Abstract
Despite 253,000 spinal cord injury (SCI) patients in the United States, little is known about how SCI affects brain networks. Spinal MRI provides only structural information with no insight into functional connectivity. Resting-state functional MRI (RS-fMRI) quantifies network connectivity through the identification of resting-state networks (RSNs) and allows detection of functionally relevant changes during disease. Given the robust network of spinal cord afferents to the brain, we hypothesized that SCI produces meaningful changes in brain RSNs. RS-fMRIs and functional assessments were performed on 10 SCI subjects. Blood oxygen-dependent RS-fMRI sequences were acquired. Seed-based correlation mapping was performed using five RSNs: default-mode (DMN), dorsal-attention (DAN), salience (SAL), control (CON), and somatomotor (SMN). RSNs were compared with normal control subjects using false-discovery rate-corrected two way t tests. SCI reduced brain network connectivity within the SAL, SMN, and DMN and disrupted anti-correlated connectivity between CON and SMN. When divided into separate cohorts, complete but not incomplete SCI disrupted connectivity within SAL, DAN, SMN and DMN and between CON and SMN. Finally, connectivity changed over time after SCI: the primary motor cortex decreased connectivity with the primary somatosensory cortex, the visual cortex decreased connectivity with the primary motor cortex, and the visual cortex decreased connectivity with the sensory parietal cortex. These unique findings demonstrate the functional network plasticity that occurs in the brain as a result of injury to the spinal cord. Connectivity changes after SCI may serve as biomarkers to predict functional recovery following an SCI and guide future therapy.
Keywords: : biomarkers, MRI, neuroplasticity, SCI
Introduction
Despite the fact that 253,000 people in the United States are living with spinal cord injury (SCI) and that $10 billion is spent annually on SCI research and medical care,1–3 there remains a significant void in meaningful interventions, and no reliable method to define brain reorganization and potential for functional recovery after SCI. There is a substantial need for accurate noninvasive imaging techniques to study how SCI affects the nervous system and help predict recovery potential.
The American Spinal Injury Association (ASIA) International Standards for the Neurological Classification of Spinal Cord Injury is the standard instrument for assessing neurological deficits after SCI.4 It differentiates between complete SCI, in which there is no motor or sensory function below the injury, and incomplete SCI, in which function persists below the injury. This measure provides a useful and objective parameter to help manage patients with an SCI, but is not sufficiently prognostic nor does it provide information on functional architecture of the brain. Standard MRI sequences of the spine provide anatomic images in acute or chronic SCI. There is typically no substantial structural abnormality of the brain after SCI, and, therefore, standard anatomic imaging offers little insight into how the spinal insult has altered brain organization.
Resting-state functional MRI (RS-fMRI) is a robust method that can define the manner in which the brain's organization is altered by SCI. This approach uses the endogenous brain activity detectable with blood oxygen level dependent (BOLD) MRI to identify areas that are interacting at rest. Spontaneous BOLD fluctuations are low-frequency oscillations that are temporally correlated within distinct functional networks.5 Resting-state correlation maps are similar to the functional maps obtained from task activations.6 Alteration of this correlation structure can define functionally relevant changes after injury to the central nervous system.7,8 This approach can also provide insights into the impact that SCI has on the brain the as a result of the abrupt loss of afferent and efferent neural input. Quantifying these changes may further enable an improved understanding of the nature of the spinal injury and predict functional recovery.
Although recent advances in spinal diffusion tensor imaging and diffusion basis spectrum imaging have shown promise as biomarkers of white matter integrity following an SCI,9–16 additional methods are needed. Although most attention and effort have focused on direct imaging of the spinal cord, there has been limited work on the effects of SCI on the brain.17–23 A large network of circuits makes up ascending spinal cord tracts to the brain and includes the dorsal column, and the spinothalamic, spinocerebellar, cuneocerebellar, and spinoreticular tracts.24 Select reports have suggested that SCI affects the brain and brain networks.17,19,21,25–29 For example, SCI has been associated with atrophy of cerebral white matter tracts, and the atrophy has been shown to correlate with performance.29
Here, we show that SCI produced significant changes in select brain RS-fMRI networks. Further, we found that complete SCI disrupts connectivity more than incomplete SCI. Finally, we show that functional connectivity between multiple brain networks changes over time after an SCI. These findings help explain physiological changes in the brain after an SCI. Further, measurement of brain network connectivity may serve as a future method to predict functional recovery in SCI patients and, thereby, guide subsequent therapy.
Methods
Participants
After obtaining approval from the Washington University Human Research Protection Organization Institutional Review Board, 10 subjects were enrolled and informed consent was obtained. Recruitment was performed by A.H.H. and W.Z.R. This study played no role in the medical care of SCI subjects. To be included in this study, participants were required to (1) have a history ASIA A, B, C or D SCI prior to enrollment; (2) be able to participate in physical examinations and surveys; (3) speak English; (4) be medically stable; and (5) tolerate an unsedated MRI. Exclusion criteria were: being ≤18 or >80 years old; pregnancy; having an MRI-incompatible device (e.g., a pacemaker); having a known diagnosis of amyotrophic lateral sclerosis, multiple sclerosis, rheumatoid arthritis, spine tumor, brain tumor, encephalopathy, traumatic brain injury, psychiatric disease, dementia, meningitis, stroke, transient ischemic attacks, neurodegenerative disorder, epilepsy, previous incident of SCI, or HIV-related myelopathy; having a history of abnormal brain MRI; and having a systemic instability or being deemed unstable to tolerate standard unsedated MRI sequencing, abnormal orientation, and cranial nerve physical examination. Control MRI data from 37 normal controls subjects (average 42.6 years, range 23–65) without any significant medical history were collected. The majority of control participants were males (20). No experimental or control subjects were diagnosed with any psychiatric condition, and none were taking chronic opioids, muscle relaxants, or other analgesics. Education level was not recorded.
General methodology
Once identified and enrolled in the study, medical records from the subject's initial SCI and recovery were reviewed. Patients with an SCI were assessed upon enrollment and every 4–6 months as tolerated. During each visit, validated clinical scores and outcomes were collected, and patients received a resting state fMRI of the brain. Seven subjects received one MRI and three subjects received two serial MRIs. Behavioral outcome measures included International Standards for the Neurological Classification of Spinal Cord Injury including data from a complete motor and sensory physical examination4,30 and Manual Motor Testing.31 RS-fMRI was obtained at the Mallinckrodt Institute of Radiology at Washington University School of Medicine.
Clinical examination and measures
Subjects underwent a detailed clinical examination by a neurological surgery provider intended to provide a comprehensive evaluation of neurological function. Examination of orientation and cranial nerves were performed to exclude significant and potentially confounding cerebral pathology. International Standards for the Neurological Classification of Spinal Cord Injury including data from a complete motor and sensory physical examination4,30 and manual motor testing31 are well-established and commonly used methods to grade neurological status after SCI.
Acquisition and preprocessing of the RS-fMRI
Imaging was acquired using a 3.0 Tesla Siemens Trio scanner (Erlagen, Germany) with a standard 32 channel head coil. A high-resolution T1-weighted magnetization-prepared rapid gradient echo was used for atlas registration. T2-weighted fast spin echo images were used for RS-fMRI atlas registration. T2* echo-planar imaging sequence responsive to BOLD contrast (flip angle 90 degrees, 4 × 4 × 4 mm voxels, echo time (TE) 27 ms, repetition time (TR) 2200 ms, bandwidth 2441 Hz) covered the entire brain while the subjects were asked to fixate on a visual cross-hair and remain awake. Three runs of 164 frames totaling 18 min were completed. Preprocessing, registration, and quality assurance measures was performed as previously described.32–34 Briefly, preprocessing included correction for asynchronous slice acquisition by compensation for slice-dependent time shifts, correction of even–odd interleaving acquisition, image debanding, intensity normalization to mode 1000, spatial smoothing with a 6 mm full-width half-maximum Gaussian kernel, and low-pass (< 0.1 Hz) filtering. Frame scrubbing was performed based on the temporal derivative of root-mean-square variance over voxels (DVARS) >0.5%.35 Nuisance regression was performed to remove signals related to 12 parameter rigid body head motion, bilateral white matter, bilateral cerebrospinal fluid, and global signal. Images were aligned by rigid body transformation to a standardized atlas space and re-sampled in 3 mm isotropic cubic voxels for further analysis.
Seed-based correlation mapping and connectivity measurements
Seed-based correlation mapping is one of the most widely adopted techniques for studying co-fluctuations in intrinsic neuronal activity, or functional connectivity.36,37 Seed-based analysis requires prior knowledge of the locations of regions of interest (ROIs). Thirty-six ROIs were derived by maximizing topographic concordance between results obtained by seed-based correlation mapping and spatial independent component analysis as described.38–40 Resting-state networks (RSNs) and ROI coordinates were selected as described in Brier and colleagues, except for seeds 26–29.32 Seeds 26 and 27 were left and right insula as previously described.32 Seeds 28 and 29 were left and right parietal opercula, respectively. Somatomotor, language, and visual RSNs were combined into a unified sensory-motor network (SMN). Five RSNs included default mode network (DMN), dorsal attention network (DAN), salience network (SAL), control network (CON), and unified somatomotor network (SMN, Fig. 1). Correlation maps were generated by extracting time courses from each of the 36 seed regions. Pearson product-moment correlation (R) is the most widely used method to measure functional connectivity within and between networks.5,36,41–44 Pearson correlations were calculated between the time course from seed ROIs and that from all other ROIs. Pearson correlations were converted to normally distributed variables, z, with application of a Fisher's z transform. Functional connectivity maps were generated for complete SCI subjects by averaging the correlation maps between subjects. Average internal and cross connectivity of the DMN, DAN, CON, SAL, and SMN RSNs were calculated for each subject as detailed by Brier and colleagues.32 The average change in correlation over time was calculated for each ROI. This was performed by subtracting the z-transformed correlation of the 2 month MRI from the ≥6 month MRI for each subject. Then change in connectivity was averaged across the three subjects.
FIG. 1.
Regions of interest (ROIs). Thirty-six ROIs were selected as seeds (red) for five resting-state networks (RSNs): default mode network (DMN), dorsal attention network (DAN), control network (CON), salience network (SAL), and unified somatomotor network (SMN).
Experimental design and statistical analysis
Data are reported as mean ± standard deviation. Figures are shown as notched box-and-whisker plots with each outlier marked “+.” Notched box plots are centered around a median, while notch indicates 95% confidence interval of the median. The box indicates the interquartile range (IQR), whiskers indicate 1.5 times the IQR. To assess for statistical differences, two sample t tests were performed. P values, after false-discovery rate multiple comparison correction' degrees of freedom; and effect-size statistics are reported in the manuscript. Statistical values with p < 0.05 indicate statistical significance.
Results
Demographics
Ten SCI subjects participated in the study (Table 1). Nine subjects were male. The average age at MRI was 46.9 ± 16.1 years and the average time between injury and study was 7.9 ± 13.3 years. There were four complete SCI patients (ASIA A) and six incomplete SCI patients (ASIA C or D). Injury levels ranged from C4 to T5. There was no difference of age for complete (38.5 ± 11.7 years) versus incomplete (52.5 ± 17.0) SCI groups (p = 0.2, t[8[ = −1.4, unpaired t test). Time from injury was also similar between complete (7.3 ± 7.3 years) and incomplete (8.3 ± 17.0) SCI groups (p = 0.9, t[8] = −0.1).
Table 1.
Demographic Data
| Age at injury (years) | Level of injury | ASIA gradea | Sex |
|---|---|---|---|
| 44 | C4 | A | M |
| 32 | C7 | A | F |
| 52 | C7 | A | M |
| 26 | T5 | A | M |
| 57 | C6 | D | M |
| 60 | C4 | D | M |
| 53 | C4 | D | M |
| 52 | C7 | C | M |
| 72 | C4 | D | M |
| 21 | C4 | D | M |
American Spinal Injury Association Grade at time of MRI.
SCI disrupts functional connectivity within and between RSNs
Connectivity between the 36 ROIs was assessed within and between DMN, DAN, CON, SAL, and SMN networks in all SCI subjects and compared with controls (Fig. 2A). Connectivity within the DMN was lower after SCI (0.23 ± 0.10) when compared with controls (0.33 ± 0.10; p = 0.04, t[45] = 2.85; Fig. 2B). DAN and CON networks were not significantly disrupted (Fig. 2C,D). Connectivity within the SAL network was reduced after SCI (0.31 ± 0.14) when compared with controls (0.46 ± 0.11; p = 0.02, t[45] = 3.93; Fig. 2E). Connectivity within the unified SMN network was reduced after SCI (0.28 ± 0.15) when compared with controls (0.41 ± 0.13; p = 0.04, t[45] = 2.76; Fig. 2F). Hence, when assessed as one group, SCI disrupts connectivity within SAL, SMN and DMN networks.
FIG. 2.
Spinal cord injury (SCI) disrupts functional connectivity within resting-state networks (RSNs). (A) Connectivity matrixes showing correlations within and between regions of interest (ROIs) clustered into five RSNs after SCI. Exemplar SCI subject (SCI, left) is compared with an average of 37 normal subjects (CONT, right). Intra-network correlations in SCI subjects are compared with those of controls for (B) default mode network (DMN), (C) dorsal attention network (DAN), (D) control network (CON), (E) salience network (SAL), and (F) somatomotor network (SMN). n = 10 SCI subjects and 37 controls (CONT). Data are shown as notched box plots where the red line indicated median, notch indicates 95% confidence interval of the median, box indicates interquartile range (IQR), whiskers indicate 1.5 times IQR, and crosses indicate outliers. Asterisk indicates different from CONT, p < 0.05, two sample t test.
Connectivity among DMN, DAN, CON, SAL, and SMN networks were assessed after SCI and compared to controls (Fig. 3). The normal anticorrelation between the CON network and the unified SMN network was disrupted after SCI (−0.16 ± 0.11) when compared with controls (−0.25 ± 0.08; p = 0.04, t[45] = −2.68; Fig. 3, bottom middle). Connectivity between other networks was not different when comparing all SCI subjects to controls. Hence, when assessed as one group, SCI disrupts the normally anticorrelated connectivity between the CON and SMN networks.
FIG. 3.
Spinal cord injury (SCI) disrupts functional connectivity between resting-state networks (RSNs). Inter-network correlations in SCI subjects are compared with those in controls among the five RSNs: default mode network (DMN), dorsal attention network (DAN), control network (CON), salience network (SAL), and somatomotor network (SMN). n = 10 SCI subjects and 37 controls (CONT). Asterisk indicates different from CONT, p < 0.05, two sample t test.
RSN connectivity after complete and incomplete SCI
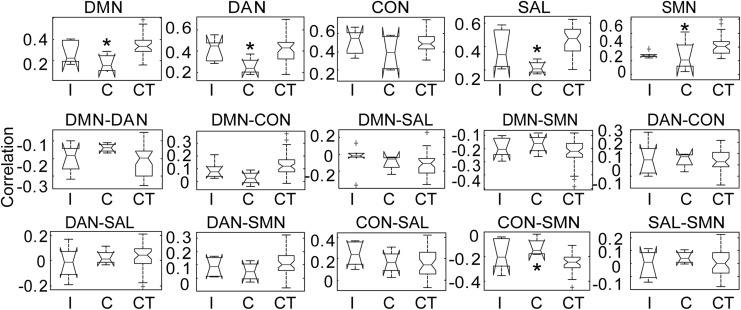
To evaluate for functionally relevant differences in network adaptation after SCI, seed-based RSN connectivity was stratified into two cohorts: complete SCI (ASIA A) and incomplete SCI (ASIA C–D). Connectivity within and among DMN, DAN, CON, SAL, and SMN networks for complete and incomplete SCI subjects was compared with controls (Fig. 4). Connectivity within and among several networks was affected by complete but not incomplete SCI when compared with controls. Connectivity within DMN was disrupted after complete SCI (0.15 ± 0.05; p = 0.006, t[39] = 3.63; Fig. 4) but not incomplete SCI (0.27 ± 0.14; p = 0.45 t[41] = 1.49) when compared with controls (0.33 ± 0.10). Connectivity within DAN was disrupted after complete SCI (0.27 ± 0.1; p = 0.04, t[39] = 2.55) but not incomplete SCI (0.42 ± 0.10; p = 0.97, t[41] = 0.04) when compared with controls (0.42 ± 0.11). CON network connectivity was not disrupted after complete nor incomplete SCI. Connectivity within the SAL network was markedly reduced after complete (0.22 ± 0.06; p = 0.003, t[39] = 4.1, Figs. 4 and 5) but not incomplete SCI (0.37 ± 0.16; p = 0.44, t[41] = 1.61) when compared with controls (0.46 ± 0.11). Finally, connectivity within the unified SMN network was similarly reduced after complete (0.23 ± 0.17; p = 0.04, t[39] = 2.55; Figs. 4 and 5) but not incomplete (0.28 ± 0.04; p = 0.37, t[41] = 2.34) SCI when compared with controls (0.41 ± 0.13). Internetwork connectivity between CON and SMN was increased after complete (−0.12 ± 0.07; p = 0.02, t[39] = −2.99) but not incomplete (−0.19 ± 0.13; p = 0.45, t[41] = −1.47) SCI when compared with controls (−0.25 ± 0.08). Connectivity between other networks was not significantly altered for either incomplete or complete SCI. Stratifying SCI based on complete or incomplete injury revealed that brain RS-fMRI metrics distinguishes between complete and incomplete SCI.
FIG. 4.
Resting-state network (RSN) correlations after incomplete (I, n = 6) and complete (C, n = 4) spinal cord injuries (SCI) were compared with those in controls (CT, n = 37). Connectivity within and among the default mode network (DMN), dorsal attention network (DAN), control network (CON), salience network (SAL), and somatomotor network (SMN) RSNs were measured. Asterisk indicates different from CT, p < 0.05, two sample t test.
FIG. 5.
Exemplar functional connectivity after complete spinal cord injury (SCI). Average functional connectivity maps are shown for select exemplar seed regions in ASIA (ASIA) A complete SCI subjects (n = 4). Left prefrontal cortex seed region showed robust connectivity with the anterior cingulate cortex and frontal lobes (top). The left insula seed was correlated with the ipsilateral and contralateral insula (middle). The left primary motor cortex correlated with the bilateral somatomotor regions (bottom).
Cerebral network connectivity changes over time after SCI
Serial RS-fMRIs were performed in incomplete SCI patients to evaluate for changes in brain network connectivity over time. Average change in connectivity between 2 months and >6 months after injury was assessed by subtracting the average of the correlation maps at two time points. Each seed displayed varying patterns of change over time, but three seeds displayed the most robust changes that reproduced bilaterally. Primary motor cortex became less correlated with primary sensory cortex over time (Fig. 6, top). The cerebellar cortex, part of the DMN, showed increased connectivity (or rather a loss of anticonnectivity) to both the primary motor and sensory cortices over time after SCI (Fig. 6, middle). The primary visual cortex became less connected with primary motor cortex, but more connected with posterior cingulate gyrus and parietal operculum over time after SCI (Fig. 6, bottom). Hence, the cerebral network connectivity reorganizes over time after an SCI.
FIG. 6.
Functional brain reorganization over time after spinal cord injury (SCI). Average change in functional connectivity between 2 months post-injury and >6 months post-injury is shown as heat maps for exemplar seed regions (n = 3 subjects). Average change in connectivity was assessed by subtracting the average of the correlation maps at two time points. The left primary motor cortex showed a robust decline in functional connectivity with the primary somatosensory cortex over time after SCI (top). The default mode network (DMN) seed, left cerebellum (Cereb) showed increased functional connectivity over time with the supplementary motor cortex, primary motor cortex, and somatosensory cortex after SCI (middle). The left visual cortex showed decreased connectivity with the primary motor cortex, but increased connectivity with the posterior cingulate gyrus and the parietal operculum (bottom).
Discussion
The research presented here has both interesting scientific and translational ramifications. Through the clinical scenario of SCI, this study reveals the substantial role that the spinal cord plays in the maintenance of the complex functional architecture of the brain. Although, sensory deprivation-induced brain reorganization has been studied after blindness and other injuries,45–49 there are limited data on brain reorganization after an SCI. Findings in this work demonstrate that fundamental loss of afferent and efferent neural input alter connectivity within and across numerous networks in the brain, which extends far beyond just somatomotor function. The magnitude of network perturbation correlates to the degree to which the spinal cord is injured. Further, the brain network reorganization changes over time. Taken as a whole, this work further elucidates the role of the spinal cord in cerebral plasticity and the malleable nature of central nervous system functional organization.
fMRI is an indirect measure of neuronal activity. RS-fMRI measures the endogenous/spontaneous brain activity using BOLD, and identifies areas that are interacting at rest. Spontaneous BOLD fluctuations are low-frequency oscillations in metabolic activity that are anatomically correlated within distinct functional networks.50 Spontaneous RS-fMRI activity generates RSNs that are similar to the functional maps obtained from traditional task-based fMRI.6
RSNs represent topographies of functionally connected regions across the brain, and these RSNs can be divided into a hierarchy of several large networks that can range in size across the cortex.13,33,34 DMN is an RSN that is more active at rest than during performance of goal-directed tasks.6,13,44,51–57 The DMN is largely anticorrelated, with systems controlling executive and attentional mechanisms.41,58,59 SMN encompasses primary and higher order motor and sensory areas.5 The visual and language networks, which span the occipital cortex6,51–54 and language-related regions33,60,61 can be incorporated with the somatomotor network to create a unified SMN network.38 DAN is involved with attentional control and recruited by tasks requiring control of spatial attention.39,53,54,62,63 CON enables the performance of tasks requiring executive control.53,64,65 SAL, also known as the core control network, is involved with processing and filtering sensory and cognitive inputs and activating executive function networks.63,64,66
Here, we show that when the central nervous system suffers deafferentation from SCI, cerebral networks are forced to reorganize. These changes informed us of the roles the spinal cord has on the normal network architecture. The results shown here demonstrate how SCI leads to plasticity in the cortex in several specific brain networks. First, SCI produces an unsurprising loss of connectivity within the somatomotor regions. Interestingly, SCI alters connectivity within DMN, a network most active at rest. However, it has been demonstrated that the DMN is often affected in many neurological disorders.67–71 SCI most robustly impairs connectivity within the SAL network, important in filtering sensory and cognitive inputs and activating executive function networks. Finally, SCI disrupts the normal anticorrelated connectivity between CON and SMN networks. Correlation with the functional status of the subjects showed that RSN connectivity can be used to discriminate between complete and incomplete SCI. This finding emphasizes how complete loss of circuits from the spinal cord to the brain produces the largest degree of network reorganization. Finally, when we looked at the change in network plasticity over time, we found that certain networks reorganize over time. Normally, the primary motor cortex is highly correlated with the primary somatosensory cortex. However, after SCI, the primary motor cortex became less connected over time with the primary sensory cortex. Meanwhile, the visual cortex was normally highly correlated with the primary motor cortex and not correlated with the parietal cortices. After SCI, the visual cortex became less connected with the primary motor cortex but more connected with the cingulate gyrus and parietal lobe, suggesting an enhancement in visual-related sensory processing after loss of spinal afferents. The cerebellum is typically anticorrelated with the primary motor and sensory cortices. However, after SCI, this anticorrelation is disrupted: there is an increase in connectivity between the cerebellum and both primary motor and sensory cortices.
Lesion experiments have provided important data on human central nervous system cytoarchitecture and networks. White- and gray-matter lesions differentially modulate mesoscale networks and have been used to study short-range connectivity within the brain. Such mesoscale experiments have shown that local white-matter tracts in the cortex maintain topographical anatomic heterogeneity, whereas gray-matter connections segregate cortical synchronization.72 Hemispheric disconnection experiments have provided an understanding of the importance of long-range subcortical connections to local cerebral networks. Disconnecting deep subcortical connections through a hemispherotomy impairs the transfer of information from large-scale distributed brain networks to the local cortex, thereby increasing local mesoscale connectivity.73 This study provides information at the macroscale level on the role of distant afferents into cerebral networks. Loss of distant afferents from a SCI reduces functional connectivity within and among multiple cerebral networks.
This study corroborates and extends the data in the literature. Most reports have focused their efforts to evaluate whether SCI affects cortical functional connectivity within primary motor and sensory cortices. Min and colleagues found increased connectivity between motor-related regions and sensory-related regions of the cortex in subjects with incomplete SCI.18 Oni-Orisan and colleagues then found subjects with distant complete SCI that decreased functional connectivity within bilateral primary motor and sensory cortices.19 Sharp and colleagues performed task-based fMRI on incomplete SCI subjects and found that patients with incomplete SCI had increased activation of ipsilateral post-central gyrus compared with controls.21 Most recently, Kaushal and colleagues performed a graph-theory study of efficiency after remote complete SCI, and found that there were changes in whole-brain connectivity and in connectivity between the cerebellum and the cortex.17 Here, we performed a seed-based correlation mapping using canonical RSNs in complete and incomplete SCI subjects. We demonstrate that when the subjects were taken as one group, SCI was shown to disrupt numerous canonical RSNs. However when the subjects were divided into two cohorts, there was a difference in RSN connectivity between complete and incomplete SCI. Finally, we show that cerebral networks reorganize over time between time windows after SCI. The findings in this study are in line with prior reports, and add to global understanding of reorganization of the cerebral cortex after a SCI.
This study has several limitations. First, the sample size of the SCI subjects was low. Although the results in this study corroborate and extend knowledge shown by other researchers,17,19,21,27,74,75 future studies with larger sample sizes must be performed as validation. Second, the sex ratios for the control groups and the SCI groups were not matched. Future studies must be prospectively matched to reduce any sex-related false-positive results. Third, education level was not recorded.
The translational and clinical relevance of the data reported here may become important in the future. There is an urgent need for an accurate and clinically workable noninvasive method to predict recovery and monitor treatments after SCI. Although clinical examination metrics serve as the standard for assessing and classifying neurological deficits in patients with SCI, these methods are not sufficiently prognostic following SCI. The implications of this research go beyond traumatic SCI, as this method could be applied toward myelopathy and spinal neoplasms.1–3,76
Acknowledgments
This study was supported by grant funding support from the American Association of Neurological Surgeon/Congress of Neurological Surgeons Joint Section on Disorders of the Spine and Peripheral Nerves (A.H.H), Neurosurgery Research and Education Foundation (A.H.H), Cervical Spine Research Society (A.H.H.), National Institute of Neurological Disorders and Stroke (NIH, K23NS084932, W.Z.R), Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NIH, U54 HD087011, J.S.S.), and the Intellectual and Developmental Disabilities Research Center at Washington University (J.S.S). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank the study coordinators and Washington University spine surgeons for their participation in the study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kadanka Z., Bednarik J., Vohanka S., Vlach O., Stejskal L., Chaloupka R., Filipovicova D., Surelova D., Adamova B., Novotny O., Nemec M., Smrcka V., and Urbanek I. (2000). Conservative treatment versus surgery in spondylotic cervical myelopathy: a prospective randomised study. Eur. Spine J. 9, 538–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagata K., Yoshimura N., Muraki S., Hashizume H., Ishimoto Y., Yamada H., Takiguchi N., Nakagawa Y., Oka H., Kawaguchi H., Nakamura K., Akune T., and Yoshida M. (2012). Prevalence of cervical cord compression and its association with physical performance in a population-based cohort in Japan: The Wakayama Spine Study. Spine 37, 1892–1898 [DOI] [PubMed] [Google Scholar]
- 3.Boogaarts H., and Bartels R.M.A. (2015). Prevalence of cervical spondylotic myelopathy. Eur. Spine J., 24, Suppl. 2, 139–141 [DOI] [PubMed] [Google Scholar]
- 4.Kirshblum S.C., Burns S.P., Biering-Sorensen F., Donovan W., Graves D.E., Jha A., Johansen M., Jones L., Krassioukov A., Mulcahey M.J., Schmidt-Read M., and Waring W. (2011). International standards for neurological classification of spinal cord injury (revised 2011). J. Spinal Cord Med. 34, 535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswal B., Yetkin F.Z., Haughton V.M., and Hyde J.S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541 [DOI] [PubMed] [Google Scholar]
- 6.Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., and Beckmann C.F. (2009). Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U.S.A. 106, 13,040–13,045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.New A.B., Robin D.A., Parkinson A.L., Duffy J.R., McNeil M.R., Piguet O., Hornberger M., Price C.J., Eickhoff S.B., and Ballard K.J. (2015). Altered resting-state network connectivity in stroke patients with and without apraxia of speech. Neuroimage Clin. 8, 429–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grefkes C., and Fink G.R. (2011). Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain 134, 1264–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J.H., Loy D.N., Liang H.F., Trinkaus K., Schmidt R.E., and Song S.K. (2007). Noninvasive diffusion tensor imaging of evolving white matter pathology in a mouse model of acute spinal cord injury. Magn. Reson. Med. 58, 253–260 [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Wang Q., Haldar J.P., Yeh F.-C., Xie M., Sun P., Tu T.-W., Trinkaus K., Klein R.S., and Cross A.H. (2011). Quantification of increased cellularity during inflammatory demyelination. Brain 134, 3590–3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naismith R.T., Xu J., Klawiter E.C., Lancia S., Tutlam N.T., Wagner J.M., Qian P., Trinkaus K., Song S.-K., and Cross A.H. (2013). Spinal cord tract diffusion tensor imaging reveals disability substrate in demyelinating disease. Neurology 80, 2201–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D., and Raichle M.E. (2010). Disease and the brain's dark energy. Nat. Rev. Neurol. 6, 15–28 [DOI] [PubMed] [Google Scholar]
- 13.Lee M.H., Hacker C.D., Snyder A.Z., Corbetta M., Zhang D., Leuthardt E.C., and Shimony J.S. (2012). Clustering of resting state networks. PLoS One 7, e40370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy R.K., Sun P., Han R.H., Griffin K.J., Wagner J.M., Yarbrough C.K., Wright N.M., Dorward I.G., Riew D.K., Kelly M.P., Santiago P., Zebala L.P., Trinkaus K., Ray W.Z., and Song S.K. (2016). Fractional anisotropy to quantify cervical spondylotic myelopathy severity. J. Neurosurg. Sci. [EPub ahead of print; PMID 27149369] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy R.K., Sun P., Xu J., Wang Y., Sullivan S., Gamble P., Wagner J., Wright N.N., Dorward I.G., Riew D., Santiago P., Kelly M.P., Trinkaus K., Ray W.Z., and Song S.K. (2016). Magnetic resonance imaging biomarker of axon loss reflects cervical spondylotic myelopathy severity. Spine (Phila Pa 1976) 41, 751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy R.K.J., Gamble P., Sun P., Wang Y., Jacobs E., Song S.-K., and Ray W.Z. (2014). 144 predicting recovery after a spinal cord injury: the role of diffusion basis spectrum imaging as a biomarker of corticospinal tract integrity. Neurosurgery 61, 207 [Google Scholar]
- 17.Kaushal M., Oni-Orisan A., Chen G., Li W., Leschke J., Ward B.D., Kalinosky B., Budde M.D., Schmit B.D., Li S.J., Muqeet V., and Kurpad S.N. (2017). Evaluation of whole-brain resting-state functional connectivity in spinal cord injury: a large-scale network analysis using network-based statistic. J. Neurotrauma 34, 1278–1282 [DOI] [PubMed] [Google Scholar]
- 18.Min Y.S., Park J.W., Jin S.U., Jang K.E., Nam H.U., Lee Y.S., Jung T.D., and Chang Y. (2015). Alteration of resting-state brain sensorimotor connectivity following spinal cord injury: a resting-state functional magnetic resonance imaging study. J. Neurotrauma 32, 1422–1427 [DOI] [PubMed] [Google Scholar]
- 19.Oni-Orisan A., Kaushal M., Li W., Leschke J., Ward B.D., Vedantam A., Kalinosky B., Budde M.D., Schmit B.D., Li S.J., Muqeet V., and Kurpad S.N. (2016). Alterations in cortical sensorimotor connectivity following complete cervical spinal cord injury: a prospective resting-state fMRI Study. PLoS One 11, e0150351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao J.S., Liu Z., Zhao C., Wei R.H., Zhao W., Yang Z.Y., and Li X.G. (2016). Longitudinal evaluation of functional connectivity variation in the monkey sensorimotor network induced by spinal cord injury. Acta. Physiol. (Oxf) 217, 164–173 [DOI] [PubMed] [Google Scholar]
- 21.Sharp K.G., Gramer R., Page S.J., and Cramer S.C. (2017). Increased brain sensorimotor network activation after incomplete spinal cord injury. J. Neurotrauma 34, 623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F.Q., Tan Y.M., Wu L., Zhuang Y., He L.C., and Gong H.H. (2015). Intrinsic functional plasticity of the sensory-motor network in patients with cervical spondylotic myelopathy. Sci. Rep. 5, 9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun P., Murphy R.K., Gamble P., George A., Song S.K., and Ray W.Z. (2017). Diffusion assessment of cortical changes, induced by traumatic spinal cord injury. Brain Sci. 7, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpenter M. (1991). Core Text of Neuroanatomy. Williams and Wilkins: Baltimore [Google Scholar]
- 25.Cramer S.C., Lastra L., Lacourse M.G., and Cohen M.J. (2005). Brain motor system function after chronic, complete spinal cord injury. Brain 128, 2941–2950 [DOI] [PubMed] [Google Scholar]
- 26.Mattia D., Cincotti F., Mattiocco M., Scivoletto G., Marciani M.G., and Babiloni F. (2006). Motor-related cortical dynamics to intact movements in tetraplegics as revealed by high-resolution EEG. Hum. Brain Mapp. 27, 510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabbah P., de S.S., Leveque C., Gay S., Pfefer F., Nioche C., Sarrazin J.L., Barouti H., Tadie M., and Cordoliani Y.S. (2002). Sensorimotor cortical activity in patients with complete spinal cord injury: a functional magnetic resonance imaging study. J. Neurotrauma 19, 53–60 [DOI] [PubMed] [Google Scholar]
- 28.Choe A.S., Belegu V., Yoshida S., Joel S., Sadowsky C.L., Smith S.A., van Zijl P.C., Pekar J.J., and McDonald J.W. (2013). Extensive neurological recovery from a complete spinal cord injury: a case report and hypothesis on the role of cortical plasticity. Front. Hum. Neurosci. 7, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freund P., Weiskopf N., Ashburner J., Wolf K., Sutter R., Altmann D.R., Friston K., Thompson A., and Curt A. (2013). MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: a prospective longitudinal study. Lancet Neurol. 12, 873–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalsi-Ryan S., Wilson J., Yang J.M., and Fehlings M.G. (2014). Neurological grading in traumatic spinal cord injury. World Neurosurg. 82, 509–518 [DOI] [PubMed] [Google Scholar]
- 31.Poole J.L. (2011). Measures of hand function: Arthritis Hand Function Test (AHFT), Australian Canadian Osteoarthritis Hand Index (AUSCAN), Cochin Hand Function Scale, Functional Index for Hand Osteoarthritis (FIHOA), Grip Ability Test (GAT), Jebsen Hand Function Test (JHFT), and Michigan Hand Outcomes Questionnaire (MHQ). Arthritis Care Res (Hoboken) 63, Suppl. 11, S189–199 [DOI] [PubMed] [Google Scholar]
- 32.Brier M.R., Thomas J.B., Snyder A.Z., Benzinger T.L., Zhang D., Raichle M.E., Holtzman D.M., Morris J.C., and Ances B.M. (2012). Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J. Neurosci. 32, 8890–8899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee M.H., Smyser C.D., and Shimony J.S. (2012). Resting-state fMRI: a review of methods and clinical applications. AJNR Am. J. Neuroradiol. 34, 1866–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leuthardt E.C., Allen M., Kamran M., Hawasli A.H., Snyder A.Z., Hacker C.D., Mitchell T.J., and Shimony J.S. (2014). Resting state BOLD fMRI – a paradigm shift in preoperative brain mapping. Stereotact. Funct. Neurosurg. 93, 427–439 [DOI] [PubMed] [Google Scholar]
- 35.Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., and Petersen S.E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cordes D., Haughton V.M., Arfanakis K., Wendt G.J., Turski P.A., Moritz C.H., Quigley M.A., and Meyerand M.E. (2000). Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am. J. Neuroradiol. 21, 1636–1644 [PMC free article] [PubMed] [Google Scholar]
- 37.Shehzad Z., Kelly A.M., Reiss P.T., Gee D.G., Gotimer K., Uddin L.Q., Lee S.H., Margulies D.S., Roy A.K., Biswal B.B., Petkova E., Castellanos F.X., and Milham M.P. (2009). The resting brain: unconstrained yet reliable. Cereb. Cortex 19, 2209–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brier M.R., Thomas J.B., Snyder A.Z., Wang L., Fagan A.M., Benzinger T., Morris J.C., and Ances B.M. (2014). Unrecognized preclinical Alzheimer disease confounds rs-fcMRI studies of normal aging. Neurology 83, 1613–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., and Raichle M.E. (2006). Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. U.S.A. 103, 10,046–10,051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox M.D., Snyder A.Z., Vincent J.L., and Raichle M.E. (2007). Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 56, 171–184 [DOI] [PubMed] [Google Scholar]
- 41.Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., and Raichle M.E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U.S.A. 102, 9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowe M.J., Mock B.J., and Sorenson J.A. (1998). Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage 7, 119–132 [DOI] [PubMed] [Google Scholar]
- 43.Xiong J., Parsons L.M., Gao J.H., and Fox P.T. (1999). Interregional connectivity to primary motor cortex revealed using MRI resting state images. Hum. Brain Mapp. 8, 151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greicius M.D., Krasnow B., Reiss A.L., and Menon V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U.S.A. 100, 253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bavelier D., and Neville H.J. (2002). Cross-modal plasticity: where and how? Nat. Rev. Neurosci. 3, 443–452 [DOI] [PubMed] [Google Scholar]
- 46.Pascual-Leone A., and Hamilton R. (2001). The metamodal organization of the brain. Prog. Brain Res. 134, 427–445 [DOI] [PubMed] [Google Scholar]
- 47.Burton H., Snyder A.Z., and Raichle M.E. (2014). Resting state functional connectivity in early blind humans. Front. Syst. Neurosci. 8, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merabet L.B., and Pascual-Leone A. (2010). Neural reorganization following sensory loss: the opportunity of change. Nat. Rev. Neurosci. 11, 44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roland J.L., Hacker C.D., Breshears J.D., Gaona C.M., Hogan R.E., Burton H., Corbetta M., and Leuthardt E.C. (2013). Brain mapping in a patient with congenital blindness – a case for multimodal approaches. Front. Hum. Neurosci. 7, 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fox M.D., and Raichle M.E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711 [DOI] [PubMed] [Google Scholar]
- 51.Beckmann C.F., DeLuca M., Devlin J.T., and Smith S.M. (2005). Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Luca M., Beckmann C.F., DeStefano N., Matthews P.M., Smith S.M. (2006). fMRI Resting State Networks Define Distinct Modes of Long-Distance Interactions in the Human Brain. Neuroimage 29, 1359–1367 [DOI] [PubMed] [Google Scholar]
- 53.Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A., Vogel A.C., Laumann T.O., Miezin F.M., Schlaggar B.L., and Petersen S.E. (2011). Functional network organization of the human brain. Neuron 72, 665–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Roffman J.L., Smoller J.W., Zollei L., Polimeni J.R., Fischl B., Liu H., and Buckner R.L. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Damoiseaux J.S., Rombouts S.A., Barkhof F., Scheltens P., Stam C.J., Smith S.M., and Beckmann C.F. (2006). Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U.S.A. 103, 13,848–13,853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van den Heuvel M., Mandl R., and Hulshoff Pol H. (2008). Normalized cut group clustering of resting-state FMRI data. PLoS One 3, e2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., and Shulman G.L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 98, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Golland Y., Golland P., Bentin S., and Malach R. (2008). Data-driven clustering reveals a fundamental subdivision of the human cortex into two global systems. Neuropsychologia 46, 540–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raichle M.E. (2006). Neuroscience. The brain's dark energy. Science 314, 1249–1250 [PubMed] [Google Scholar]
- 60.Tomasi D., and Volkow N.D. (2012). Resting functional connectivity of language networks: characterization and reproducibility. Mol. Psychiatry 17, 841–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hacker C.D., Laumann T.O., Szrama N.P., Baldassarre A., Snyder A.Z., Leuthardt E.C., and Corbetta M. (2013). Resting state network estimation in individual subjects. Neuroimage 82, 616–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corbetta M., and Shulman G.L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215 [DOI] [PubMed] [Google Scholar]
- 63.Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L. and Greicius M.D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dosenbach N.U., Visscher K.M., Palmer E.D., Miezin F.M., Wenger K.K., Kang H.C., Burgund E.D., Grimes A.L., Schlaggar B.L., and Petersen S.E. (2006). A core system for the implementation of task sets. Neuron 50, 799–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Power J.D., and Petersen S.E. (2013). Control-related systems in the human brain. Curr. Opin. Neurobiol. 23, 223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Menon V., and Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhat D.I., Indira Devi B., Bharti K., and Panda R. (2017). Cortical plasticity after brachial plexus injury and repair: a resting-state functional MRI study. Neurosurg. Focus 42, E14. [DOI] [PubMed] [Google Scholar]
- 68.Hamilton J.P., Furman D.J., Chang C., Thomason M.E., Dennis E., and Gotlib I.H. (2011). Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol. Psychiatry 70, 327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsiao F.J., Yu H.Y., Chen W.T., Kwan S.Y., Chen C., Yen D.J., Yiu C.H., Shih Y.H., and Lin Y.Y. (2015). Increased intrinsic connectivity of the default mode network in temporal lobe epilepsy: evidence from resting-state MEG recordings. PLoS One 10, e0128787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mohan A., Roberto A.J., Mohan A., Lorenzo A., Jones K., Carney M.J., Liogier-Weyback L., Hwang S., and Lapidus K.A. (2016). The significance of the default mode network (DMN) in neurological and neuropsychiatric disorders: a review. Yale J. Biol. Med. 89, 49–57 [PMC free article] [PubMed] [Google Scholar]
- 71.Tessitore A., Esposito F., Vitale C., Santangelo G., Amboni M., Russo A., Corbo D., Cirillo G., Barone P., and Tedeschi G. (2012). Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology 79, 2226–2232 [DOI] [PubMed] [Google Scholar]
- 72.Hawasli A.H., Kim D., Ledbetter N.M., Dahiya S., Barbour D.L., and Leuthardt E.C. (2016). Influence of white and gray matter connections on endogenous human cortical oscillations. Front. Hum. Neurosci. 10, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hawasli A.H., Chacko R., Szrama N.P., Bundy D.T., Pahwa M., Yarbrough C.K., Dlouhy B.J., Limbrick D.D., Barbour D.L., Smyth M.D., and Leuthardt E.C. (2017). Electrophysiological sequelae of hemispherotomy in ipsilateral human cortex. Front. Hum. Neurosci. 11, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Q., Zheng W., Chen X., Wan L., Qin W., Qi Z., Chen N., and Li K. (2017). Brain gray matter atrophy after spinal cord injury: a voxel-based morphometry study. Front. Hum. Neurosci. 11, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Isa T. (2017). The brain is needed to cure spinal cord injury. Trends Neurosci. 40, 625–636 [DOI] [PubMed] [Google Scholar]
- 76.McKinley W.O., Seel R.T., and Hardman J.T. (1999). Nontraumatic spinal cord injury: incidence, epidemiology, and functional outcome. Arch. Phys. Med. Rehabil. 80, 619–623 [DOI] [PubMed] [Google Scholar]