Abstract
Background
The purpose of our cohort study was to investigate the effects of pleural adhesions on perioperative outcomes in patients undergoing video-assisted thoracoscopic surgery (VATS) lobectomy for non-small-cell lung cancer (NSCLC).
Methods
We performed a single-center retrospective analysis on the prospectively-maintained dataset at our unit from February 2014 to November 2015. Patients were divided into two groups (Group A: presence of pleural adhesions; Group B: absence of pleural adhesions) according to our grading system of pleural adhesions when entering the chest cavity. Demographic differences in perioperative outcomes between these two groups were initially estimated. A multivariate logistic-regression analysis was then performed to confirm the predictive value of the presence of pleural adhesions.
Results
A total of 593 NSCLC patients undergoing VATS lobectomy were enrolled. The conversion and postoperative morbidity rates were 3.2% and 29.2%, respectively. There were 154 patients with pleural adhesions (Group A) and 439 patients without pleural adhesions (Group B). Group A patients had significantly higher rates of conversion to thoracotomy (9.1% vs. 1.1%; P<0.001) and surgical complications (24.0% vs. 14.4%; P=0.006) than those of Group B patients. No significant difference was found in the overall morbidity and cardiopulmonary complication rates between these two groups. The presence of pleural adhesions was also significantly associated with the prolonged length of chest tube drainage (log-rank P<0.001) and length of stay (log-rank P=0.032). Finally, the presence of pleural adhesions was identified as an independent risk factor for conversion to thoracotomy [odds ratio (OR) =5.49; P=0.003] and surgical complications (OR =1.94; P=0.033) by multivariate logistic-regression analyses.
Conclusions
Presence of pleural adhesions can predict conversion to thoracotomy and postoperative surgical complications in patients undergoing VATS lobectomy for NSCLC. Our study calls for an internationally accepted grading system for the presence of pleural adhesions to stratify the surgical risk.
Keywords: Pleural adhesions, video-assisted thoracoscopic surgery (VATS), lobectomy, non-small cell lung cancer (NSCLC)
Introduction
Rationale
Nowadays, radical surgery is considered not only as the optimal therapeutic option for early-stage non-small cell lung cancer (NSCLC) but also as the key component of multi-disciplinary treatment for more advanced NSCLC (1,2). Since the 1990s, single-lobectomy via video-assisted thoracoscopic surgery (VATS), a minimally invasive technique to gain access to the chest cavity, has been dramatically developed and widely utilized in the modern surgical modality for operable NSCLC, offering more advantages than traditional thoracotomy in terms of cosmetic wounds, pain and stress control, preservation of pulmonary functions and enhanced recovery to normal life (2,3).
However, despite advances in surgical techniques, anesthetic techniques and perioperative care, the morbidity rate still remains at 24.9–36.3% after VATS lobectomy (2-5). Besides, the unique advantages of VATS techniques will be hardly embodied once unexpected conversion is required intraoperatively (5). As an important intraoperative issue, the presence of pleural adhesions is usually the end result of an inflammatory process and presented as the pathological bonds between the pleura, the chest wall, and the intra-thoracic organs (6,7). Thoracic surgeons usually regard the dense pleural adhesions as a contraindication to VATS procedure in their early learning curves because the presence of pleural adhesions may bring a great technical challenge that correlates with conversion to thoracotomy and postoperative complications (6,8).
The most recent study reported by Kouritas et al. (6) on 144 patients undergoing lung resections demonstrates that the presence of pleural adhesions is significantly associated with the prolonged length of stay and higher pleural morbidity. However, the predictive roles of pleural adhesions for conversion to thoracotomy and other major morbidity are not well-understood according to the currently limited data (6). More importantly, the classifications of pleural adhesions still remain controversial in the literature (6,9). There is not any common grading system with clinical significance produced for the presence of pleural adhesions until now.
Objectives
In this study, the degrees of pleural adhesions were rated by a modified assessment model integrating the involvements of both filmy and dense adhesions. The primary purpose of our study was to evaluate the effects of pleural adhesions on conversion to thoracotomy and postoperative morbidity in patients undergoing VATS lobectomy for NSCLC. Our secondary goal was to assess the association between the presence of pleural adhesions and the length of stay following VATS lobectomy.
Methods
Study design
This single-center retrospective analysis was performed on a prospectively-maintained database with the medical records at our unit. It was written in compliance with the Strengthening the Reporting of Observational Studies in Epidemiology statement (Table S1) (10).
Study protocol
The study protocol was approved by the Regional Ethics Committee of Sichuan University West China Hospital (ID: 2016-255).
Participant selection
Settings
We retrospectively analyzed the clinical data of patients undergoing VATS lobectomy for NSCLC in our institution between February 2014 and November 2015. Their medical records about perioperative characteristics and outcomes were carefully reviewed and further analyzed.
Eligibility criteria
Patients who met all the following eligibility criteria would be enrolled:
The target diseases were operable primary NSCLCs;
Only standardized single-lobectomy with systematic mediastinal lymph node dissection operated by a VATS procedure would be included;
Patients should finish our entire clinical pathway during the hospitalization;
In addition, patients with loss of accurate records on estimated characteristics or outcomes would not be considered.
Follow-up
The endpoints of our study belong to in-hospital outcomes. Therefore, a follow-up would be provided for each patient until 30 days after surgery or death in the hospital.
Outcome data, measures and definitions
We recorded and defined the following perioperative characteristics and outcome data.
Preoperative variables
Baseline information included the age, gender, body mass index (BMI) and smoking history (formal/current/never-smoker).
Preoperative underlying comorbidities included the chronic obstructive pulmonary disease (COPD), tuberculosis, preoperative respiratory infection (defined as the presence of one or more of pulmonary infections, including the obstructive pneumonia, aspiration pneumonia, bronchiectasis, lung abscess and bacterial/fungal infections), asthma, hypertension, diabetes mellitus, coronary heart disease, previous malignancy and steroid use.
A multi-disciplinary team meeting would discuss the combined treatment modalities before surgery if necessary. Neoadjuvant/adjuvant therapy was a cisplatin/paclitaxel-based chemo-radiotherapy in accordance with the National Comprehensive Cancer Network Guidelines: China Editions.
Intraoperative variables
Intraoperative parameters in the comparable analyses are presented as follows: tumor location, degrees of pleural invasion (none/visceral/parietal) and pulmonary fissure completeness (4), operation time, estimated intraoperative blood loss, perioperative blood transfusion and conversion to thoracotomy.
Assessment of pleural adhesions
In many surgeons’ opinions, the strength of pleural adhesions seems to be more clinically meaningful and worth to be evaluated than in their covered area when performing the adhesiolysis. Dense pleural adhesions, which are generally originated from the protracted fibro-calcification and scar tissues at the end stage of inflammatory processes, usually force thoracic surgeons to dissect sharply with the electrocautery or energy device rather than to release down easily (6).
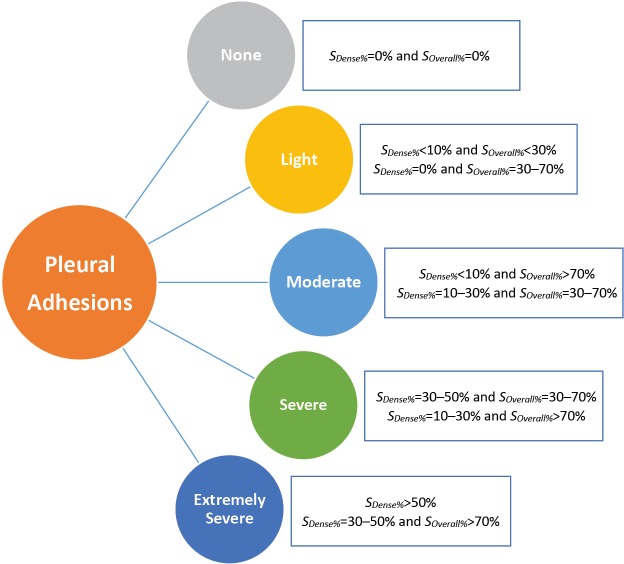
In our cohort, we established a grading system according to the extent of dense pleural adhesions requiring the sharp separation and ligation with the electrocautery or energy device, and the overall involvement of both loose and dense pleural adhesions over the lung surface, which were both calculated as the rough percentages of the pleural cavity in the operative side (SDense% and SOverall%), as shown in Figure 1. We classified all the patients into five groups of pleural adhesions according to the following classification criterion combined by above adhesion data:
Figure 1.
Classifications of the pleural adhesions. SDense%, percentage of the area of dense pleural adhesions requiring sharp dissections with the electrocautery or energy device over the inner surface of the pleural cavity; SOverall%, percentage of the area of overall pleural adhesions (both ‘dense’ and ‘loose’) over the inner surface of the pleural cavity.
None-adhesion: SDense%=0% and SOverall%=0%;
Light-adhesion: SDense%<10% and SOverall%<30%; SDense%=0% and SOverall%=30–70%;
Moderate-adhesion: SDense%<10% and SOverall%>70%; SDense%=10–30% and SOverall%=30–70%;
Severe-adhesion: SDense%=30–50% and SOverall%=30–70%; SDense%=10–30% and SOverall%>70%;
Extremely-severe-adhesion: SDense%>50%; SDense%=30–50% and SOverall%>70%;
Two board-certified surgeons with an 8-year clinical experience commonly estimated the above two percentages during VATS lobectomy and recorded these adhesion data in the operative notes. For cases where two surgeons disagreed, a consensus evaluation would be performed by a third surgeon with a 15-year experience.
Pathological variables
The following pathological parameters would be considered, including the histological subtypes, differentiation degrees (low/moderate/high), tumor invasion (T-stage), lymph node metastasis (N-stage) and TNM-stage, which were defined according to the Union for International Cancer Control seventh edition.
Outcomes of interest
The primary outcomes were conversion to thoracotomy, postoperative complications and in-hospital mortality. Overall morbidity was defined by the presence of any complication developed within 30 postoperative days or later during the same hospitalization, which was judged according to the Society of Thoracic Surgeons and the European Society of Thoracic Surgeons joint definitions (11). In-hospital mortality was defined as any death within 30 postoperative days in the hospital. In addition, postoperative pulmonary, surgical and cardiovascular complications, which had been defined according to the French Society of Thoracic and Cardiovascular Surgery database (12), were also further analyzed, respectively.
The secondary outcomes involved the length of stay and length of chest tube drainage. The length of stay was calculated from the operation day to the discharge day. The length of pleural drainage was defined as the days with chest tube drainage.
Grouping criterion
We divided all enrolled patients into two groups based on their levels of pleural adhesions. Patients with moderate to extremely severe pleural adhesions were classified as the Group A, revealing the presence of pleural adhesions. On the contrary, patients with none to light pleural adhesions were classified as the Group B, suggesting the absence of pleural adhesions.
Surgical procedure, perioperative care and discharge criteria
Our standardized lobectomy with mediastinal lymph node dissection was operated by a three-portal VATS procedure using the single-direction technique as Liu et al. (13,14) previously described, which was known as a modified ‘fissureless’ technique that avoids early dissections through the fissural parenchyma. A stapler line reinforcement was also utilized on the bronchial stumps.
Our cohort of patients was managed in compliance with a standardized clinical pathway. The institutional policies for perioperative care, including the preoperative pulmonary rehabilitation, postoperative pain control, lung recruitment assessment and postoperative chest tube management had been reported in our previous studies (2,4,5,15,16).
Patients would be discharged if they met the following criteria:
Patients were encouraged to ambulate freely after removing the chest tube;
Patients restored to proper breathing activities, instead of presenting the shortness of breath, wheezing or crackles, with an oxygen saturation >94%;
Severe complications and symptoms had been sufficiently controlled before the discharge day.
Statistical analysis
We utilized the SPSS 22.0 software to accomplish the following statistical analyses.
The continuous data were presented as the means with standard deviations and the medians with interquartile ranges (IQRs), while the categorical data were presented as the patient numbers with percentages.
We employed the Pearson’s chi-squared test or Fisher’s exact-test to compare the categorical variables and the Mann-Whitney U-test to compare the continuous variables. In addition, the effects of the presence of pleural adhesions on the length of hospital stay were assessed by the Kaplan-Meier analysis using a log-rank test.
For multivariate analysis, we would analyze the risk factors for conversion to thoracotomy based on the entire cohort. However, when exploring the predisposing factors for postoperative complications, we would exclude the converted cases and analyze the clinical data of the remaining patients undergoing completely VATS lobectomy, in order to eliminate potential confounding bias caused from a close connection between conversion to thoracotomy and postoperative outcomes.
All dichotomous variables with univariate P<0.05 would be involved into the multivariate logistic-regression models, which utilized the Hosmer-Lemeshow test for precision and the C-statistic for calibration, to identify the independent risk factors for conversion to thoracotomy and postoperative complications, respectively. Odds ratio (OR) with the corresponding 95% confidence interval (CI) would also be extrapolated.
The statistical significance would be revealed in both univariate and multivariate analysis if P<0.05.
Results
Patient characteristics and outcomes
Baseline information
During the study period, there were 593 patients undergoing VATS lobectomy for primary NSCLCs met the eligibility criteria and included in this study. Their perioperative characteristics are presented in Table 1.
Table 1. Patient characteristics and outcomes.
| Characteristics | Total (n=593) | Pleural adhesions | P value | |
|---|---|---|---|---|
| Presence (n=154) | Absence (n=439) | |||
| Basic information | ||||
| Age (years) | 0.017 | |||
| Mean ± SD | 63.1±8.4 | 64.7±6.6 | 62.5±8.9 | |
| Median (IQR) | 63 (58.0–69.0) | 64 (61.0–69.0) | 63 (57.0–69.0) | |
| Gender (Male, %) | 337 (56.8) | 98 (63.6) | 239 (54.4) | 0.058 |
| Body mass index (kg/m2) | 0.93 | |||
| Mean ± SD | 23.4±3.0 | 23.4±2.8 | 23.4±3.1 | |
| Median (IQR) | 23.3 (21.2–25.4) | 23.2 (21.6–25.6) | 23.3 (20.9–25.4) | |
| Smoking history, n (%) | 276 (46.5) | 92 (59.7) | 184 (41.9) | <0.001 |
| Preoperative comorbidities, n (%) | ||||
| Chronic obstructive pulmonary disease | 137 (23.1) | 48 (31.2) | 89 (20.3) | 0.006 |
| Tuberculosis | 55 (9.3) | 11 (7.1) | 44 (10.0) | 0.29 |
| Preoperative respiratory infection | 55 (9.3) | 21 (13.6) | 34 (7.7) | 0.030 |
| Asthma | 11 (1.9) | 2 (1.3) | 9 (2.1) | 0.74 |
| Hypertension | 203 (34.2) | 52 (33.8) | 151 (34.4) | 0.89 |
| Diabetes mellitus | 71 (12.0) | 13 (8.4) | 58 (13.2) | 0.12 |
| Coronary heart disease | 69 (11.6) | 23 (14.9) | 46 (10.5) | 0.14 |
| Previous malignancy | 48 (8.1) | 13 (8.4) | 35 (8.0) | 0.85 |
| Steroid use | 31 (5.2) | 11 (7.1) | 20 (4.6) | 0.22 |
| Combined treatment modalities, n (%) | ||||
| Neoadjuvant therapy | 52 (8.8) | 14 (9.1) | 38 (8.7) | 0.87 |
| Adjuvant chemotherapy | 188 (31.7) | 53 (34.4) | 135 (30.8) | 0.40 |
| Intraoperative parameters | ||||
| Tumor location, n (%) | 0.13 | |||
| Right upper lobe | 209 (35.2) | 59 (38.3) | 150 (34.2) | |
| Left upper lobe | 126 (21.2) | 27 (17.5) | 99 (22.6) | |
| Right lower lobe | 117 (19.7) | 28 (18.2) | 89 (20.3) | |
| Left lower lobe | 83 (14.0) | 29 (18.8) | 54 (12.3) | |
| Right middle lobe | 58 (9.8) | 11 (7.1) | 47 (10.7) | |
| Pleural invasion, n (%) | 0.51 | |||
| None | 287 (48.4) | 71 (46.1) | 216 (49.2) | |
| Visceral | 267 (45.0) | 70 (45.5) | 197 (44.9) | |
| Parietal | 39 (6.6) | 13 (8.4) | 26 (5.9) | |
| Pulmonary fissure completeness, n (%) | <0.001 | |||
| Complete | 394 (66.4) | 74 (51.9) | 320 (72.9) | |
| Incomplete | 199 (33.6) | 80 (48.1) | 119 (27.1) | |
| Perioperative blood transfusion | 6 (1.0) | 2 (1.3) | 4 (0.9) | 0.65 |
| Estimated intraoperative blood loss (mL) | 0.057 | |||
| Mean ± SD | 79.0±145.5 | 105.0±217.1 | 70.0±106.5 | |
| Median (IQR) | 50 (20.0–100.0) | 50 (20.0–100.0) | 50 (20.0–80.0) | |
| Operation time (min) | 0.089 | |||
| Mean ± SD | 114.6±61.9 | 125.9±71.2 | 110.4±57.5 | |
| Median (IQR) | 110 (80.0–145.0) | 120 (71.0–180.0) | 110 (80.0–140.0) | |
| Conversion to thoracotomy (%) | 19 (3.2) | 14 (9.1) | 5 (1.1) | <0.001 |
| Pathological parameters | ||||
| Histology, n (%) | 0.79 | |||
| Adenocarcinoma | 459 (77.4) | 118 (76.6) | 341 (77.7) | |
| Squamous cell carcinoma | 134 (22.6) | 36 (23.4) | 98 (22.3) | |
| Differentiation degree, n (%) | 0.12 | |||
| Low | 110 (18.5) | 35 (22.7) | 75 (17.1) | |
| Moderate/high | 483 (81.5) | 119 (77.3) | 364 (82.9) | |
| Tumor invasion (T-stage), n (%) | 0.54 | |||
| T1 | 254 (42.8) | 65 (42.3) | 189 (43.1) | |
| T2-3 | 339 (57.2) | 89 (57.7) | 250 (56.9) | |
| Lymph node metastasis (N-stage), n (%) | 0.24 | |||
| N0 | 481 (81.1) | 120 (77.9) | 361 (82.2) | |
| N1-2 | 112 (18.9) | 34 (22.1) | 78 (17.8) | |
| TNM-stage, n (%) | 0.10 | |||
| I | 444 (74.9) | 107 (69.5) | 337 (76.8) | |
| II–IIIa | 149 (25.1) | 47 (30.5) | 102 (23.2) | |
| Postoperative complications, n (%) | ||||
| Overall morbidity | 173 (29.2) | 47 (30.5) | 126 (28.7) | 0.67 |
| Cardiopulmonary complications | 107 (18.0) | 30 (19.5) | 77 (17.5) | 0.59 |
| Surgical complications | 100 (16.9) | 37 (24.0) | 63 (14.4) | 0.006 |
IQR, interquartile range; SD, standard deviation
Our cohort consists of 337 (56.8%) male and 256 (33.2%) female patients, with the mean age of 63.1±8.4 years (median, 63 years; IQR, 38–82 years) and mean BMI of 23.4±3.0 kg/m2 (median, 23.3 kg/m2; IQR, 21.2–25.4 kg/m2), and 276 of them were active smokers (46.5%). There were totally 402 patients (67.8%) suffered from one or more underlying comorbidities, as shown in Table 1. The malignant lesions located in the right upper lobe (n=209) accounted for the largest proportion of our cohort (35.2%). Lung adenocarcinoma and squamous cell carcinoma were diagnosed in 459 (77.4%) and 134 (22.6%) patients, respectively. The great majority of patients (n=526) were diagnosed with stage I–II NSCLCs (88.7%). Lymph node metastasis was confirmed in 112 patients postoperatively (18.9%) by the pathological criteria.
Patient percentages distributed within five ranges of pleural adhesions are shown in Figure 2. There were 259 patients with no pleural adhesion, accounting for 43.7% of all cases, followed by 180 patients with light adhesions (30.4%), 100 patients with moderate adhesions (16.9%), 44 patients with severe adhesions (7.4%) and 10 patients with extremely severe adhesions (1.7%). Therefore, 154 patients with moderate-to-extremely severe pleural adhesions were included in the Group A (26.0%), and the remaining 439 patients with none-to-light pleural adhesions were included in the Group B (74.0%).
Figure 2.
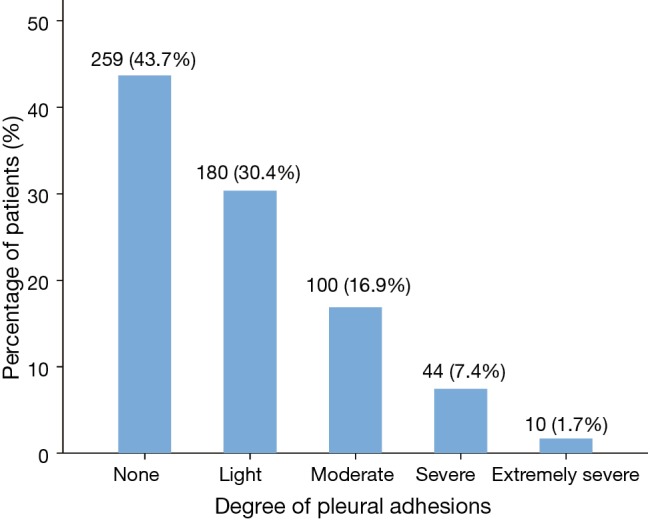
Histogram revealing the proportions of different degrees of pleural adhesions.
Perioperative outcomes
There were 19 patients converted to thoracotomy during VATS lobectomy, with a conversion rate of 3.2%. The causes of conversion were severe fibro-calcified lymph nodes in 7 (36.8%) patients, uncontrollable intraoperative bleeding in 5 (26.3%) patients, dense pleural adhesions in 4 (21.1%) patients, failure of single-lung ventilation in 2 (10.5%) patients and extensive tumor invasion in 1 (5.3%) patient.
A total of 173 patients developed one or more complications postoperatively, with an overall morbidity rate of 29.2%. Moreover, postoperative cardiopulmonary complications were developed in 107 patients, occupying the largest percentages of all types of postoperative complications (18.0%), followed by postoperative surgical complications developed in 100 (16.9%) patients. The incidences of individual complications are presented in Table 2. There was no in-hospital death (Table 1).
Table 2. Individual postoperative complications.
| Complications | Total (n=593) | Pleural adhesions | P value | |
|---|---|---|---|---|
| Presence (n=154) | Absence (n=439) | |||
| Cardiopulmonary complications, n (%) | ||||
| Pneumonia | 64 (10.8) | 24 (15.6) | 40 (9.1) | 0.026 |
| Atelectasis | 31 (5.2) | 13 (8.4) | 18 (4.1) | 0.037 |
| Pleural effusion requiring drainage | 11 (1.9) | 4 (2.6) | 7 (1.6) | 0.49 |
| Pulmonary embolism | 4 (0.7) | 2 (1.3) | 2 (0.5) | 0.28 |
| Acute respiratory distress syndrome | 3 (0.5) | 1 (0.6) | 2 (0.5) | 1.0 |
| Atrial arrhythmia | 7 (1.2) | 3 (1.9) | 4 (0.9) | 0.38 |
| Sinus irregularity needing medical care | 6 (1.0) | 4 (2.6) | 2 (0.5) | 0.069 |
| Ventricular arrhythmia | 2 (0.3) | 2 (1.3) | 0 (0.0) | 0.067 |
| Surgical complications, n (%) | ||||
| Prolonged air leak (>5 days) | 67 (11.3) | 25 (16.2) | 42 (9.6) | 0.025 |
| Subcutaneous emphysema | 44 (7.4) | 17 (11.0) | 27 (6.2) | 0.046 |
| Pneumothorax | 23 (3.9) | 12 (7.8) | 11 (2.5) | 0.003 |
| Chylothorax | 16 (2.7) | 6 (3.9) | 10 (2.3) | 0.38 |
| Wound infection | 2 (0.3) | 0 (0.0) | 2 (0.5) | 1.0 |
| Hemothorax | 1 (0.2) | 1 (0.6) | 0 (0.0) | 0.26 |
| Bronchial fistula | 1 (0.2) | 1 (0.6) | 0 (0.0) | 0.26 |
| Other complications, n (%) | ||||
| Gastrointestinal discomforts | 7 (1.2) | 2 (1.3) | 5 (1.1) | 0.88 |
| Delirium | 4 (0.7) | 0 (0.0) | 4 (0.9) | 0.58 |
| Urinary retention | 2 (0.3) | 0 (0.0) | 2 (0.5) | 1.0 |
In addition, the mean length of chest tube drainage and the length of stay of the entire cohort were 4.4±3.4 days (median, 3 days; IQR, 2–5 days) and 6.9±4.0 days (median, 6 days; IQR, 4–8 days), respectively.
Association between presence of pleural adhesions and patient characteristics
When compared to patients without pleural adhesions (Group B), patients with pleural adhesions (Group A) had a significantly higher mean age (P=0.017) and higher ratios of smoking history (P<0.001), COPD (P=0.006), preoperative respiratory infections (P=0.030) and incomplete inter-lobar fissures (P<0.001). No difference was found in the other baseline characteristics between these two groups (Table 1).
Effects of presence of pleural adhesions on perioperative complications
The patient percentages of four major perioperative events distributed in different ranges of pleural adhesions are shown in Figure 3. Both the incidences of conversion to thoracotomy and postoperative surgical complications showed a tendency to increase with the increasing severity of pleural adhesions. While the rates of overall morbidity and postoperative cardiopulmonary complications showed a little downward trend until reaching their peaks in patients with severe-to-extremely severe pleural adhesions.
Figure 3.
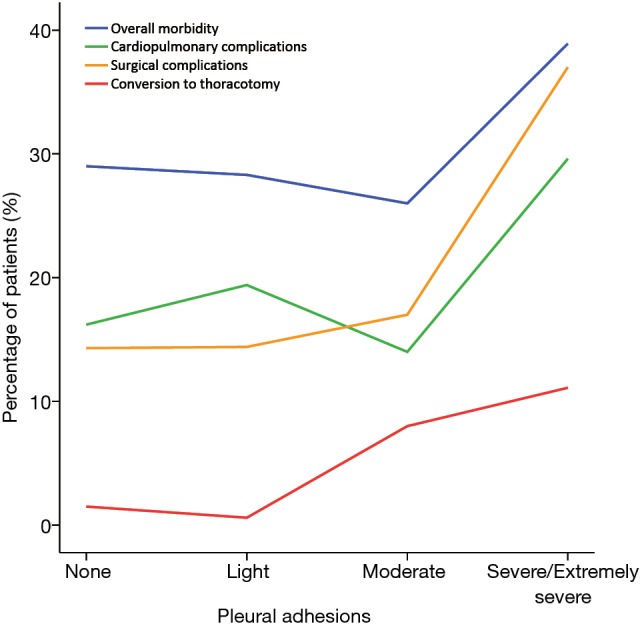
Tendency of major perioperative events with the increasing severity of pleural adhesions.
Compared to Group B patients, Group A patients had the significantly higher incidences of conversion (9.1% vs. 1.1%; P<0.001) and surgical complications (24.0% vs. 14.4%; P=0.006). However, no significant difference was found in the overall morbidity (30.5% vs. 28.7%; P=0.67) or cardiopulmonary complications (19.5% vs. 17.5%; P=0.59) between these two groups (Table 1).
We found that the incidences of prolonged air leak (PAL: 16.2% vs. 9.6%; P=0.025), pneumonia (15.6% vs. 9.1%; P=0.026), subcutaneous emphysema (11.0% vs. 6.2%; P=0.046), atelectasis (8.4% vs. 4.1%; P=0.037) and pneumothorax (7.8% vs. 2.5%; P=0.003) in Group A patients were significantly higher than those in Group B patients. No significant difference was found in any other complication between these two groups (Table 2).
Effects of presence of pleural adhesions on the length of hospital stay
A Kaplan-Meier curve revealing the length of chest tube drainage between Group A and B is presented in Figure 4. The log-rank test showed that the days with pleural drainage of Group A patients (mean, 5.4 days; 95% CI, 4.7–6.1 days) were significantly longer than those of Group B patients (mean, 4.1 days; 95% CI, 3.8–4.4 days) (log-rank P<0.001).
Figure 4.
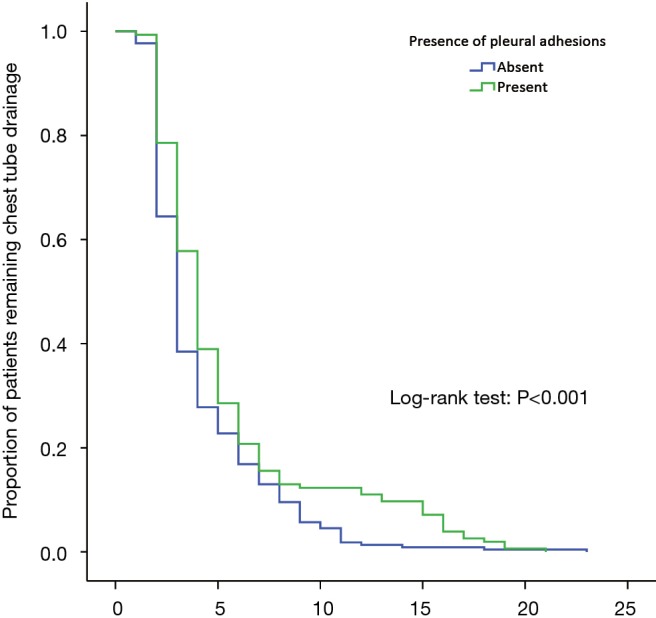
Kaplan-Meier curve for the length of chest tube drainage between patients with and without pleural adhesions.
Figure 5 shows the length of stay of Group A and B. The Kaplan-Meier analysis indicated that Group A patients (mean, 7.6 days; 95% CI, 6.8–8.4 days) had the significantly prolonged length of stay than that of Group B patients (mean, 6.6 days; 95% CI, 6.3–6.9 days) (log-rank P=0.032).
Figure 5.
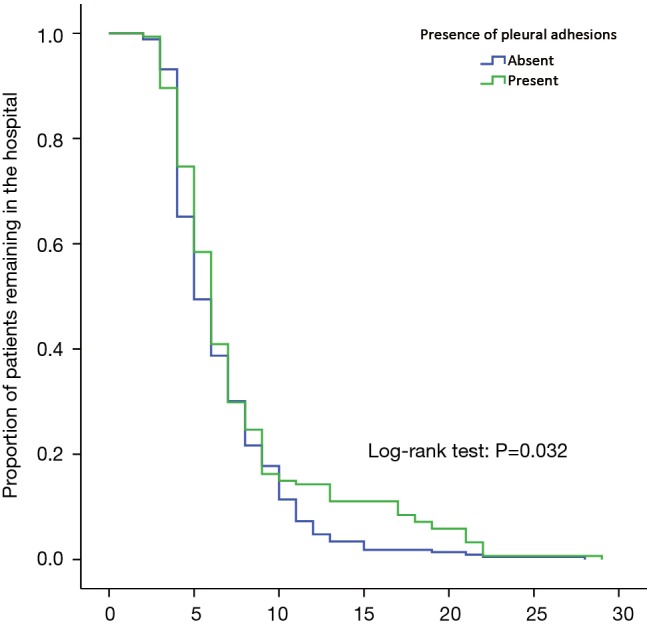
Kaplan-Meier curve for the length of stay between patients with and without pleural adhesions.
Multivariate analyses
On the basis of univariate results (Tables 1,3), we further performed a multivariate logistic-regression analysis to determine the value of the presence of pleural adhesions on serving as an independent risk factor for conversion to thoracotomy and postoperative surgical complications, respectively.
Table 3. Perioperative baseline characteristics for 19 converted patients and 574 completely VATS patients.
| Characteristics | Total (n=593) | Converted patients (n=19) | Completely VATS patients (n=574) | P value | |
|---|---|---|---|---|---|
| With PSCs (n=86) | Without PSCs (n=488) | ||||
| Basic information | |||||
| Age (years) | 0.002 | ||||
| Mean ± SD | 63.1±8.4 | 69.6±6.0 | 63.6±7.5 | 62.7±8.5 | |
| Median (IQR) | 63 (58.0–69.0) | 71 (64.0–75.0) | 62 (59.0–69.0) | 63 (57.0–69.0) | |
| Gender (Male, %) | 337 (56.8) | 16 (84.2) | 65 (75.6) | 256 (52.5) | <0.001 |
| Body mass index (kg/m2) | 0.033 | ||||
| Mean ± SD | 23.4±3.0 | 24.5±2.9 | 22.9±3.2 | 23.5±3.0 | |
| Median (IQR) | 23.3 (21.2–25.4) | 24.5 (22.8–27.3) | 22.5 (20.6–24.6) | 23.3 (21.3–25.4) | |
| Smoking history (%) | 276 (46.5) | 12 (63.2) | 55 (64.0) | 209 (42.8) | <0.001 |
| Preoperative comorbidities, n (%) | |||||
| Chronic obstructive pulmonary disease | 137 (23.1) | 11 (57.9) | 30 (34.9) | 96 (19.7) | <0.001 |
| Tuberculosis | 55 (9.3) | 4 (21.1) | 8 (9.3) | 43 (8.8) | 0.29 |
| Preoperative respiratory infection | 55 (9.3) | 8 (42.1) | 14 (16.3) | 33 (6.8) | <0.001 |
| Asthma | 11 (1.9) | 2 (10.5) | 2 (2.3) | 7 (1.4) | 0.11 |
| Hypertension | 203 (34.2) | 5 (26.3) | 37 (43.0) | 161 (33.0) | 0.15 |
| Diabetes mellitus | 71 (12.0) | 1 (5.3) | 9 (10.5) | 61 (12.5) | 0.52 |
| Coronary heart disease | 69 (11.6) | 4 (21.1) | 12 (14.0) | 53 (10.9) | 0.35 |
| Previous malignancy | 48 (8.1) | 4 (21.1) | 6 (7.0) | 38 (7.8) | 0.19 |
| Steroid use | 31 (5.2) | 3 (15.8) | 7 (8.1) | 21 (4.3) | 0.082 |
| Combined treatment modalities, n (%) | |||||
| Neoadjuvant therapy | 52 (8.8) | 2 (10.5) | 9 (10.5) | 41 (8.4) | 0.80 |
| Adjuvant chemotherapy | 188 (31.7) | 6 (31.6) | 35 (40.7) | 147 (30.1) | 0.15 |
| Intraoperative parameters | |||||
| Tumor location, n (%) | 0.93 | ||||
| Right upper lobe | 209 (35.2) | 6 (31.6) | 25 (29.1) | 150 (34.2) | |
| Left upper lobe | 126 (21.2) | 4 (21.1) | 19 (22.1) | 103 (21.1) | |
| Right lower lobe | 117 (19.7) | 5 (26.3) | 19 (22.1) | 93 (19.1) | |
| Left lower lobe | 83 (14.0) | 2 (10.5) | 12 (14.0) | 69 (14.1) | |
| Right middle lobe | 58 (9.8) | 2 (10.5) | 11 (12.8) | 45 (9.2) | |
| Pleural invasion, n (%) | 0.087 | ||||
| None | 287 (48.4) | 6 (31.6) | 35 (40.7) | 246 (50.4) | |
| Visceral | 267 (45.0) | 9 (47.4) | 45 (52.3) | 213 (43.6) | |
| Parietal | 39 (6.6) | 4 (21.1) | 6 (7.0) | 29 (5.9) | |
| Pleural adhesion, n (%) | <0.001 | ||||
| Presence | 154 (26.0) | 14 (73.7) | 27 (31.4) | 113 (23.2) | |
| Absence | 439 (74.0) | 5 (26.3) | 59 (68.6) | 375 (76.8) | |
| Pulmonary fissure completeness, n (%) | 0.001 | ||||
| Complete | 394 (66.4) | 6 (31.6) | 52 (60.5) | 336 (68.9) | |
| Incomplete | 199 (33.6) | 13 (68.4) | 34 (39.5) | 152 (31.1) | |
| Estimated intraoperative blood loss (mL) | <0.001 | ||||
| Mean ± SD | 79.0±145.5 | 535.7±535.9 | 80.0±73.2 | 60.0±63.5 | |
| Median (IQR) | 50 (20.0–100.0) | 500 (100.0–800.0) | 50 (30.0–100.0) | 50 (20.0–80.0) | |
| Operation time (min) | <0.001 | ||||
| Mean ± SD | 114.6±61.9 | 229.4±48.2 | 129.0±72.6 | 106.7±54.4 | |
| Median (IQR) | 110 (80.0–145.0) | 210 (190.0–280.0) | 120 (90.0–160.0) | 100 (70.0–140.0) | |
| Pathological parameters | |||||
| Histology, n (%) | 0.64 | ||||
| Adenocarcinoma | 459 (77.4) | 13 (68.4) | 66 (76.7) | 380 (77.9) | |
| Squamous cell carcinoma | 134 (22.6) | 6 (31.6) | 20 (23.3) | 108 (22.1) | |
| Differentiation degree, n (%) | 0.090 | ||||
| Low | 110 (18.5) | 2 (26.7) | 23 (26.7) | 85 (17.4) | |
| Moderate/high | 483 (81.5) | 17 (89.5) | 63 (73.3) | 403 (82.6) | |
| Tumor invasion (T-stage), n (%) | 0.001 | ||||
| T1 | 254 (42.8) | 4 (21.1) | 24 (27.9) | 226 (46.3) | |
| T2-3 | 339 (57.2) | 15 (78.9) | 62 (72.1) | 262 (53.7) | |
| Lymph node metastasis (N-stage), n (%) | 0.24 | ||||
| N0 | 481 (81.1) | 15 (78.9) | 64 (74.4) | 402 (82.4) | |
| N1-2 | 112 (18.9) | 4 (21.1) | 22 (25.6) | 86 (17.6) | |
| TNM-stage, n (%) | 0.10 | ||||
| I | 444 (74.9) | 13 (68.4) | 56 (65.1) | 375 (76.8) | |
| II–IIIa | 149 (25.1) | 6 (31.6) | 30 (34.9) | 113 (23.2) | |
| Postoperative complications, n (%) | <0.001 | ||||
| Overall morbidity | 173 (29.2) | 16 (84.2) | 86 (100.0) | 71 (14.5) | |
| Cardiopulmonary complications | 107 (18.0) | 10 (52.6) | 36 (41.9) | 61 (12.5) | |
| Surgical complications | 100 (16.9) | 14 (73.7) | 86 (100.0) | 0 (0.0) | |
VATS, video-assisted thoracoscopic surgery; IQR, interquartile range; PSC, postoperative surgical complication; SD, standard deviation.
Conversion to thoracotomy
There were six categorical parameters initially identified to be significantly associated with conversion to thoracotomy, as shown in Table 4. Finally, the logistic-regression model involving these variables, with a C-statistic of 0.83 (95% CI, 0.72–0.93; P<0.001), indicated that the history of PRI (OR =4.35; 95% CI, 1.46–12.97; P=0.008) and the presence of pleural adhesions (OR =5.49; 95% CI, 1.79–16.83; P=0.003) were strongly independent predictors for conversion to thoracotomy.
Table 4. Multivariate analysis on predictors for conversion to thoracotomy.
| Estimated factors | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Male gender | 2.32 | 0.62–8.62 | 0.21 |
| Age (≥65 vs. <65 years) | 2.12 | 0.76–5.92 | 0.15 |
| Chronic obstructive pulmonary disease | 2.34 | 0.83–6.61 | 0.11 |
| Preoperative respiratory infection | 4.35 | 1.46–12.97 | 0.008 |
| Presence of pleural adhesions | 5.49 | 1.79–16.83 | 0.003 |
| Incomplete pulmonary fissures | 2.32 | 0.76–6.53 | 0.14 |
CI, confidence interval.
Postoperative surgical complications
Table 5 shows eight categorical variables that correlate with the risk of surgical complications based on a cohort of 574 completely VATS lobectomies. The C-statistic for this logistic-regression model was 0.76 (95% CI, 0.69–0.83; P<0.001). Finally, we found that the history of COPD (OR =1.74; 95% CI, 1.04–2.91; P=0.035) and preoperative respiratory inflammation (OR =2.89; 95% CI, 1.34–6.20; P=0.007), presence of pleural adhesions (OR =1.94; 95% CI, 1.05–3.55; P=0.033) and operation time ≥110 min (OR =2.17; 95% CI, 1.18–4.01; P=0.013) could be predictive of postoperative surgical complications after eliminating the bias risks from other confounding factors.
Table 5. Multivariate analysis on predictors for postoperative surgical complications in 574 completely VATS patients.
| Estimated factors | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Male gender | 1.92 | 0.72–5.13 | 0.19 |
| Smoking history | 1.26 | 0.64–2.47 | 0.51 |
| Chronic obstructive pulmonary disease | 1.74 | 1.04–2.91 | 0.035 |
| Preoperative respiratory infection | 2.89 | 1.34–6.20 | 0.007 |
| Presence of pleural adhesions | 1.94 | 1.05–3.55 | 0.033 |
| Operation time (≥110 vs. <110 min) | 2.17 | 1.18–4.01 | 0.013 |
| Tumor invasion (T2-3 vs. T1) | 1.71 | 0.89–3.26 | 0.11 |
| TNM-stage (II–IIIa vs. I) | 1.06 | 0.54–2.06 | 0.87 |
VATS, video-assisted thoracoscopic surgery; CI, confidence interval.
Discussion
Key results
The main finding of the present study was that the presence of pleural adhesions was significantly associated with conversion to thoracotomy and postoperative surgical complications in patients undergoing VATS lobectomy for NSCLC. Patients with pleural adhesions had the significantly higher incidences of PAL, pneumonia, subcutaneous emphysema, atelectasis and pneumothorax than those of patients without pleural adhesions. Both the length of stay and chest tube drainage duration in patients with pleural adhesions were significantly longer than those in patients without pleural adhesions. Finally, we confirmed the validity of the presence of pleural adhesions on serving as an independent risk factor for conversion to thoracotomy and surgical complications after minimizing the confounding influence by multivariate logistic-regression analyses.
Interpretations
Pleural adhesions consist of a thin film of connective tissue, a thick fibro-calcific bridge containing small vessels and nerves, or a direct adhesion between two organ surfaces. It is commonly perceived that pleural adhesions may be more frequently encountered in the patients with a heavy smoking history and respiratory inflammations when entering the chest cavity, and meanwhile, predispose to the poor operative visualization, intraoperative bleeding, lung parenchymal damage and higher morbidity risks (6,9). Thus, a technically demanding operation will be highly expected for thoracic surgeons to get over this surgical difficulty.
In our series, the presence of pleural adhesions was found in 26.0% of all enrolled VATS cases, which occupied a lower percentage than that in patients undergoing thoracotomy in the literature (6,9). The history of COPD and respiratory infections was more frequent in the patients with pleural adhesions, revealing a significant association between pleural adhesions and inflammatory responses of the chest. In addition, we found that the patients with pleural adhesions had less-discrete pulmonary fissures. That might be because the inflammatory process rarely spared the visceral pleura within the inter-lobar fissures. Thus, as Kouritas et al. (6) suggested, the presence of pleural adhesions is not a rare finding in elective pulmonary resections.
There were only 3.2% (n=19) in our series required into conversion to thoracotomy, which compared favorably with the conversion rate of 6.2–23.0% in the literature (5). The possible reason might be that we enrolled the patients in a later period of our learning cure. Conversion rate is supposed to be dropped with the increase in VATS experiences. Diffuse adhesions obliterating the pleural cavity have been considered as a contraindication to VATS lobectomy, although they are usually not the most common cause of conversion (5,6). There were only 4 converted cases out of 19 in our cohort caused by dense pleural/hilar adhesions. Thus, the technical feasibility of VATS lobectomy can be achieved by the increase in VATS expertise.
Prior studies had shown that the presence of pleural adhesions was significantly associated with the risk of PAL, although their classification was not described in detail (17). In an earlier cohort of 59 patients undergoing thoracoscopic reduction pneumoplasty, Mineo et al. (9) proposed a simplified cut-off of larger than 30% of dense pleural adhesions over the inner area of pleural cavity to indicate the presence of pleural adhesions. The most recent study conducted by Kouritas et al. (6) calculated the involvement of each lobar surface as a percentage of the total pleural cavity (>20%) to suggest the presence of pleural adhesions. However, the influence caused by the strength of pleural adhesions was not sufficiently evaluated in that study.
In our study, we quantified the degrees of pleural adhesions based on an intraoperative assessment of the percentages of both dense pleural adhesions requiring sharp dissections and overall pleural adhesions over the inner surface of the operative pleural cavity. The presence of pleural adhesions was defined as the moderate to extremely severe adhesions obliterating the pleural cavity. Then, a comparative analysis was further conducted to show the demographic differences in perioperative outcomes between patients with and without pleural adhesions.
We found that the patients with pleural adhesions had the significantly higher rates of conversion to thoracotomy and postoperative surgical complications. Specifically, the incidences of PAL, pneumonia, subcutaneous emphysema, atelectasis and pneumothorax were all significantly higher in the patients with pleural adhesions. Besides, the length of chest tube drainage and length of stay were both significantly prolonged in the patients with pleural adhesions, possibly due to their higher probability of perioperative morbidity. Finally, after minimizing the confounding bias risks by multivariate logistic-regression analyses, the presence of pleural adhesions itself was verified as an independent risk factor for conversion to thoracotomy and surgical complications following completely VATS lobectomy. We speculate that the following three reasons may be considered when trying to explain this phenomenon.
First of all, pleural adhesions can significantly increase the surgical difficulty related to unexpected conversion, especially when performing the adhesiolysis with sharp dissections to deal with dense pleural adhesions containing vascular and nervous bundles, possibly resulting in uncontrollable intraoperative bleeding. Division of pleural adhesions between lung surface and parietal pleura can easily produce an air leak, because this procedure is usually finished by electrocautery or by blunt or sharp dissection. The incidence of alveolar air leak generally decreases within several days after surgery, but there are still 8–15% of patients developed a PAL that persists beyond the normal hospitalization (17-19). A devastating PAL, sometimes with its induced pneumothorax, can seriously impair the lung recruitment, which can further aggravate the pulmonary edema and atelectasis, resulting in the development of pneumonia. Therefore, the presence of pleural adhesions was also found to be associated with the risk of pneumonia and atelectasis.
Secondly, another important factor that may predispose to the occurrence of surgical complications is the existence of respiratory inflammations. In our series, we found a generally higher proportion of dense pleural adhesions in the active smokers and in the patients who had the history of COPD and respiratory infections, which may be attributable to the excessive exudation induced by chronic inflammations. Therefore, the smoking history and the underlying respiratory inflammations in the patients with pleural adhesions, which cause the reduced area of gas exchange and the inadequate distance from alveolar surface to capillary endothelium, can further aggravate the formation of pneumonia and several surgical complications, although their confounding influence has been minimized by multivariate analysis when evaluating the predictive roles of pleural adhesions (17,20,21).
Finally, thoracic surgeons are usually forced to manipulate more meticulously and slowly, resulting in a prolonged duration of surgery, to avoid the uncontrollable bleeding and stress responses when solving dense pleural adhesions. Therefore, a prolonged ventilation, more frequent alternation of surgical instruments and movement through the operating portals can promote an air seepage towards the tears of subcutaneous tissue around the incisions, increasing the risk of subcutaneous emphysema. Besides, a mismatch between the distorted shapes of the chest wall and the underlying lung induced by a prolonged anesthesia time can also lead to severe atelectasis, which is closely related to postoperative pneumonia (22). That may be another one explanation supported by our findings.
Generalizability
According to our study results, it may be more sufficient to incorporate the presence of pleural adhesions into an assessment model to stratify the surgical difficulty and morbidity risk. That may assist to decide the best therapeutic strategy for patients with operable NSCLC. In addition, for young surgeons, our results may also help to select the participants in a teaching program of VATS techniques or in their early learning curve, in order to avoid adverse events and train themselves more effectively.
However, we must recognize that a convincing grading system of pleural adhesions with clinical significance has not yet been produced. There are ever some assessment tools that use the extent and macroscopic appearance to provide a common language for classification of adhesions among general surgeons but fail to be widely implemented (9,23). Nowadays, thoracic surgeons still report the pleural adhesions subjectively based on their own experience and capability. Therefore, a grading system that has the ability to alert surgeons to potential perioperative events and provide tailor-made postoperative care to the patients with pleural adhesions still remains urgently needed.
Limitations
The following several major limitations in this study should be sufficiently acknowledged.
Firstly, our study was subject to the inherent limitations of any single-center retrospective analysis. Potential selection bias might complicate our findings, although a multivariate logistic-regression analysis had involved as many clinicopathological variables as possible to minimize the bias risks from potential confounding factors. Furthermore, our monocentric sample size is relatively small, resulting in a slightly low analytical power. Thus, a propensity-score matched analysis based on a large-scale cohort of patients will be more reliable in the future.
Secondly, there is no standardized grading system on the classification of pleural adhesions until now. In this study, we tried to establish a modified classification system with reference to several assessment tools reported in previous studies and stratified the patients into five different degrees of pleural adhesions (6,9). But essentially, all of them were evaluated subjectively based on the surgeons’ experience and capability.
Thirdly, the conversion and morbidity rates can also depend on the surgeons’ expertise. However, it may be difficult to analyze this artificial factor quantitatively. This is another one important limitation that cannot be ignored.
Fourthly, according to our study objectives, only VATS lobectomies were analyzed, so our findings may not be generalized to the sub-lobar resections.
Finally, complete laboratorial indexes were not available in all enrolled patients, so that we did not included them into the assessment.
Conclusions
In conclusion, the present study demonstrates that the presence of pleural adhesions can be regarded as an independent risk factor for conversion to thoracotomy and surgical complications following VATS lobectomy for NSCLC. Moreover, the presence of pleural adhesions is also significantly associated with the prolonged length of chest tube drainage and length of stay following VATS lobectomy. Our study calls for a widely accepted grading system from the community of thoracic surgeons to describe the pleural adhesions, which may be more helpful to stratify the surgical risk. More large-scale multicenter analyses are needed to further confirm and modify our findings in the future.
Table S1. STROBE statement—checklist of items that should be included in reports of cohort studies.
| Item detail | Item no. | Recommendation | Reported on page# |
|---|---|---|---|
| Title and abstract | 1 | Indicate the study’s design with a commonly used term in the title or the abstract | 416 |
| Provide in the abstract an informative and balanced summary of what was done and what was found | 416 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 417 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 417 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 417 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 417 |
| Participants | 6 | Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up | 417 |
| For matched studies, give matching criteria and number of exposed and unexposed | |||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 418 |
| Data sources/measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 418 |
| Bias | 9 | Describe any efforts to address potential sources of bias | 418–420 |
| Study size | 10 | Explain how the study size was arrived at | 417–419 (Figure 1) |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 418, 419 (Figure 1) |
| Statistical methods | 12 | Describe all statistical methods, including those used to control for confounding | 419, 420 |
| Describe any methods used to examine subgroups and interactions | |||
| Explain how missing data were addressed | |||
| If applicable, explain how loss to follow-up was addressed | |||
| Describe any sensitivity analyses | |||
| Results | |||
| Participants | 13* | Report numbers of individuals at each stage of study—e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 420, 423 (Figure 2) |
| Give reasons for non-participation at each stage | |||
| Consider use of a flow diagram | |||
| Descriptive data | 14* | Give characteristics of study participants (e.g., demographic, clinical, social) and information on exposures and potential confounders | 420–422 (Table 1) |
| Indicate number of participants with missing data for each variable of interest | |||
| Summarise follow-up time (e.g., average and total amount) | |||
| Outcome data | 15* | Report numbers of outcome events or summary measures over time | 420–423, 426, 427 (Tables 1–3) |
| Main results | 16 | Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g., 95% CI). Make clear which confounders were adjusted for and why they were included | 420, 423, 424 (Figures 3–5), 421, 422, 426, 427 (Tables 1–3) |
| Report category boundaries when continuous variables were categorized | |||
| If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | |||
| Other analyses | 17 | Report other analyses done—e.g., analyses of subgroups and interactions, and sensitivity analyses | 424, 425, 428 (Tables 4,5) |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | 425 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 429, 430 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 425, 428, 429 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 429 |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 430 |
*, give information separately for exposed and unexposed groups; CI, confidence interval. An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at http://www.strobe-statement.org.
Acknowledgements
Special thanks to the English language polishing contributions from Mr. Stanley Crawford, from the Institution of Medical English, West China Medical Center, Sichuan University, Chengdu, China.
Funding: This study was supported by Foundation of Science and Technology support plan Department of Sichuan Province (2015SZ0158).
Ethical Statement: Our regional ethics committee approved this study and waived the need for informed consent (ID: 2016-255).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Li S, Wang Z, Huang J, et al. Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: does the 'obesity paradox' really exist? Eur J Cardiothorac Surg 2017;51:817-28. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Zhou K, Du H, et al. Body surface area is a novel predictor for surgical complications following video-assisted thoracoscopic surgery for lung adenocarcinoma: a retrospective cohort study. BMC Surg 2017;17:69. 10.1186/s12893-017-0264-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laursen LØ, Petersen RH, Hansen HJ, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg 2016;49:870-5. 10.1093/ejcts/ezv205 [DOI] [PubMed] [Google Scholar]
- 4.Li S, Zhou K, Wang M, et al. Degree of pulmonary fissure completeness can predict postoperative cardiopulmonary complications and length of hospital stay in patients undergoing video-assisted thoracoscopic lobectomy for early-stage lung cancer. Interact Cardiovasc Thorac Surg 2018;26:25-33. 10.1093/icvts/ivx261 [DOI] [PubMed] [Google Scholar]
- 5.Li SJ, Zhou K, Shen C, et al. Body surface area: a novel predictor for conversion to thoracotomy in patients undergoing video-assisted thoracoscopic lung cancer lobectomy. J Thorac Dis 2017;9:2383-96. 10.21037/jtd.2017.07.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kouritas VK, Kefaloyannis E, Tcherveniakov P, et al. Do pleural adhesions influence the outcome of patients undergoing major lung resection? Interact Cardiovasc Thorac Surg 2017;25:613-9. 10.1093/icvts/ivx173 [DOI] [PubMed] [Google Scholar]
- 7.Kouritas VK, Tepetes K, Christodoulides G, et al. Permeability alterations after surgical trauma in normal rabbit peritoneum. Eur Surg Res 2010;45:113-9. 10.1159/000318146 [DOI] [PubMed] [Google Scholar]
- 8.Augustin F, Maier HT, Weissenbacher A, et al. Causes, predictors and consequences of conversion from VATS to open lung lobectomy. Surg Endosc 2016;30:2415-21. 10.1007/s00464-015-4492-3 [DOI] [PubMed] [Google Scholar]
- 9.Mineo TC, Pompeo E, Rogliani P, et al. Thoracoscopic reduction pneumoplasty for severe emphysema: do pleural adhesions affect outcome? Thorac Cardiovasc Surg 1999;47:288-92. 10.1055/s-2007-1013160 [DOI] [PubMed] [Google Scholar]
- 10.Von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806-8. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez FG, Falcoz PE, Kozower BD, et al. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg 2015;99:368-76. 10.1016/j.athoracsur.2014.05.104 [DOI] [PubMed] [Google Scholar]
- 12.Thomas PA, Berbis J, Falcoz PE, et al. National perioperative outcomes of pulmonary lobectomy for cancer: the influence of nutritional status. Eur J Cardiothorac Surg 2014;45:652-9. 10.1093/ejcts/ezt452 [DOI] [PubMed] [Google Scholar]
- 13.Zhao K, Mei J, Xia C, et al. Prolonged air leak after video-assisted thoracic surgery lung cancer resection: risk factors and its effect on postoperative clinical recovery. J Thorac Dis 2017;9:1219-25. 10.21037/jtd.2017.04.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C, Pu Q, Guo C, et al. Non-grasping en bloc mediastinal lymph node dissection for video-assisted thoracoscopic lung cancer surgery. BMC Surg 2015;15:38. 10.1186/s12893-015-0025-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Lai Y, Zhou X, et al. Short-term high-intensity rehabilitation in radically treated lung cancer: a three-armed randomized controlled trial. J Thorac Dis 2017;9:1919-29. 10.21037/jtd.2017.06.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou K, Su J, Lai Y, et al. Short-term inpatient-based high-intensive pulmonary rehabilitation for lung cancer patients: is it feasible and effective? J Thorac Dis 2017;9:4486-93. 10.21037/jtd.2017.10.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunelli A, Cassivi SD, Halgren L. Risk factors for prolonged air leak after pulmonary resection. Thorac Surg Clin 2010;20:359-64. 10.1016/j.thorsurg.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 18.Li SJ, Zhou K, Li YJ, et al. Efficacy of the fissureless technique on decreasing the incidence of prolonged air leak after pulmonary lobectomy: A systematic review and meta-analysis. Int J Surg 2017;42:1-10. 10.1016/j.ijsu.2017.04.016 [DOI] [PubMed] [Google Scholar]
- 19.Li S, Lv W, Zhou K, et al. Does the fissureless technique decrease the incidence of prolonged air leak after pulmonary lobectomy? Interact Cardiovasc Thorac Surg 2017;25:122-4. 10.1093/icvts/ivx061 [DOI] [PubMed] [Google Scholar]
- 20.Singhal S, Ferraris VA, Bridges CR, et al. Management of alveolar air leaks after pulmonary resection. Ann Thorac Surg 2010;89:1327-35. 10.1016/j.athoracsur.2009.09.020 [DOI] [PubMed] [Google Scholar]
- 21.Li SJ, Zhou XD, Huang J, et al. A systematic review and meta-analysis-does chronic obstructive pulmonary disease predispose to bronchopleural fistula formation in patients undergoing lung cancer surgery? J Thorac Dis 2016;8:1625-38. 10.21037/jtd.2016.05.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tusman G, Böhm SH, Warner DO, et al. Atelectasis and perioperative pulmonary complications in high-risk patients. Curr Opin Anaesthesiol 2012;25:1-10. 10.1097/ACO.0b013e32834dd1eb [DOI] [PubMed] [Google Scholar]
- 23.Coccolini F, Ansaloni L, Manfredi R, et al. Peritoneal adhesion index (PAI): proposal of a score for the "ignored iceberg" of medicine and surgery. World J Emerg Surg 2013;8:6. 10.1186/1749-7922-8-6 [DOI] [PMC free article] [PubMed] [Google Scholar]