Abstract
Influenza virus infections remain a significant health burden worldwide, despite available vaccines. Factors that contribute to this include a lack of broad coverage by current vaccines and continual emergence of novel virus strains. Further complicating matters, when influenza viruses infect a host, severe infections can develop when bacterial pathogens invade. Secondary bacterial infections (SBIs) contribute to a significant proportion of influenza-related mortality, with Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus pyogenes, and Haemophilus influenzae as major coinfecting pathogens. Vaccines against bacterial pathogens can reduce coinfection incidence and severity, but few vaccines are available and those that are, may have decreased efficacy in influenza virus-infected hosts. While some studies indicate a benefit of vaccine-induced immunity in providing protection against SBIs, a comprehensive understanding is lacking. In this review, we discuss the current knowledge of viral and bacterial vaccine availability, the generation of protective immunity from these vaccines, and the effectiveness in limiting influenza-associated bacterial infections.
Keywords: : influenza, secondary bacterial infections, protective immunity
Introduction
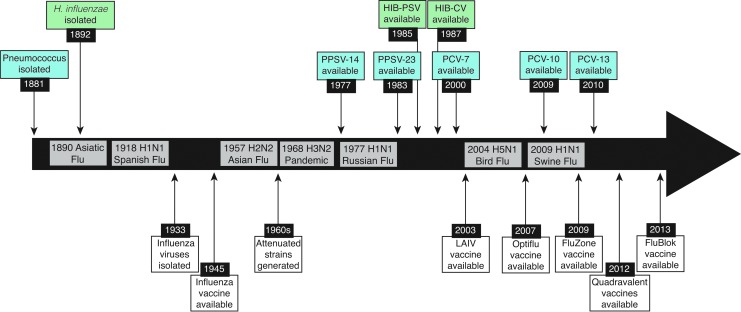
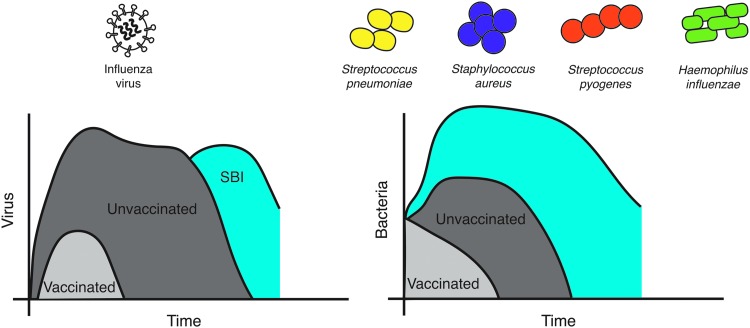
Vaccines against influenza viruses have been available for over 60 years (67,150,242) (Fig. 1), yet recent estimates indicate that ∼114–624,000 hospitalizations and 5,000–27,000 deaths are associated with influenza virus infections annually in the United States alone (199). Contributing factors include a lack of comprehensive strain coverage in vaccines (3), a large number of antigenically distinct influenza virus strains (11,13), and complications arising from underlying health conditions and/or secondary bacterial infections (SBIs) (33,102). Post-influenza SBIs are often associated with either the gram-positive pathogens Streptococcus pneumoniae, Staphylococcus aureus, or Streptococcus pyogenes, or the gram-negative pathogen Haemophilus influenzae (154,259) (Fig. 2). Together, these pathogens have accounted for 40–95% of influenza-related mortality in past pandemics (31,133,164,253), and have contributed to deaths during seasonal epidemics (202). In cases where bacterial coinfections are identified, severe pneumonia, hospitalization, and high mortality rates are observed despite antibiotic use (53,93,119,181).
FIG. 1.
Timeline of available vaccines and influenza pandemics. Timeline of the major influenza virus pandemics from 1890 to 2017. Since the first isolation of an influenza virus in 1933, numerous vaccines have been developed and approved for use (white). Vaccines for pneumococcus (blue) and Hib (green), on the other hand, took nearly a century from the first isolation in 1881 and 1892, respectively.
FIG. 2.
Time course of influenza-bacterial coinfections and major causative agents. Influenza virus infection in unvaccinated individuals (dark gray) results in rapid viral growth before the virus peaks and the infection resolves. Bacteria often invade late in the infection, which can result in a rebound of viral loads (blue). In vaccinated individuals (light gray), viral loads are reduced and the infection length is shortened considerably, leaving little opportunity for bacterial invasion. Similarly, primary bacterial infections with S. pneumoniae, S. aureus, or S. pyogenes in vaccinated individuals (light gray) result in more mild infection compared to those that are unvaccinated (dark gray). When these pathogens invade during influenza (blue), bacteria grow rapidly with little immune control.
Association between influenza virus and SBIs was first recorded by R.T.H. Laennec in his observations regarding excess mortality after an outbreak of la grippe in the 1700's (120). In current clinical settings, influenza-related SBIs can be difficult to diagnose because viruses other than influenza, including respiratory syncytial virus, parainfluenza virus, and adenoviruses are also known to initiate SBIs (62,94,95). Further complicating matters is the wide range of bacterial pathogens that are associated with community-acquired (34,94,95) and hospital-acquired (1,100) SBIs. The majority of those at risk for an SBI are the young (<1 year old) and the elderly (>65 years old) (202), and these populations notoriously have weak immune responses toward both infection and vaccination (138,155,189).
Despite the high mortality rates associated with SBIs, influenza-infected individuals may be more likely to recover without complication than succumb to an SBI (20), an observation that indicates the potential for identifying mechanisms that can overcome these deadly infections. Recent laboratory-based studies into the cause of SBIs have advanced our understanding of the viral, bacterial, and host contributions to these synergistic interactions [reviewed in (22,146, 154,201,204,215,224), among others]. Defining these host-pathogen interactions should give insight into how the host responds to an SBI. However, the extent to which vaccines and therapeutics can be used to reduce SBI severity remains under investigation, especially with respect to translating laboratory findings into the clinic. Recent work using laboratory-based models of SBI to evaluate vaccines that are either FDA approved or in development has helped identify the impact each has toward limiting these deadly diseases. Currently, the true impact of vaccines on the outcome of polymicrobial infections is difficult to evaluate, in part, because there is little systematic surveillance of bacterial coinfections during seasonal influenza (43,111). In addition, because the immune status of the human population varies tremendously and is difficult to model in the laboratory, well-designed and controlled clinical trials will be needed to determine the effects of vaccines on SBI severity. In this review, we review the use of vaccines to abrogate influenza-associated bacterial infections and discuss advances in this area, including both surprising findings and current gaps in our knowledge.
Vaccines That Target Influenza Viruses
The influenza virus is a likely target for circumventing a secondary bacterial invasion. There are a number of FDA-approved vaccines against influenza viruses that range from traditional inactivated influenza virus (IIV) preparations, live attenuated influenza viruses (LAIV), and recombinant proteins expressed by baculoviruses (77). Both IIV and LAIV vaccines are prepared by infecting either chicken eggs (71,114) or cells in culture (137,165) with master donor viruses, while recombinant protein vaccines are created without the use of influenza viruses (38,92). The main target of vaccine-induced immunity remains the viral hemagglutinin (HA) protein (256), and the hemagglutination inhibition titer is the gold standard for correlating vaccine-induced immunity with protection (15,17,81,152,176). In population studies, influenza vaccines often show variable effectiveness (180), with recent reports indicating ranges from 23% to 62% effectiveness against acute respiratory illness (28,65,66) depending, in part, on how well the vaccine matches the circulating strains (3,66,67). Continuous change in circulating influenza viruses creates a situation where vaccine formulations must be considered annually (3) and we are often unprepared to effectively mitigate the initial emergence of a pandemic strain (19,75). This gap in vaccine preparedness can subsequently affect the incidence of both primary influenza virus infections and SBIs (5,8,160,214,244), and lead to devastating consequences during both epidemic and pandemic influenza virus outbreaks.
Because the HA protein is constantly changing through antigenic drift (227), there have been efforts toward developing universal influenza vaccines that target conserved viral proteins (231). Three targets, including the ion channel matrix 2 extracellular (M2e) protein (47,172), the conserved stalk region of the HA protein (50,233,251), and the neuraminidase [NA; (36,107,109,131,210)], are of particular interest in these efforts. Each of these targets has the potential to induce broad immunity within a specific subtype and may even allow for heterosubtypic immunity (117,131, 136,206). To date, the only FDA-approved vaccine that induces immunity against a single influenza virus protein is the recombinant HA vaccine that is produced using baculovirus (38,92), and vaccines that target conserved epitopes may be able to build upon the success of this vaccine (55). One potential drawback to focusing on a single viral component for vaccination may be the absence of immunity against other viral proteins that may help limit SBIs, including the NA protein (88,147). In addition, because vaccine-induced immunity is not always sterilizing (134,257), even when the vaccine closely matches the circulating strain, the effect that a mild or moderate infection can have on the host must be considered. Indeed, vaccines that target conserved influenza virus proteins often generate antibodies that limit virus spread, rather than prevent infection (2,50,58,127, 152,245). This can yield a mild infection that may leave the vaccinated host susceptible to an SBI, further justifying studies that determine the impact of influenza vaccines on SBI severity, both in the laboratory and the clinic (14,30,126, 156,178,243).
Effect of Influenza Vaccines on SBIs
To date, only a few clinical studies have reported on the influence of vaccine-induced immunity against influenza virus on subsequent SBI severity. One of these studies used military recruits, a group known to be susceptible to S. pyogenes infections (126). This study showed that vaccination against influenza viruses limited illness associated with S. pyogenes in this population, which was a promising finding in the area of vaccine-induced prevention of SBI severity. As further evidence of influenza virus vaccine-induced prevention of SBIs in humans, reduced incidence of otitis media was observed in pediatric populations during clinical trials that preceded approval of the LAIV vaccine (14). Together, these studies encourage future meta-analyses that may help determine the impact of influenza vaccines on SBIs. However, due to the complex nature of SBIs, direct evaluation of the benefit that influenza virus vaccines has on SBIs in humans remains limited to defined patient populations where conditions can be controlled. Expanding these studies into larger populations is difficult due to an inability to control prior immunity against influenza viruses and bacterial species, including immunity induced by vaccination (156).
Experiments that evaluate vaccine-induced immunity in the context of SBIs can be difficult to control clinically, but key concepts related to vaccine-induced protection can be modeled in the laboratory. To date, the majority of vaccine studies are designed to evaluate protection against the pathogen the host was vaccinated against, and there have only been a limited number of studies evaluating influenza virus vaccines in the context of an SBI. One such study used IIV preparations to determine whether immunity against the HA protein or the NA protein was more effective in limiting SBI severity (88). Results from this study showed that immunity against either protein could limit the severity of secondary infections when S. pneumoniae was used as the secondary invader. Because the NA expressed by an influenza virus is known to increase both bacterial attachment (147) and SBI severity in animal models (186,187), and NA is one of the potential vaccine targets mentioned above, these findings justify continued efforts to evaluate the contribution of anti-NA immunity toward SBI severity.
In separate studies in mice, IIV and LAIV vaccines were evaluated for their ability to limit secondary S. pyogenes infection (30,177). In these studies, vaccine-induced immunity against influenza virus improved survival after an SBI, but did not always yield complete protection. Interestingly, these studies show that S. pyogenes can establish a lung infection in mice that were challenged with influenza viruses that matched the vaccine strain (30). This is surprising because the vaccinated mice in these studies did not show signs of infection after virus challenge, observed using weight loss, yet bacterial persistence and death following inoculation with a sublethal dose of S. pyogenes were observed in some mice (30). Because influenza vaccines are not always able to completely protect against an SBI, the immune mechanisms associated with severe SBIs in the vaccinated host could be further elucidated as a step toward translating these findings into clinical practice. Similarly, future vaccine studies could focus on defining the impact of supplementing antiviral and antibiotic options in situations when vaccine and challenge strains do not match.
Despite the limitations described above, it is clear that vaccinating against influenza viruses limits SBI severity in the majority of vaccinated animals (30,88,157). However, the LAIV vaccine has an important caveat in laboratory studies, which shows that bacterial colonization and replication may be enhanced in the nasopharynx and middle ear after LAIV delivery (158,159). Changes in commensal microbiota within the nasal passage after LAIV administration (241) may contribute to this increased colonization and replication. This observation brings into question the value of evaluating the timing of anti-influenza and antibacterial vaccines in an effort to limit colonization, particularly in pediatric populations. Indeed, carriage of H. influenzae, but not pneumococcus, may be moderately increased in children within 1 week after LAIV administration (243). Similar to the animal studies, increased pneumococcal density in children was observed, but not until 28 days after vaccination (243). Thus, the impact of influenza vaccines on subsequent bacterial infections should be considered both in the days after vaccination and at the peak of influenza season.
Vaccines That Target Bacterial Pathogens
While influenza viruses initiate SBIs, bacteria often dictate illness severity (68,132,254). As such, the role of vaccines against bacterial pathogens in limiting coinfections must be discussed. Clinical (211) and laboratory (112,153) studies demonstrate bacterial vaccine effectiveness against SBIs, and use of bacterial vaccines in this capacity dates back to the 1918 pandemic (32,59,247). In 1918, vaccines were often crude preparations of bacterial membranes that were delivered without the benefit of strict clinical trial criteria, yet protective efficacy was conferred in some (32,59), but not all (26,125,143) of these clinical experiments. Similar to influenza vaccines, the efficacy of bacterial vaccines is limited to strains included in the vaccine, which can be important when the circulating serotype is not included in the vaccine (80). Further complicating matters, studies designed to evaluate bacterial vaccines in SBIs range in their reported benefit from complete protection against an SBI (112) to no protection observed (153,157). This efficacy range shows a benefit to vaccinating against bacteria even when influenza-induced illness is observed prior to bacterial inoculation. Given that few vaccines are available for coinfecting bacteria and identifying universal epitopes that can induce protective immunity within a bacterial species is difficult, the extent that antibacterial vaccines are efficacious for SBIs is not fully appreciated. These issues are discussed in more detail below.
The primary bacterial species associated with severe SBI includes S. pneumoniae, H. influenzae, S. aureus, and S. pyogenes (Fig. 2). Vaccines are available for S. pneumoniae and H. influenzae, but not for S. aureus or S. pyogenes (Fig. 1). Vaccines against S. pneumoniae, prepared using purified polysaccharide, have been available for decades as 23-valent preparations that are recommended for adults (221). Pneumococcal conjugate vaccines have been approved for use by the FDA since 2000, initially as 7-valent preparations and more recently in a 13-valent variety (190). Together, vaccines that protect against S. pneumoniae are regularly given to adults over 60 years of age and are included in the standard vaccination schedule for children (194). Despite these vaccination efforts, pneumococcus remains the most common bacterial pathogen associated with complications after influenza virus infection (94,95,173). This is likely due to the lack of comprehensive coverage of pneumococcal serotypes within a single vaccine preparation, which can become a bigger problem when nonvaccine serotypes circulate among vaccinated populations (4,191).
Vaccines that target conserved epitopes that are expressed by multiple S. pneumoniae isolates may help improve the breadth of protection, even if the target is not necessarily universal. In an effort to increase the breadth of serotypes affected by vaccination against S. pneumoniae, the PspA protein has been studied as a vaccine candidate since 1994 (149). A recent screen of 52 different pneumococcal proteins identified another seven potential targets (PhtB, PhtD, PhtE, PrtA, NanA, PavB, and Eng) with protective capabilities (7). However, to date, there have been no published studies reporting on the contribution of vaccine-induced immunity against any of these proteins in preventing severe SBIs. When considering conserved epitopes like PspA, it is important to determine whether antibodies would overcome influenza virus-induced defects in host immunity at the time an SBI is initiated (154). Vaccines that target these conserved epitopes would be of value, but would require additional testing in animals to validate their contributions toward protection against severe SBIs.
In contrast to S. pneumoniae, vaccines against H. influenzae b (Hib), which were introduced initially as polysaccharide vaccines in 1985 (185) and then as a conjugate vaccine for use in children (128) (Fig. 1), have largely reduced Hib incidence globally (16,64,209). The fact that there are fewer Hib serotypes compared to the >90 serotypes of S. pneumoniae (190), may have helped to improve Hib vaccine effectiveness. Overall, bacterial vaccines have demonstrated a benefit in preventing primary infection with these pathogens, which in turn helps reduce the incidence and severity of SBIs (Fig. 2).
Although vaccines against S. pneumoniae and Hib are available, vaccines against S. aureus remain in development (161). S. aureus is a particular concern as it was a frequent cause of SBIs during both the 1957 and 1968 influenza pandemics (148), and it now accounts for a large number of influenza-associated childhood fatalities (262). In fact, the emergence of methicillin-resistant S. aureus (MRSA) (99,182,184), which can cause a severe and deadly necrotizing pneumonia (49,198), has contributed to this increased severity of SBIs. Recurring infections complicate therapies designed to combat S. aureus infection, and vaccinating against S. aureus antigens has had limited success (23,46,113,162). Advancing vaccine efforts against S. aureus could consider identifying and developing vaccines that induce broad immunity against conserved epitopes, and possibly using antigens from other organisms with similar epitopes to provide cross-protective immunity (260). Until a preventative measure is available, antibiotics remain the primary therapeutic option. However, these drugs often have reduced efficacy once an invasive SBI has established (103,104,222).
Similar to S. aureus, vaccines against S. pyogenes are not available clinically (234). S. pyogenes, which is also known as group A streptococcus, is a bacterial species that is associated with asymptomatic carriage of the skin and respiratory tract (240), which can cause both noninvasive and invasive diseases. Noninvasive diseases caused by S. pyogenes include pharyngitis (140), scarlet fever (140), pyoderma (40), and cellulitis (122), while invasive diseases caused by these bacteria include pneumonia (169), erysipelas (27), streptococcal toxic shock (124), and necrotizing fasciitis (35,39,261). Even after S. pyogenes is cleared, host responses against the bacteria can cause acute rheumatic fever (248) and poststreptococcal glomerulonephritis (25). It is estimated that lower respiratory tract complications are associated with 11–12% of invasive S. pyogenes infections (179) and there is a 38% case fatality rate given this diagnosis (169). While antibiotic resistance has been observed, in particular against macrolide antibiotics (76), S. pyogenes remains susceptible to penicillin (203). However, as with other SBI cases, these antibiotics are often unable to clear SBIs in hospitalized patients (103,104,222). Without clinically available vaccines against MRSA and S. pyogenes, evaluating the impact of these vaccines on SBI severity is limited to the laboratory (112).
Vaccinating Against Bacteria to Reduce SBI Severity
The effect that antibacterial vaccines have on reducing the severity of an influenza-associated SBI has been assessed for its impact on survival in animal SBI models using either S. pneumoniae or S. pyogenes as the secondary pathogen (112,153). Both the pneumococcal conjugate vaccine, Prevnar (153), and a recombinant M protein vaccine that targets S. pyogenes have been evaluated (112). In the case of Prevnar, the vaccine was <40% protective against a secondary infection, while the vaccine targeting S. pyogenes showed complete protection against SBI. Both of these studies relied on challenge with bacterial isolates that matched those included in the vaccine, which partially limits the translation of this knowledge into clinical settings where bacterial species cannot be controlled. The observed survival in these SBI models is impressive considering that influenza-induced illness was observed before inoculation with bacteria (112). This observation implies that the known virus-induced deficits in host immunity that predispose to severe SBIs (154) can be at least partially overcome in the presence of vaccine-induced antibacterial immunity. In the case of S. pyogenes, antibodies against the M protein could induce antibody-dependent cellular phagocytosis (ADCP) by macrophages, which was observed in vitro using sera from vaccinated mice (112). This potential contribution of vaccine-induced antibodies toward clearance of a secondary bacterial invader may help limit SBI severity, but further work in this area is needed to define relevant host mechanisms of protection.
Vaccine-Induced Immune Responses Affecting SBI Severity
When considering the effect of vaccine-induced immunity on SBI severity, one must understand how vaccination influences immune responses in the context of both the primary pathogen and the secondary pathogen of interest. However, to date, the majority of vaccine studies focus solely on vaccine-induced responses against the pathogen the vaccine was designed to protect against (156). Previous work with influenza vaccines in SBI models indicate that a severe SBI can develop even when the challenge strain matches the vaccine strain (30,177). Host factors that can influence SBI severity include changes in the microbiota, innate immune responses, and adaptive immune response. Recent work in the microbiome field shows that the unique group of microbes that comprise the microbiome changes during infection (29,89,195,232) and may influence SBI disease severity (192). Similarly, host responses from vaccination have the ability to directly alter the resident microbiota (175,205,241). While the role of the respiratory and gut microbiome in SBIs is of significant interest (48,142), we will focus the remainder of our discussion on contributions from innate and adaptive immunity.
Innate immune responses against influenza viruses can directly influence severity of influenza-associated SBIs. Numerous innate immune responses have been shown to play a role in SBI acquisition, viral and bacterial growth, and severity. Many of these are likely time dependent and may be correlated. Bacterial invasion and initial growth kinetics have been shown to be a consequence of viral-mediated alveolar macrophage (AM) depletion (74,223,225), while the subsequent neutrophil response is dysfunctional and these cells are unable to limit the later stages of bacterial growth (208,236). An impaired NK cell response, which may be upstream of the effects from AMs and neutrophils, has also been identified to have a role in the defective postinfluenza antibacterial defense (220). In addition, differences in the expression of cytokines, such as type I interferons (IFNs), can alter the function of these cells and enhance SBI severity (130,171,212,213). The contribution of innate immune responses is important to consider because they can be induced even when the infecting virus matches the vaccine strain, the vaccine-induced immunity is sterilizing, and the circulating virus has not mutated sufficiently to escape vaccine-induced immunity. Indeed, a live attenuated Bordetella pertussis vaccine demonstrated protection against lethal influenza virus challenges by controlling cytokine responses and reducing virus-mediated inflammation (129).
In addition to innate immunity, the dynamic interactions of an invading pathogen and the host adaptive immune response, including cell-mediated immunity (i.e., T cells and B cells) and antibodies (i.e., IgG, IgM, and IgA), have also been studied in the context of SBIs. In fact, recent work in mice shows that virus-specific antibody responses, B cell and T cell populations, and germinal centers are reduced during influenza-pneumococcal coinfection (18,255,258). Although animal models of influenza-bacterial coinfection are lethal, these immune alterations may indicate a reduction in the quality and quantity of protective immunity following coinfection in nonlethal situations. Treatment with influenza virus-specific immune serum can partially restore the response, improving survival (258), which may indicate a protective role for anti-influenza virus antibodies. Interestingly, when pneumococcal infection precedes influenza virus infection, the B cell and CD4+ T follicular helper cell responses are enhanced (255). However, the robust response is short lived with waning antibody neutralization within 30 days of infection (255). Further complicating matters, these innate and adaptive immune responses differ in unvaccinated and vaccinated individuals, which can influence the effectiveness of vaccines in SBIs.
As mentioned previously, laboratory and clinical studies have often focused on developing vaccines against single pathogens and evaluating each using challenge studies that incorporate the pathogen of interest. However, there are often off-target effects of vaccines that are underappreciated due to a lack of investigation within polymicrobial infection models (156). Because the majority of our knowledge of vaccine:challenge studies relies on outcomes using the single-pathogen approach, these responses can be considered when selecting the characteristics of vaccines that would most likely influence the severity of an SBI. This relies largely on our collective knowledge of humoral and cellular correlates of vaccine-induced protective immunity, and whether those aspects of the adaptive immune response may be specifically depleted or made ineffective during an SBI. Because postinfection innate immunity in vaccinated individuals and changes in the microbiome can be difficult to consider based on the available information, we will focus our discussion on known mechanisms of adaptive immunity, which may alter SBI severity, in particular those mediated by vaccine-induced antibodies.
Host Responses to Influenza Virus Vaccines
One main goal of vaccination is to generate neutralizing antibodies that prevent infection and, ideally, induce long-term sterilizing immunity (257). Achieving this level of protection is also useful for limiting, but not necessarily preventing, SBIs (30,177). Vaccines that stimulate neutralizing antibodies, including those against influenza viruses (249), are often most effective when these antibody titers achieve high levels. However, efficacy is often lost as antibody levels wane over time (207). In some instances, vaccine-induced immunity can wane within a single influenza season, which can affect vaccine effectiveness against viruses that circulate later in the season (63). In addition, both historical (82–84) and recent evaluation of vaccine effectiveness have resulted in the antigenic distance hypothesis (226), which predicts the current season's vaccine effectiveness based on negative interference from the previous season's vaccine. This hypothesis continues to be tested to determine how repeated vaccination alters susceptibility to influenza virus infection, with some evidence that repeated annual influenza vaccination reduces vaccine effectiveness (12,217). This may be due to mutations of the influenza virus HA that arise while growing the viruses in cell culture (219) or in eggs (218), which are both used during vaccine production (71,137). Thus, challenges remain with respect to influenza vaccines and host responses to these vaccines that must be considered in the effort to improve effectiveness, especially as it relates to the impact of vaccination on SBIs.
From the host response perspective, antibodies may have the ability to interact with Fc receptors in addition to neutralizing virus. This can assist in virus clearance when sterilizing immunity is unavailable (45,50,86,87,168). Similarly, antibodies targeting conserved epitopes can rely on Fc receptor interactions to enhance clearance from an infected host (45,50,51,58,127,245). Thus, it appears that a balance of neutralizing and nonneutralizing antibodies encompasses an optimal response against influenza virus infection. At this time, the majority of immune assays focus on the neutralizing characteristics of the antibody, as defined by their interaction with an antigen through the Fab region (183,197,249), and only more recently has there been strong consideration for the contribution of the Fc region of the antibodies toward virus clearance (21,45,72,73,96,97,166,167). This change in focus to evaluate the whole antibody toward pathogen clearance has impacted our knowledge of the contribution of conserved targets toward clearing pathogens from an infected host (45,50,51,58,79,127,168,239,245,246), which we will discuss below.
The influenza vaccine field has focused recently on the development of universal vaccines that target more conserved epitopes of the influenza virus, including the M2 ion channel (47), the HA stalk region (110), and the NA protein (131). One issue facing these vaccines is the reduced ability of vaccine-induced antibodies to directly prevent infection in the conventional way that we envision neutralizing immunity. For example, because the M2 ion channel is expressed in higher abundance on infected host cells rather than the virion itself (123,263), the infected cell becomes the target of vaccine-induced immunity (58). Thus, vaccine efficacy relies on establishing at least a mild infection for vaccine-induced antibodies to eliminate the pathogen. As discussed above, this initial infection may have profound effects on the microbiota, innate immunity, adaptive immunity, and SBI severity.
Similarly, antibodies against the HA stalk region do not interfere with HA binding to sialic acid residues through the receptor-binding region of the HA (37,57). However, in contrast to antibodies against M2, antistalk antibodies appear to directly enhance ADCP (168) when viruses concomitantly bind host cells through sialic acid residues, while linking stalk-specific antibodies to the cellular Fc receptor (50,239). Host responses that develop during this interaction, including cytokine production by macrophages, have yet to be defined and may also affect severity of an SBI. Furthermore, the intracellular trafficking of these internalized viruses brings into question whether vaccine-induced antibodies enhance virus clearance through ADCP or if this interaction may contribute to the antibody-dependent enhancement of infection responses that have sometimes been observed in humans (237,238) and large animal influenza vaccine studies (69,70,105,196,229). There are clearly benefits of targeting conserved epitopes and developing vaccines that are broadly reactive against influenza viruses. However, additional research into the cellular and molecular immune responses that develop when these antibodies are actively clearing the virus from an infected host is needed.
Finally, antibodies against the NA have been of interest for decades (54,98,107,108,170,210). Their role in protection has been assigned to limiting virus spread rather than directly neutralizing virus particles (108,109). The NA is an intriguing target when discussing SBIs as it has an enzymatic activity known to enhance SBI severity (147). In addition, because the NA expressed by influenza A viruses with pandemic potential (e.g., H5 N1) can match the NA expressed by seasonal influenza viruses (e.g., H1 N1), NA immunity may provide some protection from these pandemic viruses (131,139,206). This potential impact of vaccine-induced anti-NA immunity on a future pandemic has led to a refinement of the NA inhibition assay with an emphasis on using this assay to define anti-NA antibodies as correlates of vaccine-induced protection (56,152,163). Furthermore, a contribution of anti-NA immunity toward limiting SBI severity has been demonstrated in a murine superinfection model (88). At this point, it is unknown whether anti-NA antibodies limit SBI severity by inhibiting NA activity itself or through another host immune mechanism, but the potential for this vaccine target to limit secondary infections has been demonstrated experimentally.
Host Responses to Bacterial Vaccines
In contrast to the more recent appreciation for Fc receptors toward influenza virus clearance, ADCP as a mechanism of pathogen clearance has been appreciated for decades when discussing the effectiveness of vaccines against bacteria (24,44,61,116,193). Specifically, antibodies induced in response to S. pneumoniae are often tested in opsonophagocytosis assays (24), and vaccines in development against S. pyogenes were designed to target opsonic epitopes of the M protein (41,42,85). Thus, it is not surprising that antibodies induced against S. pyogenes are able to increase bacterial uptake by macrophages, in a manner that may help reduce SBI severity (112). However, influenza virus infections have profound effects on the macrophage population, including defects in cellular function that contribute to SBI severity (141,154,235). Thus, it seems plausible that vaccinating to induce Fc receptor-interacting antibodies that also recognize bacteria through their Fab region may help overcome virus-induced macrophage defects. Future evaluation of vaccine-induced antibodies should include efforts that determine ways to optimize Fc receptor-mediated clearance of bacteria. This would determine whether mechanisms that are known to contribute to immune-mediated protection against primary bacterial infection can be effective during an SBI or if other immune mechanisms of protection should be considered.
Alternative Interventions
In addition to vaccine-induced antibodies, the contribution of antiviral and antibiotic therapies toward limiting the severity of an SBI must be considered. From the antiviral side, neuraminidase inhibitors are effective against influenza A and B viruses (151) and can reduce the duration and severity of SBIs in animal models (144,145,222). On the other hand, antibiotics can differ in their efficacy in SBIs (103,104), a finding that is appreciated clinically (60) and experimentally with models showing that bacteriostatic antibiotics are more effective against SBIs than bactericidal antibiotics (103). Interestingly, computational models indicate a strong benefit if combination therapy were employed (222), but this scenario has not been tested in animal models or in the clinic. When considering expanding our antimicrobial repertoire, it is also important to understand how these drugs affect the microbiome, which is known to be affected by some antimicrobials (101). In addition, it is imperative that new therapies be evaluated using models of SBIs to ensure their use will be beneficial when vaccines are unavailable (e.g., during a rapidly developing pandemic) and to guide the appropriate selection and timing of therapeutic treatment.
Another potential approach that has been increasing in popularity and may represent a first-line treatment in the face of a pandemic, is immunotherapy using antibodies (52,91, 216,230). This is interesting because the use of passive antibody therapies revisits classical approaches with convalescent sera that were used to limit SBI severity during the 1918 pandemic (135). Today, therapeutic antibodies can be monoclonal (118,188,250,252) or polyclonal (52,106,200) in nature and can be used to target either the virus or the bacteria. As described above, optimal protection against influenza virus infection seems to benefit from a balance of neutralizing and nonneutralizing antibodies (6,121,239), a finding that hints that polyclonal antibody preparations may provide a more balanced approach toward therapeutic treatment. These polyclonal antibodies may be created using cocktails of monoclonal antibodies with demonstrated efficacy against their individual targets (78,90,216,228,230). Interestingly, off-target effects for these products are possible, including a global anti-inflammatory response that has been observed in studies testing human intravenous immunoglobulin (9,10,174). Studies on therapeutic antibody preparations would benefit from validation in SBI models, as both direct and indirect effects on SBI severity could be appreciated before use in the clinic.
Concluding Remarks
Several studies indicate a benefit of viral and bacterial vaccination on the potential deadly outcomes from SBIs, despite the fact that most of these vaccines were not preevaluated in SBI models. In particular, individual studies reporting decreases in otitis media and S. pyogenes infections in humans that were vaccinated against influenza (14,126) provide evidence that these vaccines can limit SBI severity. To date, how SBI severity changes in individuals vaccinated against bacterial pathogens, but not against influenza viruses is unknown. Laboratory models evaluating bacterial vaccines in the context of SBIs have shown mixed results (112,153). Thus, additional testing of these findings in the laboratory and clinic is warranted. Furthermore, as the number of bacterial vaccines increases and as more conserved targets for influenza virus vaccines are identified, there is an opportunity to determine optimal host responses that can be targeted to reduce SBI severity after vaccination.
From a clinical standpoint, work with both influenza and bacterial vaccines suggests that SBIs can be mitigated through robust vaccination. Because of this benefit, annual influenza vaccine efforts could be complemented with annual bacterial vaccines for maximum efficacy. To our knowledge, studies that evaluate SBIs in animals that have been vaccinated against both the challenge virus and the challenge bacteria have not been reported, which may be due to technical difficulties in modeling this clinically relevant scenario in the laboratory. Examining the potential of complementary vaccination may be best suited for computational investigation, which is more amenable to assessing complex phenomena and clinical situations. Currently, bacterial vaccines are indicated for the young and the elderly (115,190), with minimal use in healthy adults. If these vaccines were tested for their simultaneous effects on SBI severity, rapidly characterizing their impact could help preparedness for future epidemics and, importantly, pandemics where viral vaccines may not be available. Gaps in vaccine coverage for S. pneumoniae isolates and an absence of vaccines against S. aureus and S. pyogenes leave us with incomplete bacterial vaccine coverage. Future exploration in antivirals, antibiotics, and immunotherapies must continue to consider how vaccine-induced immunity affects host responses that contribute to severe SBIs as part of preclinical validation studies.
Acknowledgments
The authors received support from NIH Grants 1 R56 AI125324 01 (A.M.S.), 5 R44 AI117976 02 (VCH), and 5 P20 GM103443 16 (V.C.H.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Aiken AM, Mturi N, Njuguna P, et al. . Risk and causes of paediatric hospital-acquired bacteraemia in Kilifi District Hospital, Kenya: a prospective cohort study. Lancet 2011;378:2021–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Air GM, and Laver WG. The neuraminidase of influenza virus. Proteins 1989;6:341–356 [DOI] [PubMed] [Google Scholar]
- 3.Alan H, Ian B, Nancy C, et al. . Improving the selection and development of influenza vaccine viruses - Report of a WHO informal consultation on improving influenza vaccine virus selection, Hong Kong SAR, China, 18–20 November 2015. Vaccine 2017;35:1104–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allemann A, Frey PM, Brugger SD, et al. . Pneumococcal carriage and serotype variation before and after introduction of pneumococcal conjugate vaccines in patients with acute otitis media in Switzerland. Vaccine 2017;35:1946–1953 [DOI] [PubMed] [Google Scholar]
- 5.Ampofo K, Herbener A, Blaschke AJ, et al. . Association of 2009 pandemic influenza A (H1 N1) infection and increased hospitalization with parapneumonic empyema in children in Utah. Pediatr Infect Dis J 2010;29:905–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ana-Sosa-Batiz F, Vanderven H, Jegaskanda S, et al. . Influenza-specific antibody-dependent phagocytosis. PLoS One 2016;11:e0154461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson RJ, Guru S, Weeratna R, et al. . In vivo screen of genetically conserved Streptococcus pneumoniae proteins for protective immunogenicity. Vaccine 2016;34:6292–6300 [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1 N1) - United States, May-August 2009. MMWR Morb Mortal Wkly Rep 2009;58:1071–1074 [PubMed] [Google Scholar]
- 9.Anthony RM, Nimmerjahn F, Ashline DJ, et al. . Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 2008;320:373–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayry J, Bansal K, Kazatchkine MD, et al. . DC-SIGN and alpha2,6-sialylated IgG Fc interaction is dispensable for the anti-inflammatory activity of IVIg on human dendritic cells. Proc Natl Acad Sci U S A 2009;106:E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedford T, Riley S, Barr IG, et al. . Global circulation patterns of seasonal influenza viruses vary with antigenic drift. Nature 2015;523:217–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belongia EA, Skowronski DM, McLean HQ, et al. . Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017;16:1–14 [DOI] [PubMed] [Google Scholar]
- 13.Belshe RB. The need for quadrivalent vaccine against seasonal influenza. Vaccine 2010;28 Suppl 4:D45–D53 [DOI] [PubMed] [Google Scholar]
- 14.Belshe RB, and Gruber WC. Prevention of otitis media in children with live attenuated influenza vaccine given intranasally. Pediatr Infect Dis J 2000;19:S66–S71 [DOI] [PubMed] [Google Scholar]
- 15.Belshe RB, Gruber WC, Mendelman PM, et al. . Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis 2000;181:1133–1137 [DOI] [PubMed] [Google Scholar]
- 16.Bisgard KM, Kao A, Leake J, et al. . Haemophilus influenzae invasive disease in the United States, 1994–1995: near disappearance of a vaccine-preventable childhood disease. Emerg Infect Dis 1998;4:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Black S, Nicolay U, Vesikari T, et al. . Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011;30:1081–1085 [DOI] [PubMed] [Google Scholar]
- 18.Blevins LK, Wren JT, Holbrook BC, et al. . Coinfection with Streptococcus pneumoniae negatively modulates the size and composition of the ongoing influenza-specific CD8+ T cell response. J Immunol 2014;193:5076–5087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borse RH, Shrestha SS, Fiore AE, et al. . Effects of vaccine program against pandemic influenza A(H1 N1) virus, United States, 2009–2010. Emerg Infect Dis 2013;19:439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brook I. Acute sinusitis in children. Pediatr Clin North Am 2013;60:409–424 [DOI] [PubMed] [Google Scholar]
- 21.Brown EP, Dowell KG, Boesch AW, et al. . Multiplexed Fc array for evaluation of antigen-specific antibody effector profiles. J Immunol Methods 2017;443:33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis 2006;6:303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bubeck WJ, and Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med 2008;205:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burton RL, and Nahm MH. Development of a fourfold multiplexed opsonophagocytosis assay for pneumococcal antibodies against additional serotypes and discovery of serological subtypes in Streptococcus pneumoniae serotype 20. Clin Vaccine Immunol 2012;19:835–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carapetis JR, Steer AC, Mulholland EK, et al. . The global burden of group A streptococcal diseases. Lancet Infect Dis 2005;5:685–694 [DOI] [PubMed] [Google Scholar]
- 26.Cecil RL, and Vaughan HF. Results of prophylactic vaccination against pneumonia at Camp Wheeler. J Exp Med 1919;29:457–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celestin R, Brown J, Kihiczak G, et al. . Erysipelas: a common potentially dangerous infection. Acta Dermatovenerol Alp Panonica Adriat 2007;16:123–127 [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Early estimates of seasonal influenza vaccine effectiveness—United States, January 2013. MMWR Morb Mortal Wkly Rep 2013;62:32–35 [PMC free article] [PubMed] [Google Scholar]
- 29.Chaban B, Albert A, Links MG, et al. . Characterization of the upper respiratory tract microbiomes of patients with pandemic H1 N1 influenza. PLoS One 2013;8:e69559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaussee MS, Sandbulte HR, Schuneman MJ, et al. . Inactivated and live, attenuated influenza vaccines protect mice against influenza: Streptococcus pyogenes super-infections. Vaccine 2011;29:3773–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chien YW, Klugman KP, and Morens DM. Bacterial pathogens and death during the 1918 influenza pandemic. N Engl J Med 2009;361:2582–2583 [DOI] [PubMed] [Google Scholar]
- 32.Chien YW, Klugman KP, and Morens DM. Efficacy of whole-cell killed bacterial vaccines in preventing pneumonia and death during the 1918 influenza pandemic. J Infect Dis 2010;202:1639–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chowell G, Echevarria-Zuno S, Viboud C, et al. . Epidemiological characteristics and underlying risk factors for mortality during the autumn 2009 pandemic wave in Mexico. PLoS One 2012;7:e41069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cilloniz C, Ewig S, Menendez R, et al. . Bacterial co-infection with H1 N1 infection in patients admitted with community acquired pneumonia. J Infect 2012;65:223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole JN, Barnett TC, Nizet V, et al. . Molecular insight into invasive group A streptococcal disease. Nat Rev Microbiol 2011;9:724–736 [DOI] [PubMed] [Google Scholar]
- 36.Couch RB, Kasel JA, Gerin JL, et al. . Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J Infect Dis 1974;129:411–420 [DOI] [PubMed] [Google Scholar]
- 37.Cox F, Kwaks T, Brandenburg B, et al. . HA antibody-mediated FcgammaRIIIa activity is both dependent on FcR engagement and interactions between HA and sialic acids. Front Immunol 2016;7:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cox MM, Izikson R, Post P, et al. . Safety, efficacy, and immunogenicity of Flublok in the prevention of seasonal influenza in adults. Ther Adv Vaccines 2015;3:97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 2000;13:470–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Currie BJ. Group A streptococcal infections of the skin: molecular advances but limited therapeutic progress. Curr Opin Infect Dis 2006;19:132–138 [DOI] [PubMed] [Google Scholar]
- 41.Dale JB. Multivalent group A streptococcal vaccine designed to optimize the immunogenicity of six tandem M protein fragments. Vaccine 1999;17:193–200 [DOI] [PubMed] [Google Scholar]
- 42.Dale JB, Chiang EY, Liu S, et al. . New protective antigen of group A streptococci. J Clin Invest 1999;103:1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damasio GA, Pereira LA, Moreira SD, et al. . Does virus-bacteria coinfection increase the clinical severity of acute respiratory infection? J Med Virol 2015;87:1456–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daniels CC, Kim KH, Burton RL, et al. . Modified opsonization, phagocytosis, and killing assays to measure potentially protective antibodies against pneumococcal surface protein A. Clin Vaccine Immunol 2013;20:1549–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Vries RD, Nieuwkoop NJ, Pronk M, et al. . Influenza virus-specific antibody dependent cellular cytoxicity induced by vaccination or natural infection. Vaccine 2017;35:238–247 [DOI] [PubMed] [Google Scholar]
- 46.DeLeo FR, and Otto M. An antidote for Staphylococcus aureus pneumonia? J Exp Med 2008;205:271–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng L, Cho KJ, Fiers W, et al. . M2e-based universal influenza A vaccines. Vaccines (Basel) 2015;3:105–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deriu E, Boxx GM, He X, et al. . Influenza virus affects intestinal microbiota and secondary salmonella infection in the gut through type I interferons. PLoS Pathog 2016;12:e1005572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diep BA, Le VT, Badiou C, et al. . IVIG-mediated protection against necrotizing pneumonia caused by MRSA. Sci Transl Med 2016;8:357ra124. [DOI] [PubMed] [Google Scholar]
- 50.DiLillo DJ, Palese P, Wilson PC, et al. . Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J Clin Invest 2016;126:605–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DiLillo DJ, Tan GS, Palese P, et al. . Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med 2014;20:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dixit R, Herz J, Dalton R, et al. . Benefits of using heterologous polyclonal antibodies and potential applications to new and undertreated infectious pathogens. Vaccine 2016;34:1152–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dominguez-Cherit G, Lapinsky SE, Macias AE, et al. . Critically Ill patients with 2009 influenza A(H1 N1) in Mexico. JAMA 2009;302:1880–1887 [DOI] [PubMed] [Google Scholar]
- 54.Downie JC. Neuraminidase- and hemagglutinin-inhibiting antibodies in serum and nasal secretions of volunteers immunized with attenuated and inactivated influenza B-Eng-13-65 virus vaccines. J Immunol 1970;105:620–626 [PubMed] [Google Scholar]
- 55.Dunkle LM, Izikson R, Patriarca P, et al. . Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017;376:2427–2436 [DOI] [PubMed] [Google Scholar]
- 56.Eichelberger MC, Couzens L, Gao Y, et al. . Comparability of neuraminidase inhibition antibody titers measured by enzyme-linked lectin assay (ELLA) for the analysis of influenza vaccine immunogenicity. Vaccine 2016;34:458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ekiert DC, Bhabha G, Elsliger MA, et al. . Antibody recognition of a highly conserved influenza virus epitope. Science 2009;324:246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El BK, Descamps F, De FM, et al. . Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J Immunol 2011;186:1022–1031 [DOI] [PubMed] [Google Scholar]
- 59.Ely CF, Lloyd BJ, Hitchcock CD, et al. . Influenza as seen at the puget sound navy yard. JAMA 1919;72:24–28 [Google Scholar]
- 60.English BK. Limitations of beta-lactam therapy for infections caused by susceptible Gram-positive bacteria. J Infect 2014;69 Suppl 1:S5–S9 [DOI] [PubMed] [Google Scholar]
- 61.Fabbrini M, Sammicheli C, Margarit I, et al. . A new flow-cytometry-based opsonophagocytosis assay for the rapid measurement of functional antibody levels against Group B Streptococcus. J Immunol Methods 2012;378:11–19 [DOI] [PubMed] [Google Scholar]
- 62.Falsey AR, Becker KL, Swinburne AJ, et al. . Bacterial complications of respiratory tract viral illness: a comprehensive evaluation. J Infect Dis 2013;208:432–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferdinands JM, Fry AM, Reynolds S, et al. . Intraseason waning of influenza vaccine protection: evidence from the US influenza vaccine effectiveness network, 2011–2012 through 2014–2015. Clin Infect Dis 2017;64:544–550 [DOI] [PubMed] [Google Scholar]
- 64.Fernandez J, Levine OS, Sanchez J, et al. . Prevention of Haemophilus influenzae type b colonization by vaccination: correlation with serum anti-capsular IgG concentration. J Infect Dis 2000;182:1553–1556 [DOI] [PubMed] [Google Scholar]
- 65.Flannery B, Chung JR, Thaker SN, et al. . Interim estimates of 2016–2017 seasonal influenza vaccine effectiveness—United States, February 2017. MMWR Morb Mortal Wkly Rep 2017;66:167–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flannery B, Clippard J, Zimmerman RK, et al. . Early estimates of seasonal influenza vaccine effectiveness - United States, January 2015. MMWR Morb Mortal Wkly Rep 2015;64:10–15 [PMC free article] [PubMed] [Google Scholar]
- 67.Francis T, Salk JE, and Quilligan JJ. Experience with vaccination against influenza in the spring of 1947: a preliminary report. Am J Public Health Nations Health 1947;37:1013–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gabrilovich MI, Huff MD, McMillen SM, et al. . Severe necrotizing tracheobronchitis from panton-valentine leukocidin-positive MRSA pneumonia complicating influenza A-H1 N1-09. J Bronchol Interv Pulmonol 2017;24:63–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gauger PC, Loving CL, Khurana S, et al. . Live attenuated influenza A virus vaccine protects against A(H1 N1)pdm09 heterologous challenge without vaccine associated enhanced respiratory disease. Virology 2014;471-473C:93–104 [DOI] [PubMed] [Google Scholar]
- 70.Gauger PC, Vincent AL, Loving CL, et al. . Enhanced pneumonia and disease in pigs vaccinated with an inactivated human-like (delta-cluster) H1 N2 vaccine and challenged with pandemic 2009 H1 N1 influenza virus. Vaccine 2011;29:2712–2719 [DOI] [PubMed] [Google Scholar]
- 71.Gerdil C. The annual production cycle for influenza vaccine. Vaccine 2003;21:1776–1779 [DOI] [PubMed] [Google Scholar]
- 72.Gerhard W. The role of the antibody response in influenza virus infection. Curr Top Microbiol Immunol 2001;260:171–190 [DOI] [PubMed] [Google Scholar]
- 73.Gerhard W, Mozdzanowska K, Furchner M, et al. . Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev 1997;159:95–103 [DOI] [PubMed] [Google Scholar]
- 74.Ghoneim HE, Thomas PG, and McCullers JA. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J Immunol 2013;191:1250–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Girard MP, Tam JS, Assossou OM, et al. . The 2009 A (H1 N1) influenza virus pandemic: a review. Vaccine 2010;28:4895–4902 [DOI] [PubMed] [Google Scholar]
- 76.Green MD, Beall B, Marcon MJ, et al. . Multicentre surveillance of the prevalence and molecular epidemiology of macrolide resistance among pharyngeal isolates of group A streptococci in the USA. J Antimicrob Chemother 2006;57:1240–1243 [DOI] [PubMed] [Google Scholar]
- 77.Grohskopf LA, Sokolow LZ, Broder KR, et al. . Prevention and Control of Seasonal Influenza with Vaccines. MMWR Recomm Rep 2016;65:1–54 [DOI] [PubMed] [Google Scholar]
- 78.Group PIW, Multi-National PIIST, Davey RT Jr., et al. . A randomized, controlled trial of ZMapp for ebola virus infection. N Engl J Med 2016;375:1448–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Henry Dunand CJ, Leon PE, Huang M, et al. . Both neutralizing and non-neutralizing human H7 N9 influenza vaccine-induced monoclonal antibodies confer protection. Cell Host Microbe 2016;19:800–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hicks LA, Harrison LH, Flannery B, et al. . Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis 2007;196:1346–1354 [DOI] [PubMed] [Google Scholar]
- 81.Hobson D, Curry RL, Beare AS, et al. . The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972;70:767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoskins TW, Davies JR, Allchin A, et al. . Controlled trial of inactivated influenza vaccine containing the a-Hong Kong strain during an outbreak of influenza due to the a-England-42-72 strain. Lancet 1973;2:116–120 [DOI] [PubMed] [Google Scholar]
- 83.Hoskins TW, Davies JR, Smith AJ, et al. . Influenza at Christ's Hospital: March, 1974. Lancet 1976;1:105–108 [DOI] [PubMed] [Google Scholar]
- 84.Hoskins TW, Davies JR, Smith AJ, et al. . Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ's Hospital. Lancet 1979;1:33–35 [DOI] [PubMed] [Google Scholar]
- 85.Hu MC, Walls MA, Stroop SD, et al. . Immunogenicity of a 26-valent group A streptococcal vaccine. Infect Immun 2002;70:2171–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huber VC, Lynch JM, Bucher DJ, et al. . Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol 2001;166:7381–7388 [DOI] [PubMed] [Google Scholar]
- 87.Huber VC, McKeon RM, Brackin MN, et al. . Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vaccine Immunol 2006;13:981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huber VC, Peltola V, Iverson AR, et al. . Contribution of vaccine-induced immunity toward either the HA or the NA component of influenza viruses limits secondary bacterial complications. J Virol 2010;84:4105–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ichinohe T, Pang IK, Kumamoto Y, et al. . Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A 2011;108:5354–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Irani V, Guy AJ, Andrew D, et al. . Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol Immunol 2015;67:171–182 [DOI] [PubMed] [Google Scholar]
- 91.Itoh Y, Yoshida R, Shichinohe S, et al. . Protective efficacy of passive immunization with monoclonal antibodies in animal models of H5 N1 Highly pathogenic avian influenza virus infection. PLoS Pathog 2014;10:e1004192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Izikson R, Leffell DJ, Bock SA, et al. . Randomized comparison of the safety of Flublok((R)) versus licensed inactivated influenza vaccine in healthy, medically stable adults >/ = 50 years of age. Vaccine 2015;33:6622–6628 [DOI] [PubMed] [Google Scholar]
- 93.Jain S, Kamimoto L, Bramley AM, et al. . Hospitalized patients with 2009 H1 N1 influenza in the United States, April-June 2009. N Engl J Med 2009;361:1935–1944 [DOI] [PubMed] [Google Scholar]
- 94.Jain S, Self WH, Wunderink RG, et al. . Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N Engl J Med 2015;373:415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jain S, Williams DJ, Arnold SR, et al. . Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015;372:835–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jegaskanda S, Job ER, Kramski M, et al. . Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J Immunol 2013;190:1837–1848 [DOI] [PubMed] [Google Scholar]
- 97.Jegaskanda S, Reading PC, and Kent SJ. Influenza-specific antibody-dependent cellular cytotoxicity: toward a universal influenza vaccine. J Immunol 2014;193:469–475 [DOI] [PubMed] [Google Scholar]
- 98.Johansson BE, Bucher DJ, and Kilbourne ED. Purified influenza virus hemagglutinin and neuraminidase are equivalent in stimulation of antibody response but induce contrasting types of immunity to infection. J Virol 1989;63:1239–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnston BL. Methicillin-resistant Staphylococcus aureus as a cause of community-acquired pneumonia—a critical review. Semin Respir Infect 1994;9:199–206 [PubMed] [Google Scholar]
- 100.Karauzum H, Ferry T, de BS, et al. . Comparison of adhesion and virulence of two predominant hospital-acquired methicillin-resistant Staphylococcus aureus clones and clonal methicillin-susceptible S. aureus isolates. Infect Immun 2008;76:5133–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karkman A, Lehtimaki J, and Ruokolainen L. The ecology of human microbiota: dynamics and diversity in health and disease. Ann N Y Acad Sci 2017;1399:78–92 [DOI] [PubMed] [Google Scholar]
- 102.Karlsson EA, Marcelin G, Webby RJ, et al. . Review on the impact of pregnancy and obesity on influenza virus infection. Influenza Other Respi Viruses 2012;6:449–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karlstrom A, Boyd KL, English BK, et al. . Treatment with protein synthesis inhibitors improves outcomes of secondary bacterial pneumonia after influenza. J Infect Dis 2009;199:311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karlstrom A, Heston SM, Boyd KL, et al. . Toll-like receptor 2 mediates fatal immunopathology in mice during treatment of secondary pneumococcal pneumonia following influenza. J Infect Dis 2011;204:1358–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khurana S, Loving CL, Manischewitz J, et al. . Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Transl Med 2013;5:200ra114. [DOI] [PubMed] [Google Scholar]
- 106.Khurana S, Suguitan AL, Jr., Rivera Y, et al. . Antigenic fingerprinting of H5 N1 avian influenza using convalescent sera and monoclonal antibodies reveals potential vaccine and diagnostic targets. PLoS Med 2009;6:e1000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kilbourne ED, Christenson WN, and Sande M. Antibody response in man to influenza virus neuraminidase following influenza. J Virol 1968;2:761–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kilbourne ED, Laver WG, Schulman JL, et al. . Antiviral activity of antiserum specific for an influenza virus neuraminidase. J Virol 1968;2:281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kilbourne ED, Pokorny BA, Johansson B, et al. . Protection of mice with recombinant influenza virus neuraminidase. J Infect Dis 2004;189:459–461 [DOI] [PubMed] [Google Scholar]
- 110.Klausberger M, Tscheliessnig R, Neff S, et al. . Globular head-displayed conserved influenza H1 hemagglutinin stalk epitopes confer protection against heterologous H1 N1 virus. PLoS One 2016;11:e0153579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klein EY, Monteforte B, Gupta A, et al. . The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses 2016;10:394–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klonoski JM, Hurtig HR, Juber BA, et al. . Vaccination against the M protein of Streptococcus pyogenes prevents death after influenza virus:S. pyogenes super-infection. Vaccine 2014;32:5241–5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kobayashi SD, and DeLeo FR. A MRSA-terious enemy among us: boosting MRSA vaccines. Nat Med 2011;17:168–169 [DOI] [PubMed] [Google Scholar]
- 114.Kon TC, Onu A, Berbecila L, et al. . Influenza vaccine manufacturing: effect of inactivation, splitting and site of manufacturing. Comparison of influenza vaccine production processes. PLoS One 2016;11:e0150700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kondo K, Suzuki K, Washio M, et al. . Effectiveness of 23-valent pneumococcal polysaccharide vaccine and seasonal influenza vaccine for pneumonia among the elderly - Selection of controls in a case-control study. Vaccine 2017;35:4806–4810 [DOI] [PubMed] [Google Scholar]
- 116.Kotloff KL, Corretti M, Palmer K, et al. . Safety and immunogenicity of a recombinant multivalent group a streptococcal vaccine in healthy adults: phase 1 trial. JAMA 2004;292:709–715 [DOI] [PubMed] [Google Scholar]
- 117.Krammer F, Pica N, Hai R, et al. . Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol 2013;87:6542–6550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Krause JC, Tsibane T, Tumpey TM, et al. . A broadly neutralizing human monoclonal antibody that recognizes a conserved, novel epitope on the globular head of the influenza H1 N1 virus hemagglutinin. J Virol 2011;85:10905–10908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kumar A, Zarychanski R, Pinto R, et al. . Critically ill patients with 2009 influenza A(H1 N1) infection in Canada. JAMA 2009;302:1872–1879 [DOI] [PubMed] [Google Scholar]
- 120.Laennec RTH. 1923. Translation of selected passages from De l'Auscultation Mediate, p. 88–95. Williams Wood & Co., New York, NY [Google Scholar]
- 121.Laidlaw BJ, Decman V, Ali MA, et al. . Cooperativity between CD8+ T cells, non-neutralizing antibodies, and alveolar macrophages is important for heterosubtypic influenza virus immunity. PLoS Pathog 2013;9:e1003207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lamagni TL, Darenberg J, Luca-Harari B, et al. . Epidemiology of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol 2008;46:2359–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lamb RA, Zebedee SL, and Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell 1985;40:627–633 [DOI] [PubMed] [Google Scholar]
- 124.Lappin E, and Ferguson AJ. Gram-positive toxic shock syndromes. Lancet Infect Dis 2009;9:281–290 [DOI] [PubMed] [Google Scholar]
- 125.Leary T. The use of influenza vaccine in the present epidemic. Am J Public Health (N Y) 1918;8:754–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee SE, Eick A, Bloom MS, et al. . Influenza immunization and subsequent diagnoses of group A streptococcus-illnesses among U.S. Army trainees, 2002–2006. Vaccine 2008;26:3383–3386 [DOI] [PubMed] [Google Scholar]
- 127.Lee YN, Lee YT, Kim MC, et al. . Fc receptor is not required for inducing antibodies but plays a critical role in conferring protection after influenza M2 vaccination. Immunology 2014;143:300–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lepow M. Clinical trials of the Haemophilus influenzae type b capsular polysaccharide-diphtheria toxoid conjugate vaccine. Pediatr Infect Dis J 1987;6:804–807 [DOI] [PubMed] [Google Scholar]
- 129.Li R, Lim A, Phoon MC, et al. . Attenuated Bordetella pertussis protects against highly pathogenic influenza A viruses by dampening the cytokine storm. J Virol 2010;84:7105–7113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li W, Moltedo B, and Moran TM. Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of gammadelta T cells. J Virol 2012;86:12304–12312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu WC, Lin CY, Tsou YT, et al. . Cross-Reactive Neuraminidase-inhibiting antibodies elicited by immunization with recombinant neuraminidase proteins of H5 N1 and pandemic H1 N1 influenza A viruses. J Virol 2015;89:7224–7234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Loffler B, Niemann S, Ehrhardt C, et al. . Pathogenesis of Staphylococcus aureus necrotizing pneumonia: the role of PVL and an influenza coinfection. Expert Rev Anti Infect Ther 2013;11:1041–1051 [DOI] [PubMed] [Google Scholar]
- 133.Louria DB, Blumenfeld HL, Ellis JT, et al. . Studies on influenza in the pandemic of 1957–1958. II. Pulmonary complications of influenza. J Clin Invest 1959;38:213–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Loving CL, Lager KM, Vincent AL, et al. . Efficacy in pigs of inactivated and live attenuated influenza virus vaccines against infection and transmission of an emerging H3 N2 similar to the 2011–2012 H3 N2v. J Virol 2013;87:9895–9903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Luke TC, Kilbane EM, Jackson JL, et al. . Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5 N1 treatment? Ann Intern Med 2006;145:599–609 [DOI] [PubMed] [Google Scholar]
- 136.Ma JH, Yang FR, Yu H, et al. . An M2e-based synthetic peptide vaccine for influenza A virus confers heterosubtypic protection from lethal virus challenge. Virol J 2013;10:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Manini I, Domnich A, Amicizia D, et al. . Flucelvax (Optaflu) for seasonal influenza. Expert Rev Vaccines 2015;14:789–804 [DOI] [PubMed] [Google Scholar]
- 138.Manzoli L, Ioannidis JP, Flacco ME, et al. . Effectiveness and harms of seasonal and pandemic influenza vaccines in children, adults and elderly: a critical review and re-analysis of 15 meta-analyses. Hum Vaccin Immunother 2012;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marcelin G, Bland HM, Negovetich NJ, et al. . Inactivated seasonal influenza vaccines increase serum antibodies to the neuraminidase of pandemic influenza A(H1 N1) 2009 virus in an age-dependent manner. J Infect Dis 2010;202:1634–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martin JM, and Green M. Group A streptococcus. Semin Pediatr Infect Dis 2006;17:140–148 [DOI] [PubMed] [Google Scholar]
- 141.Marvin SA, Russier M, Huerta CT, et al. . Influenza virus overcomes cellular blocks to productively replicate, impacting macrophage function. J Virol 2017;91: pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McAleer JP, and Kolls JK. Contributions of the intestinal microbiome in lung immunity. Eur J Immunol 2017. [Epub ahead of print]; DOI: 10.1002/eji.201646721 [DOI] [PMC free article] [PubMed]
- 143.McCoy GW, Murray VB, and Teeter AL. The failure of a bacterial vaccine as a prophylactic against influenza. JAMA 1918;71:1997 [Google Scholar]
- 144.McCullers JA. Effect of antiviral treatment on the outcome of secondary bacterial pneumonia after influenza. J Infect Dis 2004;190:519–526 [DOI] [PubMed] [Google Scholar]
- 145.McCullers JA. Preventing and treating secondary bacterial infections with antiviral agents. Antivir Ther 2011;16:123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McCullers JA. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol 2014;12:252–262 [DOI] [PubMed] [Google Scholar]
- 147.McCullers JA, and Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis 2003;187:1000–1009 [DOI] [PubMed] [Google Scholar]
- 148.McCullers JA, and Huber VC. Correlates of vaccine protection from influenza and its complications. Hum Vaccin Immunother 2012;8:34–44 [DOI] [PubMed] [Google Scholar]
- 149.McDaniel LS, Ralph BA, McDaniel DO, et al. . Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb Pathog 1994;17:323–337 [DOI] [PubMed] [Google Scholar]
- 150.McLean WM, and Francis T. Influenza virus vaccine production. Problems 1950:157–159 [Google Scholar]
- 151.Meijer A, Rebelo-de-Andrade H, Correia V, et al. . Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors, 2012–2013. Antiviral Res 2014;110:31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Memoli MJ, Shaw PA, Han A, et al. . Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1 N1 virus healthy human challenge model. MBio 2016;7:e00417-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Metzger DW, Furuya Y, Salmon SL, et al. . Limited efficacy of antibacterial vaccination against secondary serotype 3 pneumococcal pneumonia following influenza infection. J Infect Dis 2015;212:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Metzger DW, and Sun K. Immune dysfunction and bacterial coinfections following influenza. J Immunol 2013;191:2047–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Michiels B, Govaerts F, Remmen R, et al. . A systematic review of the evidence on the effectiveness and risks of inactivated influenza vaccines in different target groups. Vaccine 2011;29:9159–9170 [DOI] [PubMed] [Google Scholar]
- 156.Mina MJ. Generalized herd effects and vaccine evaluation: impact of live influenza vaccine on off-target bacterial colonisation. J Infect 2017;74 Suppl 1:S101–S107 [DOI] [PubMed] [Google Scholar]
- 157.Mina MJ, Klugman KP, and McCullers JA. Live attenuated influenza vaccine, but not pneumococcal conjugate vaccine, protects against increased density and duration of pneumococcal carriage after influenza infection in pneumococcal colonized mice. J Infect Dis 2013;208:1281–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mina MJ, Klugman KP, Rosch JW, et al. . Live attenuated influenza virus increases pneumococcal translocation and persistence within the middle ear. J Infect Dis 2015;212:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mina MJ, McCullers JA, and Klugman KP. Live attenuated influenza vaccine enhances colonization of Streptococcus pneumoniae and Staphylococcus aureus in mice. MBio 2014;5:e01040-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Miroballi Y, Baird JS, Zackai S, et al. . Novel influenza A(H1 N1) in a pediatric healthcare facility in New York City during the first wave of the 2009 pandemic. Arch Pediatr Adolesc Med 2010;164:24–30 [DOI] [PubMed] [Google Scholar]
- 161.Missiakas D, and Schneewind O. Staphylococcus aureus vaccines: deviating from the carol. J Exp Med 2016;213:1645–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mocca CP, Brady RA, and Burns DL. Role of antibodies in protection elicited by active vaccination with genetically inactivated alpha hemolysin in a mouse model of Staphylococcus aureus skin and soft tissue infections. Clin Vaccine Immunol 2014;21:622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Monto AS, Petrie JG, Cross RT, et al. . Antibody to influenza virus neuraminidase: an independent correlate of protection. J Infect Dis 2015;212:1191–1199 [DOI] [PubMed] [Google Scholar]
- 164.Morens DM, Taubenberger JK, and Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008;198:962–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Moro PL, Winiecki S, Lewis P, et al. . Surveillance of adverse events after the first trivalent inactivated influenza vaccine produced in mammalian cell culture (Flucelvax((R))) reported to the vaccine adverse event reporting system (VAERS), United States, 2013–2015. Vaccine 2015;33:6684–6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mozdzanowska K, Feng J, and Gerhard W. Virus-neutralizing activity mediated by the Fab fragment of a hemagglutinin-specific antibody is sufficient for the resolution of influenza virus infection in SCID mice. J Virol 2003;77:8322–8328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mozdzanowska K, Maiese K, Furchner M, et al. . Treatment of influenza virus-infected SCID mice with nonneutralizing antibodies specific for the transmembrane proteins matrix 2 and neuraminidase reduces the pulmonary virus titer but fails to clear the infection. Virology 1999;254:138–146 [DOI] [PubMed] [Google Scholar]
- 168.Mullarkey CE, Bailey MJ, Golubeva DA, et al. . Broadly neutralizing hemagglutinin stalk-specific antibodies induce potent phagocytosis of immune complexes by neutrophils in an fc-dependent manner. MBio 2016;7:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Muller MP, Low DE, Green KA, et al. . Clinical and epidemiologic features of group a streptococcal pneumonia in Ontario, Canada. Arch Intern Med 2003;163:467–472 [DOI] [PubMed] [Google Scholar]
- 170.Murphy BR, Kasel JA, and Chanock RM. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N Engl J Med 1972;286:1329–1332 [DOI] [PubMed] [Google Scholar]
- 171.Nakamura S, Davis KM, and Weiser JN. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest 2011;121:3657–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Neirynck S, Deroo T, Saelens X, et al. . A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med 1999;5:1157–1163 [DOI] [PubMed] [Google Scholar]
- 173.Nelson JC, Jackson M, Yu O, et al. . Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine 2008;26:4947–4954 [DOI] [PubMed] [Google Scholar]
- 174.Nimmerjahn F, and Ravetch JV. The antiinflammatory activity of IgG: the intravenous IgG paradox. J Exp Med 2007;204:11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Oh JZ, Ravindran R, Chassaing B, et al. . TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity 2014;41:478–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ohmit SE, Petrie JG, Cross RT, et al. . Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J Infect Dis 2011;204:1879–1885 [DOI] [PubMed] [Google Scholar]
- 177.Okamoto S, Kawabata S, Fujitaka H, et al. . Vaccination with formalin-inactivated influenza vaccine protects mice against lethal influenza Streptococcus pyogenes superinfection. Vaccine 2004;22:2887–2893 [DOI] [PubMed] [Google Scholar]
- 178.Okamoto S, Matsuoka S, Takenaka N, et al. . Intranasal immunization with a formalin-inactivated human influenza A virus whole-virion vaccine alone and intranasal immunization with a split-virion vaccine with mucosal adjuvants show similar levels of cross-protection. Clin Vaccine Immunol 2012;19:979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Olsen RJ, Ashraf M, Gonulal VE, et al. . Lower respiratory tract infection in cynomolgus macaques (Macaca fascicularis) infected with group A Streptococcus. Microb Pathog 2010;49:336–347 [DOI] [PubMed] [Google Scholar]
- 180.Osterholm MT, Kelley NS, Sommer A, et al. . Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012;12:36–44 [DOI] [PubMed] [Google Scholar]
- 181.Palacios G, Hornig M, Cisterna D, et al. . Streptococcus pneumoniae coinfection is correlated with the severity of H1 N1 pandemic influenza. PLoS One 2009;4:e8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Palavecino E. Community-acquired methicillin-resistant Staphylococcus aureus infections. Clin Lab Med 2004;24:403–418 [DOI] [PubMed] [Google Scholar]
- 183.Pavlova S, D'Alessio F, Houard S, et al. . Workshop report: immunoassay standardisation for “universal” influenza vaccines. Influenza Other Respir Viruses 2017;11:194–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Peacock JE, Jr., Moorman DR, Wenzel RP, et al. . Methicillin-resistant Staphylococcus aureus: microbiologic characteristics, antimicrobial susceptibilities, and assessment of virulence of an epidemic strain. J Infect Dis 1981;144:575–582 [DOI] [PubMed] [Google Scholar]
- 185.Peltola H, Kayhty H, Sivonen A, et al. . Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics 1977;60:730–737 [PubMed] [Google Scholar]
- 186.Peltola VT, Boyd KL, McAuley JL, et al. . Bacterial sinusitis and otitis media following influenza virus infection in ferrets. Infect Immun 2006;74:2562–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Peltola VT, Murti KG, and McCullers JA. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J Infect Dis 2005;192:249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pendzialek J, Roose K, Smet A, et al. . Bispecific T cell engaging antibody constructs targeting a universally conserved part of the viral M2 ectodomain cure and prevent influenza A virus infection. Antiviral Res 2017;141:155–164 [DOI] [PubMed] [Google Scholar]
- 189.Pfleiderer M, Trouvin JH, Brasseur D, et al. . Summary of knowledge gaps related to quality and efficacy of current influenza vaccines. Vaccine 2014;32:4586–4591 [DOI] [PubMed] [Google Scholar]
- 190.Pichichero ME. Ten-Year Study of Acute Otitis Media in Rochester, NY. Pediatr Infect Dis J 2016;35:1027–1032 [DOI] [PubMed] [Google Scholar]
- 191.Pichichero ME, Khan MN, and Xu Q. Next generation protein based Streptococcus pneumoniae vaccines. Hum Vaccin Immunother 2016;12:194–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Planet PJ, Parker D, Cohen TS, et al. . Lambda interferon restructures the nasal microbiome and increases susceptibility to Staphylococcus aureus superinfection. MBio 2016;7:e01939-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Plested JS, and Granoff DM. Vaccine-induced opsonophagocytic immunity to Neisseria meningitidis group B. Clin Vaccine Immunol 2008;15:799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Principi N, and Esposito S. Prevention of community-acquired pneumonia with available pneumococcal vaccines. Int J Mol Sci 2016;18:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Qin N, Zheng B, Yao J, et al. . Influence of H7 N9 virus infection and associated treatment on human gut microbiota. Sci Rep 2015;5:14771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rajao DS, Loving CL, Gauger PC, et al. . Influenza A virus hemagglutinin protein subunit vaccine elicits vaccine-associated enhanced respiratory disease in pigs. Vaccine 2014;32:5170–5176 [DOI] [PubMed] [Google Scholar]
- 197.Rajendran M, Nachbagauer R, Ermler ME, et al. . Analysis of anti-influenza virus neuraminidase antibodies in children, adults, and the elderly by ELISA and enzyme inhibition: evidence for original antigenic sin. MBio 2017;8:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rasigade PP, Laurent F, Hubert P, et al. . Lethal necrotizing pneumonia caused by an ST398 Staphylococcus aureus strain. Emerg Infect Dis 2010;16:1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Reed C, Chaves SS, Daily KP, et al. . Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One 2015;10:e0118369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rinaldi C, Penhale WJ, Stumbles PA, et al. . Modulation of innate immune responses by influenza-specific ovine polyclonal antibodies used for prophylaxis. PLoS One 2014;9:e89674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Robinson KM, Kolls JK, and Alcorn JF. The immunology of influenza virus-associated bacterial pneumonia. Curr Opin Immunol 2015;34:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rothberg MB, Haessler SD, and Brown RB. Complications of viral influenza. Am J Med 2008;121:258–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rudolph K, Bruce MG, Bruden D, et al. . Epidemiology of Invasive Group A Streptococcal Disease in Alaska, 2001 to 2013. J Clin Microbiol 2016;54:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rynda-Apple A, Robinson KM, and Alcorn JF. Influenza and bacterial superinfection: illuminating the immunologic mechanisms of disease. Infect Immun 2015;83:3764–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Salk HM, Simon WL, Lambert ND, et al. . Taxa of the nasal microbiome are associated with influenza-specific IgA response to live attenuated influenza vaccine. PLoS One 2016;11:e0162803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sandbulte MR, Jimenez GS, Boon AC, et al. . Cross-reactive neuraminidase antibodies afford partial protection against H5 N1 in mice and are present in unexposed humans. PLoS Med 2007;4:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sasaki S, Sullivan M, Narvaez CF, et al. . Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest 2011;121:3109–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Schliehe C, Flynn EK, Vilagos B, et al. . The methyltransferase Setdb2 mediates virus-induced susceptibility to bacterial superinfection. Nat Immunol 2015;16:67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Schmitt HJ, von KR, Hassenpflug B, et al. . Haemophilus influenzae type b disease: impact and effectiveness of diphtheria-tetanus toxoids-acellular pertussis (-inactivated poliovirus)/H. influenzae type b combination vaccines. Pediatr Infect Dis J 2001;20:767–774 [DOI] [PubMed] [Google Scholar]
- 210.Schulman JL, Khakpour M, and Kilbourne ED. Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J Virol 1968;2:778–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shafinoori S, Ginocchio CC, Greenberg AJ, et al. . Impact of pneumococcal conjugate vaccine and the severity of winter influenza-like illnesses on invasive pneumococcal infections in children and adults. Pediatr Infect Dis J 2005;24:10–16 [DOI] [PubMed] [Google Scholar]
- 212.Shahangian A, Chow EK, Tian X, et al. . Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest 2009;119:1910–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Shepardson KM, Larson K, Morton RV, et al. . Differential type I interferon signaling is a master regulator of susceptibility to postinfluenza bacterial superinfection. MBio 2016;7:e00506-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Shieh WJ, Blau DM, Denison AM, et al. . 2009 pandemic influenza A (H1 N1): pathology and pathogenesis of 100 fatal cases in the United States. Am J Pathol 2010;177:166–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Short KR, Habets MN, Hermans PW, et al. . Interactions between Streptococcus pneumoniae and influenza virus: a mutually beneficial relationship? Future Microbiol 2012;7:609–624 [DOI] [PubMed] [Google Scholar]
- 216.Shriver Z, Trevejo JM, and Sasisekharan R. Antibody-based strategies to prevent and treat influenza. Front Immunol 2015;6:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Skowronski DM, Chambers C, De Serres G, et al. . Serial vaccination and the antigenic distance hypothesis: effects on influenza vaccine effectiveness during A(H3 N2) epidemics in Canada, 2010–2011 to 2014–2015. J Infect Dis 2017;215:1059–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Skowronski DM, Janjua NZ, De SG, et al. . Low 2012–2013 influenza vaccine effectiveness associated with mutation in the egg-adapted H3 N2 vaccine strain not antigenic drift in circulating viruses. PLoS One 2014;9:e92153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Skowronski DM, Sabaiduc S, Chambers C, et al. . Mutations acquired during cell culture isolation may affect antigenic characterisation of influenza A(H3 N2) clade 3C.2a viruses. Euro Surveill 2016;21:30112. [DOI] [PubMed] [Google Scholar]
- 220.Small CL, Shaler CR, McCormick S, et al. . Influenza infection leads to increased susceptibility to subsequent bacterial superinfection by impairing NK cell responses in the lung. J Immunol 2010;184:2048–2056 [DOI] [PubMed] [Google Scholar]
- 221.Smart LE, Dougall AJ, and Girdwood RW. New 23-valent pneumococcal vaccine in relation to pneumococcal serotypes in systemic and non-systemic disease. J Infect 1987;14:209–215 [DOI] [PubMed] [Google Scholar]
- 222.Smith AM. Quantifying the therapeutic requirements and potential for combination therapy to prevent bacterial coinfection during influenza. J Pharmacokinet Pharmacodyn 2017;44:81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Smith AM, Adler FR, Ribeiro RM, et al. . Kinetics of coinfection with influenza A virus and Streptococcus pneumoniae. PLoS Pathog 2013;9:e1003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Smith AM, and McCullers JA. Secondary bacterial infections in influenza virus infection pathogenesis. Curr Top Microbiol Immunol 2014;385:327–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Smith AM, and Smith AP. A critical, nonlinear threshold dictates bacterial invasion and initial kinetics during influenza. Sci Rep 2016;6:38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Smith DJ, Forrest S, Ackley DH, et al. . Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci U S A 1999;96:14001–14006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Smith DJ, Lapedes AS, De Jong JC, et al. . Mapping the antigenic and genetic evolution of influenza virus. Science 2004;305:371–376 [DOI] [PubMed] [Google Scholar]
- 228.Sondermann P, and Szymkowski DE. Harnessing Fc receptor biology in the design of therapeutic antibodies. Curr Opin Immunol 2016;40:78–87 [DOI] [PubMed] [Google Scholar]
- 229.Souza CK, Rajao DS, Loving CL, et al. . Age at vaccination and timing of infection do not alter vaccine-associated enhanced respiratory disease in influenza a virus-infected pigs. Clin Vaccine Immunol 2016;23:470–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sparrow E, Friede M, Sheikh M, et al. . Passive immunization for influenza through antibody therapies, a review of the pipeline, challenges and potential applications. Vaccine 2016;34:5442–5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Stanekova Z, and Vareckova E. Conserved epitopes of influenza A virus inducing protective immunity and their prospects for universal vaccine development. Virol J 2010;7:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Steed AL, Christophi GP, Kaiko GE, et al. . The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 2017;357:498–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Steel J, Lowen AC, Wang T, et al. . Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio 2010;1:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Steer AC, Carapetis JR, Dale JB, et al. . Status of research and development of vaccines for Streptococcus pyogenes. Vaccine 2016;34:2953–2958 [DOI] [PubMed] [Google Scholar]
- 235.Sun K, and Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med 2008;14:558–564 [DOI] [PubMed] [Google Scholar]
- 236.Sun K, and Metzger DW. Influenza infection suppresses NADPH oxidase-dependent phagocytic bacterial clearance and enhances susceptibility to secondary methicillin-resistant Staphylococcus aureus infection. J Immunol 2014;192:3301–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tamura M, Webster RG, and Ennis FA. Antibodies to HA and NA augment uptake of influenza A viruses into cells via Fc receptor entry. Virology 1991;182:211–219 [DOI] [PubMed] [Google Scholar]
- 238.Tamura M, Webster RG, and Ennis FA. Subtype cross-reactive, infection-enhancing antibody responses to influenza A viruses. J Virol 1994;68:3499–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tan GS, Leon PE, Albrecht RA, et al. . Broadly-reactive neutralizing and non-neutralizing antibodies directed against the H7 influenza virus hemagglutinin reveal divergent mechanisms of protection. PLoS Pathog 2016;12:e1005578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tanz RR, and Shulman ST. Chronic pharyngeal carriage of group A streptococci. Pediatr Infect Dis J 2007;26:175–176 [DOI] [PubMed] [Google Scholar]
- 241.Tarabichi Y, Li K, Hu S, et al. . The administration of intranasal live attenuated influenza vaccine induces changes in the nasal microbiota and nasal epithelium gene expression profiles. Microbiome 2015;3:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Taylor AR, Sharp DG, McLean IW Jr., et al. . Concentration and purification of influenza virus for the preparation of vaccines. J Immunol 1945;50:291–316 [Google Scholar]
- 243.Thors V, Christensen H, Morales-Aza B, et al. . the effects of live attenuated influenza vaccine on nasopharyngeal bacteria in healthy 2 to 4 year olds. A randomized controlled trial. Am J Respir Crit Care Med 2016;193:1401–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Truelove SA, Chitnis AS, Heffernan RT, et al. . Comparison of patients hospitalized with pandemic 2009 influenza A (H1 N1) virus infection during the first two pandemic waves in Wisconsin. J Infect Dis 2011;203:828–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Van den Hoecke S, Ehrhardt K, Kolpe A, et al. . Hierarchical and redundant roles of activating FcgammaRs in protection against influenza disease by M2e-specific IgG1 and IgG2a antibodies. J Virol 2017;91:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Vanderven HA, Ana-Sosa-Batiz F, Jegaskanda S, et al. . What lies beneath: antibody dependent natural killer cell activation by antibodies to internal influenza virus proteins. EBioMedicine 2016;8:277–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.von Sholly AI, and Park WH., VII Report on the prophylactic vaccination of 1536 persons against acute respiratory diseases, 1919–1920. J Immunol 1921;6:103–115 [Google Scholar]
- 248.Wald ER, Dashefsky B, Feidt C, et al. . Acute rheumatic fever in western Pennsylvania and the tristate area. Pediatrics 1987;80:371–374 [PubMed] [Google Scholar]
- 249.Wang B, Russell ML, Brewer A, et al. . Single radial haemolysis compared to haemagglutinin inhibition and microneutralization as a correlate of protection against influenza A H3 N2 in children and adolescents. Influenza Other Respir Viruses 2017;11:283–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang R, Song A, Levin J, et al. . Therapeutic potential of a fully human monoclonal antibody against influenza A virus M2 protein. Antiviral Res 2008;80:168–177 [DOI] [PubMed] [Google Scholar]
- 251.Wang TT, Tan GS, Hai R, et al. . Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci U S A 2010;107:18979–18984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang TT, Tan GS, Hai R, et al. . Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog 2010;6:e1000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Weinberger DM, Simonsen L, Jordan R, et al. . Impact of the 2009 influenza pandemic on pneumococcal pneumonia hospitalizations in the United States. J Infect Dis 2012;205:458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Williams DJ, Hall M, Brogan TV, et al. . Influenza coinfection and outcomes in children with complicated pneumonia. Arch Pediatr Adolesc Med 2011;165:506–512 [DOI] [PubMed] [Google Scholar]
- 255.Wolf AI, Strauman MC, Mozdzanowska K, et al. . Coinfection with Streptococcus pneumoniae modulates the B cell response to influenza virus. J Virol 2014;88:11995–12005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wong SS, and Webby RJ. Traditional and new influenza vaccines. Clin Microbiol Rev 2013;26:476–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wright PF, Hoen AG, Ilyushina NA, et al. . Correlates of immunity to influenza as determined by challenge of children with live, attenuated influenza vaccine. Open Forum Infect Dis 2016;3:ofw108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wu Y, Tu W, Lam KT, et al. . Lethal coinfection of influenza virus and Streptococcus pneumoniae lowers antibody response to influenza virus in lung and reduces numbers of germinal center B cells, T follicular helper cells, and plasma cells in mediastinal lymph Node. J Virol 2015;89:2013–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wuerth BA, Bonnewell JP, Wiemken TL, et al. . Trends in pneumonia mortality rates and hospitalizations by Organism, United States, 2002–2011(1). Emerg Infect Dis 2016;22:1624–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yeaman MR, Filler SG, Schmidt CS, et al. . Applying convergent immunity to innovative vaccines targeting Staphylococcus aureus. Front Immunol 2014;5:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Young MH, Aronoff DM, and Engleberg NC. Necrotizing fasciitis: pathogenesis and treatment. Expert Rev Anti Infect Ther 2005;3:279–294 [DOI] [PubMed] [Google Scholar]
- 262.Yu KO, Randolph AG, Agan AA, et al. . Staphylococcus aureus alpha-toxin response distinguishes respiratory virus-methicillin-resistant S. aureus coinfection in children. J Infect Dis 2016;214:1638–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zebedee SL, Richardson CD, and Lamb RA. Characterization of the influenza virus M2 integral membrane protein and expression at the infected-cell surface from cloned cDNA. J Virol 1985;56:502–511 [DOI] [PMC free article] [PubMed] [Google Scholar]