
The paramyxoviruses and pneumoviruses have been recently reclassified (2,3). Previously, the Paramyxoviridae family of viruses included subfamilies Paramyxovirinae and Pneumovirinae. However, in 2016 the International Committee on the Taxonomy of Viruses recommended that the paramyxoviruses and pneumoviruses be split into distinct families (Paramyxoviridae and Pneumoviridae). The reclassification was made for several reasons. First, the polymerase genes of pneumoviruses are more closely related to those of filoviruses than those of paramyxoviruses. Second, pneumoviruses differ from paramyxoviruses by possession of an M2 gene that encodes two unique proteins. Third, the ribonucleoprotein (RNP) complexes of pneumoviruses and paramyxoviruses are structurally distinct (2).
The most current taxonomy listings and virus names can be found online at www.ictvonline.org Table 1 includes examples of Paramyxoviridae and Pneumoviridae family members. The Paramyxoviridae family currently contains seven genera, including Morbillivirus, Respirovirus, and Rubulavirus. The Pneumoviridae family contains the genera Metapneumovirus and Orthopneumovirus.
Table 1.
Examples of Members of the Families Paramyxoviridae and Pneumoviridae
| Family | Genus | Speciesa |
|---|---|---|
| Paramyxoviridae | Aquaparamyxovirus | Salmon aquaparamyxovirus (Atlantic salmon paramyxovirus, AsaPV) |
| Avulavirus | Avian avulavirus 1 (Newcastle disease virus, NDV) | |
| Ferlavirus | Reptilian ferlavirus (Fer-de-Lance virus, FDLV) | |
| Henipavirus | Hendra henipavirus (Hendra virus, HeV) | |
| Nipah henipavirus (Nipah virus, NiV) | ||
| Cedar virus (CedV) | ||
| Morbillivirus | Measles morbillivirus (measles virus, MeV) | |
| Canine morbillivirus (canine distemper virus, CDV) | ||
| Rinderpest morbillivirus (rinderpest virus, RPV) | ||
| Respirovirus | Human respirovirus 1 (human parainfluenza virus 1, HPIV1) | |
| Murine respirovirus (Sendai virus, SeV) | ||
| Human respirovirus 3 (human parainfluenza virus 3, HPIV3) | ||
| Bovine respirovirus 3 (bovine parainfluenza virus 3, BPIV3) | ||
| Rubulavirus | Mumps rubulavirus (mumps virus, MuV) | |
| Mammalian rubulavirus 5 (parainfluenza virus 5, PIV5; previously named simian virus 5, SV5) | ||
| Human rubulavirus 2 (human parainfluenza virus 2, HPIV2) | ||
| Human rubulavirus 4 (human parainfluenza virus 4, HPIV4) | ||
| Simian rubulavirus (simian virus 41, SV-41) | ||
| Pneumoviridae | Metapneumovirus | Human metapneumovirus (HMPV) |
| Avian metapneumovirus (AMPV) | ||
| Orthopneumovirus | Human orthopneumovirus (human respiratory syncytial virus, HRSV) | |
| Bovine orthopneumovirus (bovine respiratory syncytial virus, BRSV) | ||
| Murine orthopneumovirus (murine pneumonia virus; previously pneumonia virus of mice, PVM) |
Renamed virus species that use non-Latinized binomial names similar to nomenclature already implemented for six of the eight families of Mononegavirales are listed first (ICTV code 2016.011aM). Names of exemplar virus members and abbreviations are listed in parentheses.
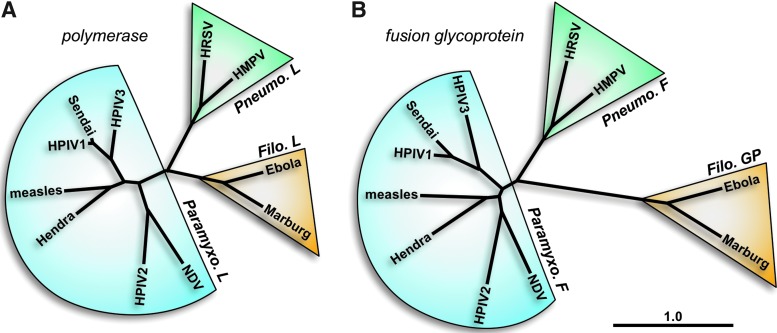
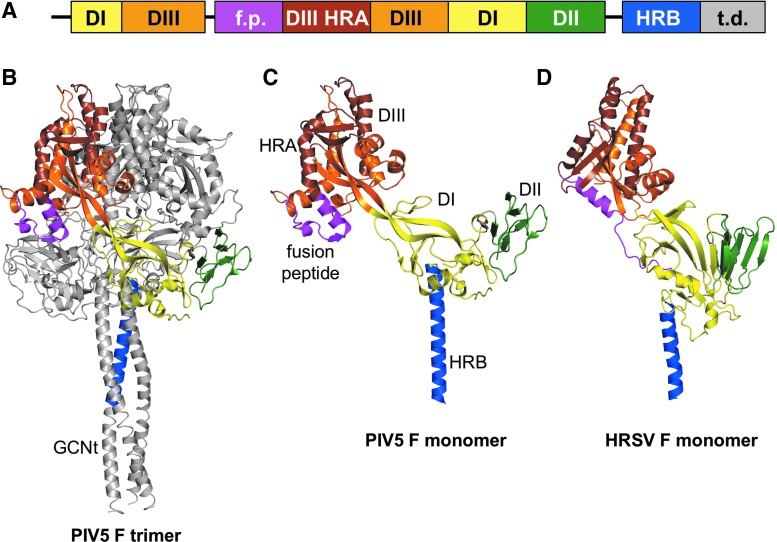
The paramyxoviruses and pneumoviruses have a variety of similarities and differences. The polymerase (L) proteins of viruses from these two families are well separated phylogenetically, having an almost equal phylogenetic relationship with members of the Filoviridae family (e.g., Ebola virus) as with each other (Fig. 1A). On the other hand, the fusion (F) surface glycoproteins of pneumoviruses are much more closely related phylogenetically to those from paramyxoviruses than they are to the glycoproteins (GP) of filoviruses (Fig. 1B). Accordingly, the structures of the F proteins of the paramyxovirus PIV5 and the pneumovirus human respiratory syncytial virus (HRSV or RSV) in their prefusion forms are similar to each other and quite distinct from Ebola virus GP (48,54,96). In addition to having similar domain structures (Fig. 2), the PIV5 and HRSV F proteins have similar intermediate structures that can be inhibited by heptad repeat-derived peptides (46,69,71) and form similar six-helix bundle structures in their postfusion forms (6,56,84). As the F surface glycoprotein is a primary antigen of the pneumoviruses and paramyxoviruses, similar vaccine strategies for both virus families may prove successful.
FIG. 1.
Phylogenetic relationships between representative members of the Paramyxoviridae, Pneumoviridae, and Filoviridae families. Amino acid sequences of the polymerase (A) and surface glycoprotein (B) genes were used to compare virus families. Taxonomy is shown according to the 2016 release by the International Committee on the Taxonomy of Viruses. The phylogenetic tree was generated with CLC Main Workbench (CLC bio). The scale bar represents branch length as base substitutions per site. Virus names are abbreviated as follows: human metapneumovirus (HMPV), human parainfluenza virus 1 (HPIV1), human parainfluenza virus 2 (HPIV2), human parainfluenza virus 3 (HPIV3), human respiratory syncytial virus (HRSV), and Newcastle disease virus (NDV). L protein sequences are as follows: Ebolavirus Zaire (NP_066251), Marburg virus 1980 (YP_001531159), Hendra virus (NP_047113), HMPV CAN97-83 (YP_012613), HPIV1 Z (CAA272273), HPIV2 Toshiba (P26676), HPIV3 (ZLNZP3), HRSV B1 (NP_056924), measles virus Ichinose-B95a (NP_056924), NDV B1 (NP_071471), and Sendai virus Enders (AAA69579). GP protein sequences are from Ebolavirus Zaire (AAB81004) and Marburg virus Popp (CAA48507). F protein sequences are from Hendra virus (NP_047111.2), HMPV TN/92-4 (ABM67j072), HPIV1 C39 (P12605), HPIV2 (NP_598404), HPIV3 (AAB21447.1), HRSV B 9320 (AAR14266), measles virus Edmonston (AF266288_6), NDV LaSota (AAC28374.1), and Sendai virus Z (AAB06281.1).
FIG. 2.
Prefusion structures of the fusion (F) proteins of PIV5 and HRSV. (A) Ectodomain structure of the F protein. Domains are as follows: domain I (DI, yellow), domain II (DII, green), domain III (DIII, orange), domain III heptad repeat A (DIII HRA, brown), fusion peptide (f.p., magenta), heptad repeat B (HRB, blue), and trimerization domain (t.d.). (B, C) Trimer and monomer structure of the PIV5 F protein in its prefusion form. (D) Monomer structure of the HRSV F protein in its prefusion form. Domains in B–D are color coded as in the domain structure (A). Structures of PIV5 F (96) and HRSV F (54) were rendered with MacPYMOL using coordinates 2B9B and 4JHW, respectively.
Virion Structure
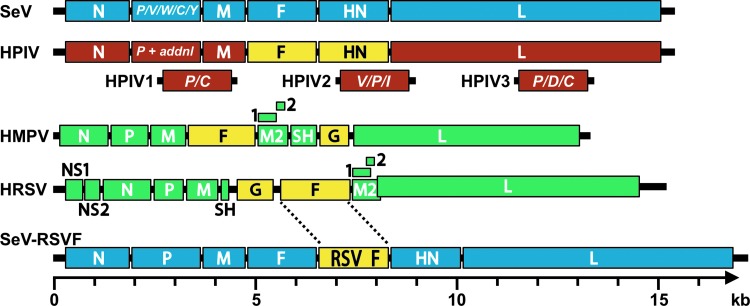
As members of the order Mononegavirales, the paramyxoviruses and pneumoviruses have nonsegmented, linear, single-stranded negative-sense RNA genomes (Fig. 3). Their viral RNA (vRNA) genomes are encapsidated by nucleoproteins (N). The genetic cores of these viruses are RNP complexes that consist of vRNA, N protein, phosphoprotein (P), and the large (L) polymerase protein (19). The matrix (M) protein has two compact beta-rich domains connected by a linker (59). The M protein facilitates virus assembly through interactions with the RNP, plasma membrane, and envelope glycoproteins (75,87). While the F glycoproteins of the paramyxoviruses and pneumoviruses are phylogenetically and structurally similar (Fig. 2), the hemagglutinin–neuraminidase (HN), hemagglutinin (H), and glycoprotein (G, for HMPV and HRSV) surface attachment proteins differ in sequence, structure, and binding properties (13,31,47,55,97). For both virus families, the F protein and the attachment protein are exposed on the surfaces of virions and infected cells, help promote virus entry, and provide targets for vaccine development.
FIG. 3.
Schematic diagrams of paramyxovirus and pneumovirus genomes. Genomes shown include Sendai virus (SeV, blue), a generic human parainfluenza virus genome (HPIV, brown), human metapneumovirus (HMPV, green), human respiratory syncytical virus (HRSV green) and an RSV F vaccine vectored by Sendai virus (SeV-RSVF, blue with yellow insert). The additional (termed “addnl” in the figure) proteins expressed from the P genes of HPIV1, HPIV2, and HPIV3 are shown in brown below the generic HPIV genome. Surface glycoproteins of the human viruses are shown in yellow. In each genome, the 3′ leader is on the left terminus and the 5′ trailer is on the right. The intergenic junctions are not shown but contain transcription stop, intergenic, and transcription start sequences. In the HMPV and HRSV genomes, the M2 gene contains two overlapping products, M2-1 and M2-2. The RSV M2 and L genes overlap. Genomes are drawn to scale (bottom), except for the generic HPIV genome.
Diseases Caused by the Paramyxoviruses and Pneumoviruses in Infants and Young Children
Lower respiratory tract (LRT) infection is the leading cause of death in low-income nations and the third-leading cause of mortality worldwide (www.who.int) (62). Approximately half of the respiratory viral hospitalizations of U.S. children annually are caused by RSV (63,000), HMPV (21,000), and HPIV1, HPIV2, and HPIV3 (21,000) (18). RSV infects most children by age 2 and may cause more than 100,000 deaths worldwide annually (30,61,77). Acute RSV bronchiolitis associates with recurrent respiratory symptoms in approximately one third of the infected children (78–80). Chronic diseases include asthma, which afflicts over 8% of the United States at an annual cost of $56 billion (57). HPIV1 is the leading cause of severe croup (laryngotracheobronchitis), and HPIV3 is a leading cause of bronchiolitis and pneumonia (14,27,90).
The Measles Virus Vaccine, a Successful Vaccine for Older Children
Impressive clinical successes in the paramyxovirus field include development of the human mumps and measles virus vaccines. These licensed vaccines are usually formulated as elements of the mumps, measles, and rubella (MMR) pediatric vaccine product, widely distributed throughout the world (44). When communities of individuals reject MMR vaccinations, new measles epidemics and associated morbidities can arise, demonstrating the significant positive influence of the MMR vaccine on human health (25). The MMR vaccine is usually delivered intramuscularly with priming and booster doses.
Measles virus vaccines have changed over the years. One of the first measles virus vaccines was a formalin-inactivated (FI) product. A noted risk with this product was that when children were naturally exposed to measles virus after vaccination, they could suffer enhanced immunopathology (33). Today, live attenuated vaccines are used. Vaccine success is high (>95%) and clinicians hope that the vaccine will eventually eliminate measles virus from the human population. It is noteworthy that the MMR vaccine is usually recommended only for older infants or children over the age of 1 year. These children no longer harbor protective maternal antibodies at high titer, and are therefore particularly vulnerable to measles virus infections (44).
The mumps component of the MMR vaccine is a live attenuated virus developed by the serial passage of wild-type virus. Following vaccination, durable neutralizing antibodies are induced and protection from infection is virtually complete (10).
Past and Current Strategies for Pneumovirus and Paramyxovirus Vaccine Development
The past and current strategies for pneumovirus and paramyxovirus vaccine development for the youngest infants are many, and will be described only briefly in this report. Despite convincing studies using research models, and numerous phase I clinical studies, few of these vaccines have advanced to phase III clinical trials (9,11).
RSV vaccines have been heavily studied, because RSV infections cause high-frequency morbidity and mortality in infants. One strategy that has yielded many candidates for phase I testing has been the attenuation of wild-type human RSV (7,15,23,40,42,51,67,72,83,94). This was originally accomplished by selecting cold-adapted mutants (e.g., RSV cps2, NCT01852266, and NCT01968083-2013) or by deletion of whole genes (e.g., MEDI ΔM2-2, NCT01459198-2011 and RSV LID ΔM2-2, NCT02040831; NCT02237209-2014) or by combinations of both strategies (e.g., RSV LID cp ΔM2-2, NCT02948127-2016). Mutations were also introduced deliberately into genes for internal and/or external proteins (e.g., RSV LID ΔM2-2 1030s, NCT02952339; NCT02794870-2016, RSV ΔNS2/Δ1313/I1314L, NCT03227029; NCT01893554-2013, RSV D46/NS2/N/ΔM2-2-HindIII, NCT03099291; NCT03102034-2017, and RSV 276, NCT03227029). When candidate vaccines were advanced for clinical testing, they were often abandoned or tagged for further mutation if (1) the vaccine was overattenuated and did not induce a robust immune response, or (2) the vaccine was underattenuated and associated with adverse events such as wheezing or reversions to a less-attenuated phenotype (38,52). The production of sufficient vaccine virus for distribution has been another key challenge with this approach.
Another popular strategy has been to produce recombinant vaccines by expressing RSV proteins in a non-RSV viral construct. Examples of live viral delivery vehicles have included a bovine PIV-type 3/HPIV3 chimera (b/hPIV-3) (49,74) and Sendai virus (SeV) (1,32,34,36,68,81,85,86,98–100). For example, MedImmune advanced a b/hPIV-3 construct carrying a recombinant RSV F gene. Immune responses toward this vaccine were unfortunately lower than expected and there was evidence of RSV F gene instability (88,95). New strategies using the b/hPIV-3 construct are now being developed (50). A chimpanzee-derived adenovirus vector, ChAd155-RSV, has also been tested in adults (NCT 2491463-2015) and is currently being investigated in a phase II study in RSV seropositive infants aged 12–23 months (NCT02927873-2016). The SeV platform has been advanced at St. Jude Children's Research Hospital (1,32). For example, a recom-binant SeV-expressing RSV F has been proven effective in small animals and nonhuman primates, and is expected to advance to a phase I clinical trial soon (34–36,68,86,98–100).
Recombinant constructs may be used as vaccines directly, or may be used to produce purified RSV protein vaccines. As an example, Novavax is testing a baculovirus-derived RSV F protein vaccine using recombinant nanoparticle technology. This vaccine was immunogenic in both alum adjuvanted and unadjuvanted preparations in elderly subjects, but the unadjuvanted preparation failed to demonstrate disease prevention in a pivotal phase III trial in the elderly (as described by Fries, L. at RSV 2016, 29 September 2016, Bariloche, Argentina). Currently, a second phase II trial is being conducted in 300 elderly subjects in Australia comparing the immunogenicity of vaccine with no adjuvant, alum adjuvant, and a novel adjuvant called Martrix M-1 (NCT 030263348). Immunogenicity and safety of the alum adjuvanted preparation in women of childbearing age (4,26) and in pregnant women were demonstrated (please see http://novavax.com/download/files/presentations/FIGO_7OCT2015_AA_P2_Data_10_14_15_FINAL(1).pdf), and a phase III trial of the alum-adjuvanted vaccine in pregnant mothers for the protection of infants is ongoing (NCT02624947-2015). Medimmune developed a novel RSV sF vaccine adjuvanted to GLA (glucopyranosyl lipid A)—MEDI7510 (20,21) that entered into a 2-year phase II study in healthy elderly patients (NCT02508194). The trial was terminated at the end of year 1, and drug development discontinued due to lack of efficacy (22).
Other developers (e.g., Vaccine Research Center) are testing prefusion F proteins derived from mammalian cells (28,37,45,50). A study to evaluate the safety, reactogenicity, and immunogenicity of the GlaxoSmithKline RSV investigational vaccine (GSK3003891a) in healthy pregnant women and infants born to vaccinated mothers, using a novel prefusion form of RSV F protein, was cancelled due to instability of the PreF during manufacturing (NCT03191383). Debates continue as to which protein vaccine is best, and which form of protein should be used (e.g., prefusion or postfusion F) (4,45,53–56,84,101). Today, dozens of RSV vaccine candidates are under development (9).
Recombinant vectors have also been used to target paramyxoviruses or pneumoviruses other than RSV. For example, Mok et al. (58) have used Venezuelan equine encephalitis virus replicon particles, and Russell et al. (70) have used SeV recombinants to present the F protein of HMPV. SeV additionally serves as a Jennerian vaccine against HPIV1, because the two viruses are closely similar, both by protein sequences and by protein conformations (16,66,82). Similarly, BPIV3 or BPIV3 chimeras have been tested as Jennerian vaccines for HPIV3 (5,29,39,41,73). Newer strategies include RNA-based vaccines, such as the HMPV/PIV3 vaccine produced by Moderna, mRNA-1653 (17). Finally, we note that cocktail vaccines have been studied, so that several pathogens can be represented in a single vaccine formulation (e.g., a mixture of three SeV recombinants protected against RSV, HPIV1, HPIV2, and HPIV3 in a cotton rat model) (34,100).
Hurdles That Must Be Overcome
It is perhaps surprising that the vast research dedicated to the paramyxovirus and pneumovirus vaccine fields has not generated licensed products for the youngest infants. One hurdle to be considered is that maternal antibodies may weaken vaccines in young infants, but experiences in other fields and with vaccine models (36) show that vaccines can be efficacious shortly after birth. We consider two additional hurdles as follows.
Lack of community awareness
When a young infant suffers from a respiratory virus infection, the disease is often inaccurately termed “flu.” The reality is that acute lower respiratory viral infections in the youngest children are most often caused by paramyxoviruses or pneumoviruses (61,77). Furthermore, infections with the paramyxoviruses and pneumoviruses are not routinely reported to the Centers for Disease Control and Prevention or other regulatory agencies, meaning that communities cannot easily tabulate the number of infections per year or the disease outcomes [agencies contemplate making RSV infections notifiable in select states on a trial basis to assist future evaluation of candidate vaccines (43)]. The vast morbidity and mortality caused by infections with the paramyxoviruses and pneumoviruses perhaps go unrecognized by the public eye, and the value of potential protective vaccines is not understood. Instead, the risk of vaccine development may be perceived as too high, and research funds may be diverted elsewhere.
Vaccine-induced inflammation in the respiratory tract: beneficial or pathogenic?
The paramyxoviruses and pneumoviruses are somewhat unique in that they often strike the respiratory tracts of the youngest infants. If a vaccine can induce antibodies and effectors (e.g., plasma cells and T cells) that target virus in the respiratory tract, this may have the desired effect of inhibiting virus replication in the lung. However, by definition, entry of cells into tissues is termed “inflammation,” a condition that is generally feared in the context of the pediatric respiratory tract. The situation differs from vaccination with other products such as the polio vaccine, after which cellular responses in the mucosa are expected and tolerated (64).
A fear of inflammation in the pediatric respiratory tract is warranted, because excessive cell influx into the airways can block respiration. An experience of the 1960s provides an example. When an FI-RSV vaccine was tested in a clinical trial, vaccinated children fared worse than their control counterparts following natural exposure to RSV (reminiscent of the immunopathology associated with the FI-measles vaccine previously described). There were numerous hospitalizations and two deaths caused by the vaccine (12). The explanation for the FI-RSV vaccine outcome remains unknown. Perhaps, because there were no neutralizing antibodies induced by the vaccine, virus trafficked to the LRT unperturbed; innate and adaptive immune cells then infiltrated the LRT and constricted the airways (60).
To avoid consequences similar to those observed with FI-vaccines, new respiratory virus vaccines must induce balanced inflammatory responses. A robust, acute, local immune response in respiratory tissues may be desired to support rapid virus clearance and to avoid tissue damage [and consequent, enhanced inflammatory responses (65)], but the initial cell recruitment into respiratory tissues must not be so great as to constrict the airways. How robust should the response be? This question is difficult to answer, in part because responding cells are heterogeneous in nature, and in part because a cell population that might benefit one individual might harm another. Outcomes will depend on the age, size, and lung constitution/environment of each vaccinee.
When particular cytokines, CD4+ T cells, or eosinophils (to name a few factors) are upregulated locally or systemically by vaccination with a new candidate respiratory virus vaccine, concerns are raised in the research community that can discourage advanced vaccine development. Debates then continue as to the benefits/risks of particular immune effector populations, and models/assays by which effectors should be measured (8,24,63,76,89,91–93). Currently, such debates are unresolved, but their eventual resolution may greatly accelerate the development of paramyxovirus and pneumovirus vaccines for the pediatric arena.
Conclusions
Mumps and measles virus vaccines mark two successes in the paramyxovirus field, but these are often recommended for children age 1 or older. Neither the paramyxovirus nor pneumovirus field has yet to produce a licensed vaccine for the youngest infants. While numerous promising vaccine candidates exist, major hurdles are also present. The public is often unaware of the diseases caused by paramyxoviruses and pneumoviruses, and therefore does not appreciate the importance of associated vaccines. Also, researchers must be assured that vaccine-induced inflammation in the respiratory tract will be effective without constricting the airways. These significant hurdles must be overcome if vaccines for the paramyxoviruses and pneumoviruses are to be developed for the youngest children.
Acknowledgments
Work was supported by NIH NCI P30 CA21765 and ALSAC.
Author Disclosure Statement
Julia L. Hurwitz and Charles J. Russell are authors of a patent describing a Sendai virus-based vaccine vector. No competing financial interests exist.
References
- 1.Adderson E, Branum K, Sealy RE, et al. Safety and immunogenicity of an intranasal Sendai virus-based human parainfluenza virus type 1 vaccine in 3- to 6-year-old children. Clin Vaccine Immunol 2015;22:298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afonso CL, Amarasinghe GK, Banyai K, et al. Taxonomy of the order Mononegavirales: update 2016. Arch Virol 2016;161:2351–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amarasinghe GK, Bao Y, Basler CF, et al. Taxonomy of the order Mononegavirales: update 2017. Arch Virol 2017;162:2493–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.August A, Glenn GM, Kpamegan E, et al. A phase 2 randomized, observer-blind, placebo-controlled, dose-ranging trial of aluminum-adjuvanted respiratory syncytial virus F particle vaccine formulations in healthy women of childbearing age. Vaccine 2017;35:3749–3759 [DOI] [PubMed] [Google Scholar]
- 5.Bailly JE, McAuliffe JM, Durbin AP, et al. A recombinant human parainfluenza virus type 3 (PIV3) in which the nucleocapsid N protein has been replaced by that of bovine PIV3 is attenuated in primates. J Virol 2000;74:3188–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker KA, Dutch RE, Lamb RA, et al. Structural basis for paramyxovirus-mediated membrane fusion. Mol Cell 1999;3:309–319 [DOI] [PubMed] [Google Scholar]
- 7.Boyoglu-Barnum S, Todd SO, Meng J, et al. Mutating the CX3C motif in the G protein should make a live respiratory syncytial virus vaccine safer and more effective. J Virol 2017;91:e02059-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandenburg AH, Kleinjan A, van Het LB, et al. Type 1-like immune response is found in children with respiratory syncytial virus infection regardless of clinical severity. J Med Virol 2000;62:267–277 [PubMed] [Google Scholar]
- 9.Broadbent L, Groves H, Shields MD, et al. Respiratory syncytial virus, an ongoing medical dilemma: an expert commentary on respiratory syncytial virus prophylactic and therapeutic pharmaceuticals currently in clinical trials. Influenza Other Respir Viruses 2015;9:169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunell PA, Brickman A, and Steinberg S. Evaluation of a live attenuated mumps vaccine (Jeryl Lynn). With observations on the optimal time for testing serologic response. Am J Dis Child 1969;118:435–440 [DOI] [PubMed] [Google Scholar]
- 11.Buczkowski H, Muniraju M, Parida S, et al. Morbillivirus vaccines: recent successes and future hopes. Vaccine 2014;32:3155–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chin J, Magoffin RL, Shearer LA, et al. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol 1969;89:449–463 [DOI] [PubMed] [Google Scholar]
- 13.Colf LA, Juo ZS, and Garcia KC. Structure of the measles virus hemagglutinin. Nat Struct Mol Biol 2007;14:1227–1228 [DOI] [PubMed] [Google Scholar]
- 14.Counihan ME, Shay DK, Holman RC, et al. Human parainfluenza virus-associated hospitalizations among children less than five years of age in the United States. Pediatr Infect Dis J 2001;20:646–653 [DOI] [PubMed] [Google Scholar]
- 15.Crowe JE, Jr., Randolph V, and Murphy BR. The live attenuated subgroup B respiratory syncytial virus vaccine candidate RSV 2B33F is attenuated and immunogenic in chimpanzees, but exhibits partial loss of the ts phenotype following replication in vivo. Virus Res 1999;59:13–22 [DOI] [PubMed] [Google Scholar]
- 16.Dave VP, Allan JE, Slobod KS, et al. Viral cross-reactivity and antigenic determinants recognized by human parainfluenza virus type 1-specific cytotoxic T-cells. Virology 1994;199:376–383 [DOI] [PubMed] [Google Scholar]
- 17.DeFrancesco L. The “anti-hype” vaccine. Nat Biotechnol 2017;35:193–197 [DOI] [PubMed] [Google Scholar]
- 18.Edwards KM, Zhu Y, Griffin MR, et al. Burden of human metapneumovirus infection in young children. N Engl J Med 2013;368:633–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egelman EH, Wu SS, Amrein M, et al. The Sendai virus nucleocapsid exists in at least four different helical states. J Virol 1989;63:2233–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falloon J, Ji F, Curtis C, et al. A phase 1a, first-in-human, randomized study of a respiratory syncytial virus F protein vaccine with and without a toll-like receptor-4 agonist and stable emulsion adjuvant. Vaccine 2016;34:2847–2854 [DOI] [PubMed] [Google Scholar]
- 21.Falloon J, Talbot HK, Curtis C, et al. Dose selection for an adjuvanted respiratory syncytial virus F protein vaccine for older adults based on humoral and cellular immune responses. Clin Vaccine Immunol 2017;24:e00157-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falloon J, Yu J, Esser MT, et al. An adjuvanted, postfusion F protein-based vaccine did not prevent respiratory syncytial virus illness in older adults. J Infect Dis 2017;216:1362-1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedewald WT, Forsyth BR, Smith CB, et al. Low-temperature-grown RS virus in adult volunteers. JAMA 1968;204:690–694 [PubMed] [Google Scholar]
- 24.Garofalo RP, Patti J, Hintz KA, et al. Macrophage inflammatory protein-1alpha (not T helper type 2 cytokines) is associated with severe forms of respiratory syncytial virus bronchiolitis. J Infect Dis 2001;184:393–399 [DOI] [PubMed] [Google Scholar]
- 25.Gastanaduy PA, Budd J, Fisher N, et al. A measles outbreak in an underimmunized Amish community in Ohio. N Engl J Med 2016;375:1343–1354 [DOI] [PubMed] [Google Scholar]
- 26.Glenn GM, Fries LF, Thomas DN, et al. A randomized, blinded, controlled, dose-ranging study of a respiratory syncytial virus recombinant fusion (F) nanoparticle vaccine in healthy women of childbearing age. J Infect Dis 2016;213:411–422 [DOI] [PubMed] [Google Scholar]
- 27.Glezen WP, Frank AL, Taber LH, et al. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J Infect Dis 1984;150:851–857 [DOI] [PubMed] [Google Scholar]
- 28.Graham BS. Vaccines against respiratory syncytial virus: the time has finally come. Vaccine 2016;34:3535–3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg DP, Walker RE, Lee MS, et al. A bovine parainfluenza virus type 3 vaccine is safe and immunogenic in early infancy. J Infect Dis 2005;191:1116–1122 [DOI] [PubMed] [Google Scholar]
- 30.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009;360:588–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashiguchi T, Kajikawa M, Maita N, et al. Crystal structure of measles virus hemagglutinin provides insight into effective vaccines. Proc Natl Acad Sci U S A 2007;104:19535–19540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurwitz JL, Soike KF, Sangster MY, et al. Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Vaccine 1997;15:533–540 [DOI] [PubMed] [Google Scholar]
- 33.Iankov ID, Pandey M, Harvey M, et al. Immunoglobulin g antibody-mediated enhancement of measles virus infection can bypass the protective antiviral immune response. J Virol 2006;80:8530–8540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones B, Zhan X, Mishin V, et al. Human PIV-2 recombinant Sendai virus (rSeV) elicits durable immunity and combines with two additional rSeVs to protect against hPIV-1, hPIV-2, hPIV-3, and RSV. Vaccine 2009;27:1848–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones BG, Sealy RE, Rudraraju R, et al. Sendai virus-based RSV vaccine protects African green monkeys from RSV infection. Vaccine 2012;30:959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones BG, Sealy RE, Surman SL, et al. Sendai virus-based RSV vaccine protects against RSV challenge in an in vivo maternal antibody model. Vaccine 2014;32:3264–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joyce MG, Zhang B, Ou L, et al. Iterative structure-based improvement of a fusion-glycoprotein vaccine against RSV. Nat Struct Mol Biol 2016;23:811–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karron RA, Luongo C, Thumar B, et al. A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children. Sci Transl Med 2015;7:312ra175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karron RA, Makhene M, Gay K, et al. Evaluation of a live attenuated bovine parainfluenza type 3 vaccine in two- to six-month-old infants. Pediatr Infect Dis J 1996;15:650–654 [DOI] [PubMed] [Google Scholar]
- 40.Karron RA, Wright PF, Crowe JE Jr., et al. Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus vaccines in chimpanzees and in human adults, infants, and children. J Infect Dis 1997;176:1428–1436 [DOI] [PubMed] [Google Scholar]
- 41.Karron RA, Wright PF, Hall SL, et al. A live attenuated bovine parainfluenza virus type 3 vaccine is safe, infectious, immunogenic, and phenotypically stable in infants and children. J Infect Dis 1995;171:1107–1114 [DOI] [PubMed] [Google Scholar]
- 42.Kim HW, Arrobio JO, Pyles G, et al. Clinical and immunological response of infants and children to administration of low-temperature adapted respiratory syncytial virus. Pediatrics 1971;48:745–755 [PubMed] [Google Scholar]
- 43.Kim L, Rha B, Abramson JS, et al. Identifying gaps in respiratory syncytial virus disease epidemiology in the United States prior to the introduction of vaccines. Clin Infect Dis 2017;65:1020–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kowalzik F, Faber J, and Knuf M. MMR and MMRV vaccines. Vaccine 2017. [Epub ahead of print]; DOI: 10.1016/j.vaccine.2017.07.051 [DOI] [PubMed] [Google Scholar]
- 45.Krarup A, Truan D, Furmanova-Hollenstein P, et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat Commun 2015;6:8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambert DM, Barney S, Lambert AL, et al. Peptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusion. Proc Natl Acad Sci U S A 1996;93:2186–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawrence MC, Borg NA, Streltsov VA, et al. Structure of the haemagglutinin-neuraminidase from human parainfluenza virus type III. J Mol Biol 2004;335:1343–1357 [DOI] [PubMed] [Google Scholar]
- 48.Lee JE, Fusco ML, Hessell AJ, et al. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature 2008;454:177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang B, Munir S, Amaro-Carambot E, et al. Chimeric bovine/human parainfluenza virus type 3 expressing respiratory syncytial virus (RSV) F glycoprotein: effect of insert position on expression, replication, immunogenicity, stability, and protection against RSV infection. J Virol 2014;88:4237–4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang B, Ngwuta JO, Herbert R, et al. Packaging and prefusion stabilization separately and additively increase the quantity and quality of respiratory syncytial virus (RSV)-neutralizing antibodies induced by an RSV fusion protein expressed by a parainfluenza virus vector. J Virol 2016;90:10022–10038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luongo C, Winter CC, Collins PL, et al. Respiratory syncytial virus modified by deletions of the NS2 gene and amino acid S1313 of the L polymerase protein is a temperature-sensitive, live-attenuated vaccine candidate that is phenotypically stable at physiological temperature. J Virol 2013;87:1985–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malkin E, Yogev R, Abughali N, et al. Safety and immunogenicity of a live attenuated RSV vaccine in healthy RSV-seronegative children 5 to 24 months of age. PLoS One 2013;8:e77104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLellan JS, Chen M, Joyce MG, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 2013;342:592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McLellan JS, Chen M, Leung S, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 2013;340:1113–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLellan JS, Ray WC, and Peeples ME. Structure and function of respiratory syncytial virus surface glycoproteins. Curr Top Microbiol Immunol 2013;372:83–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLellan JS, Yang Y, Graham BS, et al. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J Virol 2011;85:7788–7796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohapatra SS, and Boyapalle S. Epidemiologic, experimental, and clinical links between respiratory syncytial virus infection and asthma. Clin Microbiol Rev 2008;21:495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mok H, Tollefson SJ, Podsiad AB, et al. An alphavirus replicon-based human metapneumovirus vaccine is immunogenic and protective in mice and cotton rats. J Virol 2008;82:11410–11418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Money VA, McPhee HK, Mosely JA, et al. Surface features of a Mononegavirales matrix protein indicate sites of membrane interaction. Proc Natl Acad Sci U S A 2009;106:4441–4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murphy BR, Prince GA, Walsh EE, et al. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J ClinMicrobiol 1986;24:197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010;375:1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nair H, Simoes EA, Rudan I, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet 2013;381:1380–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Openshaw PJM, Chiu C, Culley FJ, et al. Protective and harmful immunity to RSV infection. Annu Rev Immunol 2017;35:501–532 [DOI] [PubMed] [Google Scholar]
- 64.Pasetti MF, Simon JK, Sztein MB, et al. Immunology of gut mucosal vaccines. Immunol Rev 2011;239:125–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Penkert RR, Surman SL, Jones BG, et al. Vitamin A deficient mice exhibit increased viral antigens and enhanced cytokine/chemokine production in nasal tissues following respiratory virus infection despite the presence of FoxP3+ T cells. Int Immunol 2016;28:139–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Power UF, Ryan KW, and Portner A. Sequence characterization and expression of the matrix protein gene of human parainfluenza virus type 1. Virology 1992;191:947–952 [DOI] [PubMed] [Google Scholar]
- 67.Rostad CA, Stobart CC, Gilbert BE, et al. A recombinant respiratory syncytial virus vaccine candidate attenuated by a low-fusion F protein is immunogenic and protective against challenge in cotton rats. J Virol 2016;90:7508–7518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Russell CJ, and Hurwitz JL. Sendai virus as a backbone for vaccines against RSV and other human paramyxoviruses. Expert Rev Vaccines 2016;15:189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Russell CJ, Jardetzky TS, and Lamb RA. Membrane fusion machines of paramyxoviruses: capture of intermediates of fusion. EMBO J 2001;20:4024–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russell CJ, Jones BG, Sealy RE, et al. A Sendai virus recombinant vaccine expressing a gene for truncated human metapneumovirus (hMPV) fusion protein protects cotton rats from hMPV challenge. Virology 2017;509:60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Russell CJ, Kantor KL, Jardetzky TS, et al. A dual-functional paramyxovirus F protein regulatory switch segment: activation and membrane fusion. J Cell Biol 2003;163:363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schickli JH, Kaur J, and Tang RS. Nonclinical phenotypic and genotypic analyses of a phase 1 pediatric respiratory syncytial virus vaccine candidate MEDI-559 (rA2cp248/404/1030DeltaSH) at permissive and non-permissive temperatures. Virus Res 2012;169:38–47 [DOI] [PubMed] [Google Scholar]
- 73.Schmidt AC, McAuliffe JM, Huang A, et al. Bovine parainfluenza virus type 3 (BPIV3) fusion and hemagglutinin-neuraminidase glycoproteins make an important contribution to the restricted replication of BPIV3 in primates. J Virol 2000;74:8922–8929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmidt AC, McAuliffe JM, Murphy BR, et al. Recombinant bovine/human parainfluenza virus type 3 (B/HPIV3) expressing the respiratory syncytial virus (RSV) G and F proteins can be used to achieve simultaneous mucosal immunization against RSV and HPIV3. J Virol 2001;75:4594–4603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmitt AP, Leser GP, Waning DL, et al. Requirements for budding of paramyxovirus simian virus 5 virus-like particles. J Virol 2002;76:3952–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sealy RE, Surman SL, and Hurwitz JL. CD4+ T cells support establishment of RSV-specific IgG and IgA antibody secreting cells in the upper and lower murine respiratory tract following RSV infection. Vaccine 2017;35:2617–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi T, McAllister DA, O'Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017;390:946–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sigurs N. A cohort of children hospitalised with acute RSV bronchiolitis: impact on later respiratory disease. Paediatr Respir Rev 2002;3:177–183 [DOI] [PubMed] [Google Scholar]
- 79.Sigurs N, Bjarnason R, Sigurbergsson F, et al. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000;161:1501–1507 [DOI] [PubMed] [Google Scholar]
- 80.Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005;171:137–141 [DOI] [PubMed] [Google Scholar]
- 81.Slobod KS, Shenep JL, Lujan-Zilbermann J, et al. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine 2004;22:3182–3186 [DOI] [PubMed] [Google Scholar]
- 82.Smith FS, Portner A, Leggiadro RJ, et al. Age-related development of human memory T-helper and B-cell responses toward parainfluenza virus type-1. Virology 1994;205:453–461 [DOI] [PubMed] [Google Scholar]
- 83.Stobart CC, Rostad CA, Ke Z, et al. A live RSV vaccine with engineered thermostability is immunogenic in cotton rats despite high attenuation. Nat Commun 2016;7:13916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swanson KA, Settembre EC, Shaw CA, et al. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci U S A 2011;108:9619–9624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takimoto T, Hurwitz JL, Coleclough C, et al. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J Virol 2004;78:6043–6047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takimoto T, Hurwitz JL, Zhan X, et al. Recombinant Sendai virus as a novel vaccine candidate for respiratory syncytial virus. Viral Immunol 2005;18:255–266 [DOI] [PubMed] [Google Scholar]
- 87.Takimoto T, Murti KG, Bousse T, et al. Role of matrix and fusion proteins in budding of Sendai virus. J Virol 2001;75:11384–11391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang RS, Spaete RR, Thompson MW, et al. Development of a PIV-vectored RSV vaccine: preclinical evaluation of safety, toxicity, and enhanced disease and initial clinical testing in healthy adults. Vaccine 2008;26:6373–6382 [DOI] [PubMed] [Google Scholar]
- 89.Varga SM, Wissinger EL, and Braciale TJ. The attachment (G) glycoprotein of respiratory syncytial virus contains a single immunodominant epitope that elicits both Th1 and Th2 CD4+ T cell responses. J Immunol 2000;165:6487–6495 [DOI] [PubMed] [Google Scholar]
- 90.Weinberg GA, Hall CB, Iwane MK, et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr 2009;154:694–699 [DOI] [PubMed] [Google Scholar]
- 91.Welliver RC, Kaul A, and Ogra PL. Cell-mediated immune response to respiratory syncytial virus infection: relationship to the development of reactive airway disease. J Pediatr 1979;94:370–375 [DOI] [PubMed] [Google Scholar]
- 92.Welliver RC., Sr. The immune response to respiratory syncytial virus infection: friend or foe? Clin Rev Allergy Immunol 2008;34:163–173 [DOI] [PubMed] [Google Scholar]
- 93.Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med 1981;305:841–846 [DOI] [PubMed] [Google Scholar]
- 94.Wright PF, Karron RA, Belshe RB, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis 2000;182:1331–1342 [DOI] [PubMed] [Google Scholar]
- 95.Yang CF, Wang CK, Malkin E, et al. Implication of respiratory syncytial virus (RSV) F transgene sequence heterogeneity observed in phase 1 evaluation of MEDI-534, a live attenuated parainfluenza type 3 vectored RSV vaccine. Vaccine 2013;31:2822–2827 [DOI] [PubMed] [Google Scholar]
- 96.Yin HS, Wen X, Paterson RG, et al. Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature 2006;439:38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yuan P, Swanson KA, Leser GP, et al. Structure of the Newcastle disease virus hemagglutinin-neuraminidase (HN) ectodomain reveals a four-helix bundle stalk. Proc Natl Acad Sci U S A 2011;108:14920–14925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhan X, Hurwitz JL, Krishnamurthy S, et al. Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine 2007;25:8782–8793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhan X, Slobod KS, Jones BG, et al. Sendai virus recombinant vaccine expressing a secreted, unconstrained respiratory syncytial virus fusion protein protects against RSV in cotton rats. Int Immunol 2015;27:229–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhan X, Slobod KS, Krishnamurthy S, et al. Sendai virus recombinant vaccine expressing hPIV-3 HN or F elicits protective immunity and combines with a second recombinant to prevent hPIV-1, hPIV-3 and RSV infections. Vaccine 2008;26:3480–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang W, Choi Y, Haynes LM, et al. Vaccination to induce antibodies blocking the CX3C-CX3CR1 interaction of respiratory syncytial virus G protein reduces pulmonary inflammation and virus replication in mice. J Virol 2010;84:1148–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]