Abstract
Polyfunctional CD8+ T cells play a critical role in controlling viremia during AIDS lentiviral infections. However, for most HIV-infected individuals, virus-specific CD8+ T cells exhibit loss of polyfunctionality, including loss of IL2, TNFα, and IFNγ. Using the feline immunodeficiency virus (FIV) model for AIDS lentiviral persistence, our laboratory has demonstrated that FIV-activated Treg cells target CD8+ T cells, leading to a reduction in IL2 and IFNγ production. Furthermore, we have demonstrated that Treg cells induce expression of the repressive transcription factor, Foxp3, in CD8+ T cells. Based upon these findings, we asked if Treg-induced Foxp3 could bind to the IL2, TNFα, and IFNγ promoter regions in virus-specific CD8+ T cells. Following coculture with autologous Treg cells, we demonstrated decreased mRNA levels of IL2 and IFNγ at weeks 4 and 8 postinfection and decreased TNFα at week 4 postinfection in virus-specific CD8+ T cells. We also clearly demonstrated Treg cell-induced Foxp3 expression in virus-specific CD8+ T cells at weeks 1, 4, and 8 postinfection. Finally, we documented Foxp3 binding to the IL2, TNFα, and IFNγ promoters at 8 weeks and 6 months postinfection in virus-specific CD8+ T cells following Treg cell coculture. In summary, the results here clearly demonstrate that Foxp3 inhibits IL2, TNFα, and IFNγ transcription by binding to their promoter regions in lentivirus-specific CD8+ T cells. We believe this is the first description of this process during the course of AIDS lentiviral infection.
Keywords: : animal models, T cell immunity, cell mediated immunity, T regulatory cell, polyfunctional T cell, CD8+ T cell
Introduction
A rapid rise in CD8+ T lymphocytes displaying an activated phenotype is observed during early HIV infection and the quality of the CD8+ T cell response is associated with a decrease in plasma viremia.1–3 A small subset of HIV-infected individuals called elite controllers (ECs) exhibit a low viral set point, low viral load, and appear to control virus naturally, in the absence of anti-retroviral therapy (reviewed O'connell).4 Compared with HIV progressors, CD8+ T cells from ECs exhibit polyfunctional responses to HIV antigen, potent suppression of HIV replication, and enhanced proliferation, indicating that robust CD8+ T cell function is important in controlling HIV.5–7 However, for most individuals (HIV progressors) despite this early, vigorous CD8+ T cell antiviral response, the virus is not eliminated, and establishes a persistent infection with a relatively high viral set point and viral load.5–7
Establishment of persistent infection relies upon a complex series of virus and host factors. One important factor is the early and progressive loss of antigen-specific T cell response during the course of AIDS lentiviral infection.8–10 In addition to HIV infection, the phenomenon of CD8+ T cell hyporesponsiveness to viral antigens is well documented for a number of viral infections, including hepatitis C virus, and lymphocytic choriomeningitis virus (LCMV) in mice.11,12 Investigations using the LCMV model have offered insights into the mechanisms underlying progressive antigen-specific CD8+ T cell dysfunction. These studies suggest that CD8+ T cell immune dysfunction is not an all-or-none phenomenon, but represents progressive loss of function.13,14 The loss of antigen-stimulated CD8+ T cell cytokine secretion is first manifested by the depression and then loss of IL2, followed by loss of TNFα, and finally loss of IFNγ, resulting in complete dysfunction as manifested by nonresponsiveness to viral antigens.14 Collectively, these findings suggest that an understanding of the molecular events contributing to CD8+ T cell dysfunction during the progression of AIDS lentiviral infection may be key to enhanced vaccination and reservoir elimination strategies.
Using the feline immunodeficiency virus (FIV) model for AIDS lentiviral persistence, our laboratory investigates Treg cell, CD4+, and CD8+ T cell interactions from the very early stage of infection through long-term infection. Treg cells from FIV+ cats are activated during the course of FIV infection, meaning that they potently suppress autologous T cell function when compared with Treg cells from uninfected control cats. Treg cells from FIV+ cats suppress IL2 and IFNγ production in CD8+ T cells early and progressively during the course of infection.15–18 The mechanism of suppression is TGFβ dependent, with a membrane bound form of TGFβ displayed upon FIV-activated Treg cell surfaces ligating TGFβRII upon target effector cells.19 Following coculture with activated Treg cells, the TGFβ signaling cascade leads to the induction of the repressive transcription factor forkhead box P3 (Foxp3), in the target effector cells. The induction of Foxp3 in CD4+ T helper cells leads to the conversion of these cells to “induced” Treg cells, which exhibit Treg cell phenotype and function.15 Furthermore, we have clearly demonstrated that Foxp3 is induced in CD8+ T cell targets following Treg cell/CD8+ T cell coculture and that Foxp3 binds the IL2 promoter in CD8+ T cells.20
Based upon our previous work using the FIV model of lentiviral persistence, we hypothesized that Treg-induced Foxp3 contributes to the progressive loss of CD8+ T cell function in a manner reminiscent of the LCMV mechanism described above. In this study, we demonstrate that mRNA levels of IL2, TNFα, and IFNγ decreased in virus-specific CD8+ T cells following ex vivo Treg/CD8+ T cell coculture. Furthermore, we report the induction of Foxp3 in virus-specific CD8+ T cells following ex vivo Treg/CD8+ T cell coculture. Most importantly, we demonstrate that Foxp3 binds the IL2, TNFα, and IFNγ promoter regions in virus-specific CD8+ T cells from FIV+ cats following Treg cell coculture. We believe this is the first report demonstrating Treg induced Foxp3 binding of all three of these promoter regions in virus-specific CD8+ T cells during the course of lentiviral infection. These results help explain, in part, the progressive CD8+ T cell dysfunction that is associated with persistent lentiviral infection.
Materials and Methods
Cats and FIV infection
Specific pathogen-free (SPF) cats were obtained from Liberty Laboratories (Liberty Corners, NJ) and housed at the Laboratory Animal Resource Facility at the College of Veterinary Medicine, North Carolina State University. Cats were inoculated with 1 × 105 TCID50 cell-free NCSU1 FIV as described by Bucci et al.3 FIV infection was confirmed using a commercially available ELISA Kit (IDEXX Laboratories, Inc., Westbrook, ME) and by provirus detection by polymerase chain reaction (PCR) using primers specific for the FIV-p24 GAG sequence as described previously.18,21 Age-matched, noninfected control cats were housed separately from FIV-infected cats. Control cats were sham infected with the same type of culture medium (sterile) used to culture the virus. All protocols were approved by the North Carolina State University Institutional Animal Care and Use Committee.
Sample collection and preparation
Single-cell suspensions were prepared from popliteal or submandibular peripheral lymph nodes (PLNs) obtained through surgical biopsies or following euthanasia, by gently and repeatedly injecting sterile cell culture media (CTL) into the tissue using an 18G needle until the cells were released from the tissue. Cell viability was determined by Trypan Blue dye exclusion.
CD8+ coculture and carboxyfluorescein succinimidyl ester cell proliferation assays
Both anti-feline CD4 and anti-feline CD8 monoclonal antibodies were developed by our feline lentivirus research group as described previously.22 The feline anti-CD25 monoclonal antibody was developed by Ohno as described previously.23 Single cells from LNs were suspended at 1 × 108 cells/ml in HBSS with 2% FBS and stained with anti-feline CD8 PE antibody (clone 3.357) at 4°C for 30 min. EasySep® PE Selection Cocktail was added at 100 μL/ml of cell suspension at room temperature for 15 min, and EasySep Magnetic Nanoparticles were added at 50 μL/ml at RT for 10 min. CD8+PE+ cells were separated by using the magnet provided in the kit (STEMCELL, Vancouver, BC, Canada). The rest of the cell suspension was stained with mouse anti-feline CD4 APC antibody to isolate CD4+ cells by using the EasySep APC Selection Kit (STEMCELL). Isolated CD4+ cells were then stained with mouse anti-feline CD25 FITC antibody to sort CD4+ CD25+ double-positive Treg cells using the MoFlo XDP high-speed cell sorter (Beckman Coulter). DAPI (BioLegend) was used as the cell viability dye to ensure that we obtained live cells at the end of each of the sorts. CD8+ T cells were resuspended in prewarmed phosphate-buffered saline (PBS)/0.1% bovine serum albumin and stained with 10 μM carboxyfluorescein succinimidyl ester (CFSE) dye from the Cell Trace™ CFSE Cell Proliferation Kit (Life Technologies). CD8+ T cells were returned to LN culture without CD4+CD25+ Treg cells, and stimulated in vitro with UV-inactivated FIV-NCSU1. The cells were cocultured for 72 h. Following stimulation, the virus-specific proliferating CFSEint/low cells and nonspecific CD8+ T cell CFSEhigh (internal control) were isolated by resorting using a high-speed cell sorter. For all of the coculture studies presented here, CD8+ lymphocytes were cocultured at a 1:1 (Treg: CD8+) ratio with autologous CD4+CD25+ Treg cells for 24 h. After coculture, the cells were washed and then resorted into CD8+ populations for analysis by quantitative PCR (qPCR) or chromatin immunoprecipitation (ChIP). The purity of magnetic bead-sorted cells was ≥95% and Moflo XDP-sorted cell populations was >99%.
RNA extraction, RT, and real-time PCR quantification
Total RNA was extracted from cells using the PureLink™ RNA Micro Kit (Life Technologies).The concentration was quantified using a Nano Drop Spectrophotometer. qPCR was performed for mRNA using the qScript cDNA Synthesis Kit (Quanta Biosciences). Fifteen microliter reactions were incubated for 5 min at 22°C, 40 min at 42°C, and 5 min at 85°C to inactivate the reverse transcriptase. Feline-specific primers as shown in Table 1 were used to detect the Foxp3, IL2, TNFα, and IFNγ mRNA levels using LightCycler 480 System (Roche) qPCR. GAPDH mRNA expression was used as a normalizing control. For qPCR experiments, a ΔΔCt ratio was used to quantify relative mRNA expression. Eight microliter of diluted cDNA, 10 μl PerfeCTa SYBR Green SuperMix Reaction Mix (Quanta Biosciences), 1 μl forward primer, and 1 μl reverse primer were run in triplicates under the following cycling conditions: hot start enzyme (Qiagen) activation at 95°C for 5 min, denatured at 94°C for 45 s, annealed at 60°C for 45 s, and elongated at 72°C for 1 min with 35 cycles, and final extension at 72°C for 10 min.
Table 1.
List of Primers Used for Quantitative Polymerase Chain Reaction
| Primer target | Forward | Reverse |
|---|---|---|
| Foxp3 | 5′-GCCTGCCACCTGGAATCAAC-3′ | 5′-GTGTGCTGGGCTTGGGA-3′ |
| IL2 | 5′-ACAGTGCACCTGCTTCAAGCTCT-3′ | 5′-CCTGGAGAGTTTGGGGTTCTCAGG-3′ |
| TNFα | 5′-ATGCCCTCCTGGCCAATGGCG-3′ | 5′-TAGACCTGCCCGGACTCGGC-3′ |
| IFNγ | 5′-TGGTGGGTCGCTTTTCGTAG-3′ | 5′-GAAGGAGACAATTTGGCTTTGAA-3′ |
| GAPDH | 5′-GGAGAAGGCTGGGGCTCAC-3′ | 5′-GGTGCAGGAGGCATTGCTGA-3′ |
Chromatin immunoprecipitation assay
The ChIP was performed using the ChromaFlash High-Sensitivity ChIP Kit (EpiGentek). The protocol was followed according to the manufacturer's specifications. In brief, anti-Foxp3 (Abcam), anti-RNA polymerase II (positive control), and nonimmune IgG (negative control) antibodies were first bound to Assay Strip Wells. The sorted cells were crosslinked by adding CTL media containing formaldehyde to a final concentration of 1% with incubation at room temperature (20–25°C) for 10 min on a rocking platform (50–100 rpm). To each tube, prewarmed 1.25 M Glycine (1:10) was added to a final concentration of 125 mM and incubated at room temperature for 5 min. After washing with ice-cold PBS, Working Lysis Buffer was added to resuspend the cell pellet and incubated on ice for 10 min. After carefully removing the supernatant, Working ChIP Buffer was added to resuspend the chromatin pellet and the chromatin sheared by sonication. ChIP samples were centrifuged at 12,000 rpm at 4°C for 10 min after shearing and the supernatant was transferred to a new vial. The ChIP samples were added to the wells bound with antibodies, positive control, or negative control. The reaction wells were incubated at 4°C overnight. ChIP samples were then washed according to the protocol and subjected to reverse crosslinking at 42°C for 30 min, 60°C for 45 min. DNA release was at 95°C for 15 min in a thermocycler. Finally, the DNA samples were purified by spin column for qPCR using the ChIP primers shown in Table 2. The relative expression of the target gene was calculated. The relative expression was calculated by using a ratio of amplification efficiency of the ChIP sample over that of nonimmune IgG, FE% = 2(IgG Ct–Sample Ct) × 100%.
Table 2.
Primers Used for Chromatin Immunoprecipitation
| Primer target | Forward | Reverse |
|---|---|---|
| IL2 promoter-1 | 5′-ACTCAACTTGCATCCCCTTG-3′ | 5′-ACCCAGGAAAGGATTTGCAT-3′ |
| IL2 promoter-2 | 5′-TGCTCCACATGTTCAACACA-3′ | 5′-CCCACACTTAGGTGGCAGTT-3′ |
| TNFα promoter-1 | 5′-AGGGTTGCTTTCACTCCCAC-3′ | 5′-GGGAGCTTGAGAGAAGGCTG-3′ |
| TNFα promoter-2 | 5′-GAGCTCATGGGTTTCTCCAC-3′ | 5′-AGCTTCTGCTGACTGGGTGT-3′ |
| IFNγ promoter-1 | 5′-GCTTTCAAAGGATCCCACAA-3′ | 5′-TTTGTGGCATTTTGGTGTTG-3′ |
| IFNγ promoter-2 | 5′-CTTCCTCACCACCTTGGTCT-3′ | 5′-AGGGGTGCTCCAACCTTTAC-3′ |
Data analysis
Data (Figs. 2–4) are presented in the text as the mean + standard error of the mean and were analyzed using an unpaired t-test with significance set at p < .05. All data were analyzed using GraphPad Prism software.
FIG. 2.
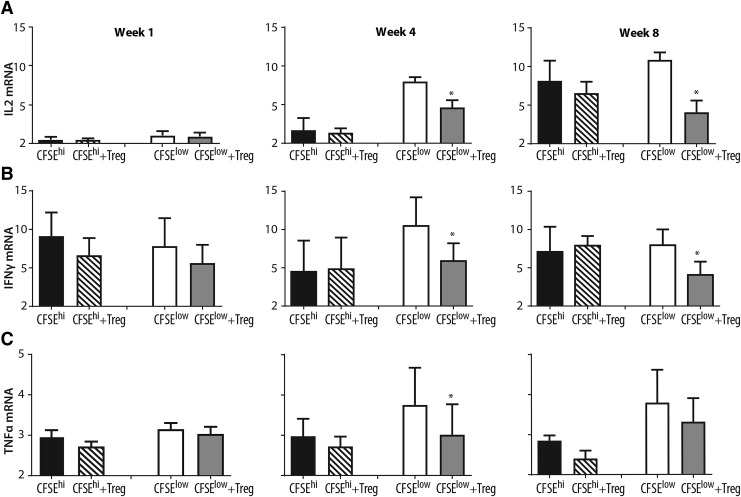
IL2, IFNγ, and TNFα mRNA from virus-specific CD8+ T cells following autologous CD4+CD25+ Treg cell coculture. Virus-nonspecific and virus-specific CD8+ T cells from FIV+ cats were sorted as described in Figure 1. Virus-nonspecific CD8+ T cells (black bars, CFSEhi) from FIV+ cats were cultured with autologous Treg cells (striped bars, CFSEhi+Treg) and virus-specific CD8+ T cells from FIV+ cats (white bars, CFSElow) were sorted with autologous Treg cells (gray bars, CFSElow+Treg). Cells were isolated and cultured at 1 week (left column), 4 weeks (middle column), and 8 weeks (right column) postinfection. (A) Following Treg cell coculture, IL2 mRNA is decreased in virus-specific CD8+ T cells at 4 and 8 weeks postinfection. (B) Following Treg cell coculture, IFNγ mRNA is decreased in virus-specific CD8+ T cells at 4 and 8 weeks postinfection. (C) TNFα is decreased in virus-specific CD8+ T cells at 4 weeks postinfection following Treg cell coculture (n = 3–7 cats for each time point). Bars represent the mean + SEM. Data were analyzed using an unpaired t-test with significance set at p < .05, asterisks. SEM, standard error of the mean.
FIG. 3.
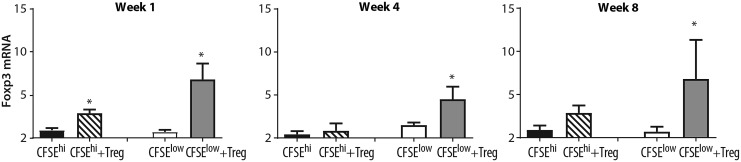
Foxp3 mRNA expression is increased in virus-specific CD8+ T cells following coculture with lentivirus-activated Treg cells. Virus-nonspecific and virus-specific CD8+ T cells from FIV+ cats were sorted as described in Figure 1. Virus-nonspecific CD8+ T cells (black bars, CFSEhi) from FIV+ cats were cultured with autologous Treg cells (striped bars, CFSEhi+Treg) and virus-specific CD8+ T cells from FIV+ cats (white bars, CFSElow) were sorted with autologous Treg cells (gray bars, CFSElow+Treg). Cells were isolated and cultured at 1 week (left), 4 weeks (middle), and 8 weeks (right) postinfection. Following Treg coculture, Foxp3 is increased in virusspecific CD8+ T cells at all time points and in nonspecific CD8+ T cells at week 1 postinfection (n = 4–6 cats for each time point). Bars represent the mean + SEM. Data were analyzed using an unpaired t-test with significance set at p < .05, asterisks.
FIG. 4.
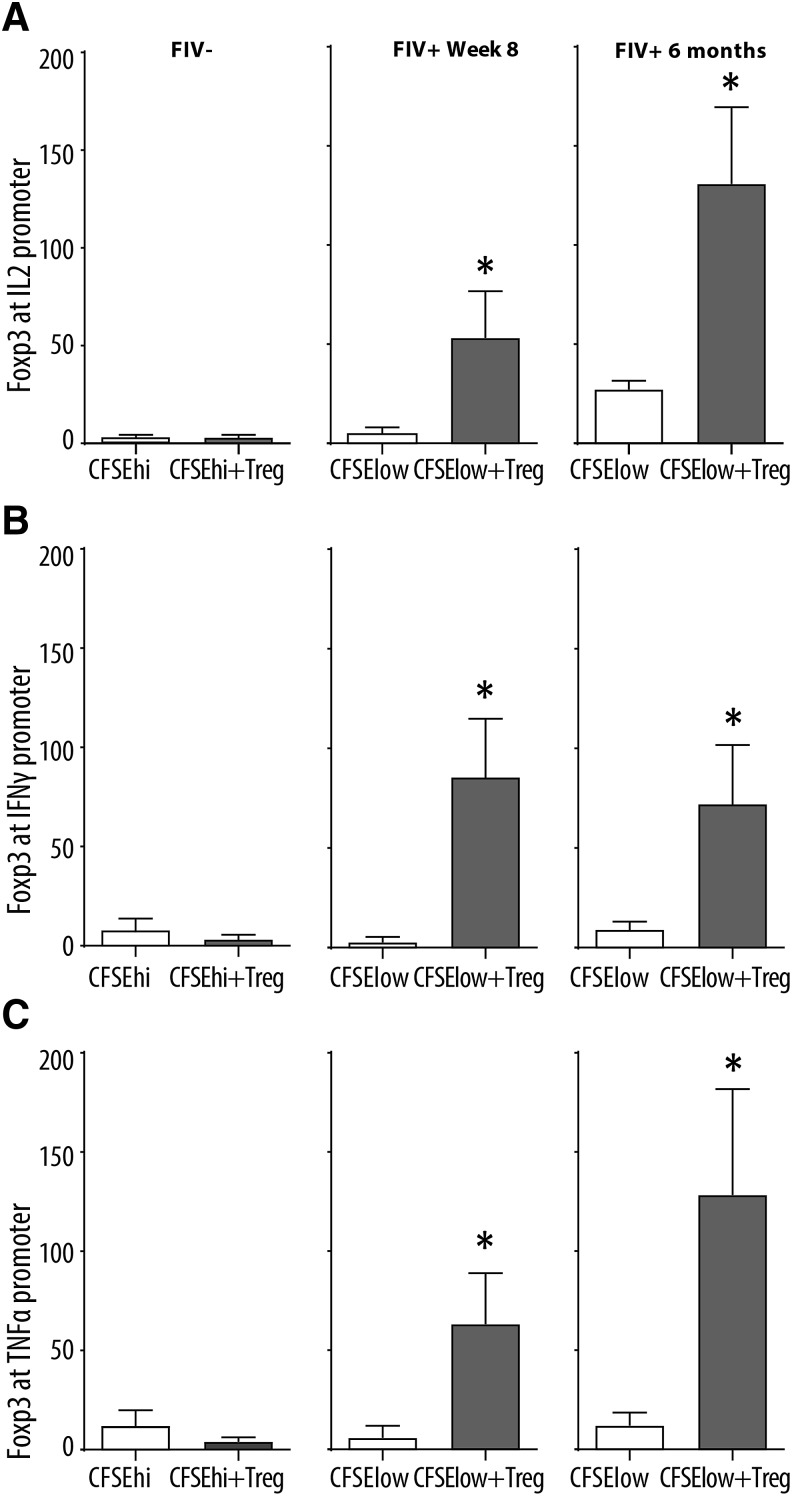
Foxp3 binds the IL2, IFNγ, and TNFα promoters in virus-specific CD8+ T cells. CD8+ T cells from FIV- cats were cocultured with autologous CD4+CD25+ Treg cells (left column). Virus-specific CD8+ T cells from FIV+ cats 8 weeks (center column) and 6 months (right column) were either untreated (white bars, CFSElow) or cocultured with autologous CD4+CD25+ Treg cells (CFSElow+Treg). After 24 h, Foxp3 ChIP followed by quantitative polymerase chain reaction for IL2, IFNγ, and TNFα promoters were performed. Results show increased Foxp3 binding to the IL2, IFNγ, and TNFα promoters and (A–C) after coculture with autologous T reg cells at both 8 weeks and 6 months postinfection for FIV+ cats (n = 5–7 cats). FIV negative cats exhibited almost no Foxp3 binding to the various promoters following autologous Treg cell coculture. Bars represent the mean + SEM. Data were analyzed using an unpaired t-test with significance set at p < .05, asterisks. ChIP, chromatin immunoprecipitation.
Results
Isolation of virus-specific CD8+ T cells from FIV+ cats
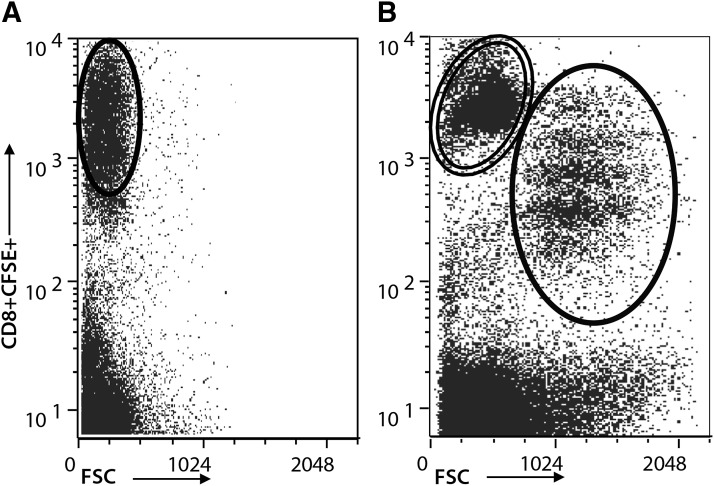
SPF cats were infected with FIV-NCSU1 and an equal number of cats were sham infected with sterile cell culture medium as described in the Methods section. Two PLN (submandibular and popliteal) were collected at 1, 4, and 8 weeks postinfection during the acute stage of infection and between 6 and 12 months postinfection. CD4+CD25+ Treg cells were depleted from LN suspensions and kept separately in culture. Isolated CD8+ T cells were CFSE labeled, returned to culture, and stimulated with virus in vitro as described in the Methods section. Following stimulation, virus-specific CD8+ T cells (CFSEint/low) were isolated from nonspecific CD8+ T cells (CFSEhi) for subsequent coculture experiments with autologous CD4+CD25+ Treg cells. Figure 1A shows nonspecific CD8+ T cells in FIV- control cats after FIV stimulation. Figure 1B shows the proliferation of CD8+ T cells from FIV+ cats through approximately four generations, in response to viral stimulation.
FIG. 1.
Isolation of virus-specific CD8+ T cells from FIV+ cats. CD8+ T cells were isolated from peripheral LNs of FIV- control cats (A) and FIV+ cats (B). The cells were CFSE stained and returned to CD4+CD25+ depleted LN cultures. The cultures were stimulated with UV inactivated FIV for 72 h and resorted. CD8+ T cells from FIV- control cats exhibited some CFSE dilution in culture (circled, A). Dead cells were gated by DAPI exclusion (not shown). Virus-specific CD8+ T cells from FIV+ cats were identified by CFSE dilution and increased forward scatter (single circle, B) as compared with virus-nonspecific CD8+ T cells that did not divide in response to FIV (double circle, B). CFSE, carboxyfluorescein succinimidyl ester; LN, lymph node; FIV, feline immunodeficiency virus.
IL2, IFNγ, and TNFα mRNA levels from virus-specific CD8+ T cells were decreased following autologous CD4+CD25+ Treg cell coculture
Following Treg cell coculture, CD8+ T cells were reisolated and analyzed by qPCR for IL2, TNFα, and IFNγ mRNA expression at 1, 4, and 8 weeks postinfection. CFSEhi (nonvirus specific) CD8+ T cells were compared with virus-specific CD8+ T cells. There was no change observed in the mRNA levels of IL2, IFNγ, and TNFα in nonspecific CD8+ T cells (CFSEhi) upon coculture with autologous Treg cells. As reported in Figure 2, TNFα mRNA expression was decreased in virus-specific CD8+ T cells from FIV+ cats following autologous Treg cell coculture at 4 weeks postinfection. IL2 and IFNγ mRNA expression were decreased in virus-specific CD8+ T cells following autologous Treg cell coculture at both 4 and 8 weeks postinfection.
Foxp3 mRNA levels were increased in virus-specific CD8+ T cells following autologous CD4+CD25+ Treg cell coculture
Virus-specific CD8+ cells (CFSEint/low) from FIV+ cats and nonvirus-specific (CFSEhi) were cocultured with or without autologous CD4+CD25+ Treg suppressor cells for 24 h. After coculture, cells were resorted by high-speed cell sorting and analyzed by qPCR for Foxp3 mRNA expression. Following coculture with autologous Treg cells, increased Foxp3 mRNA levels were observed in virus-specific CD8+ T cells at 1, 4, and 8 weeks postinfection in FIV+ cats (Fig. 3A–C). Foxp3 induction was also noted in nonvirus-specific (CFSEhi) CD8+ T cells at 1 and 8 weeks postinfection, but to a much lesser degree than virus-specific CD8+ T cells. These results demonstrate that lentivirus-activated Tregs in FIV+ cats are able to induce Foxp3 mRNA expression in virus-specific CD8+ T cell targets.
Foxp3 binds the IL2, IFNγ, and TNFα promoter regions in virus-specific CD8+ T cells
We performed ChIP on virus-specific CD8+ T cells using a feline-specific anti-Foxp3 Ab, followed by qPCR for the IL2, TNFα, and IFNγ promoters. FIV- cats did not show any significant change in Foxp3 binding to the cytokine promoter regions before and after coculture (Fig. 4A–C). Our data clearly show Foxp3 binding to the IL2, TNFα, and IFNγ promoters at 8 weeks and 6 months (Fig. 4A–C) postinfection in virus-specific CD8+ T cells following Treg cell coculture.
Discussion
The purpose of the study here was to explore the molecular mechanisms associated with Treg-mediated suppression of virus-specific CD8+ T cells. Specifically, we looked at IL2 as an indicator of proliferative capacity in combination with TNFα and IFNγ as an indicator of antiviral function. In the murine LCMV system, chronic infection leads to a predictable inactivation of virus-specific CD8+ T cell responses. Fuller et al. clearly showed the sequential decrease and then loss of IL2, TNFα, and IFNγ in virus-specific CD8+ T cells.13 More importantly, CD8+ T cell antiviral function was restored following a reduction in LCMV viral load.13 Polyfunctional CD8+ T cells have been recognized as important to controlling virus in HIV ECs and in other HIV patient cohorts.24,25 A recent study by Deng et al. clearly showed that chronically infected HIV patients retain broad-spectrum CTL responses capable of eliminating viral reservoirs.26 However, these CTLs were dysfunctional, requiring extensive priming to eliminate autologous CD4+ reservoirs in vitro and in humanized mice in vivo. In the pivotal study by Shan et al. utilizing HIV latency-reversing drugs, CD8+ T cells were unable to kill infected CD4+ T cells following latency reversal alone; however, following Gag stimulation before latency reversal, autologous CD8+ T cells efficiently killed virus-infected CD4+ targets.27 Taken together, these results suggest that dysfunctional virus-specific CD8+ T cells are maintained during the course of AIDS lentiviral infection and may be rescued under the right conditions. Because feline CD8+ T cell tetramers are currently not available, we identified virus-specific CD8+ T cells through proliferation in response to FIV, ex vivo (Fig. 1). Our investigations, in this study, indicate a reduction in IL2, TNFα, and IFNγ mRNA in virus-specific CD8+ T cells, following Treg cell coculture, as early as 4 weeks postinfection (Fig. 2). The reduction in IL2 and IFNγ mRNA persisted in virus-specific CD8+ T cells at 8 weeks postinfection, whereas TNFα exhibited a trend of decreased mRNA, but did not reach significance.
Infection with a recombinant SIV/HIV virus (R5-SHIV) in macaques leads to the accumulation of Treg cells in LNs and Treg cell depletion restores CD8+ T cell responses to Gag epitopes.28 Miles et al. recently described accumulation of Treg cells and TGFβ-dependent suppression of follicular Th cells in LN follicles, and in a previous investigation, the same group clearly demonstrated that a lower number of SIV-specific CTLs within LN follicles was associated with increased SIV RNA.29,30 Using the FIV lentiviral model, we have clearly demonstrated that activated Treg cells display membrane-bound TGFβ (mTGFβ), activated Th, and CD8+ T cells upregulate TGFβRII, and that Treg-mediated suppression is TGFβ dependent.16,19 Treg-mediated signaling through the T cell TGFβRII leads to downstream Smad phosphorylation and increased Foxp3 expression.16 We have previously documented TGFβ-dependent Foxp3 induction in CD4+ and CD8+ T cells following Treg cell coculture.15,17 We have demonstrated that Treg-induced Foxp3 expression in CD4+ T helper target cells leads to the induction of Treg cell suppressor function in the CD4+ T helper targets.15 We have also described Treg-induced Foxp3 binding to the CD8+ T cell IL2 promoter in virus-nonspecific CD8+ T cells.20 Although others have demonstrated that CD8+Foxp3+ cells are indeed suppressors, our laboratory was unable to document that CD8+ lymphocytes exhibited suppressor function following Treg coculture, despite the induction of CD8+ Foxp3 expression.17,31,32 In this study, we clearly demonstrate Treg-induced Foxp3 mRNA in virus-specific CD8+ T cells at 1, 4, and 8 weeks postinfection (Fig. 3). It is interesting to note that Foxp3 mRNA in virus-specific CD8+ T cells is induced quite early during the course of lentiviral infection. When taken together with Figure 2, the results suggest an inverse correlation between Foxp3 mRNA and IL2, TNFα, and IFNγ mRNA in virus-specific CD8+ T cells, following Treg cell coculture. These results also suggest a short lag period between Foxp3 mRNA upregulation (1 week postinfection, Fig. 2) and inhibition of cytokine mRNA (4 weeks postinfection, Fig. 3) as Foxp3 protein binds these promoter regions in the nucleus.
The transcription factor Foxp3 serves as a “master molecule” for Treg function. Foxp3 alters gene expression by binding to specific promoters and regulating their transcription.20 For example, Foxp3 binds the IL2 promoter and decreases transcription of IL2 while increasing the expression of the high-affinity IL2 receptor alpha (CD25).33 In CD4+ T cells, Foxp3 binding to the IL2, TNFα, and IFNγ promoter regions has been described.34,35 Transient expression of Foxp3 in activated CD8+ lymphocytes is most likely a normal regulatory feedback mechanism.36,37 However, less is known about the role persistent Foxp3 signaling might play in modulating CD8+ T cell function.17,37 Based upon the collective evidence described above (Figs. 2, 3), we asked if Treg-induced Foxp3 could bind to the IL2, TNFα, and IFNγ promoter regions in virus-specific CD8+ T cells from FIV+ cats. We were able to document Foxp3 binding to the IL2, TNFα, and IFNγ promoters at 8 weeks and 6 months postinfection in virus-specific CD8+ T cells following Treg cell coculture (Fig. 4).
There were several limitations to this study. The assessment of mRNA (Figs. 2, 3) and the ChIP assays required a relatively high number of virus-specific CD8+ T cells. Therefore, we did not examine surface CD8+ T cell phenotype or polyfunctionality by flow cytometry for these studies. Based upon the mRNA and ChIP data presented in this study, and our previous studies examining Treg-mediated inhibition of cytokine secretion in FIV+ cats, it is likely that there was a reduction in virus-specific CD8+ T cell polyfunctionality following autologous Treg cell coculture.15–19 We were limited by the total number of lymphocytes available after harvesting PLNs. Therefore, we chose 1, 4, and 8 weeks postinfection for mRNA assessment and 8 weeks post and 6 months postinfection to perform the Foxp3 ChIP assays. As shown in Figure 2, TNFα mRNA was not decreased at 8 weeks postinfection. However, Figure 4 demonstrated a high but variable degree of Foxp3 binding to the TNFα promoter region 8 weeks postinfection. Taken together, these results suggest a more complex relationship between Foxp3 and TNFα expression.
Our previous findings demonstrate that lentivirus-activated Treg cells are activated during the course of FIV infection and upregulate mTGFβ, and that CD4+ and CD8+ T cells upregulate TGFβRII during the course of infection.15,16,19 Furthermore, we have demonstrated that Foxp3 induction is mediated through TGFβ signaling in both CD4+ and CD8+ T cells and that Foxp3 binds the IL2 promoter in virus nonspecific CD8+ T cells.15,17,20 The results here clearly demonstrate that lentivirus-activated Treg cells interact with virus-specific CD8+ T cells to induce Foxp3 expression. Furthermore, these results demonstrate that Foxp3 inhibits IL2, TNFα, and IFNγ transcription by binding to these gene promoter regions in lentivirus-specific CD8+ T cells. We believe this is the first description of this process during the course of lentiviral infection. More importantly, these findings demonstrate a potential mechanism for the progressive loss of function in virus-specific CD8+ T cells. Heightened Treg cell suppressor function has been clearly documented for lentiviral infection, including FIV, SIV, and HIV.16,27,29 However, Treg cell activation is not unique to lentiviral infection. Treg cells are activated during the course of other types of chronic infections, such as hepatitis C virus and Leishmania infection and neoplastic diseases such as melanoma.11,38,39 Therefore, it is likely that the mechanisms described in this study may also contribute to T cell immune dysfunction in other diseases with heightened Treg cell function. The studies by Deng et al. and Shan et al. mentioned above, suggest that CD8+ T cell dysfunction is reversible and that augmenting antiviral activity in these cells is possible.26,27 Our previous studies and the studies presented here demonstrate that lentivirus-activated Treg cells mediate CD8+ T cell dysfunction through Foxp3 induction. Based upon these findings, our laboratory is exploring mechanisms for blocking Foxp3 promoter binding in an effort to augment CD8+ T cell antiviral function. These results may yield new therapeutic approaches for enhancing virus-specific CD8+ T cell function in vaccination and latency reversal strategies.
Acknowledgments
The authors would like to thank Laura Edwards, Dan Bogan, and Linda English for their excellent technical assistance. This research was supported by the Creative and Novel Ideas in HIV Research (CNIHR) Program through a supplement to the University of Alabama at Birmingham (UAB) Center For AIDS Research funding (P30 AI027767). This funding was made possible by collaborative efforts of the Office of AIDS Research, the National Institute of Allergy and Infectious Diseases, and the International AIDS Society. This publication resulted (in part) from research supported by the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR), an NIH funded program P30 AI50410.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lopez M, Soriano V, Lozano S, et al. : No major differences in the functional profile of HIV Gag and Nef-specific CD8+ responses between long-term nonprogressors and typical progressors. AIDS Res Hum Retroviruses 2008;24:1185–1195 [DOI] [PubMed] [Google Scholar]
- 2.Jagannathan P, Osborne CM, Royce C, et al. : Comparisons of CD8+ T cells specific for human immunodeficiency virus, hepatitis C virus, and cytomegalovirus reveal differences in frequency, immunodominance, phenotype, and interleukin-2 responsiveness. J Virol 2009;83:2728–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bucci J, Gebhard D, Childers T, et al. : The CD8+ phenotype mediating antiviral activity in FIV-infected cats is characterized by reduced surface expression of the CD8 beta chain. J Infect Dis 1998;178:968–977 [DOI] [PubMed] [Google Scholar]
- 4.O'Connell KA, Bailey JR, Blankson JN: Elucidating the elite: Mechanisms of control in HIV-1 infection. Trends Pharmacol Sci 2009;30:631–637 [DOI] [PubMed] [Google Scholar]
- 5.Riou C, Burgers WA, Mlisana K, et al. : Differential impact of magnitude, polyfunctional capacity, and specificity of HIV-specific CD8+ T cell responses on HIV set point. J Virol 2014;88:1819–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betts MR, Nason MC, West SM, et al. : HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006;107:4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Migueles SA, Laborico AC, Shupert WL, et al. : HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol 2002;3:1061–1068 [DOI] [PubMed] [Google Scholar]
- 8.Kelker HC, Seidlin M, Vogler M, et al. : Lymphocytes from some long-term seronegative heterosexual partners of HIV-infected individuals proliferate in response to HIV antigens. AIDS Res Hum Retroviruses 1992;8:1355–1359 [DOI] [PubMed] [Google Scholar]
- 9.Reddy MM, Englard A, Brown D, et al. : Lymphoproliferative responses to human immunodeficiency virus antigen in asymptomatic intravenous drug abusers and in patients with lymphadenopathy or AIDS. J Infect Dis 1987;156:374–376 [DOI] [PubMed] [Google Scholar]
- 10.Wahren B, Morfeldt-Mansson L, Biberfeld G, et al. : Characteristics of the specific cell-mediated immune response in human immunodeficiency virus infection. J Virol 1987;61:2017–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boettler T, Spangenberg HC, Neumann-Haefelin C, et al. : T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol 2005;79:7860–7867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zajac AJ, Blattman JN, Murali-Krishna K, et al. : Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 1998;188:2205–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller MJ, Khanolkar A, Tebo AE, et al. : Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J Immunol 2004;172:4204–4214 [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan M, Frauwirth KA: Peripheral tolerance in CD8+ T cells. Cytokine 2009;46:147–159 [DOI] [PubMed] [Google Scholar]
- 15.Miller MM, Petty CS, Tompkins MB, et al. : CD4+CD25+ T regulatory cells activated during feline immunodeficiency virus infection convert T helper cells into functional suppressors through a membrane-bound TGFbeta/GARP-mediated mechanism. Virol J 2014;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fogle JE, Mexas AM, Tompkins WA, et al. : CD4(+)CD25(+) T regulatory cells inhibit CD8(+) IFN-gamma production during acute and chronic FIV infection utilizing a membrane TGF-beta-dependent mechanism. AIDS Res Hum Retroviruses 2010;26:201–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fogle JE, Tompkins WA, Tompkins MB: CD4+CD25+ T regulatory cells from FIV+ cats induce a unique anergic profile in CD8+ lymphocyte targets. Retrovirology 2010;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mexas AM, Fogle JE, Tompkins WA, et al. : CD4+CD25+ regulatory T cells are infected and activated during acute FIV infection. Vet Immunol Immunopathol 2008;126:263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petty CS, Tompkins MB, Tompkins WA: Transforming growth factor-beta/transforming growth factor-betaRII signaling may regulate CD4+CD25+ T-regulatory cell homeostasis and suppressor function in feline AIDS lentivirus infection. J Acquir Immune Defic Syndr 2008;47:148–160 [DOI] [PubMed] [Google Scholar]
- 20.Miller MM, Akaronu N, Thompson EM, et al. : Modulating DNA methylation in activated CD8+ T cells inhibits regulatory T cell-induced binding of Foxp3 to the CD8+ T Cell IL-2 promoter. J Immunol 2015;194:990–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritchey JW, Levy JK, Bliss SK, et al. : Constitutive expression of types 1 and 2 cytokines by alveolar macrophages from feline immunodeficiency virus-infected cats. Vet Immunol Immunopathol 2001;79:83–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tompkins MB, Gebhard DH, Bingham HR, et al. : Characterization of monoclonal antibodies to feline T lymphocytes and their use in the analysis of lymphocyte tissue distribution in the cat. Vet Immunol Immunopathol 1990;26:305–317 [DOI] [PubMed] [Google Scholar]
- 23.Ohno K, Goitsuka R, Kitamura K, et al. : Production of a monoclonal antibody that defines the alpha-subunit of the feline IL-2 receptor. Hybridoma 1992;11:595–605 [DOI] [PubMed] [Google Scholar]
- 24.McKinnon LR, Kaul R, Kimani J, et al. : HIV-specific CD8+ T-cell proliferation is prospectively associated with delayed disease progression. Immunol Cell Biol 2012;90:346–351 [DOI] [PubMed] [Google Scholar]
- 25.Buckheit RW, 3rd, Salgado M, Silciano RF, et al. : Inhibitory potential of subpopulations of CD8+ T cells in HIV-1-infected elite suppressors. J Virol 2012;86:13679–13688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng K, Pertea M, Rongvaux A, et al. : Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 2015;517:381–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shan L, Deng K, Shroff NS, et al. : Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 2012;36:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santosuosso M, Righi E, Hill ED, et al. : R5-SHIV induces multiple defects in T cell function during early infection of rhesus macaques including accumulation of T reg cells in lymph nodes. PLoS One 2011;6:e18465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miles B, Miller SM, Folkvord JM, et al. : Follicular regulatory T cells impair follicular T helper cells in HIV and SIV infection. Nat Commun 2015;6:8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connick E, Folkvord JM, Lind KT, et al. : Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. J Immunol 2014;193:5613–5625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nigam P, Velu V, Kannanganat S, et al. : Expansion of FOXP3+ CD8 T cells with suppressive potential in colorectal mucosa following a pathogenic simian immunodeficiency virus infection correlates with diminished antiviral T cell response and viral control. J Immunol 2010;184:1690–1701 [DOI] [PubMed] [Google Scholar]
- 32.George J, Cofano EB, Lybarger E, et al. : Early short-term antiretroviral therapy is associated with a reduced prevalence of CD8(+)FoxP3(+) T cells in simian immunodeficiency virus-infected controller rhesus macaques. AIDS Res Hum Retroviruses 2011;27:763–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fontenot JD, Gavin MA, Rudensky AY: Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003;4:330–336 [DOI] [PubMed] [Google Scholar]
- 34.Beavis PA, Gregory B, Green P, et al. : Resistance to regulatory T cell-mediated suppression in rheumatoid arthritis can be bypassed by ectopic foxp3 expression in pathogenic synovial T cells. Proc Natl Acad Sci U S A 2011;108:16717–16722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen C, Rowell EA, Thomas RM, et al. : Transcriptional regulation by Foxp3 is associated with direct promoter occupancy and modulation of histone acetylation. J Biol Chem 2006;281:36828–36834 [DOI] [PubMed] [Google Scholar]
- 36.Morgan ME, van Bilsen JH, Bakker AM, et al. : Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol 2005;66:13–20 [DOI] [PubMed] [Google Scholar]
- 37.Hoji A, Coro A, Ng HL, et al. : Proliferation and foxp3 expression in virus-specific memory CD8+ T lymphocytes. AIDS Res Hum Retroviruses 2008;24:1087–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belkaid Y, Piccirillo CA, Mendez S, et al. : CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 2002;420:502–507 [DOI] [PubMed] [Google Scholar]
- 39.Baumgartner J, Wilson C, Palmer B, et al. : Melanoma induces immunosuppression by up-regulating FOXP3(+) regulatory T cells. J Surg Res 2007;141:72–77 [DOI] [PMC free article] [PubMed] [Google Scholar]