Abstract
Background
In non-small cell lung cancer (NSCLC), circulating tumor cells (CTC) are shed and circulate to the peripheral blood through the pulmonary vein. Previously, CTC count in pulmonary venous blood (pvCTC) was shown to significantly increase after surgical manipulation. Therefore, we assessed the correlation between the changes in the pvCTC count (ΔpvCTC) and clinical outcomes.
Methods
Consecutive patients with peripheral-type, NSCLC, who underwent lobectomy or bi-lobectomy through open thoracotomy, were enrolled prospectively. Before and after lobectomy, 2.5 mL of blood was drawn from the associated lobar pulmonary vein (PV), and was served for the quantitative evaluation of CTC using the CellSearch® system. The cut-off point of ΔpvCTC was determined according to clinical outcomes and ΔpvCTC using receiver operation characteristic (ROC) curve. Then the correlation between ΔpvCTC and clinical outcomes was evaluated by Kaplan-Meier analyses and log-rank test. In addition, the correlation between ΔpvCTC and perioperative variables was assessed.
Results
A total of 30 patients were enrolled, tumor recurrence occurred in 11 patients over a median follow-up of 64.4 months. Of these, 7 patients had distant metastasis and 4 had local recurrence. The median ΔpvCTC was 49 cells/2.5 mL, and pvCTC-count was increased during surgical manipulation in 24 patients (80%). We divided patients into two groups based on ΔpvCTC with the cut-off value as 119 cells/2.5 mL according to ROC curve. Significant shorter time to distant metastasis (TDM) (P=0.0123) was observed in high ΔpvCTC group (ΔpvCTC ≥119 cells/2.5 mL) than low ΔpvCTC group (ΔpvCTC <119 cells/ 2.5mL). Neither disease-free survival (DFS) nor overall survival (OS) was significantly correlated with ΔpvCTC.
Conclusions
Increasing pvCTC count during surgical manipulation was significantly correlated with postoperative distant metastasis in completely resected NSCLC patients. Significant shorter TDM was observed in patient with high ΔpvCTC group.
Keywords: Circulating tumor cells (CTCs), non-small cell lung cancer (NSCLC), surgical manipulation
Introduction
In non-small cell lung cancer (NSCLC), the incidence of tumor recurrence with distant metastasis is observed in approximately 25% of cases, even though complete resection was achieved (1). Distant metastasis after curative surgery may cause that the primary tumor may shed tumor cells to the blood stream through pulmonary vein (PV). Detection of circulating tumor cells (CTCs) may play an important role in the prediction of distant metastasis. Indeed, the CTC count determined with CellSearch® system is well known as a prognostic factor in various cancers (2-5).
We conducted a series of prospective studies to assess the clinical impacts of CTCs using the CellSearch® system in NSCLC (6-9). First, we demonstrated that CTCs were detected in the peripheral blood of 30.4% with NSCLC and that the presence of CTCs in the peripheral blood was significantly correlated with the presence of distant metastases (6). Second, we conducted a preliminary prospective study for the CTC counts in the peripheral (peri-CTC) and pulmonary venous blood (pvCTC) in 30 consecutive patients undergoing surgery for NSCLC (7). We demonstrated that pvCTCs were detected in most patients (29 of 30, 96.7%), and that peri-CTCs were also detected in some patients (5 of 30, 16.7%). Based on these results, we concluded that CTCs could be more readily detected in the PV than in the peripheral blood. Third, we conducted a prospective study measuring the pvCTC count in patients before and after surgical manipulation of NSCLC (9), which revealed that the pvCTC count was significantly increased by surgical manipulation. This latter result suggested that surgical manipulation led to a spill of tumor cells. Now we hypothesized that increasing pvCTC count would be a risk factor for postoperative tumor recurrence. This study was a follow-up of our previous study (9), thus, we investigated the correlation between the changes in the pvCTC count during surgery (ΔpvCTC) and the clinical outcome based on our hypothesis.
Methods
Study design and patient selection
This prospective study was approved by the Institutional Review Board of Hyogo College of Medicine (No. 715). Patients with peripheral-type NSCLC were eligible if admitted for lobectomy or bi-lobectomy via open thoracotomy at the Department of Thoracic Surgery, Hyogo College of Medicine Hospital, from August 2009 through October 2010. Informed consent was obtained for all patients before surgery, and we collected all relevant clinical data, including the history, as well as the physical and radiological examination results.
For the evaluation of tumor progression, whole-body computed tomography (CT), brain magnetic resonance imaging (MRI), and positron emission tomography (PET) scanning were routinely conducted before surgery. The clinical (c-) stage and pathological (p-) were determined with the seventh edition of the Tumor-Node-Metastasis (TNM) classification proposed by the International Association for the Study of Lung Cancer (10). Pleural invasion (pl), lymphatic invasion (ly), and vessel invasion (v) of primary tumor in the resected lung were evaluated under light microscopy. The radiological tumor findings were divided into three groups based on thin-section CT, as previously reported (11,12): pure solid nodules (consolidation appearance only), pure ground-glass nodules (ground-glass appearance only), and part-solid ground-glass nodules (both consolidation and ground-glass appearances).
Follow-up and assessment of clinical outcomes
Postoperative clinical follow-up was performed every 6 months. PET/CT or thin-section CT and brain MRI were performed for systemic evaluation every year for at least 5 years after surgery. Evidence of recurrence or death was obtained from the medical records. Local recurrence was defined as ipsilateral intrathoracic lymph node metastasis, recurrence at the surgical stump, or dissemination in the ipsilateral thoracic cavity. Distant metastasis was defined as the lack of these findings.
Surgical procedure and blood sampling
After thoracotomy, the PV responsible for lobar drainage was exposed and punctured with a 23-gauge needle before surgical manipulation for lobectomy, and 2.5 mL of blood was drawn. Another 2.5 mL of blood was drawn from the drainage PV just after completion of lobectomy. For each blood sample, 5.0 mL of dilution buffer was added, and the total 7.5 mL diluted samples were stored in a CellSaver® tube (Veridex LLC, Raritan, NJ, USA) at room temperature, for CTC count within 72 hours of sampling. During surgery, the sequence of vessel ligation whether the pulmonary artery (PA) or PV was ligated first was determined by the operating surgeon and the differences in outcomes assessed.
Evaluation of CTCs
CTCs were isolated from pulmonary venous blood using the CellSearch® system (Veridex LLC), and the number of CTCs was determined according to the manufacturer’s protocol (6-9). All evaluations were performed independently by two authors (K Yoneda and F Tanaka) without knowledge of the clinical characteristics. Both assessors had completed a cell interpretation proficiency assessment for the identification of CTCs that was managed by Veridex LLC.
Statistical methods
In evaluating the prognostic significance of high ΔpvCTC, disease-free survival (DFS) and ΔpvCTC were applied to the receiver operation characteristic (ROC) method. The cut-off value was determined as that corresponding to the maximum log-rank statistical analyses. DFS was the time from the day of surgery to recurrence and patients without recurrence were censored at the time point known for not having recurrence. Time to distant metastasis (TDM) was the time from the day of surgery to recurrence including distant metastasis and patients without recurrence were censored at the time point known not for having recurrence. Overall survival (OS) was the time from the day of surgery to death and patients without death were censored at the time point known not having death.
All statistical analyses were performed using GraphPad Prism version 5.0 for Windows (GraphPad Software, San Diego, CA, USA) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical function to frequently used biostatistics. The comparison of survival curve was used for the log-rank test. Fisher’s exact test was employed to compare between two groups. Differences were considered statistically significant when the P value was <0.05.
Results
Patient characteristics and distribution of ΔpvCTC
A total of 30 patients were enrolled in the study, and their baseline characteristics are detailed in Table 1. In the radiological examination, c-stage I (n=23; 76.7%) and pure solid appearance on the radiological findings (n=21; 70.0%) were majority. Histologically, adenocarcinoma was the main tumor type (n=22; 73.3%). Complete resection and systemic nodal dissection were achieved without diagnostic wedge lung resection in all cases. In most cases, PA were ligated first (n=22; 73.3%), with fewer PV ligated first (n=8; 26.7%). After pathological examination of resected specimens, p-stage I and p-stage II–III were in 17 (56.7%) and 13 (43.3%) respectively, and pleural invasion (pl), lymphatic invasion (ly), and vessel invasion (v) were found in 17(56.7%), 20 (66.7%), 22 (73.3%) respectively.
Table 1. Patient characteristics.
| Characteristics | Values (n=30) |
|---|---|
| Age [mean, median (range)] (years) | 67.0, 68.0 (53.0–84.0) |
| Sex, n (%) | |
| Male | 18 (60.0%) |
| Female | 12 (40.0%) |
| Side of primary tumor, n (%) | |
| Right upper lobe | 16 (53.3%) |
| Right middle lobe | 2 (6.7%) |
| Right lower lobe | 5 (16.7%) |
| Left upper lobe | 6 (20.0%) |
| Left lower lobe | 1 (3.3%) |
| Tumor size [mean, median (range)] (cm) | 3.0, 3.3 (1.5–6.0) |
| Radiological tumor findings, n (%) | |
| Pure GGN | 1 (3.3%) |
| Part-solid GGN | 8 (26.7%) |
| Pure solid | 21 (70.0%) |
| Tumor histology, n (%) | |
| Adenocarcinoma | 22 (73.3%) |
| Squamous cell carcinoma | 6 (20.0%) |
| Others | 2 (6.7%) |
| c-T factor | |
| cT1 | 11 (36.7%) |
| cT2–3 | 19 (63.3%) |
| c-N factor | |
| cN0 | 25 (83.3%) |
| cN1–2 | 5 (16.7%) |
| c-stage | |
| c-stage I | 23 (76.7%) |
| c-stage II–III | 7 (23.3%) |
| p-T factor | |
| pT1 | 6 (20.0%) |
| pT2–4 | 24 (80.0%) |
| p-N factor | |
| pN0 | 22 (73.3%) |
| pN1–2 | 8 (26.7%) |
| p-stage | |
| p-stage I | 17 (56.7%) |
| p-stage II–III | 13 (43.3%) |
| Pleural invasion (pl) | |
| Negative | 13 (43.3%) |
| Positive | 17 (56.7%) |
| Lymphatic invasion (ly) | |
| Negative | 10 (33.3%) |
| Positive | 20 (66.7%) |
| Vessel invasion (v) | |
| Negative | 8 (26.7%) |
| Positive | 22 (73.3%) |
| Sequence of vessel ligation | |
| PA→PV | 22 (73.3%) |
| PV→PA | 8 (26.7%) |
| Duration of operation time {mean, median [range]} (min) | 136, 123 [69–235] |
| Amount of blood loss {mean, median [range]} (mL) | 76, 50 [0–290] |
| ÄpvCTC | |
| >1,000 | 1 (3.3%) |
| 101–1,000 | 8 (26.7%) |
| 11–100 | 9 (30.0%) |
| 1–10 | 6 (20.0%) |
| 0 | 2 (6.7%) |
| <0 | 4 (13.3%) |
GGN, ground glass nodule; c, clinical; p, pathological; PA, pulmonary artery; PV, pulmonary vein; ÄpvCTC, the changes in the CTC count in pulmonary venous blood during surgery.
The distribution of ΔpvCTC is shown in Table 1 and Figure 1. After surgical manipulation, pvCTC count was increased in 24 (80.0%) patients and decreased in 4 (13.3%), but no changes were observed in 2 (6.7%) patients. The distribution of the ΔpvCTC is as follows: >1,000 in 1 (3.3%), 101–1,000 in 8 (26.7%), 11–100 in 9 (30.0%), 1–10 in 6 (20.0%), 0 in 2 (6.7%), and <0 in 4 (13.3%).
Figure 1.
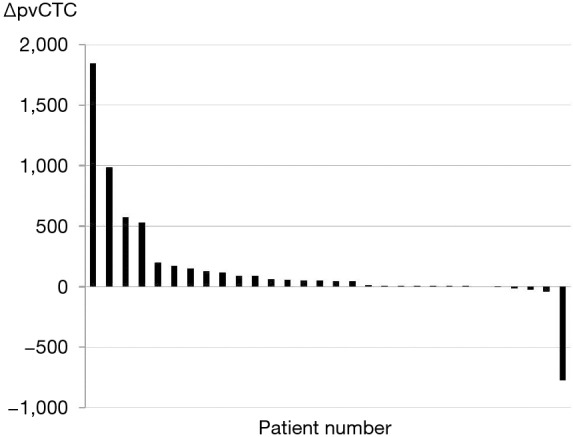
Waterfall plot showing changes in the pvCTC count before and after surgical manipulation. pvCTC, circulating tumor cell (CTC) count in pulmonary venous blood.
Clinical outcomes
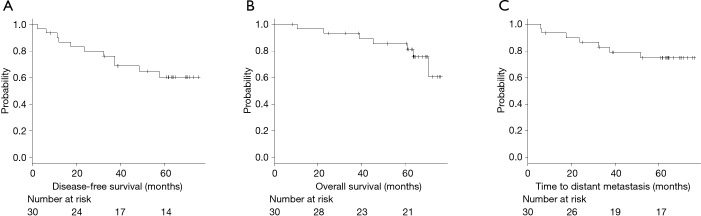
The median follow-up period was 64.4 months (range, 8–77 months), and tumor recurrence occurred in 11 (36.7%) patients; of these, distant metastasis occurred in 7 (23.3%). In addition, 4 of the 30 (13.3%) patients died of cancer, whereas 3 (10%) died of other causes. The overall 5-year DFS rate, TDM rate, and OS rate were 60.4%, 74.9% and 81.2%, respectively (Figure 2).
Figure 2.
Kaplan-Meier curves of disease-free survival (A), overall survival (B), and the time to distant metastasis (C) in all patients (n=30).
Correlation between ΔpvCTC and clinical outcome
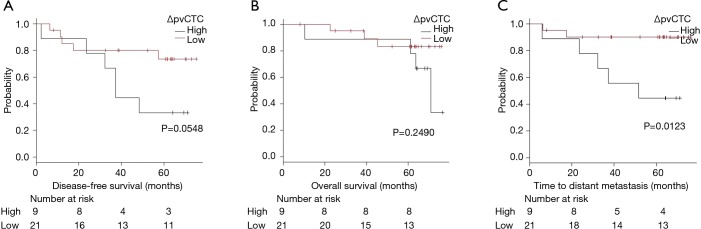
As ROC method was performed to determine the cut-off point of ΔpvCTC, it revealed that the cut-off point was 119 CTCs/2.5 mL (Figure 3). According to ROC method, we divided patients into two groups as high ΔpvCTC group and low ΔpvCTC group. High ΔpvCTC group was defined as patients with 119 or more pvCTCs (n=9), low ΔpvCTC group was defined as patients with less than 119 pvCTCs (n=21).
Figure 3.
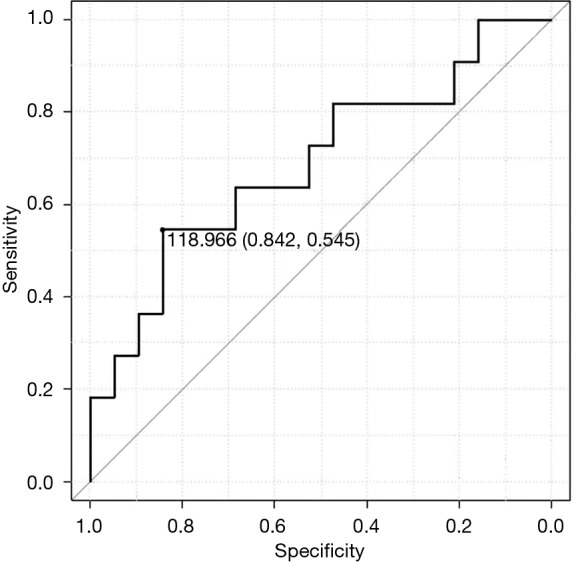
Receiver operating characteristic curve for the ΔpvCTC for disease-free survival. ΔpvCTC, change in circulating tumor cell (CTC) count in pulmonary venous blood from before to after surgery.
The survival curve for DFS, TDM and OS between high ΔpvCTC group and low ΔpvCTC group are shown in Figure 4. Significant shorter TDM was observed in the high ΔpvCTC group than in the low ΔpvCTC group (P=0.0123). No other survival significance was observed.
Figure 4.
Kaplan-Meier curves of disease-free survival (A), overall survival (B), and the time to distant metastasis (C) between high ΔpvCTC group (ΔpvCTC ≥119; n=9) and low ΔpvCTC group (ΔpvCTC <119; n=21). ΔpvCTC, change in circulating tumor cell (CTC) count in pulmonary venous blood from before to after surgery.
Correlations between perioperative variables and the high ΔpvCTC group
The correlations between perioperative variables and high ΔpvCTC group were analyzed (Table 2). Pure solid appearance on the radiological findings and pleural invasion (pl) were significantly correlated with high ΔpvCTC group. Even though all patients of high ΔpvCTC group were performed PA ligation before PV ligation (PA first ligation), significant correlation was not observed. No significant correlations were also observed in other perioperative variables.
Table 2. Comparison of perioperative variables according to ÄpvCTC.
| Variables | Low ÄpvCTC group (ÄpvCTC <119; n=21) | High ÄpvCTC group (ÄpvCTC ≥119; n=9) | P value |
|---|---|---|---|
| Sex | 0.7036 | ||
| Male | 12 | 6 | |
| Female | 9 | 3 | |
| Age (years) | 0.6944 | ||
| Lower <67 | 9 | 5 | |
| Higher ≥67 | 12 | 4 | |
| Histology | 0.1954 | ||
| Adenocarcinoma | 17 | 5 | |
| Others | 4 | 4 | |
| Side | 1.0000 | ||
| Right | 16 | 7 | |
| Left | 5 | 2 | |
| Lobe | 0.6662 | ||
| Upper | 16 | 6 | |
| Middle and lower | 5 | 3 | |
| Primary tumor diameter (mm) | 0.2293 | ||
| ≤30 | 11 | 2 | |
| >30 | 10 | 7 | |
| Radiological tumour findings | 0.0289 | ||
| Pure GGN and part-solid GGN | 9 | 0 | |
| Pure solid | 12 | 9 | |
| c-T factor | 0.4189 | ||
| cT1 | 9 | 2 | |
| cT2–3 | 12 | 7 | |
| c-N factor | 0.6220 | ||
| cN0 | 18 | 7 | |
| cN1–2 | 3 | 2 | |
| c-stage | 0.1954 | ||
| Stage I | 17 | 5 | |
| Stage II–III | 4 | 4 | |
| p-T factor | 0.1405 | ||
| pT1 | 6 | 0 | |
| pT2–4 | 15 | 9 | |
| p-N factor | 0.6662 | ||
| pN0 | 16 | 6 | |
| pN1–2 | 5 | 3 | |
| p-stage | 0.1232 | ||
| Stage I | 14 | 3 | |
| Stage II–III | 7 | 6 | |
| Sequence of vessel ligation | 0.0665 | ||
| PA→PV | 13 | 9 | |
| PV→PA | 8 | 0 | |
| Duration of operation time (min) | 0.4270 | ||
| <123 | 12 | 3 | |
| ≥123 | 9 | 6 | |
| Blood loss during operation (mL) | 1.0000 | ||
| <50 | 10 | 4 | |
| ≥50 | 11 | 5 | |
ÄpvCTC, the changes in the CTC count in pulmonary venous blood during surgery; CTC, circulating tumor cell; GGN, ground glass nodule; c, clinical; p, pathological; PA, pulmonary artery; PV, pulmonary vein.
Discussion
Several studies regarding pvCTC have been published (13-16). Sienel and co-workers showed that the detection rate of pvCTCs in patients before surgical manipulation was about 20% with operable NSCLC, and pvCTC was correlated with a poor prognosis (13). In this present study, we analyzed the correlation between ΔpvCTC and clinical outcome based on our previous study (9). As a result, we succeed in showing a significant correlation between the ΔpvCTC and only TDM. Significant correlation was not observed with DFS or OS. Of the 11 patients with tumor recurrence, distant metastasis occurred in 7 and local recurrence or lymph node metastasis occurred in 4. We thought that the distant metastasis might correlate with surgical manipulation, but local recurrence or dissemination of the ipsilateral thoracic cavity could have been correlated with another cause. In particular, the mechanism of developing lymph node metastasis remained unclear. OS might be influenced by tumor recurrence and therapeutic response. In fact, therapeutic response is known to be a major prognostic factor in patients with postoperative tumor recurrence (17). Based on these results, we argue that surgical manipulation may promote distant metastasis, and that it may be responsible for a worse prognosis.
A pure solid appearance on imaging is known as a poor prognostic factor in NSCLC (12). Suzuki and co-workers showed that occult lymph node metastasis was present in 24% of patients with c-T1a adenocarcinoma that had pure solid appearances, and that vascular and lymphatic invasion each occurred in approximately 50% of cases (11). Since pure solid appearance is the risk factor of the postoperative metastasis (12), it might be reasonable that significant correlation between pure solid appearance and high ΔpvCTC group. As a result, it possibly suggests that tumor spillage due to surgical manipulation occurs more readily in patients with pure solid tumors. Given this role as a poor prognostic factor, we should consider to avoid tumor spillage in patient with pure solid tumor.
Pleural invasion also correlated with high ΔpvCTC group in this cohort. Pleural invasion is well known to aggressive and invasive factor in NSCLC (18,19). Jiwangga and co-workers reported the presence of pleural invasion was a predictive marker of postoperative pleural seeding and bilateral lung metastases in stage I NSCLC (20). In contrast, pleural invasion could be more frequently observed in advanced NSCLC especially depended on T factor (18). Even though significant correlation observed between pleural invasion and high ΔpvCTC group, it might be suspected that it was strongly influenced by multiple factors including T factor.
Regarding the sequence of vessel ligation during lobectomy, it is theoretically recommended to ligate PV prior to PA. Indeed, the efficacy of ligating PV first to avoid tumor spillage during surgery for NSCLC was reported in several literatures (21,22). Even though several studies regarding the sequence of vessel ligation were conducted in NSCLC, significant correlation with clinical outcome remained unproven (22-27). For example, Kozak and co-workers divided patients into two groups: group A underwent ligation of the PA branch first (n=215) and group V underwent ligation of PV first (n=170). Although long-term survival was not influenced by the sequence of vessel ligation, hematogenous metastasis occurred with a higher incidence in group A than group V (20.5% vs. 14.7%, P=0.18) (25). In the present cohort of 30 patients, high ΔpvCTC group consisted of only patients with ligated PA first. Moreover, no distant metastasis was observed in patients with ligated PV first. Even though we failed to show a significant correlation between the sequence of vessel ligation and the ΔpvCTC, we should continue to consider the influence of the sequence of vessel ligation in NSCLC patients.
Among the studies of CTC in NSCLC, several studies have suggested that the presence of cluster cells is related to metastasis (14,15). Funaki and co-workers reported that the cluster cells were detected in both peripheral and pulmonary venous blood after surgical manipulation, and the presence of clustered CTCs was considered a poor prognostic factor, and it could be speculated that the cluster cell may be able to survive longer, directly access another organ, and establish distant metastasis. Moreover, a correlation with epithelial-mesenchymal transition (EMT) and clustered CTCs has also been reported (28-30), which might explain the early recurrence and poor prognosis when compared with non-clustered CTCs. The CellSearch® system used in this study is only able to capture cells positive for the epithelial cell adhesion molecule, so neither the presence of clustered CTCs nor the EMT could be evaluated.
There are some limitations regarding this study. First, the number of patient in this cohort was too small to achieve strong evidence. Since, sampling PV blood prior to surgical manipulation had a risk of intraoperative bleeding, the recruitment of patients was difficult in this cohort. Thus, it would be difficult to conduct a prospective study with large number. Second, the patient population of this cohort was heterogeneous, especially, the sequence of vessel ligation was committing to surgeons, as a result, PV first ligation was performed in only 8 (26.7%) patients. These heterogeneity of patient characteristics was possibility to affect several results of this study. More precise eligible criteria should be considered to establish further investigation.
In conclusion, we first showed the clinical meanings of ΔpvCTC, in which increasing pvCTC count during surgical manipulation significantly correlated the postoperative distant metastasis in completely resected NSCLC patients. Thus, TDM was significantly shorter in patient with ΔpvCTC ≥119 cells/2.5 mL in this prospective study.
Acknowledgements
Funding: This work was supported by the Japan Society for the Promotion of Science (Grants-in-Aid for Scientific Research, KAKENHI C 08099610).
Ethical Statement: This prospective study was approved by the Institutional Review Board of Hyogo College of Medicine (No. 715). Informed consent was obtained for all patients before surgery.
Footnotes
Conflicts of Interest: Seiki Hasegawa has received research funding from Eli Lilly, Taiho Pharmaceuticals, and Ono Pharmaceuticals. The other authors have no conflicts of interest to declare.
References
- 1.Yoshino I, Yohena T, Kitajima M, et al. Survival of non-small cell lung cancer patients with postoperative recurrence at distant organs. Ann Thorac Cardiovasc Surg 2001;7:204-9. [PubMed] [Google Scholar]
- 2.Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213-21. 10.1200/JCO.2007.15.8923 [DOI] [PubMed] [Google Scholar]
- 3.Cohen SJ, Punt CJ, Iannotti N, et al. Prognostic significance of circulating tumour cells in patients with metastatic colorectal cancer. Ann Oncol 2009;20:1223-9. 10.1093/annonc/mdn786 [DOI] [PubMed] [Google Scholar]
- 4.Cristofanilli M. Circulating tumour cells, disease progression, and survival in metastatic breast cancer. Semin Oncol 2006;33:S9-14. 10.1053/j.seminoncol.2006.03.016 [DOI] [PubMed] [Google Scholar]
- 5.Resel Folkersma L, San Jose Manso L, Galante Romo I, et al. Prognostic significance of circulating tumor cell count in patients with metastatic hormone-sensitive prostate cancer. Urology 2012;80:1328-32. 10.1016/j.urology.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 6.Tanaka F, Yoneda K, Kondo N, et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res 2009;15:6980-6. 10.1158/1078-0432.CCR-09-1095 [DOI] [PubMed] [Google Scholar]
- 7.Okumura Y, Tanaka F, Yoneda K, et al. Circulating tumor cells in pulmonary venous blood of primary lung cancer patients. Ann Thorac Surg 2009;87:1669-75. 10.1016/j.athoracsur.2009.03.073 [DOI] [PubMed] [Google Scholar]
- 8.Naito T, Tanaka F, Ono A, et al. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J Thorac Oncol 2012;7:512-9. 10.1097/JTO.0b013e31823f125d [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto M, Tanaka F, Yoneda K, et al. Significant increase in circulating tumor cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interact Cardiovasc Thorac Surg 2014;18:775-83. 10.1093/icvts/ivu048 [DOI] [PubMed] [Google Scholar]
- 10.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. 10.1097/JTO.0b013e31812f3c1a [DOI] [PubMed] [Google Scholar]
- 11.Suzuki K, Kusumoto M, Watanabe S, et al. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg 2006;81:413-9. 10.1016/j.athoracsur.2005.07.058 [DOI] [PubMed] [Google Scholar]
- 12.Hattori A, Matsunaga T, Hayashi T, et al. Prognostic Impact of the Findings on Thin-Section Computed Tomography in Patients with Subcentimeter Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:954-62. 10.1016/j.jtho.2017.02.015 [DOI] [PubMed] [Google Scholar]
- 13.Sienel W, Seen-Hibler R, Mutschler W, et al. Tumor cells in the tumor draining vein of patients with non-small cell lung cancer: detection rate and clinical significance. Eur J Cardiothorac Surg 2003;23:451-6. 10.1016/S1010-7940(02)00865-5 [DOI] [PubMed] [Google Scholar]
- 14.Funaki S, Sawabata N, Aburatani A, et al. Significance of tumor vessel invasion in determining the morphology of isolated tumor cells in the pulmonary vein in non-small-cell lung cancer. Eur J Cardiothorac Surg 2013;43:1126-30. 10.1093/ejcts/ezs553 [DOI] [PubMed] [Google Scholar]
- 15.Sawabata N, Funaki S, Hyakutake T, et al. Perioperative circulating tumor cells in surgical patients with non-small cell lung cancer: does surgical manipulation dislodge cancer cells thus allowing them to pass into the peripheral blood? Surg Today 2016;46:1402-9. 10.1007/s00595-016-1318-4 [DOI] [PubMed] [Google Scholar]
- 16.Yao X, Williamson C, Adalsteinsson VA, et al. Tumor cells are dislodged into the pulmonary vein during lobectomy. J Thorac Cardiovasc Surg 2014;148:3224-31.e1. 10.1016/j.jtcvs.2014.06.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song IH, Yeom SW, Heo S, et al. Prognostic factors for post-recurrence survival in patients with completely resected Stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:262-7. 10.1093/ejcts/ezt333 [DOI] [PubMed] [Google Scholar]
- 18.Jiang L, Liang W, Shen J, et al. The impact of visceral pleural invasion in node-negative non-small cell lung cancer: a systematic review and meta-analysis. Chest 2015;148:903-11. 10.1378/chest.14-2765 [DOI] [PubMed] [Google Scholar]
- 19.Adachi H, Tsuboi M, Nishii T, et al. Influence of visceral pleural invasion on survival in completely resected non-small-cell lung cancer. Eur J Cardiothorac Surg 2015;48:691-7. 10.1093/ejcts/ezu515 [DOI] [PubMed] [Google Scholar]
- 20.Jiwangga D, Cho S, Kim K, et al. Recurrence Pattern of Pathologic Stage I Lung Adenocarcinoma With Visceral Pleural Invasion. Ann Thorac Surg 2017;103:1126-31. 10.1016/j.athoracsur.2016.09.052 [DOI] [PubMed] [Google Scholar]
- 21.Cho Y, Hida Y, Kaga K, et al. Brain metastases secondary to tumor emboli from primary lung cancer during lobectomy. Ann Thorac Surg 2008;86:312-3. 10.1016/j.athoracsur.2008.01.029 [DOI] [PubMed] [Google Scholar]
- 22.Aylwin JA. Avoidable vascular spread in resection for bronchial carcinoma. Thorax 1951;6:250-67. 10.1136/thx.6.3.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Refaely Y, Sadetzki S, Chetrit A, et al. The sequence of vessel interruption during lobectomy for non-small cell lung cancer: is it indeed important? The J Thorac Cardiovasc Surg 2003;125:1313-20. 10.1016/S0022-5223(03)00022-9 [DOI] [PubMed] [Google Scholar]
- 24.Kurusu Y, Yamashita J, Hayashi N, et al. The sequence of vessel ligation affects tumor release into the circulation. J Thorac Cardiovasc Surg 1998;116:107-13. 10.1016/S0022-5223(98)70248-X [DOI] [PubMed] [Google Scholar]
- 25.Kozak A, Alchimowicz J, Safranow K, et al. The impact of the sequence of pulmonary vessel ligation during anatomic resection for lung cancer on long-term survival--a prospective randomized trial. Adv Med Sci 2013;58:156-63. 10.2478/v10039-012-0061-3 [DOI] [PubMed] [Google Scholar]
- 26.Toufektzian L, Attia R, Polydorou N, et al. Does the sequence of pulmonary vasculature ligation have any oncological impact during an anatomical lung resection for non-small-cell lung cancer? Interact Cardiovasc Thorac Surg 2015;20:260-4. 10.1093/icvts/ivu361 [DOI] [PubMed] [Google Scholar]
- 27.Song PP, Zhang W, Zhang B, et al. Effects of different sequences of pulmonary artery and vein ligations during pulmonary lobectomy on blood micrometastasis of non-small cell lung cancer. Oncol Lett 2013;5:463-8. 10.3892/ol.2012.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aceto N, Bardia A, Miyamoto DT, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014;158:1110-22. 10.1016/j.cell.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou JM, Krebs M, Ward T, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol 2011;178:989-96. 10.1016/j.ajpath.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013;339:580-4. 10.1126/science.1228522 [DOI] [PMC free article] [PubMed] [Google Scholar]