Abstract
Spinal cord injury (SCI) results in lesions that destroy tissue and disrupt spinal tracts, producing deficits in locomotor and autonomic function. We previously demonstrated that motoneurons and the muscles they innervate show pronounced atrophy after SCI, and these changes are prevented by treatment with testosterone. Here, we assessed whether the testosterone active metabolites estradiol and dihydrotestosterone have similar protective effects after SCI. Young adult female rats received either sham or T9 spinal cord contusion injuries and were treated with estradiol, dihydrotestosterone, both, or nothing via Silastic capsules. Basso-Beattie-Bresnahan locomotor testing was performed weekly and voiding behavior was assessed at 3 weeks post-injury. Four weeks after SCI, lesion volume and tissue sparing, quadriceps muscle fiber cross-sectional area, and motoneuron dendritic morphology were assessed. Spontaneous locomotor behavior improved after SCI, but hormone treatments had no effect. Voiding behavior was disrupted after SCI, but was significantly improved by treatment with either estradiol or dihydrotestosterone; combined treatment was maximally effective. Treatment with estradiol reduced lesion volume, but dihydrotestosterone alone and estradiol combined with dihydrotestosterone were ineffective. SCI-induced decreases in motoneuron dendritic length were attenuated by all hormone treatments. SCI-induced reductions in muscle fiber cross-sectional areas were prevented by treatment with either dihydrotestosterone or estradiol combined with dihydrotestosterone, but estradiol treatment was ineffective. These findings suggest that deficits in micturition and regressive changes in motoneuron and muscle morphology seen after SCI are ameliorated by treatment with estradiol or dihydrotestosterone, further supporting a role for steroid hormones as neurotherapeutic agents in the injured nervous system.
Keywords: : dendrites, morphology, neuroprotection, steroids
Introduction
Spinal cord injury (SCI) experienced by humans is a devastating medical problem with high mortality and long-term morbidity. According to the National SCI Statistical Center, it is estimated that the annual incidence of SCI is 17,500 new cases.1 The number of SCI patients in the United States who were alive in 2017 is between 245,000 and 353,000, with an estimated lifetime cost of $1.6-$4.8 million each.
The pathophysiology of SCI involves both immediate and secondary effects. After the initial mechanical deformation, a protracted period of progressive damage occurs, causing spreading of the lesion and further segmental destruction.2 A variety of mechanisms contribute to this progressive secondary injury, including excitotoxicity,3 free radical generation,4 protease activation,5 and inflammation,6–8 resulting in the death of motoneurons, interneurons, and glial cells in the spinal cord.2,7,9 Similarly, damage to spinal nerves resulting in laceration and avulsion of spinal roots (e.g., cauda equina injury with high impact motor vehicle accidents)10 can lead to the death of motoneurons and pre-ganglionic autonomic neurons in the spinal cord, resulting in autonomic and motor dysfunction.11
Death is not the only outcome for injured spinal motoneurons, and importantly, remaining motoneurons after such insults show a variety of morphological and functional changes. For example, denervation of motoneurons can result in dendritic reorganization12 or atrophy.13,14 Similarly, after peripheral axotomy, motoneurons show functional and biochemical changes,15,16 as well as dendritic atrophy.17–19 We have previously shown that following SCI, surviving motoneurons innervating the quadriceps muscles had extensive reductions in dendritic lengths.20,21 The muscles innervated by these motoneurons were similarly atrophic, with reductions in weight and muscle fiber cross-sectional area.20,21
The SCI-induced atrophy of motoneuron dendrites and of the muscles these motoneurons innervate can be prevented by treatment with testosterone,20 effects consistent with the wide array of protective and therapeutic effects of testosterone.22 For example, testosterone is neuroprotective from oxidative stress,23 and protects against cell death.24 Testosterone accelerates both axon regeneration and functional recovery following axotomy.25 Testosterone treatment attenuates synaptic stripping seen after motoneuron injury, preserving central input to the motoneurons.26 Similarly, neuronal death induces dendritic atrophy and a concomitant deficit in excitability in spinal motoneurons, and treatment with testosterone attenuates these regressive changes in morphology and function.27–32 Treatment with testosterone improves motor function in SCI patients. Patients treated with testosterone had higher American Spinal Injury Association Impairment Scale discharge motor scores, a result ascribed to either improved strength through the anabolic effects of testosterone on skeletal muscle or its neuroprotective/neuroregenerative effects.33
The neuroprotective/neurotherapeutic effects of testosterone treatment may not necessarily be through the actions of testosterone per se, as testosterone is readily converted into its two primary metabolites, dihydrotestosterone via the enzyme 5α-reductase or estradiol by the enzyme aromatase. Both of these testosterone metabolites have been shown to have neuroprotective/neurotherapeutic effects after motoneuron injury.34–37 For injury after SCI specifically, treatment with estradiol previously has been reported to improve motor function, reduce inflammation, reduce apoptotic cell death, reduce lesion size, increase white matter sparing, and promote earlier cytokine release and astroglial response.8, 38–42
Given the protective effects of testosterone we have previously observed, in this experiment we assessed if the active metabolites of testosterone, estradiol and dihydrotestosterone, have similar protective/therapeutic effects on the sequelae of SCI.
Methods
Young adult female rats (Sprague-Dawley; Harlan) approximately 12 weeks old were maintained on a 12:12 light/dark cycle with food and water freely available. All surgical interventions and post-operative animal care were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council) and were approved by the Indiana University Institutional Animal Care and Use Committee.
Spinal cord contusion and hormone treatment
Rats were anesthetized by injection of ketamine (61.5 mg/kg)/xylazine (8.6 mg/kg), intraperitoneally. A T9 laminectomy was performed to expose the underlying thoracic spinal cord segment(s), and animals received a severe (a 10 g weight dropped from a height of 25 mm) contusion injury using an NYU impactor. Spinal cord injury at the T9 vertebra (T10-11 spinal cord) was intended to preserve central pattern generators at L1-2 required for locomotor function and the relevant motoneurons for our analysis. The muscle and skin were then closed in layers using 5-0 suture and stainless steel wound clips, respectively. Post-operative care followed previous protocols.43,44
Immediately following contusion injury, rats were implanted with subcutaneous Silastic capsules (3.18 mm O.D., 1.57 mm I.D.)45 that were either empty (“SCI”) or filled with hormones. One group of injured rats were implanted with dihydrotestosterone (5α-androstan-17β -ol-3-one; Steraloids, Newport, RI; 30 mm; “SCI+D”). Such implants produce plasma titers of dihydrotestosterone in the normal physiological range, and have previously been demonstrated to reduce motoneuron atrophy induced by the death of neighboring motoneurons.30,34 Alternatively, some injured rats were implanted with estradiol [1,3,5(10)-estratrien-3, 17β-diol; Steraloids; 10% in cholesterol; 10 mm; “SCI+E”]. Such implants generate serum levels of estradiol similar to the average peak estradiol levels in intact cycling females in the proestrous phase,46,47 completely prevent the effect of ovariectomy on uterine weight,48 and reduce motoneuron atrophy induced by the death of neighboring motoneurons.34 Another group of injured rats was implanted with both dihydrotestosterone and estradiol capsules (“SCI+E+D”). The implant design and use followed those of Smith and colleagues,45 who demonstrated empirically that desired plasma levels of hormone are established within 1 h of implantation, and remain constant through durations of treatment similar to that used in this study (4 weeks; see below). An additional group of age-matched, sham-injured females served as normal controls. Because some of the animals in the study (overall n = 47) were not included in all analyses due to technical or histological failure, group sizes for each analysis are reported individually below.
Locomotor behavior
The Basso-Beattie-Bresnahan (BBB) locomotor rating scale49 was used to assess hindlimb locomotor recovery. BBB testing was performed weekly up to 4 weeks after SCI according to our standard protocol.50 Briefly, animals (SCI, n = 5; SCI+E, n = 7; SCI+D, n = 8; SCI+E+D, n = 6) were allowed to freely roam in an open field (diameter: 42 inches) and observed for 4 min by two trained observers lacking knowledge of the experimental groups. The scores were assigned on a scale of 0-21, which is based on movements including joint movement, hindlimb movements, stepping, forelimb and hindlimb coordination, trunk position and stability, paw placement, and tail position.
Metabolic cage test
At 3 weeks after SCI, measurement of micturition patterns was performed using metabolic cages according to methods described previously.51 Briefly, animals (SCI, n = 5; SCI+E, n = 7; SCI+D, n = 7; SCI+E+D, n = 5; an additional group of normal animals served as naïve controls, n = 8) were placed in a metabolic cage (Braintree Scientific, Braintree, MA) for measurement of voiding patterns. The voided urine was collected by a computerized system to record micturition volume and frequency. Animals were kept in this cage for 16 h with ample water and food during the period of urine collection and measurement. The micturition pattern analysis included void frequency (voids/h) and void volume (mL/void). The total volume of expelled urine was not included because of variations in water intake between individual animals.
Histological and histochemical processing
Four weeks after injury, a length of time sufficient to observe SCI-induced effects on motoneuron morphology,20,21 animals were re-anesthetized, and the left vastus lateralis muscle of the quadriceps was exposed and injected with horseradish peroxidase conjugated to the cholera toxin B subunit (BHRP; 2 μL, 0.2%; List Biological, Inc.). BHRP labeling permits population-level quantitative analysis of motoneuron somal and dendritic morphologies.52,53 Forty-eight hours after BHRP injection, a period that ensures optimal labeling of motoneurons,52,53 animals were weighed, re-anesthetized, and were then perfused intracardially with saline followed by cold fixative (1% paraformaldehyde/1.25% glutaraldehyde).
Lesion reconstruction
To assess potential effects on lesion volume and tissue sparing, spinal cords were carefully dissected to preserve the surrounding dura mater, and 15 mm thoracic spinal cord segments including the lesion epicenter were removed, post-fixed overnight in the same fixative as used for perfusion, and transferred to sucrose phosphate buffer (30% w/v, pH 7.4). Thoracic segments were then embedded in gelatin, frozen, and sectioned transversely at 40 μm; alternate sections were collected into two series, and mounted on gelatin-coated slides. One series was stained for myelin using Luxol fast blue and the other series was stained with cresyl violet and eosin for assessing lesion and spared tissue volume as described previously.20 The cross-sectional areas of lesion or spared white and gray matter for each animal (SCI, n = 11; SCI+E, n = 7; SCI+D, n = 8; SCI+E+D, n = 6) were measured in sections located 400 μm apart and spanning the entire rostrocaudal extent of the lesion using a video-based morphometry system (Stereo Investigator; MBF Bioscience, Williston, VT) at a final magnification of 202 × . An unbiased estimation of the percentage of spared tissue was calculated using the Cavalieri method.54 The total volume of spared white and gray matter was calculated by summing their individual subvolumes.55 Individual subvolumes of spared tissue were calculated by multiplying the cross-sectional area A × d, where d represents the distance between sections (400 μm). Septae or fibrous bands of tissue observed within and/or spanning areas of cystic cavitation were not considered to represent spared tissue. The percent total volume of spared white and gray matter was calculated by dividing the total volume of spared white and gray matter by the total tissue volume of the corresponding region ( × 100), respectively. Estimation of total and percent total lesion volume (which included areas of cavitation and fibrosis) was determined using identical procedures.
Motoneuron number and morphology
The vastus lateralis muscle is innervated by motoneurons located in column 3 of the lateral motor column in the L2 spinal segment.56–58 Following perfusion, the lumbar portion of the spinal cord of each animal (sham, n = 7; SCI, n = 11; SCI+E, n = 6; SCI+D, n = 6; SCI+E+D, n = 6) was removed, post-fixed 5 h in the same fixative as used for perfusion, then transferred to sucrose phosphate buffer (10% w/v, pH 7.4) overnight for cryoprotection. Spinal cords were then embedded in gelatin, frozen, and sectioned transversely at 40 μm; all sections were collected into four alternate series. One series was stained with thionin for use in cell counts. For visualization of BHRP, the three remaining series were immediately reacted using a modified tetramethyl benzidine protocol,59 mounted on gelatin-coated slides, and counterstained with thionin.
Motoneuron counts
To assess potential effects on motoneuron loss after SCI, counts of motoneurons in the quadriceps motor pool were performed. Motoneurons innervating the quadriceps muscles do not form a discrete nucleus, but instead are contained within the large continuous populations of motoneurons located within the lateral motor column. Thus, to identify the appropriate area within the lateral motor column for motoneuron counts in the unreacted series, we used a method similar to that of Byers and colleagues.20 Briefly, for each animal, the range of sections in which motoneurons labeled with BHRP after injection into the vastus lateralis muscle were present in the reacted series was identified, then motoneuron counts were performed in the appropriate matching sections in the unreacted series. For each animal, estimates of the total number of motoneurons in the left and right lateral motor columns were obtained using the optical dissector method as previously described.31 Counts were made at 937.5 × under brightfield illumination. Motoneurons are easily recognizable as large, darkly staining, multipolar cells. A counting frame (110 μm × 80 μm) was moved systematically throughout an area of each ventral horn (approximately 500 μm × 500 μm, defined by the actual distribution of BHRP-labeled somata from all of the animals used in the study) in each section within the identified range. Only motoneurons in which there was a clear nucleus and nucleolus were counted, provided they did not contact the forbidden lines of the counting frame; motoneuron nucleoli were counted as they appeared while focusing through the z axis, and nucleoli in the first focal plane (i.e., “tops”) were excluded to avoid double counting. The length of the dissector was approximately 16 μm, which was adequate for visualizing nucleoli in multiple focal planes. Motoneuron counts were derived from a mean of 7.8 sections spaced 480 μm apart and distributed uniformly through the entire rostrocaudal extent of the quadriceps motoneuron pool range. This sampling scheme produced an overall average estimated coefficient of error of 0.09. Cell counts for each animal were corrected for the proportion of sections sampled.
Using similar methods, the number of BHRP-labeled motoneurons was assessed in all sections of the reacted series through the entire rostrocaudal extent of their distribution for all animals. Counts of labeled quadriceps motoneurons were made under brightfield illumination, where somata could be visualized and cytoplasmic inclusion of BHRP reaction product confirmed.
Soma area
To assess potential atrophic changes in motoneurons after SCI, soma areas were measured. The area of quadriceps motoneuron somata was assessed in at least one set of alternate sections (160 μm apart) using the Nucleator method.60 A set of four rays emanating from a point randomly chosen within each BHRP-labeled motoneuron soma was drawn and oriented randomly. Soma areas of an average of 22.4 motoneurons were measured for each animal using Stereo Investigator at a final magnification of 780 × . The overall average estimated coefficient of error was 0.02. Soma areas within each animal were then averaged for statistical analysis.
Dendritic length
To assess potential atrophic changes in motoneurons after SCI, dendritic lengths and distributions were measured. For each animal, dendritic lengths in a single representative set of alternate sections were measured under darkfield illumination. Beginning with the first section in which BHRP-labeled fibers were present, labeling through the entire rostrocaudal extent of the quadriceps motoneuron dendritic field was assessed in every third section (480 μm apart) in three dimensions using a computer-based morphometry system (Neurolucida; MBF Bioscience, Williston, VT) at a final magnification of 250 × . No attempt was made to identify BHRP-labeled fibers as either dendrites or axons. Average dendritic length per labeled motoneuron was estimated by summing the measured dendritic lengths of the series of sections, multiplying by three to correct for sampling, then dividing by the total number of labeled motoneurons in that series. This method does not attempt to assess the actual total dendritic length of labeled motoneurons,61 but has been shown to be a sensitive and reliable indicator of changes in dendritic morphology in normal development,52,62,63 after changes in dendritic interactions62 and afferent input,12,64,65 and after injury28–32 including SCI.20,21
Dendritic distribution
To assess potential redistributions of dendrites across treatment groups, for each animal the composite dendritic arbor created in the length analysis was divided using a set of axes oriented radially around the center of the collective labeled somata. These axes divided the spinal cord into 12 bins of 30° each. The portion of each animal's dendritic arbor per labeled motoneuron contained within each location was then determined. This method provides a sensitive measure of dendritic redistribution in response to changes in dendritic interactions,62 afferent input,12,64 and SCI.20
Dendritic extent
The comparability of BHRP labeling across groups was assessed by quantifying both the rostrocaudal and the radial extent of quadriceps motoneuron dendritic arbors. The rostrocaudal extent of the dendritic arbor was determined by recording the rostrocaudal distance spanned by quadriceps motoneuron dendrites for each animal. The maximal radial extent of the arbor in the transverse plane also was measured for each animal, using the same radial axes and resultant 30° bins used for the dendritic distribution analysis. For each bin, the linear distance between the center of the quadriceps motor pool and the most distal BHRP-filled process was measured. Radial dendritic extent is independent of overall dendritic length and reflects the maximal linear distance (in the transverse plane) of BHRP transport to the most distal dendritic processes.
Muscle fiber morphology
To assess potential atrophic changes in muscle after SCI, the target musculature of the quadriceps motoneurons also was examined (sham, n = 7; SCI, n = 11; SCI+E, n = 7; SCI+D, n = 8; SCI+E+D, n = 6). The right vastus lateralis muscles were removed immediately after perfusion, weighed, post-fixed overnight in the same fixative as used for perfusion, and then transferred to sucrose phosphate buffer (10% w/v, pH 7.4). Muscles were then rinsed in distilled water and flash-frozen in 2-methylbutane. Segments from the middle of the body of the muscle were then sectioned (45 μm) on a cryostat at −20°C transversely for examination of muscle fiber cross-sectional area and thaw-mounted onto glass slides. Muscle fiber cross-sectional area, a correlate of muscle strength, was assessed after staining with Milligan's trichrome stain. Cross-sectional muscle fiber areas were measured under brightfield illumination using Stereo Investigator at a final magnification of 510 × . Muscle fiber cross-sectional areas were derived from five sections spaced 450 μm apart and distributed uniformly through the muscle segment. A counting frame (200 μm × 200 μm) was placed randomly over each section and the cross-sectional area of muscle fibers falling within the area of interest was measured using the Nucleator method.60 To obtain accurate measures of muscle fiber cross-sectional area, only en face profiles were traced. An average of 41.4 muscle fibers was measured for each animal. This sampling scheme produced an overall average estimated coefficient of error of 0.01. Fiber areas within each animal were then averaged for statistical analysis.
All data were analyzed by analyses of variance (ANOVAs; one-way or repeated measures as appropriate) followed by post hoc analyses using Fisher's least significant difference (LSD). Digital light micrographs were obtained using an MDS 290 digital camera system (Eastman Kodak Company, Rochester, NY). Brightness and contrast of these images were adjusted in Adobe Photoshop (Adobe Systems, San Jose, CA).
Results
Locomotor behavior
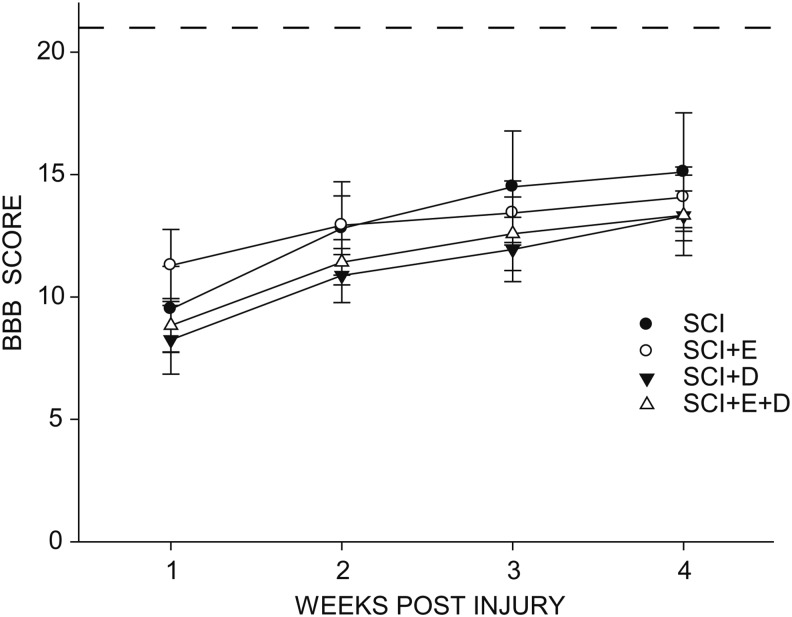
SCI reduced locomotor performance as measured by the BBB rating scale (Fig. 1). At 1 week post-injury, rats in the untreated SCI group showed occasional weight-supported plantar steps, but no forelimb-hindlimb coordination. The BBB scores improved over the 4 post-injury weeks [F(3,66) = 62.30, p < 0.0001; Fig. 1]. BBB scores plateaued at 3 weeks post-injury with rats regaining some function, typically showing consistent weight-supported plantar steps and forelimb-hindlimb coordination. Treatment with estradiol or dihydrotestosterone, either alone or in combination, had no effect on BBB score over the post-injury period, compared with the SCI-only group [group × treatment F(9,66) = 1.38, nonsignificant (ns)], and BBB scores did not differ across groups at 4 weeks post injury [F(3,22) = 0.28, ns].
FIG. 1.
Spinal cord injury (SCI) reduced locomotor performance in all groups, with improvement plateauing at 3 weeks post-injury. Treatment with hormones had no effect on locomotor performance. SCI, n = 5; SCI+estradiol (SCI+E), n = 7; SCI+dihydrotestosterone (SCI+DHT), n = 8; SCI+ estradiol and dihydrotestosterone (SCI+E+DHT), n = 6. The dashed line represents a perfect Basso-Beattie-Bresnahan (BBB) score of 21.
Micturition behavior
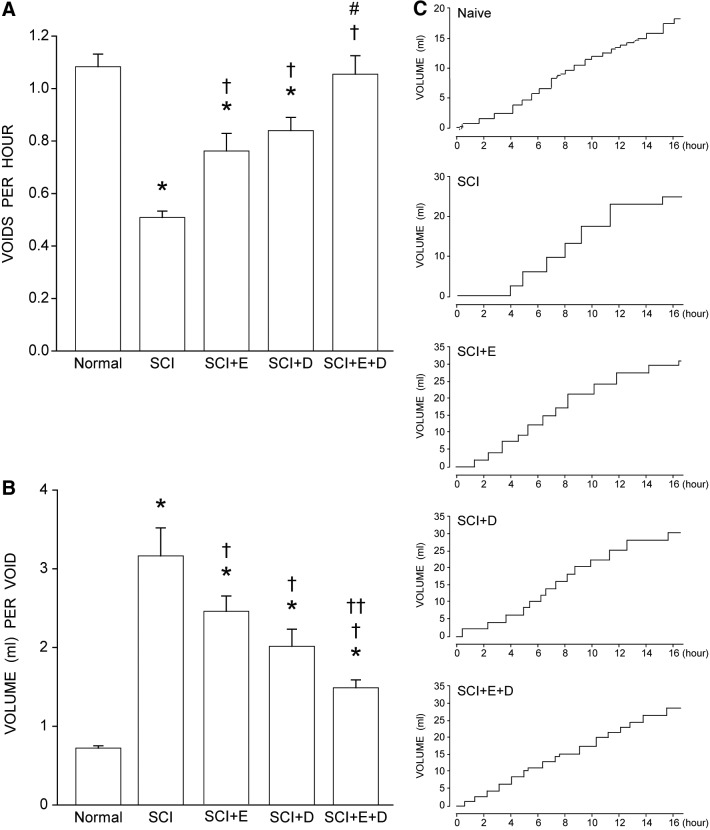
Differences in void frequency were present across groups [F(4,27) = 16.08, p < 0.0001; Fig. 2A]. Void frequency in the untreated SCI group (0.51 ± 0.02 voids/h, mean ± standard error of the mean) was significantly reduced (53%), compared with that of normal animals (1.08 ± 0.05 voids/h; LSD, p < 0.0001). Void frequency also was reduced in SCI animals treated with estradiol (30%, 0.76 ± 0.07 voids/h) or dihydrotestosterone (22%, 0.84 ± 0.05 voids/h; LSDs, p < 0.01), compared with that of normal animals, but not to the degree seen in untreated SCI animals. Compared with untreated SCI animals, void frequency in animals treated with either estradiol or dihydrotestosterone after SCI was increased by 50% and 65%, respectively (LSD; p < 0.005). Treatment with estradiol combined with dihydrotestosterone after SCI further increased void frequency by 107% (1.06 ± 0.07 voids/h) compared with untreated SCI animals (LSD, p < 0.0001), and was significantly greater than the void frequency seen in animals treated with either hormone alone (an average of 32%; LSDs, p < 0.02). Void frequency in animals treated with estradiol combined with dihydrotestosterone after SCI did not differ from that of normal animals (LSD, ns).
FIG. 2.
(A) Following spinal cord injury (SCI), void frequency decreased in untreated animals. Treatment with either estradiol (SCI+E) or dihydrotestosterone (SCI+D) improved voiding frequency; treatment with both hormones (SCI+E+D) improved voiding frequency to normal levels. (B) Following SCI, untreated animals had the highest volume per void. Treatment with either estradiol or dihydrotestosterone reduced void volume with the greatest decrease in animals treated with both hormones. Bar heights represent means ± standard error of the mean. *indicates significantly different from normal; †indicates significantly different from untreated SCI; # indicates significantly different from SCI + E and SCI+D; ††indicates significantly different from SCI+E. Normal (naïve), n = 8; SCI, n = 5; SCI+E, n = 7; SCI+DHT, n = 7; SCI+E+DHT, n = 5. (C) Representative smoothed metabolic cage traces illustrating micturition patterns. Normal (naive) animals typically have short latencies and small void volumes. Three weeks post-injury, untreated SCI animals have long latencies and high void volumes resulting in a step-like pattern. Animals that received either estradiol (SCI+E) or dihydrotestosterone (SCI+D) have a smoother pattern; treatment with both hormones (SCI+E+D) resulted in a micturition pattern that closely approximated the normal pattern.
Similarly, differences in void volume were present across groups [F(4,27) = 23.59, p < 0.0001; Fig. 2B]. Average void volume in the untreated SCI group (3.16 ± 0.36 mL/void) was significantly increased (338%), compared with that of normal animals (0.72 ± 0.03 mL/void; LSD, p < 0.0001). Void volume also was increased in SCI animals treated with estradiol (239%, 2.45 ± 0.19 mL/void) or dihydrotestosterone (179%, 2.02 ± 0.22 mL/void), compared with that of normal animals (LSDs, p < 0.0001), but not to the degree seen in untreated SCI animals. Compared with those of untreated SCI animals, void volumes in SCI animals treated with either estradiol or dihydrotestosterone were decreased by 23% and 36% respectively (LSDs, p < 0.015). Treatment with estradiol combined with dihydrotestosterone after SCI further decreased void volume by 53% (1.49 ± 0.10 mL/void), compared with untreated SCI animals (LSD, p < 0.0001), and was lower than the void volume seen in animals treated with either hormone alone (an average of 33%; estradiol combined with dihydrotestosterone vs. estradiol; LSD, p < 0.003; estradiol combined with dihydrotestosterone vs. dihydrotestosterone; LSD, marginal p < 0.075). Void volume in animals treated with estradiol combined with dihydrotestosterone after SCI was significantly increased, compared with that of normal animals (106%; LSD, p < 0.01).
The changes in void frequency and volume resulted in altered micturition patterns. Normal (naïve) animals typically have short latencies between voids and small void volumes, resulting in a linear cumulative pattern (Fig. 2C). Three weeks after SCI, the micturition pattern in untreated animals showed long latencies between voids and concomitant increases in void volumes, compared with those of normal animals, resulting in a pronounced step-like pattern. The changes in void frequency and void volume in SCI animals treated with either estradiol or dihydrotestosterone described above resulted in smoother cumulative micturition patterns; treatment with estradiol combined with dihydrotestosterone resulted in micturition patterns that closely approximated the normal pattern.
Lesion volume and white matter sparing
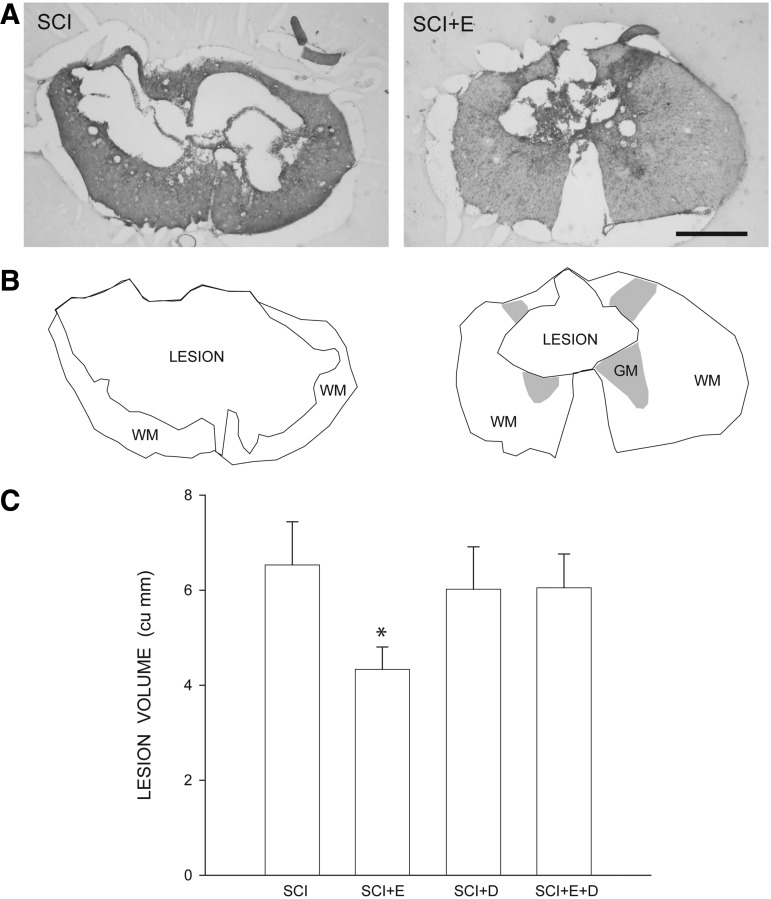
Contusive SCI resulted in large, centrally-located cystic cavities, with thin rims of spared tissue surrounding the cavity (Fig. 3A, 3B). In SCI groups, faint staining with Luxol fast blue indicated that contusion injury resulted in areas of demyelination immediately surrounding the lesion cavity. ANOVA revealed a significant main effect of group on lesion volume [F(3,28) = 2.97, p < 0.05; Fig. 3C]. Lesion volumes in estradiol-treated animals (4.35 ± 0.47 mm3) were significantly smaller than those of all other groups (LSDs, p < 0.05). Lesion volumes in blank-implanted animals (6.30 ± 0.44 mm3) did not differ from those of dihydrotestosterone (6.02 ± 0.63 mm3) or estradiol combined with dihydrotestosterone-treated animals (6.05 ± 0.39 mm3; LSDs, ns). Importantly, despite the large size of the lesions, they did not extend into the L2 level, and thus did not compromise the quadriceps motoneuron populations directly.
FIG. 3.
Histological and stereological analysis of spinal cord spared tissue and lesion volume after contusive spinal cord injury (SCI) with or without hormone treatment. (A) Representative sections through the lesion epicenter of an untreated animal (SCI) and an estradiol-treated animal (SCI+E) stained with cresyl violet and eosin, showing large centrally located cystic cavities with rims of spared tissue surrounding the cavity. Scale bar = 500 μm. (B) Stereo Investigator drawings from the same sections showing the lesion area (including regions of cavitation and fibrosis), residual white matter (WM), and spared gray matter (GM). (C) Lesion volume was reduced in SCI animals treated with estradiol. Bar heights represent means ± standard error of the mean. *indicates significantly different from untreated SCI; SCI, n = 11; SCI+estradiol (SCI+E), n = 7; SCI+dihydrotestosterone (SCI+DHT), n = 8; SCI+ estradiol and dihydrotestosterone (SCI+E+DHT), n = 6.
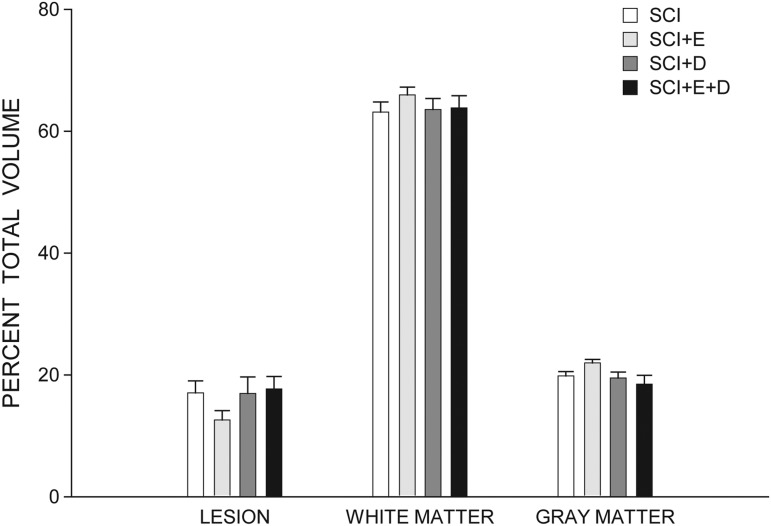
To correct for changes in spinal cord contour that were associated with cavitation after spinal cord contusion, the percent lesion volume and spared tissue were also determined (Fig. 4). Estradiol-treated SCI animals had smaller percent lesion volumes (12.42 ± 1.54%) and concomitantly larger percentages of spared tissue (87.58 ± 1.53%), compared with those of untreated SCI animals [17.18 ± 1.22% and 82.82 ± 1.22% respectively; Fs(1,16) > 5.92, ps < 0.03]. In contrast, compared with untreated SCI animals, no differences in percent lesion volumes or percentages of spared tissue were present in dihydrotestosterone- [16.94 ± 2.74% and 83.06 ± 2.74% respectively; Fs(1,17) < 0.01, ns] or estradiol-combined-with-dihydrotestosterone–treated animals [17.71 ± 2.04% and 82.29 ± 2.04% respectively; Fs(1,15) < 0.06, ns].
FIG. 4.
Percent total volumes of lesion and spared white and gray matters across groups. Bar heights represent means ± standard error of the mean. Spinal cord injury (SCI; white bars), n = 11; SCI+estradiol (SCI+E, light gray bars), n = 7; SCI+dihydrotestosterone (SCI+DHT, medium gray bars), n = 8; SCI+ estradiol and dihydrotestosterone (SCI+E+DHT, dark gray bars), n = 6.
Motoneuron counts
In sham animals, the number of motoneurons within the identified quadriceps range averaged 1913.14 (± 290.83). Contusive SCI with or without hormone treatment had no effect on the number of quadriceps motoneurons [SCI, 2434.91 ± 219.71; SCI+estradiol, 1900.00 ± 130.49; SCI+dihydrotestosterone, 1848.00 ± 325.14; SCI+estradiol and dihydrotestosterone, 1772.00 ± 348.48; F(4,31) = 1.24, ns].
Motoneuron morphometry
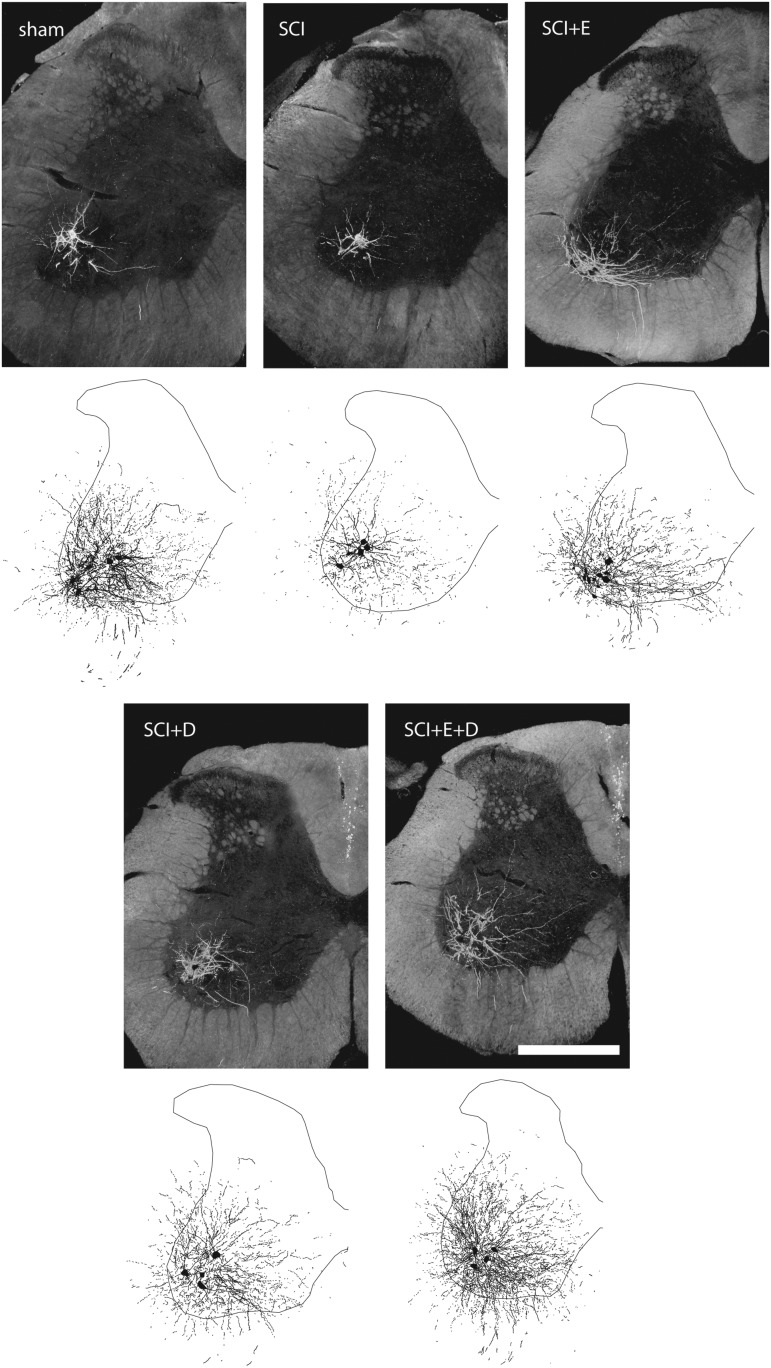
Injection of BHRP into the left vastus lateralis successfully labeled ipsilateral quadriceps motoneurons in all groups (Fig. 5). Labeled motoneurons were located in the lateral motor column in the L2 spinal segment.31,58 Dendritic arbors were strictly unilateral, with extensive ramification along the ventrolateral edges of the gray matter and in the lateral funiculus, as well as throughout the ventral horn. An average of 38.04 ± 4.88 motoneurons per animal were labeled with BHRP, and did not differ by group [F(4,31) = 0.57, ns].
FIG. 5.
Darkfield digital micrographs and matching computer-generated composites of transverse hemisections through the lumbar spinal cords of a sham animal (A, F), an injured animal given a blank implant (spinal cord injury [SCI]; B, G), an estradiol-treated injured animal (SCI+E; C, H), a dihydrotestosterone-treated injured animal (SCI+D; D, I), and an injured animal treated with both hormones (SCI+E+D; E, J), after BHRP injection into the left vastus lateralis muscle. Computer-generated composites of BHRP-labeled somata and processes were drawn at 480 μm intervals through the entire rostrocaudal extent of the quadriceps motor pool; these composites were selected because they are representative of their respective group average dendritic lengths. Scale bar = 500 μm.
Soma area
In sham animals, quadriceps motoneuron somata were typical in size (1131.59 ± 121.23 μm2), and did not differ from those of SCI (976.67 ± 54.70 μm2), SCI+estradiol (996.89 ± 26.47 μm2), SCI+dihydrotestosterone (1094.00 ± 46.31 μm2), or SCI+estradiol and dihydrotestosterone animals [1072.96 ± 105.57 μm2; F(4,31) = 0.78, ns].
Dendritic length
Following contusion injury, quadriceps motoneurons underwent marked dendritic atrophy (Fig. 6). Dendritic length decreased by 51% (4033.97 ± 639.19 μm in SCI animals, compared with 8199.59 ± 1053.87 μm for sham animals; LSD, p < 0.001; overall test for the effect of group on arbor per cell F(4,31) = 4.12, p < 0.01]. Treatment with hormones attenuated SCI-induced dendritic atrophy, and dendritic lengths in hormone-treated SCI groups did not differ from those of sham animals (LSDs, ns). Dendritic lengths in hormone-treated SCI groups were longer than those of untreated SCI animals (SCI+estradiol, 6337.89 ± 729.18 μm, 57% longer; SCI+dihydrotestosterone, 6696.20 ± 939.62 μm, 66% longer; SCI+estradiol and dihydrotestosterone, 6531.30 ± 801.19 μm, 62% longer; LSDs, p < .05).
FIG. 6.
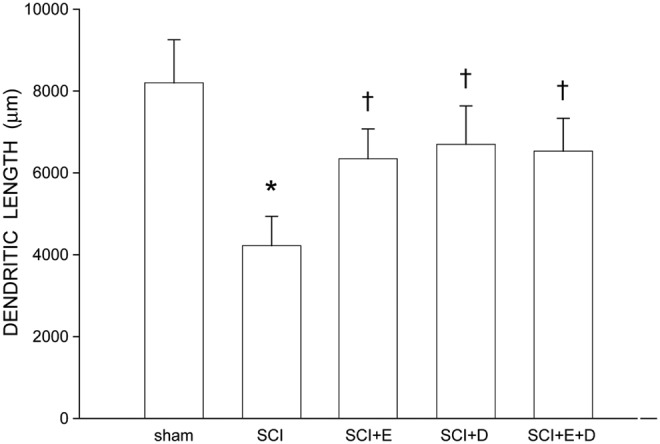
Dendritic lengths of quadriceps motoneurons of sham animals (n = 7) and injured animals that were either untreated (spinal cord injury [SCI]), n = 11, or treated with estradiol (SCI+E), n = 6; SCI+dihydrotestosterone (SCI+DHT), n = 6; or estradiol and dihydrotestosterone combined (SCI+E+DHT), n = 6. Following contusion injury, surviving quadriceps motoneurons lost over 50% of their dendritic length. Treatment with hormones attenuated this dendritic atrophy. Bar heights represent means ± standard error of the mean. *indicates significantly different from sham animals, †indicates significantly different from untreated SCI.
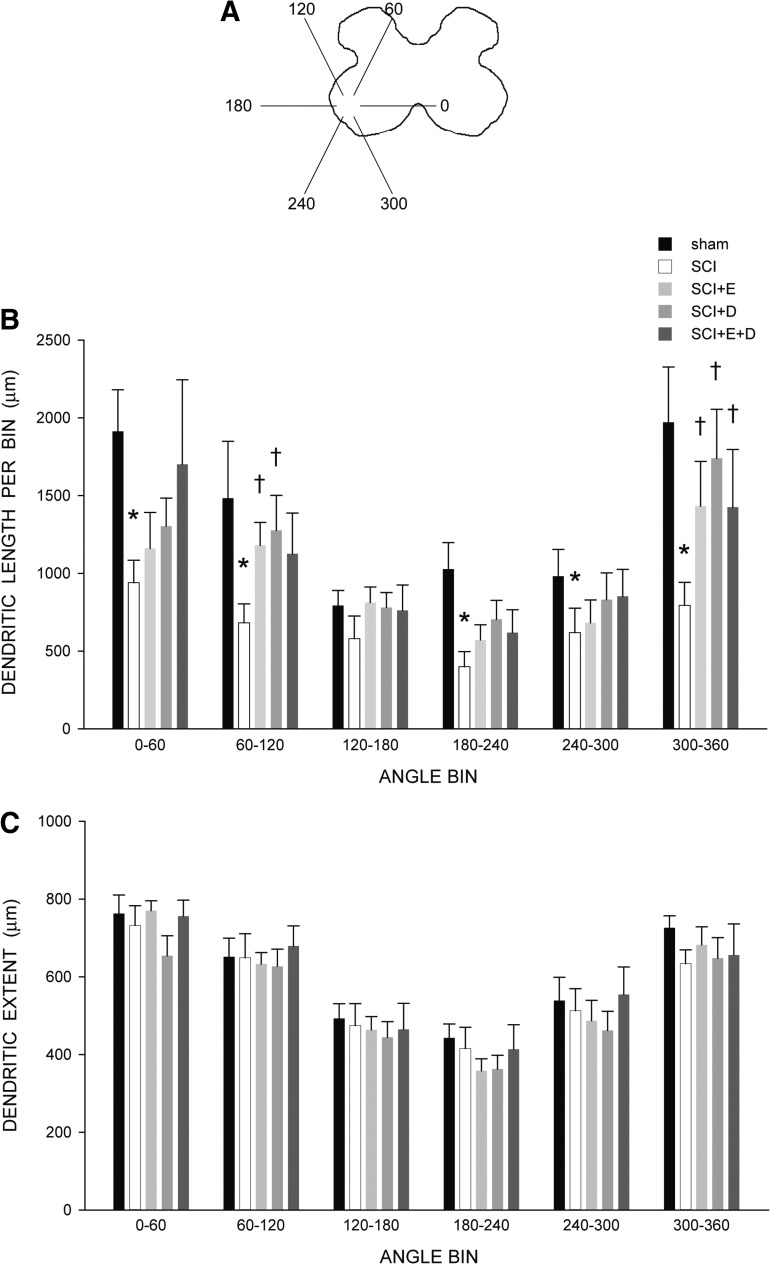
The distribution of dendrites was nonuniform across radial bins, and a repeated-measures ANOVA revealed a significant effect of radial location on dendritic length [F(11,341) = 18.50, p < 0.0001; Fig. 7B]. Consistent with the results of the arbor per cell analysis, there was also a significant effect of group [F(4,341) = 4.08, p < 0.01]. Reductions in dendritic length occurred throughout the radial distribution in SCI animals, compared with sham animals (an average of 46%, 0° to 300°), and were especially pronounced ventromedially (60%; 300° to 360°), resulting in a significant group × location interaction [F(1,176) = 2.89, p < 0.002]. Treatment with hormones attenuated SCI-induced reductions in dendritic length per bin, and there were no group differences [F(3,231) = 1.27, ns] and no group × location interaction [F(33,231) = 0.61, ns] between hormone-treated SCI animals and sham animals. The pronounced reduction in dendritic length seen ventromedially in untreated SCI animals was particularly attenuated in hormone-treated SCI groups, with dendritic lengths from 300° to 360° being longer than those of untreated SCI animals by an average of almost 80% [Fs(1,15) > 5.45, ps < 0.04].
FIG. 7.
(A) Drawing of spinal gray matter divided into radial sectors for measure of quadriceps motoneuron dendritic distribution and maximal radial extent. (B) Quadriceps motoneuron dendritic arbors display a non-uniform distribution, with the majority of the arbor located between 300° and 120°. Following contusion injury, surviving quadriceps motoneurons in untreated animals (spinal cord injury [SCI]) had reduced dendritic lengths throughout the radial distribution, especially ventromedially (60%, 300° to 360°). Treatment with hormones attenuated these reductions. *indicates significantly different from sham animals, †indicates significantly different from untreated SCI. (C) Following contusion injury, dendritic extent measures of surviving quadriceps motoneurons did not differ across groups, demonstrating a comparable degree of dendritic labeling. For graphic purposes, measures of dendritic length and extent have been collapsed into six bins of 60° each. Bar heights represent means ± standard error of the mean. Sham animals (n = 7); untreated SCI (white bars), n = 11; SCI+estradiol (SCI+E, light gray bars), n = 6; SCI+dihydrotestosterone (SCI+DHT, medium gray bars), n = 6; SCI+ estradiol and dihydrotestosterone (SCI+E+DHT, dark gray bars), n = 6.
Dendritic extent
Consistent with the nonuniform dendritic distribution of quadriceps motoneurons apparent in Figure 5, radial dendritic extent differed across bins (Fig. 7C), and repeated-measures ANOVA revealed a significant effect of location [F(11,341) = 41.35, p < 0.0001]. However, radial dendritic extent did not differ across groups [F(4,341) = 0.16, ns; Fig. 7C]. Rostrocaudal dendritic extent also did not differ across groups [F(4,31) = 1.55, ns], spanning 4480.00 ± 447.13 μm in sham animals, 4305.45 ± 434.42 μm in SCI animals, 3653.33 ± 86.82 μm in SCI+estradiol animals, 3386.67 ± 358.76 μm in SCI+dihydrotestosterone animals, and 3226.67 ± 606.34 μm in SCI+estradiol and dihydrotestosterone animals.
Muscle fiber size
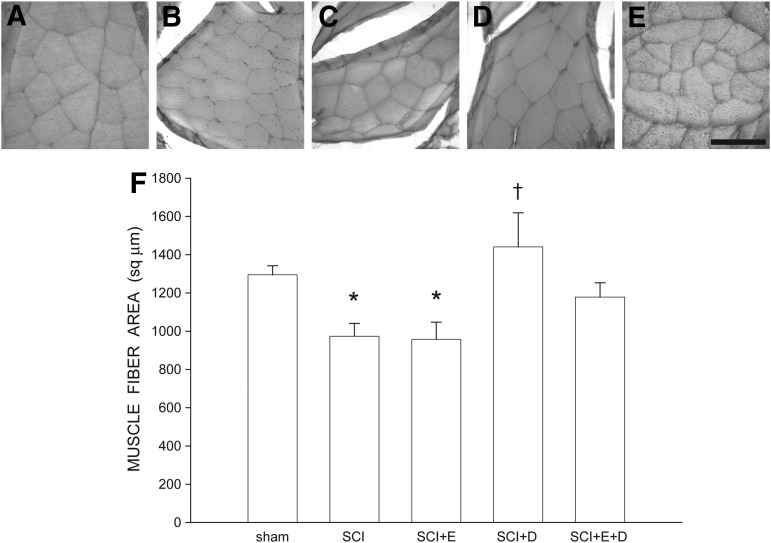
Overall body weight was not affected: animals weighed an average of 259.36 ± 4.6 g at the perfusion, and this did not differ between groups [F(4,34) = 3.47, ns]. This lack of difference is important, as body weight can influence muscle fiber cross-sectional area. Muscle fiber size was affected by contusion injury (Fig. 8). Cross-sectional area of vastus lateralis muscle fibers decreased by 25% [974.03 ± 67.18 μm2 in SCI animals, compared with 1295.12 ± 47.51 μm2 for sham animals, LSD, p < 0.03; overall test for the effect of group on muscle fiber area F(4,34) = 4.22, p < 0.007]. Similarly, muscle fiber area was decreased in SCI+estradiol animals by 26% [958.10 ± 89.33 μm2, LSD p < 0.04). However, treatment with dihydrotestosterone, either alone or in combination with estradiol, attenuated SCI-induced muscle fiber atrophy. Cross-sectional area of vastus lateralis muscle fibers in SCI+dihydrotestosterone animals (1441.01 ± 178.24 μm2) were 47.9% larger than those of SCI animals (LSD p < 0.002), and did not differ from those of sham animals (LSD, ns). Similarly, cross-sectional area of vastus lateralis muscle fibers in SCI+estradiol and dihydrotestosterone animals (1178.53 ± 74.59 μm2) were 21.0% larger than those of SCI animals, but this difference did not reach statistical significance; muscle fiber area in SCI+estradiol and dihydrotestosterone animals did not differ from those of sham animals (LSD, ns).
FIG. 8.
Digital micrographs of cross-sections through vastus lateralis muscles fibers from a sham animal (A), and injured animals that were either untreated (B), or treated with estradiol (C), dihydrotestosterone (D), or estradiol and dihydrotestosterone (E). Scale bar = 100 μm. (F) Following contusion injury, muscle fiber size in untreated animals was reduced 25%, and by 26% in animals treated with estradiol. Treatment with dihydrotestosterone, either alone or in combination with estradiol, attenuated reductions in fiber area. Bar heights represent means ± standard error of the mean. *indicates significantly different from sham animals, †indicates significantly different from untreated spinal cord injury (SCI). Sham animals (n = 7); untreated (SCI), n = 11, SCI+estradiol (SCI+E), n = 7; SCI+dihydrotestosterone (SCI+DHT), n = 8; SCI+ estradiol and dihydrotestosterone (SCI+E+DHT), n = 6.
Discussion
Testosterone treatment protects motoneurons and the muscles they innervate from SCI-induced atrophy.20 In this experiment, we assessed if the active metabolites of testosterone, estradiol and dihydrotestosterone, have similar protective/therapeutic effects on the sequelae of SCI. We found that treatment with estradiol and dihydrotestosterone, alone or in combination, had differential effects in ameliorating SCI-induced deficits. In some cases, both metabolites were protective after SCI, for example, reversing deficits in voiding behavior as well as attenuating motoneuron dendritic atrophy. In contrast, only estradiol was effective in reducing lesion volume, but only dihydrotestosterone attenuated muscle fiber atrophy. These diverse effects may provide new targets for therapeutic interventions aimed at enhancing recovery of function after SCI.
Locomotor behavior
Due to a naturally recovering reflex in rats, spinal cord injured rats regain some ability to walk within the weeks following injury. For this reason, BBB scores typically improve for the first few weeks after SCI, plateauing at a score that indicates the extent of permanent damage. In the present study, BBB scores improved post-injury, with rats regaining some function, typically showing consistent weight-supported hindpaw plantar steps and forelimb-hindlimb coordination. Treatment with estradiol, dihydrotestosterone, or both hormones combined had no effect on locomotor performance. Several previous studies have reported improved locomotor performance after SCI in animals treated with estradiol.8,38,41,42,66–68 However, others have failed to find such effects,69 or when using low doses of estradiol.8,38,42 The lack of effect on BBB scores in estradiol-treated animals in our study could be the result of the low dosage of estradiol we delivered, which was designed to be in the normal physiological range. Other differences in factors such as timing (pre- vs. post-treated) or route of hormone treatment (single injection vs. continuous infusion by pellets, osmotic pumps, or Silastic implants), type of injury (contusion vs. crush injury), sex, or animal substrains across studies also could be contributing to the inconsistencies seen with estradiol treatment.70
Micturition behavior
The storage and elimination of urine requires the spinal cord to integrate information from the brain, bladder, and urethra.71–73 The micturition reflex is mediated by a bulbospinal pathway passing through the pontine micturition center (Barrington's nucleus) in the rostral brainstem.74 SCI disrupts connections between the bladder and brainstem areas involved in micturition, leading to reduced void frequency and an accompanying increase in void volume.75 In the current study, treatment with estradiol reduced lesion volume and thus it is possible that the beneficial effects on voiding behavior we observed could potentially reflect estradiol-mediated tissue sparing or protection of descending tracts. However, similar treatment effects on voiding were present after treatment with dihydrotestosterone alone or in combination with estradiol, despite there being no reductions in lesion size or increases in tissue sparing in these groups. Thus, it is unlikely that the beneficial effects of steroid treatment on voiding we observed are likely a result of differential, long-term tissue or tract sparing.
Voiding also requires the integration of autonomic and somatic pathways within the lumbosacral cord,76 and the SCI-induced bladder areflexia recovers through a slow re-emergence of involuntary reflex micturition mediated by these spinal reflex pathways.75 Treatment with hormones resulted in improvement in both void frequency and void volumes after SCI, resulting in micturition patterns that more closely approximated the normal pattern. Because these improvements were seen in groups with lesions comparable to those of untreated animals (and thus with similar disruptions in descending tracts), it is possible that the hormones could be acting locally on the autonomic and somatic pathways within the lumbosacral cord. For example, our results showing protective effects of gonadal hormone treatment on the somatic motoneurons innervating the quadriceps muscle suggest that one possible explanation for the protective effects we observed on micturition could be through a similar hormonal protection of the autonomic motoneurons controlling urethral sphincter function. In fact, testosterone has been demonstrated to have potent effects on the size of autonomic neuron somata in the pelvic ganglia that control the bladder.77 Alternatively, changes in estrogen exposure can influence bladder innervation or activity,78,79 and the dorsal root ganglia innervating the bladder,80 as well as the bladder itself, express estrogen receptors α and β.81 Void frequency and volume in SCI animals treated with either estradiol or dihydrotestosterone were significantly improved over that of untreated SCI animals, and SCI animals treated with both hormones showed the best improvement. This suggests that estradiol and dihydrotestosterone might act additively, perhaps through action at different components of the lumbosacral reflex pathway. These results suggest that the best treatment strategy might include a combinatorial approach using both estradiol and dihydrotestosterone.
Spinal cord lesions
Following contusion, the focal injuries delivered to the T9 spinal cord developed into large lesions that spanned multiple thoracic spinal segments. Treatment with estradiol or dihydrotestosterone produced dramatically different effects on SCI lesions. Four weeks of treatment with dihydrotestosterone had no effect on lesion volume or tissue sparing, a result similar to what we previously found after treatment with testosterone.20 Thus, it appears that treatment with androgenic hormones is not effective in reducing lesion volume after SCI.
Following SCI, female mice show better recovery than males,82,83 an effect thought to be due to the neuroprotective effects of estrogens. In the present study, treatment with estradiol was effective in reducing lesion volume, a result consistent with previous findings.8,38–40,42,66–68 This reduction in lesion size is thought to be the result of reducing inflammation, reactive astrogliosis, decreased immune response, apoptotic cell death, or reductions in oxidative stress.8,38,39,42,67,68
Importantly, the reduction in lesion size we observed was produced through a physiological dose of estradiol, a result similar to that reported by Samantary and colleagues39 with low doses of estradiol. The efficacy of low dosages indicates that estradiol could be a promising therapeutic agent for treating SCI.39 Further, in the present study, estradiol was administered after trauma, modeling a clinically relevant situation.
In the present study, the effect of estradiol on decreasing lesion volume was not present when estradiol was co-administered with dihydrotestosterone. This negation of the protective effect of estradiol is similar to that reported by Hauben and colleagues,83 wherein treatment of female rats with dihydrotestosterone prior to SCI impaired recovery. Given that androgens have been demonstrated to regulate many of the same neuroprotective effects seen with estradiol treatment—such as protecting against cell death,24 upregulating GFAP84,85 or mediating the central glial response after injury86—this negation with combined treatment after SCI warrants further study. One plausible mechanism for this negation with combined treatment could be through an androgen-mediated immunosuppression.87 Regardless, given that testosterone is routinely metabolized into both estrogenic and androgenic metabolites, this negation could underlie the failure of testosterone treatment to affect SCI lesion volume we previously reported.20
Changes in motoneuron morphology are not direct effects of lesion
While extensive, spinal lesions did not extend into the lumbar spinal cord, thus sparing the gray matter and resident motoneurons. Counts of either Nissl-stained or BHRP-labeled motoneurons in SCI animals did not differ from those of sham animals, confirming the protection of quadriceps motoneurons from direct damage due to SCI-induced lesions. Similarly, soma size of quadriceps motoneurons was not significantly affected by SCI. Soma size regresses after direct insult to motoneurons, for example after axotomy88,89 or death of neighboring motoneurons,28–30 and thus the lack of significant effects on soma size suggests that the quadriceps motoneurons were not directly damaged by SCI-induced lesions. Direct insult to motoneurons, for example through axotomy89 or induced death of neighboring neurons,31 can result in dendritic atrophy in surviving motoneurons. However, as described above, because the lesion did not infiltrate the L2 level and the number of Nissl-stained or BHRP-labeled quadriceps motoneurons was not affected by SCI, we do not believe the reductions in dendritic length we observed reflect such direct effects.
Dendritic atrophy after SCI
Afferent input to motoneurons is important for the maintenance of dendritic morphology, and deafferentation often results in dendritic retraction. Following deafferentation via damage to the dorsal horn,90 spinal cord hemisection,91 or cortical ablation,14 spinal motoneurons undergo dendritic atrophy. Activity in afferent pathways is an important factor in maintaining dendritic morphology. For example, cold block of the spinal cord causes dendritic morphological changes to develop within 4 h.92
It is likely that the dendritic atrophy we observed following SCI in untreated animals reflects deafferentation resulting from the loss of descending motor and propriospinal tracts. In rats, the majority of corticospinal tract axons terminate dorsomedially, principally in laminae III-VI,93,94 while those of the rubrospinal tract terminate in laminae V and VI.95,96 In contrast, reticular formation projections to lumbar levels of the spinal cord, especially from the reticularis pontis oralis, terminate ventromedially, principally in lamina VIII.97,98 Propriospinal projections into the lumbar levels also terminate ventromedially, extending into both laminae VII and VIII.99 Following SCI, quadriceps motoneurons in untreated animals showed dendritic atrophy in all of these locations. Interestingly, while reduced dendritic length was present throughout the dendritic distribution, it was particularly pronounced in the ventromedial region, where quadriceps motoneuron dendrites normally have a dense ramification into lamina VIII. This pattern of dendritic atrophy after SCI is similar to what we observed previously.20 Because both reticulospinal and propriospinal projections are concentrated in this area, the extensive lesions present after SCI could have produced a major denervation of dendrites in this area, resulting in the pronounced dendritic atrophy we observed. We have previously reported that following spinal transection and the concomitant loss of descending pathways, spinal motoneurons undergo marked local reductions in dendritic arbor, especially in this same region of the ventral horn.12 This loss is of particular significance after SCI, as descending reticulospinal fibers course through the ventral and lateral funiculi,97,100 and disruption of these tracts results in hindlimb motor deficits.101,102
Protection of motoneuron dendrites with estradiol and dihydrotestosterone
SCI-induced atrophy of quadriceps motoneuron dendrites was attenuated in estradiol-treated, dihydrotestosterone-treated, and estradiol-combined-with-0dihydrotestosterone–treated animals. Hormone treatment could have attenuated dendritic atrophy by increasing the number of spared or regenerating axons that traverse the lesion. Such axon sparing has been reported previously with trophic factor treatment (e.g., glia cell line–derived neurotrophic factor),103 and both androgens and estrogens have been shown to regulate trophic factors such as brain-derived neurotrophic factor (BDNF).104,105 After SCI, treatment with estradiol resulted in significant reductions in lesion volume, and thus the attenuation in motoneuron dendritic atrophy present in these animals could reflect a sparing of propriospinal and supraspinal axons. However, similar effects on dendritic length were present after treatment with dihydrotestosterone alone or in combination with estradiol, despite there being no reductions in lesion size or increases in tissue sparing in these groups (see above). Thus, it is unlikely that the beneficial effects of steroid treatment on the morphology of quadriceps motoneurons we observed are a result of differential, long-term tissue or tract sparing.
We have previously speculated that the attenuation in SCI-induced dendritic atrophy we observed could have been produced by a hormone-mediated sprouting of quadriceps motoneuron dendrites locally onto remaining afferents.20 Sprouting could potentially maintain motor activation, supporting exercise training effects on locomotor function after SCI.13,106 Such an effect of hormones on attenuating dendritic atrophy and supporting motoneuron activation has in fact been directly demonstrated.27,31 The mechanisms responsible for this sprouting are not clear, but gonadal hormones have been shown to regulate the expression of cytoskeletal proteins (e.g., β-tubulin,107–110 actin and microtubule-associated protein 2,111), as well as neuritin, a critical downstream mediator of the ability of gonadal hormones to increase neurite outgrowth.112–114 Sprouting could be driven by direct action on the motoneurons or via indirect action on afferents. Thus, as for the effects seen with micturition (see above), it is possible that a hormone-mediated protection of local spinal circuitry below the level of the lesion could be responsible for the motoneurons dendritic protection we observed. One possible protected spinal population could be the short axon propriospinal neurons, which provide the largest source of input to lumbar spinal motoneurons.115–117
Changes in these afferents could underlie the regressive changes we have observed in motoneurons after SCI. Afferent input to motoneurons is important for the maintenance of their dendritic morphology, and deafferentation of motoneurons results in dendritic retraction.14,90,91 Activity in afferent pathways is also an important factor in maintaining motoneuron dendritic morphology, and regressive changes rapidly develop after its inhibition.92 The number of propriospinal neurons proximal to an SCI is severely depleted after a thoracic SCI, but short axon propriospinal neurons within the lumbosacral enlargement show no qualitative loss at 2 weeks.118 We have demonstrated dendritic changes in propriospinal neurons after SCI,119 and such changes could result in alterations in the activity of these neurons, leading to the atrophy we observed in lumbar motoneurons. Should such potential reductions in propriospinal neurons be prevented by hormonal treatment, the rescue of the major afferent source to motoneurons could underlie the beneficial effects of hormone treatment on motoneuron dendrities we have observed. Electrophysiological and anatomical tracing studies could begin to address this question.
Comparability of BHRP labeling
Previous studies have demonstrated that neither axonal transport120 nor dendritic transport as demonstrated by the rostrocaudal or radial extent of dendritic labeling29,61,63,64 of BHRP are affected by hormone levels. Thus, in the present study, we believe that the differences we observed across treatment groups reflect true dendritic atrophy in quadriceps motoneurons of untreated SCI animals, which is attenuated by treatment with hormones. The possibility that confounds arising from SCI could affect retrograde transport is also an important consideration, as such an artifact could potentially result in apparent alterations in dendritic morphology. However, no differences in either radial or rostrocaudal extents of quadriceps motoneuron dendrites in the SCI groups, compared with normal values, were observed. Therefore, because BHRP was transported equivalently to the most distal, highest order dendritic processes across groups, we believe that the dendritic labeling across groups was comparable and that the shorter dendritic lengths we observed in the untreated SCI animals reflect true dendritic atrophy.
Muscle atrophy after SCI
The regressive changes we observed in fiber diameter are typical after SCI in muscles innervated by motoneurons below the level of the lesion, especially in weight-bearing muscles such as the quadriceps.121,122 This atrophy can result from either denervation due to loss of motoneurons or damage to the ventral roots, or disuse consequent to decreases in muscle activation potentially due to the loss of synaptic input to remaining motoneurons.123 In the current study, the atrophy we observed cannot be ascribed to an effect of denervation, as we observed no changes in quadriceps motoneuron number, or the number of BHRP-labeled quadriceps motoneurons between sham animals and untreated SCI animals. Thus, the decreased fiber size we observed most likely reflect a disuse atrophy, potentially resulting after damage to descending and propriospinal projections and/or the reductions in quadriceps motoneuron dendritic length we observed. Such reductions in quadriceps motoneuron dendritic length result in attenuation of motor activation, reducing response amplitudes in the femoral nerve generated by dorsal root afferent stimulation.31 Alternatively, disuse atrophy also may result from changes in muscle length or loading conditions that could decrease protein synthesis and increase protein degradation.124,125
Protection of muscle with dihydrotestosterone
In the current study, estradiol treatment was ineffective in preventing the atrophy in fiber size seen after SCI. This lack of effect was expected, as previous work in our laboratory has not found estrogenic effects on quadriceps muscle size.34 Although estrogens have a variety of effects in skeletal muscle (e,g., downregulation of proinflammatory cytokines, enhancing insulin-like growth factor 1, expression, or satellite cell activation and proliferation126), their effects on muscle fiber cross-sectional area vary in different muscles and in different directions. For example, ovariectomy increases the size of muscle fibers in the extensor digitorum longus,127 has no effect in the plantaris,128 and can either increase,127 decrease,129 or have no effect130 on soleus muscle fibers. Estradiol replacement after ovariectomy has been reported to increase muscle fiber size in the gastrocnemius,130 decrease it in the extensor digitorum longus127 and plantaris,128 or either increase129 or decrease127 fiber size in the soleus. Differences in factors such as muscle-specific effects, route of hormone treatment (injection vs. Silastic implants), frequency of treatment or dosage, duration of treatment, or animal substrains across studies likely contribute to the inconsistencies seen with estradiol treatment. As with locomotor performance, the lack of effect on muscle fiber size in estradiol-treated animals in our study could be the result of the low dosage of estradiol we delivered, which was designed to be in the normal physiological range.
In contrast, treatment with dihydrotestosterone, either alone or in combination with estradiol, attenuated SCI-induced muscle fiber atrophy. As estradiol had no effect on muscle fiber size when administered alone, the effect seen in animals treated with estradiol combined with dihydrotestosterone is likely simply due to dihydrotestosterone. This effect on muscle fiber size is similar to what we observed previously with treatment with testosterone following SCI,20 and is consistent with the known protein anabolic effects of androgens general skeletal muscle tissue.131,132 Thus, treatment with dihydrotestosterone might have supported muscle protein synthesis and decreased protein degradation, and the resultant decrease in protein turnover could have prevented muscle atrophy.
Alternatively, dihydrotestosterone could have potentially altered mobility or activity in the treated animals, resulting in the preservation of both muscle and the related spinal cord circuitry and motoneuron dendritic morphology. This is quite plausible, as limb exercise after spinal cord transection during postnatal development has in fact been shown to prevent dendritic atrophy in spinal motoneurons.13 Further, exercise is known to elevate the expression of neurotrophic factors (e.g., BDNF) that can promote dendritic and axonal regrowth.133 Further studies utilizing behavioral or electrophysiological methods could address this hypothesis directly.
Neuroprotective mechanisms of androgens and estrogens
Androgens and estrogens have been shown to have powerful neuroprotective effects.35,134 For example, both testosterone and estradiol protect against cell death,24,42 promote functional recovery,25,41 and stimulate motoneuron axonal growth after peripheral nerve injury.135,136 The mechanisms through which androgens and estrogens act are multiple, and include regulation of apoptosis,22,38 injury-induced upregulation of GFAP,39,85 and mediation of the glial response.8,86 Proteins thought to be involved in neuroprotection also are regulated by androgens and estrogens, including proteins with antioxidant or pro-inflammatory functions,8,23,137 and the neurotrophin BDNF.104,105 As discussed above, gonadal hormones also have been shown to be powerful regulators of cytoskeletal elements crucial to dendrites and dendritic spines, mediated through the expression of proteins such as β-tubulin, actin, and microtubule-associated protein 2.107–111
Estrogens and androgens have protective/therapeutic effects after SCI, but additional questions remain. The protective effects we observed are in some cases “tissue specific.” For example, motoneuron dendrites are protected by treatment with either estradiol or the androgens testosterone and dihydrotestosterone, but protective effects in lesion size and spared tissue appear to be estrogenic, while effects in the quadriceps muscle are strictly androgenic. Such tissue specificity could arise through local differences in metabolic enzymes or differences in receptor subtypes.132 Blocking the aromatase or 5α-reductase enzymes involved in the production of estradiol and dihydrotestosterone, respectively,138 could help determine if the conversion of testosterone to its active metabolites is required to see the protective effects of hormone treatment. Alternatively, if the neuroprotective effects of testosterone, dihydrotestosterone, and estradiol are dependent on receptor-mediated mechanisms, this could be addressed by blocking the androgen or estrogen receptors.34,132 The actions of testosterone and dihydrotestosterone are mediated by the same receptor,132,139,140 but estrogens can act through several receptor subtypes,111 requiring ultimately targeting these receptor subtypes and their downstream pathways as sites of action. Non-genomic actions of gonadal steroids also have also been identified, and gonadal steroids and their cognate receptors may exert their effects through signaling pathways that do not directly or immediately involve transactivation of genomic DNA.132,141–144 Finally, even receptor-independent effects have been identified (e.g., antioxidant effects after SCI through downregulating the formation of reactive oxygen species in the lesioned and adjacent segments of the spinal cord).67 Establishing which of these mechanisms, proteins, and pathways are involved in the hormone-mediated protection of behavioral function and motoneuron dendrites from SCI-induced atrophy will be valuable contributions to developing new neurotherapeutic strategies.
Conclusion
The present results indicate that treatment with estradiol or dihydrotestosterone attenuates deficits in micturition and regressive changes in motoneuron and muscle morphology seen after SCI. Because these effects were seen independent of lesion size, our results suggest that these hormonal effects could potentially be the result of local action on spinal circuitry below the level of the lesion. Together, these results indicate that the use of gonadal hormones is an effective treatment after SCI, directed by the particular therapeutic goals.
Acknowledgments
We wish to thank Iman Mahoui for technical assistance. This work was supported by grants from Indiana Spinal Cord and Brain Injury Research Fund (ISCBIRF) and by Indiana University's Office of the Vice Provost for Research through the Faculty Research Support Program to D.R.S, and NIH R01NS100531-01A1, Merit Review Award I01 BX002356 from the U.S. Department of Veterans Affairs, Craig H Neilsen Foundation 296749, Indiana Spinal Cord and Brain Injury Research Foundation, and Mari Hulman George Endowment Funds to X.M.X.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.National SCI Statistical Center. Available at: www.nscisc.uab.edu Accessed December1, 2017
- 2.Liu X.Z., Xu X.M., Hu R., Du C., McDonald J.W., Dong H.X., Wu Y.J., Fan G.S., Jacquin M.F., Hsu C.Y., and Choi D.W. (1997). Neuronal and glial apoptosis after traumatic spinal cord injury. J. Neurosci. 17, 5395–5406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu D., Thangnipon W., and McAdoo D.J. (1991). Excitatory amino acids rise to toxic levels upon impact injury to the rat spinal cord. Brain Res. 547, 344–348 [DOI] [PubMed] [Google Scholar]
- 4.Diaz-Ruiz A., Ibarra A., Perez-Severiano F., Guizar-Sahagun G., Grijalva I., and Rios C. (2002). Constitutive and inducible nitric oxide synthase activities after spinal cord contusion in rats. Neurosci. Lett. 319, 129–132 [DOI] [PubMed] [Google Scholar]
- 5.Wang C.X., Olshowka J.A., and Wrathall J.R. (1997). Increase of interleukin-1beta mRNA and protein in the spinal cord following experimental traumatic injury in the rat. Brain Res. 759, 190–196 [DOI] [PubMed] [Google Scholar]
- 6.Liu N.K., Titsworth W.L., and Xu X.M. (2009). Phospholipase A2 in CNS disorders: Implication on traumatic spinal cord and brain injuries, in: Handbook of Neurochemistry and Molecular Neurobiology. Lajtha A. (ed). Springer: New York, pps. 321–341 [Google Scholar]
- 7.Liu N.K. and Xu X.M. (2010). Phospholipase A2 and its molecular mechanism after spinal cord injury. Mol. Neurobiol. 41, 197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritz M.F. and Hausmann O.N. (2008). Effect of 17B-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 1203, 177–188 [DOI] [PubMed] [Google Scholar]
- 9.National Institute of Neurological Disorders and Stroke. (2005). Spinal cord injury: Emerging concepts. Available at: www.ninds.nih.gov/news_and_events/proceedings/sci_report.htm Accessed December1, 2017
- 10.Moschilla G., Song S., and Chakera T. (2001). Post-traumatic lumbar nerve root avulsion. Austral. Radiol. 45, 281–284 [DOI] [PubMed] [Google Scholar]
- 11.Hoang T.X., Nieto J., Tillakaratne N.J.K., and Havton L.A. (2003). Autonomic and motor neuron death is progressive and parallel in a lumbosacral ventral root avulsion model of cauda equina injury. J. Comp. Neurol. 467, 477–486 [DOI] [PubMed] [Google Scholar]
- 12.Hebbeler S.L. and Sengelaub D.R. (2003). Development of a sexually dimorphic neuromuscular system in male rats after spinal transection: morphologic changes and implications for estrogen sites of action. J. Comp. Neurol. 467, 80–96 [DOI] [PubMed] [Google Scholar]
- 13.Gazula V.R., Roberts M., Luzzio C., Jawad A.F., and Kalb R. (2004). Effects of limb exercise after spinal cord injury on motor neuron dendrite structure. J. Comp. Neurol. 476, 130–145 [DOI] [PubMed] [Google Scholar]
- 14.Standler N. and Bernstein J.J. (1984). Dendritic alteration of spinal motoneurons after ablation of somatomotor cortex. Exp. Neurol. 83, 264–273 [DOI] [PubMed] [Google Scholar]
- 15.Bisby M.A. and Tetzlaff W. (1992). Changes in cytoskeletal protein synthesis following axon injury and during regeneration. Mol. Neurobiol. 6, 107–123 [DOI] [PubMed] [Google Scholar]
- 16.Titmus M.J. and Faber D.S. (1990). Axotomy-induced alterations in the electrophysiological characteristics of neurons. Prog. Neurobiol. 35, 1–51 [DOI] [PubMed] [Google Scholar]
- 17.Brännström T., Havton L., and Kellerth J.O. (1992). Changes in size and dendritic arborization patterns of adult cat spinal α-motoneurons following permanent axotomy. J. Comp. Neurol. 318, 439–451 [DOI] [PubMed] [Google Scholar]
- 18.O'Hanlon G.M. and Lowrie M.B. (1995). Nerve injury in adult rats causes abnormalities in the motoneuron dendritic field that differ from those seen following neonatal nerve injury. Exp. Brain Res. 103, 243–250 [DOI] [PubMed] [Google Scholar]
- 19.Sumner B.E.H. and Watson W.E. (1971). Retraction and expansion of the dendritic tree of motor neurons of adult rats induced in vivo. Nature 233, 273–275 [DOI] [PubMed] [Google Scholar]
- 20.Byers J.S., Huguenard A.L., Kuruppu D, Liu N.K., Xu X.M., and Sengelaub D.R. (2012). Neuroprotective effects of testosterone on muscle and motoneurons morphology following spinal cord injury. J. Comp. Neurol. 520, 2683–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu N.K., Byers J.S., Lam T., Lu Q.B., Sengelaub D.R., and Xu X.M. (2014). Inhibition of cPLA2 has neuroprotective effects on motoneuron and muscle atrophy following spinal cord injury. J. Neurotrauma 2014. November 11; Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fargo K.N., Foecking E.M., Jones K.J., and Sengelaub D.R. (2009). Neuroprotective actions of androgens on motoneurons. Front. Neuroendocrinol. 30, 130–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahlbom E., Prins G.S., and Ceccatelli S. (2001). Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 892, 255–262 [DOI] [PubMed] [Google Scholar]
- 24.Pike C.J. (2001). Testosterone attenuates ß-amyloid toxicity in cultured hippocampal neurons. Brain Res. 919, 160–165 [DOI] [PubMed] [Google Scholar]
- 25.Jones K.J., Brown T.J., and Damaser M. (2001). Neuroprotective effects of gonadal steroids on regenerating peripheral motoneurons. Brain Res. Rev. 37, 372–382 [DOI] [PubMed] [Google Scholar]
- 26.Jones K.J., Durica T.E., and Jacob S.K. (1997). Gonadal steroid preservation of central synaptic input to hamster facial motoneurons following peripheral axotomy. J. Neurocytol. 26, 257–266 [DOI] [PubMed] [Google Scholar]
- 27.Fargo K.N., Foster A.M., and Sengelaub D.R. (2009). Neuroprotective effect of testosterone treatment on motoneuron recruitment following the death of nearby motoneurons. Dev. Neurobiol. 69, 825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fargo K.N. and Sengelaub D.R. (2004). Testosterone manipulation protects motoneurons from dendritic atrophy after contralateral motoneuron depletion. J. Comp. Neurol. 469, 96–106 [DOI] [PubMed] [Google Scholar]
- 29.Fargo K.N. and Sengelaub D.R. (2004). Exogenous testosterone prevents motoneuron atrophy induced by contralateral motoneuron depletion. J. Neurobiol. 60, 348–359 [DOI] [PubMed] [Google Scholar]
- 30.Fargo K.N. and Sengelaub D.R. (2007). Androgenic, but not estrogenic, protection of motoneurons from somal and dendritic atrophy induced by the death of neighboring motoneurons. Dev. Neurobiol. 67, 1094–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Little C.M., Coons K.D., and Sengelaub D.R. (2009). Neuroprotective effects of testosterone on the morphology and function of somatic motoneurons following the death of neighboring motoneurons. J. Comp. Neurol. 512, 359–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson R.E., Coons K.D., and Sengelaub D.R. (2009). Neuroprotective effects of testosterone on dendritic morphology following partial motoneuron depletion: efficacy in female rats. Neurosci. Lett. 465, 123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark M.J., Petroski G.F., Mazurek M.O., Hagglund K.J., Sherman A.K., Lammy A.B., Childers M.K., and Acuff M.E. (2008). Testosterone replacement therapy and motor function in men with spinal cord injury. Am. J. Phys. Med. Rehabil. 87, 281–284 [DOI] [PubMed] [Google Scholar]
- 34.Cai Y., Chew C., Muñoz F., and Sengelaub D.R. (2016). Neuroprotective effects of testosterone metabolites and dependency on receptor action on the morphology of somatic motoneurons following the death of neighboring motoneurons. Dev. Neurobiol. 77, 691–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foecking E.M., Fargo K.N., Brown T.J., Sengelaub D.R., and Jones K.J. (2015). Gonadal steroids in regeneration and repair of neuromuscular systems, in: Neural Regeneration, So K.F. and Xu X.M. (eds). Elsevier: London, pps. 129–152 [Google Scholar]
- 36.Huppenbauer C.B., Tanzer L., DonCarlos L.L., and Jones K.J. (2005). Gonadal steroid attenuation of developing hamster facial motoneuron loss by axotomy: equal efficacy of testosterone, dihydrotestosterone, and 17-beta estradiol. J. Neurosci. 25, 4004–4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanzer L. and Jones K.J. (1997). Gonadal steroid regulation of hamster facial nerve regeneration: effects of dihydrotestosterone and estradiol. Exp. Neurol. 146, 258–264 [DOI] [PubMed] [Google Scholar]
- 38.Kachadroka S., Hall A.M., Niedzielko T.L., Chongthammakun S., and Floyd C.L. (2010). Effect of endogenous androgens on 17 beta-estradiol-mediated protection after spinal cord injury in male rats. J. Neurotrauma 27, 611–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samantaray S., Das A., Matzelle D.C., Yu S.P., Wei L., Varma A., Ray S.K., and Banik N.L. (2016). Administration of low dose estrogen attenuates gliosis and protects neurons in acute spinal cord injury in rats. J. Neurochem. 136, 1064–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sribnick E.A., Wingrave J.M., Matzelle D.D., Wilford G.G., Ray S.K., and Banik N.L. (2005). Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury rats. J. Neurosci. Res. 82, 283–293 [DOI] [PubMed] [Google Scholar]
- 41.Sribnick E.A., Samantaray S., Das A., Smith J., Matzelle D.D., Ray S.K., and Banik N.L. (2010). Postinjury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J. Neurosci. Res. 88, 1738–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yune T.Y., Kim S.J., Lee S.M., Lee Y.K., Oh Y.J., Kim Y.C., Markelonis G.J., and Oh T.H. (2004). Systemic administration of 17 beta-estradiol reduces apoptotic cell death and improves functional recovery following traumatic spinal cord injury in rats. J. Neurotrauma 21, 293–306 [DOI] [PubMed] [Google Scholar]
- 43.Bamber N.I., Huaying L., Xiaobin L., Oudenga M., Aebischer P., and Xu X.M. (2001). Neurotrophins BDNF and NT-3 promote axonal re-entry into the distal host spinal cord through Schwann cell-seeded mini-channels. Eur. J. Neurosci. 13, 257–268 [PubMed] [Google Scholar]
- 44.Xu X.M., Zhang S.X., Li H., Aebischer P., and Bunge M.B. (1999). Regrowth of axons into the distal spinal cord through Schwann cell-seeded mini-channels implanted into the hemisected adult spinal cord. Eur. J. Neurosci. 11, 1723–1740 [DOI] [PubMed] [Google Scholar]
- 45.Smith E.R., Damassa D.A., and Davidson J.M. (1977). Hormone Administration: Peripheral and Intracranial Implants, in: Methods in Psychobiology, Meyer R.D. (ed). Academic Press: New York, pps. 259–279 [Google Scholar]
- 46.Overpeck J.G., Colson S.H., Hohmann J.R., Applestine M.S., and Reilly J.F. (1978). Concentrations of circulating steroids in normal prepubertal and adult male and female humans, chimpanzees, rhesus monkeys, rats, mice, and hamsters: a literature survey. J. Toxicol. Environ. Health 4, 785–803 [DOI] [PubMed] [Google Scholar]
- 47.Sfikakis A., Christina S., Sitaras N., and Varonos D. (1978). Implication of the estrous cycle on conditioned avoidance behavior in the rat. Physiol. Behav. 21, 441–446 [DOI] [PubMed] [Google Scholar]
- 48.Garrett J.E. and Wellman C.L. (2009). Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. J. Neurosci. 162, 195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basso D.M., Beattie M.S., and Bresnahan J.C. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 [DOI] [PubMed] [Google Scholar]
- 50.Liu N.K, Zhang Y.P., Titsworth W.L., Jiang X., Han S., Lu P.H., Shields C.B., and Xu X.M. (2006). A novel role of phospholipase A(2) in mediating spinal cord secondary injury. Ann. Neurol. 59, 606–619 [DOI] [PubMed] [Google Scholar]
- 51.Lee Y.S., Lin C.Y., Jiang H.H., DePaul M., Lin V.W., and Silver J. (2013). Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J. Neurosci. 33, 10591–10606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldstein L.A., Kurz E.M., and Sengelaub D.R. (1990). Androgen regulation of dendritic growth and retraction in the development of a sexually dimorphic spinal nucleus. J. Neurosci. 10, 935–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurz E.M., Sengelaub D.R., and Arnold A.P. (1986). Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science 232, 395–398 [DOI] [PubMed] [Google Scholar]
- 54.Michel R.P. and Cruz-Orive L.M. (1988). Application of the Cavalieri principle and vertical sections method to lung: estimation of volume and pleural surface area. J. Microsc. 150, 117–136 [DOI] [PubMed] [Google Scholar]
- 55.Oorschot D.E. (1994). Are you using neuronal densities, synaptic densities or neurochemical densities as your definitive data? There is a better way to go. Prog. Neurobiol. 44, 247–433 [DOI] [PubMed] [Google Scholar]
- 56.Al-Majed A.A., Brushart T.M., and Gordon T. (2000). Electrical stimulation accelerates and increases expression of BDNF and trkB rnRNA in regenerating rat femoral motoneurons. Eur. J. Neurosci. 12, 4381–4390 [PubMed] [Google Scholar]
- 57.Brushart T.M. and Seiler W.A. (1987). Selective reinnervation of distal motor stumps by peripheral axons. Exp. Neurol. 97, 290–300 [DOI] [PubMed] [Google Scholar]
- 58.Nicolopoulos-Stournaras S. and Iles J.F. (1983). Motor neuron columns in the lumbar spinal cord of the rat. J. Comp. Neurol. 217, 75–85 [DOI] [PubMed] [Google Scholar]
- 59.Mesulam M.M. (1982). Tracing Neural Connections with Horseradish Peroxidase. Wiley: Chichester [Google Scholar]
- 60.Gundersen H.J.G. (1988). The nucleator. J. Microsc. 151, 3–21 [DOI] [PubMed] [Google Scholar]
- 61.Kurz E.M., Brewer R.G., and Sengelaub D.R. (1991). Hormonally mediated plasticity of motoneuron morphology in the adult rat spinal cord: a cholera toxin-HRP study. J. Neurobiol. 22, 976–988 [DOI] [PubMed] [Google Scholar]
- 62.Goldstein L.A., Kurz E.M., Kalkbrenner A.E., and Sengelaub D.R. (1993). Changes in dendritic morphology of rat spinal motoneurons during development and after unilateral target deletion. Dev. Brain Res. 73, 151–163 [DOI] [PubMed] [Google Scholar]
- 63.Goldstein L.A. and Sengelaub D.R. (1994). Differential effects of dihydrotestosterone and estrogen on the development of motoneuron morphology in a sexually dimorphic rat spinal nucleus. J. Neurobiol. 25, 878–892 [DOI] [PubMed] [Google Scholar]
- 64.Hebbeler S.L., Verhovshek T., and Sengelaub D.R. (2002). N-methyl-D-aspartate receptor blockade inhibits estrogenic support of dendritic growth in a sexually dimorphic rat spinal nucleus. J. Comp. Neurol. 451, 142–152 [DOI] [PubMed] [Google Scholar]
- 65.Kalb R.G. (1994). Regulation of motor neuron dendrite growth by NMDA receptor activation. Development 120, 3063–3071 [DOI] [PubMed] [Google Scholar]
- 66.Chaovipoch P., Jelks K.A., Gerhold L.M., West E.J., Chongthammakun S., and Floyd C.L. (2006). 17 beta-estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J Neurotrauma 23, 830–852 [DOI] [PubMed] [Google Scholar]
- 67.Mosquera L., Colon J.M., Santiago J.M., Torrado A.I., Melendez M., Segarra A.C., Rodriguez-Orengo J.F., and Miranda J.D. (2014). Tamoxifen and estradiol improved locomotor function and increased spared tissues in rats after spinal cord injury: Their antioxidant effect and role of estrogen receptor alpha. Brain Res. 1561, 11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siriphorn A., Dunham K.A., Chompoopong S., and Floyd C.L. (2012). Postinjury administration of 17β-estradiol induces protection in the gray and white matter with associated functional recovery after cervical spinal cord injury in male rats. J. Comp. Neurol. 520, 2630–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swartz K.R., Fee D.B., Joy K.M., Roberts K.N., Sun S., Scheff N.N., Wilson M.E., and Scheff S.W. (2007). Gender differences in spinal cord injury are not estrogen-dependent. J. Neurotrauma 24, 473–480 [DOI] [PubMed] [Google Scholar]
- 70.Kwon B.K., Okon E., Hillyer J., Mann C., Baptiste D., Weaver L.C., Fehlings M.G., and Tetzlaff W. (2011). A systematic review of noninvasive pharmacologic neuroprotective treatments for acute spinal cord injury. J. Neurotrauma 28, 1545–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cruz C.D. and Cruz F. (2011). Spinal cord injury and bladder dysfunction: new ideas about an old problem. Scientific World J. 11, 214–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Groat W.C., Araki I., Vizzard M.A., Yoshiyama M., Yoshimura N., Sugaya K., Tai C., and Roppolo J.R. (1998). Developmental and injury induced plasticity in the micturition reflex pathway. Behav. Brain Res. 92, 127–140 [DOI] [PubMed] [Google Scholar]
- 73.Fowler C.J., Griffiths D., and de Groat W.C. (2008). The neural control of micturition. Nat. Rev. Neurosci. 9, 453– 466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Groat W.C. (1990). Central neural control of the lower urinary tract. Ciba Found. Symp. 151, 27–44 [DOI] [PubMed] [Google Scholar]
- 75.de Groat W.C., Kawatani M., Hisamitsu T., Chen C.L., Ma C.P., Thor K., Steers W., and Roppolo J.R. (1990). Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J. Auton. Nerv. Syst. 30 (suppl), S71–S77 [DOI] [PubMed] [Google Scholar]
- 76.Marson L. (1997). Identification of central nervous system neurons that innervate the bladder body, bladder base, or external urethral sphincter of female rats: a transneuronal tracing study using pseudorabies virus. J. Comp. Neurol. 29, 584–602 [PubMed] [Google Scholar]
- 77.Keast J.R. and Saunders R.J. (1998). Testosterone has potent, selective effects on the morphology of pelvic autonomic neurons which control the bladder, lower bowel and internal reproductive organs. Neuroscience 85, 543–546 [DOI] [PubMed] [Google Scholar]
- 78.Johnson O.L. and Berkley K.J. (2002). Estrous influences on micturition thresholds of the female rat before and after bladder inflammation. Am. J. Physiol. 282, R289–R294 [DOI] [PubMed] [Google Scholar]
- 79.Ratz P.H., McCammon K.A., Altstatt D., Blackmore P.F., Shenfeld O.Z., and Schlossberg S.M. (1999). Differential effects of sex hormones and phytoestrogens on peak and steady state contractions in isolated rabbit detrusor. J. Urol. 162, 1821–1828 [PubMed] [Google Scholar]
- 80.Bennett H.L., Gustafsson J.A., and Keast J.R. (2003). Estrogen receptor expression in lumbosacral dorsal root ganglion cells innervating the female rat urinary bladder. Auton. Neurosci. 105, 90–100 [DOI] [PubMed] [Google Scholar]
- 81.Salmi S., Santti R., Gustafsson J.A., and Makela S. (2001). Co-localization of androgen receptor with estrogen receptor beta in the lower urinary tract of the male rat. J. Urol. 166, 674–677 [PubMed] [Google Scholar]
- 82.Farooque M., Suo Z., Arnol P.M., Wulser M.J., Chou C-T., Vancura R.W., Fowler S., and Festoff B.W. (2006). Gender-related differences in recovery of locomotor function after spinal cord injury in mice. Spinal Cord 44, 182–187 [DOI] [PubMed] [Google Scholar]
- 83.Hauben E., Mizrahi T., Agranov E., and Schwartz M. (2002). Sexual dimorphism in the spontaneous recovery from spinal cord injury: a gender gap in beneficial autoimmunity? Eur. J. Neurosci. 16, 1731–1740 [DOI] [PubMed] [Google Scholar]
- 84.Coers S., Tanzer L., and Jones K.J. (2002). Testosterone treatment attenuates the effects of facial nerve transection on glial fibrillary acidic protein (GFAP) levels in the hamster facial motor nucleus. Metab. Brain. Dis. 17, 55–63 [DOI] [PubMed] [Google Scholar]
- 85.Jones K.J., Kinderman N.B., and Oblinger M.M. (1997). Alterations in glial fibrillary acidic protein (GFAP) mRNA levels in the hamster facial motor nucleus: effects of axotomy and testosterone. Neurochem. Res. 22, 1359–1366 [DOI] [PubMed] [Google Scholar]
- 86.Jones K.J., Coers S., Storer P.D., Tanzer L., and Kinderman N.B. (1999). Androgenic regulation of the central glia response following nerve damage. J. Neurobiol. 40, 560–573 [DOI] [PubMed] [Google Scholar]
- 87.Grossman C.J. (1984). Regulation of the immune system by sex steroids. Endocr. Rev. 5, 435-455 [DOI] [PubMed] [Google Scholar]
- 88.Ma J., Novikov L.N., Wiberg M., and Kellerth J.O. (2001). Delayed loss of spinal motoneurons after peripheral nerve injury in adult rats: a quantitative morphological study. Exp. Brain Res. 139, 216–223 [DOI] [PubMed] [Google Scholar]
- 89.Yang L.Y., Verhovshek T., and Sengelaub D.R. (2004). BDNF and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology 145, 161–168 [DOI] [PubMed] [Google Scholar]
- 90.Bernstein J.J. and Standler N. (1983). Dendritic alteration of rat spinal motoneurons after dorsal horn mince: computer reconstruction of dendritic fields. Exp. Neurol. 82, 532–540 [DOI] [PubMed] [Google Scholar]
- 91.Bernstein J.J., Wacker W., and Standler N. (1984). Spinal motoneuron dendritic alteration after spinal cord hemisection in the rat. Exp. Neurol. 83, 548–554 [DOI] [PubMed] [Google Scholar]
- 92.Castro-Moure F. and Goshgarian H.G. (1997). Morphological plasticity induced in the phrenic nucleus following cervical cold block of descending respiratory drive. Exp. Neurol. 147, 299–310 [DOI] [PubMed] [Google Scholar]
- 93.Brösamle C. and Schwab M.E. (1997). Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J. Comp. Neurol. 386, 293–303 [DOI] [PubMed] [Google Scholar]
- 94.Brown L.T. (1971). Projections and termination of the corticospinal tract in rodents. Exp. Brain Res. 13, 432–450 [DOI] [PubMed] [Google Scholar]
- 95.Brown L.T. (1974). Rubrospinal projections in the rat. J. Comp. Neurol. 154, 169–188 [DOI] [PubMed] [Google Scholar]
- 96.Waldron H.A. and Gwyn D.G. (1969). Descending nerve tracts in the spinal cord of the rat. I. Fibers from the midbrain. J. Comp. Neurol. 137, 143–154 [DOI] [PubMed] [Google Scholar]
- 97.Jones B.E. and Yang T.Z. (1985). The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J. Comp. Neurol. 242, 56–92 [DOI] [PubMed] [Google Scholar]
- 98.Motorina M.V. (1977). Distribution of reticulospinal fibers and their terminations in lumbar segments of the rat spinal cord. J. Evol. Biochem. Physiol. 12, 520–527 [PubMed] [Google Scholar]
- 99.Menétey D., De Pommery J., and Roudier F. (1985). Propriospinal fibers reaching the lumbar enlargement in the rat. Neurosci. Lett. 58, 257–261 [DOI] [PubMed] [Google Scholar]
- 100.Martin G.F., Vertes R.P., and Waltzer R. (1985). Spinal projections of the gigantocelluar reticular formation in the rat. Evidence for projections from different areas to laminae I and II and lamina IX. Exp. Brain Res. 58, 154–162 [DOI] [PubMed] [Google Scholar]
- 101.Loy D.N., Talbott J.F., Onifer S.M., Mills M.D., Burke D.A., Dennison J.B., Fajardo L.C., Magnuson D.S.K. and Whittemore S.R. (2002). Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp. Neurol. 177, 575–580 [DOI] [PubMed] [Google Scholar]
- 102.Magnuson D.S.K., Trinder T.C., Zhang Y.P., Burke D., Morassutti D.J., and Shields C.B. (1999). Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp. Neurol. 156, 191–204 [DOI] [PubMed] [Google Scholar]
- 103.Iannotti C., Zhang Y.P., Shields C.B., Han Y., Burke D.A., and Xu X.M. (2004) A neuroprotective role of glial cell line-derived neurotrophic factor following moderate spinal cord contusion injury. Exp. Neurol. 189, 317–332 [DOI] [PubMed] [Google Scholar]
- 104.Solum D.T. and Handa R.J. (2002). Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J. Neurosci. 22, 2650–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Verhovshek T., Cai Y., Osborne M.C., and Sengelaub D.R. (2010). Androgen regulates brain-derived neurotrophic factor in spinal motoneurons and their target musculature. Endocrinology 151, 253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Raineteau O. and Schwab M.E. (2001). Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2, 263–273 [DOI] [PubMed] [Google Scholar]
- 107.Jones K.J. and Oblinger M.M. (1994). Androgenic regulation of tubulin gene expression in axotomized hamster facial motoneurons. J. Neurosci. 14, 3620–3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jones K.J., Storer P.D., Drengler S.M., and Oblinger M.M. (1999). Differential regulation of cytoskeletal gene expression in hamster facial motoneurons: Effects of axotomy and testosterone treatment. J. Neurosci. Res. 57, 817–823 [PubMed] [Google Scholar]
- 109.Brown T.J., Storer P., Oblinger M., and Jones K.J. (2001). Androgenic enhancement of ßII-tubulin mRNA in spinal motoneurons following sciatic nerve injury. Rest. Neurol. Neurosci. 18, 191–198 [PubMed] [Google Scholar]
- 110.Matsumoto A., Arai Y., Urano A., and Hyodo S. (1994). Androgen regulates gene expression of cytoskeletal proteins in adult rat motoneurons. Horm. Behav. 28, 357–366 [DOI] [PubMed] [Google Scholar]
- 111.Hansberg-Pastor V., González-Arenas A., Piña-Medina A.G., and Camacho-Arroyo I. (2015). Sex hormones regulate cytoskeletal proteins involved in brain plasticity. Front. Psychiatry 6, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marron T.U., Guerini V., Rusmini P., Sau D., Brevini T.A., Martini L., and Poletti A. (2005). Androgen-induced neurite outgrowth is mediated by neuritin in motor neurons. J. Neurochem. 92, 10–20 [DOI] [PubMed] [Google Scholar]
- 113.Fargo K.N., Alexander T.D., Tanzer L., Poletti A., and Jones K.J. (2008). Androgen regulates neuritin mRNA in an in vivo model of steroid-enhanced peripheral nerve regeneration. J. Neurotrauma 25, 561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fargo K.N., Galbiati M., Foecking E.M., Poletti A., and Jones K.J. (2008). Androgen regulation of axon growth and neurite extension in motoneurons. Horm. Behav. 53, 716–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rustioni A., Kuypers H.G., and Holstege G. (1971). Propiospinal projections from the ventral and lateral funiculi to the motoneurons in the lumbosacral cord of the cat. Brain Res. 34, 255–275 [DOI] [PubMed] [Google Scholar]
- 116.Sterling P. and Kuypers H.G. (1968). Anatomical organization of the brachial spinal cord of the cat. 3. The propriospinal connections. Brain Res. 7, 419–443 [DOI] [PubMed] [Google Scholar]
- 117.Szentagothai J. (1951). Short propriospinal neurons and intrinsic connections of the spinal gray. Acta Morphol. Acad. Sci. Hung. 1, 81–94 [Google Scholar]
- 118.Conta A.C. and Stelzner D.J. (2004). Differential vulnerability of propriospinal tract neurons to spinal cord contusion injury. J. Comp. Neurol. 479, 347–359 [DOI] [PubMed] [Google Scholar]
- 119.Deng L., Ruan Y., Chen C., Frye C., Xiong W., Jin X., Jones K.J., Sengelaub D., and Xu X.M. (2016). Characterization of dendritic morphology and neurotransmitter phenotype of thoracic descending propriospinal neurons after complete spinal cord transection and GDNF treatment. Exp. Neurol. 277, 103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leslie M., Forger N.G., and Breedlove S.M. (1991). Does androgen affect axonal transport of cholera toxin HRP in spinal motoneurons? Neurosci. Lett. 126:199–202 [DOI] [PubMed] [Google Scholar]
- 121.Giangregorio L. and McCartney N. (2006). Bone loss and muscle atrophy in spinal cord injury: Epidemiology, fracture prediction, and rehabilitation strategies. J. Spinal Cord Med. 29, 489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peckham P.H., Mortimer J.T., and Marsolais E.B. (1976). Alteration in the force and fatigability of skeletal muscle in quadriplegic humans following exercise induced by chronic electrical stimulation. Clin. Orthop. 114, 326–333 [PubMed] [Google Scholar]
- 123.Gordan T. and Mao J. (1994). Muscle atrophy and procedures for training after spinal cord injury. Phys. Ther. 74, 50–60 [DOI] [PubMed] [Google Scholar]
- 124.Goldspink D.F. (1978). The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J. Physiol. (Lond.) 264, 267–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Williams P.E. and Goldspink G. (1973). The effect of immobilization on the longitudinal growth of striated muscle fibers. J. Anat. 116, 45–55 [PMC free article] [PubMed] [Google Scholar]
- 126.Tiidus P.M., Lowe D.A., and Brown M.N. (2013). Estrogen replacement and skeletal muscle: mechanisms and population health. J. Appl. Physiol. 115, 569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Suzuki S. and Yamamuro T. (1985). Long-term effects of estrogen on rat skeletal muscle. Exp. Neurol. 87, 291–299 [DOI] [PubMed] [Google Scholar]
- 128.Piccone C.M., Brazeau G.A., and McCormick K.M. (2005). Effect of oestrogen on myofibre size and myosin expression in growing rats. Exp. Physiol. 90, 87–93 [DOI] [PubMed] [Google Scholar]
- 129.Weigt C., Hertrampf T., Flenker U., Hülsemann F., Kurnaz P., Fritzemeier K.H., and Diel P. (2015). Effects of estradiol, estrogen receptor subtype-selective agonists and genistein on glucose metabolism in leptin resistant female Zucker diabetic fatty (ZDF) rats. J. Steroid Biochem. Mol. Biol. 154, 12–22 [DOI] [PubMed] [Google Scholar]
- 130.Sciote J.J., Horton M.J., Zyman Y., and Pascoe G. (2001). Differential effects of diminished oestrogen and androgen levels on development of skeletal muscle in hypogonadal mice. Acta Physiol. Scand. 172, 179–187 [DOI] [PubMed] [Google Scholar]
- 131.Kochakian C.D. (1975). Definition of androgens and protein anabolic steroids. Pharmac. Therap. B 1, 149–177 [DOI] [PubMed] [Google Scholar]
- 132.Gao W. (2010). Androgen receptor as a therapeutic target. Adv. Drug Deliv. Rev. 62, 1277–1284 [DOI] [PubMed] [Google Scholar]
- 133.Wilhelm J.C., Xu M., Cucoranu D., Chmielewski S., Holmes T., Lau K.S., Bassell G.J., and English A.W. (2012). Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J. Neurosci. 32, 5002–5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brotfain E., Gruenbaum S.E., Boyko M., Kutz R., Zlotnik A., and Klein M. (2016). Neuroprotection by estrogen and progesterone in traumatic brain injury and spinal cord injury. Curr. Neuropharmacol. 14, 641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Islamov R.R., Hendricks W.A., Katwa L.C., McMurray R.J., Pak E.S., Spanier N.S., and Murashov A.K. (2003). Effect of 17 beta-estradiol on gene expression in lumbar spinal cord following sciatic nerve crush injury in ovariectomized mice. Brain Res. 966, 65–75 [DOI] [PubMed] [Google Scholar]
- 136.Kujawa K.A., Kinderman N.B., and Jones K.J. (1989). Testosterone-induced acceleration of recovery from facial paralysis following crush axotomy of the facial nerve in male hamsters. Exp. Neurol. 105, 80–85 [DOI] [PubMed] [Google Scholar]
- 137.Nilsen J. (2008). Estradiol and neurodegenerative oxidative stress. Front. Neuroendocrinol. 29, 463–475 [DOI] [PubMed] [Google Scholar]
- 138.Verhovshek T., Buckley K.E., Sergent M.A., and Sengelaub D.R. (2010). Testosterone metabolites differentially maintain adult morphology in a sexually dimorphic neuromuscular system. Dev. Neurobiol. 70, 206–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chang C., Kokontis J., and Liao S.T. (1988). Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science 240, 324–326 [DOI] [PubMed] [Google Scholar]
- 140.Chang C., Kokontis J., and Liao S.T. (1988). Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. Proc. Natl. Acad. Sci. U.S.A. 85, 7211–7215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sen A., Prizant H., and Hammes S.R. (2011). Understanding extranuclear (nongenomic) androgen signaling: what a frog oocyte can tell us about human biology. Steroids 76, 822–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thomas P. (2012). Rapid steroid hormone actions initiated at the cell surface and the receptors that mediate them with an emphasis on recent progress in fish models. Gen. Comp. Endocrinol. 175, 367–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Frye C.A. (2001). The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res. Rev. 37, 201–222 [DOI] [PubMed] [Google Scholar]
- 144.Lange C.A., Gioeli D., Hammes S.R., and Marker P.C. (2007). Integration of rapid signaling events with steroid hormone receptor action in breast and prostate cancer. Annu. Rev. Physiol. 69, 171–199 [DOI] [PubMed] [Google Scholar]