Abstract
Hydroxychloroquine (HCQ)-induced cardiomyopathy is one of the rare but severe complications following prolonged HCQ use. However, the exact mechanism of HCQ cardiotoxicity remains unclear and it is difficult to identify risk factors. We present a case of twin sisters who both suffered from systemic lupus erythematosus (SLE) and HCQ-induced cardiomyopathy presenting with decompensated heart failure (HF), suggesting that genetic predisposition might be a factor in HCQ-induced cardiomyopathy.
Keywords: Hydroxychloroquine (HCQ), cardiomyopathy, heart failure (HF), twins
Introduction
Hydroxychloroquine (HCQ) is one of the antimalarial drugs that have been used for decades to treat rheumatic diseases (1). Retinal toxicity, neuromyopathy, and cardiac toxicity are recognized toxicities following prolonged use (1). HCQ-induced cardiomyopathy is one of the rare but severe complications which could be reversible with early withdrawal of the drug (2). However, the exact mechanism of HCQ cardiotoxicity remains unclear (1) and the role of heredity has not been studied due to the rare occurrence of the disease. We present a case of twin sisters who both suffered from systemic lupus erythematosus (SLE) and HCQ-induced cardiomyopathy presenting with decompensated heart failure (HF).
Case presentation
A 55-year-old female was admitted for congestive HF at our medical center in February 2017. A transthoracic echocardiogram (TTE) performed in November 2016 at outside hospital revealed normal left ventricular (LV) size, thickness, and a depressed LV ejection fraction (EF) of 35%. Angiography showed normal coronary arteries. She was diagnosed with non-ischemic cardiomyopathy and started on HF medications including hydrochlorothiazide, aldactone, and carvedilol. However, her symptom was not improved and was admitted for further management.
The patient’s medical history was significant for SLE diagnosed in 2008. She had been treated with prednisone 4 mg twice a day and HCQ 200 mg twice a day with an estimated cumulative dose of 1,314 g.
Upon admission, a repeat TTE (Figure 1) was performed and showed mildly to moderately increased LV thickness (1.4 cm). LV systolic function was now severely decreased with an estimated EF of 20%. There was a large apical thrombus measuring 2 cm × 1 cm in the absence of apical aneurysm (Figure 1A, arrow).
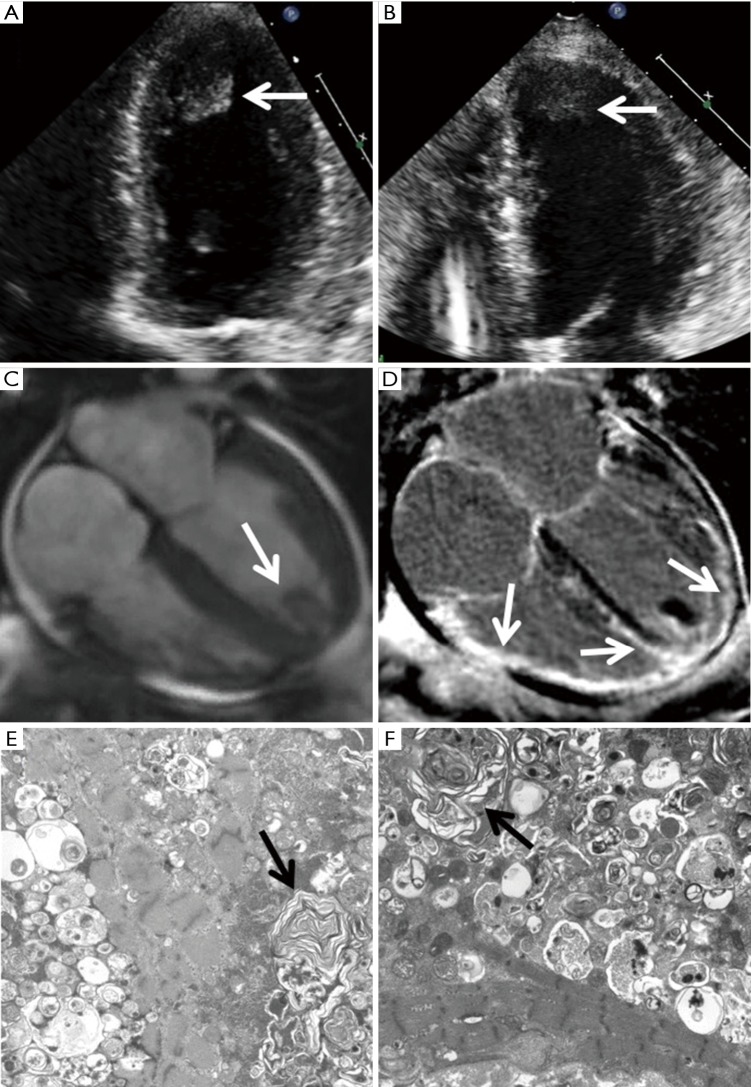
Figure 1.
HCQ-induced cardiomyopathy in twin sisters. The TTEs of the two sisters (A,B) show similar manifestation of LV hypertrophy with severely depressed systolic function and a large apical thrombus (arrow). A cardiac magnetic resonance was performed on the first sister and showed LV hypertrophy with severely decreased biventricular function (C) and confirmed the apical thrombus (arrow). Late gadolinium enhancement imaging showed extensive epicardial to mid myocardial enhancement involving the entire sub-basal mid to apical LV and the right ventricle (D, arrows). Electron microscopy of myocardial biopsies in two sisters (E,F) demonstrate myocardium with degenerative changes and numerous lamellar body inclusions (arrows). HCQ, hydroxychloroquine; TTE, transthoracic echocardiogram; LV, left ventricular.
A cardiac magnetic resonance imaging (CMR) exam was performed and demonstrated mildly increased LV thickness with severely decreased biventricular function and a 2 cm × 1.2 cm protruding thrombus at the apical LV, consistent with TTE (Figure 1C, arrow). In addition, late gadolinium enhancement (LGE) imaging showed extensive epicardial to mid myocardial enhancement involving the entire sub-basal to apical LV and RV walls (Figure 1D, arrows).
The fat pad biopsy of the patient was negative for amyloid. An endomyocardial biopsy was performed. Electron microscopy showed lamellated inclusions and degenerative changes, which was consistent with HCQ toxicity (Figure 1E, arrow).
The patient was started on milrinone treatment and HCQ was discontinued after the biopsy result became available. Intravenous diuresis and angiotensin converting enzyme inhibitor were added to her medical regimen. ICD was implanted for primary prevention.
One month after discharge, the patient was asymptomatic, and her repeat TTE showed that both LV and RV functions had improved with LVEF of 25–30%. The LV apical thrombus had decreased to 1 cm × 1 cm. Eight months after presentation, her repeat TTE showed normal LV wall thickness of 1.0 cm, an LVEF of 50%, and no evidence for LV thrombus.
The patient’s family history was notable for a twin sister with early onset SLE and HCQ treatment for 48 years. The second sister also had severe multi-vessel coronary artery disease and chronic stable angina with multiple drug-eluting stents implanted. In March 2017, 1 month after the first sister was admitted to our hospital, the second sister was admitted with new-onset HF. A TTE showed similar manifestations as the first sister with mildly increased LV thickness, a newly reduced EF of 25–30% (compared to a previous LV thickness of 1.0 cm and an EF of 55–60% 1 year ago) as well as a new apical LV thrombus (Figure 1B, arrow).
Given the very similar presentations and imaging findings on TTE as her twin sister, the second sister underwent endomyocardial biopsy immediately which showed findings consistent with HCQ toxicity (Figure 1F, arrow). Her HCQ was stopped. However, 2 days after the biopsy, she developed severe crushing chest pain and ST elevations in electrocardiogram leads III and aVR. Catheterization did not reveal a culprit vessel and she was medically managed. Her symptom of dyspnea and chest pressure were not improved, and unfortunately, she succumbed to death while undergoing heart transplant evaluation.
Discussion
We presented a case of twin sisters both with history of SLE and HCQ-induced cardiomyopathy that manifested as normal LV size, mildly increased LV thickness, severely depressed LV function, and the presence of an apical thrombus. Antimalarial-related cardiotoxicity most commonly manifests clinically as a diffusely thickened restrictive or dilated cardiomyopathy or with conduction system abnormalities including atrioventricular block and bundle branch block (3). The mechanism of cardiotoxicity was still unclear (4).
The echocardiographic findings of our patients were mild concentric LV myocardial thickening and severely depressed LV function. The clinical presentation was decompensated HF. The symmetrically thickened myocardium with decreased function is difficult to differentiate from other infiltrative diseases including amyloid, sarcoidosis, and Fabry disease based on TTE. CMR has played an important role in the assessment of biventricular function and in the evaluation of the extent of myocardial hypertrophy and fibrosis. CMR has excellent sensitivity and specificity for cardiac amyloidosis (5); however, in the setting of myocarditis, the pattern of delayed enhancement may not further differentiate the different causes of myocarditis. The patient presented with severe epi- to mid-myocardial LGE that is consistent with myocarditis, but the typical pathological changes including lamellar bodies as well as curvilinear bodies found in endomyocardial biopsy is determinant for establishing the diagnosis of HCQ-induced cardiomyopathy (6).
As in a number of cases, HCQ-induced cardiac impairment is often underestimated at the early stages and patients often present first with decompensated HF (3,7). It has been reported that cardiac function can recover after the cessation of the drug (2). In our first case, the improvement of cardiac function was observed after stopping HCQ and treating with HF medications.
Screening to detect antimalarial-related cardiotoxicity early is prudent. The monitoring of ECG and echocardiography is recommended for patients with long term treatment of HCQ (6). The risk factors for HCQ-induced cardiotoxicity have not been identified due to limited number of cases reported (1). The duration of use and cumulative dose may play a role, however, toxicities were seen with mean duration of drug administration ranged from 2 to 35 years including cases with only 4 months of administration of HCQ (4). Genetic polymorphism was recently reported for the first time as a cause of change in the enzyme activity in chloroquine metabolism (8). We present the first case of twin sisters both suffered from HCQ-induced cardiomyopathy, with near identical clinical presentation and TTE findings, suggesting that genetic predisposition might be a factor in HCQ-induced cardiomyopathy.
Acknowledgements
None.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Soong TR, Barouch LA, Champion HC, et al. New clinical and ultrastructural findings in hydroxychloroquine-induced cardiomyopathy--a report of 2 cases. Hum Pathol 2007;38:1858-63. 10.1016/j.humpath.2007.06.013 [DOI] [PubMed] [Google Scholar]
- 2.Abbasi S, Tarter L, Farzaneh-Far R, et al. Hydroxychloroquine: a treatable cause of cardiomyopathy. J Am Coll Cardiol 2012;60:786. 10.1016/j.jacc.2011.12.060 [DOI] [PubMed] [Google Scholar]
- 3.Joyce E, Fabre A, Mahon N. Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: key diagnostic features and literature review. Eur Heart J Acute Cardiovasc Care 2013;2:77-83. 10.1177/2048872612471215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tonnesmann E, Kandolf R, Lewalter T. Chloroquine cardiomyopathy - a review of the literature. Immunopharmacol Immunotoxicol 2013;35:434-42. 10.3109/08923973.2013.780078 [DOI] [PubMed] [Google Scholar]
- 5.Vogelsberg H, Mahrholdt H, Deluigi CC, et al. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. J Am Coll Cardiol 2008;51:1022-30. 10.1016/j.jacc.2007.10.049 [DOI] [PubMed] [Google Scholar]
- 6.Yogasundaram H, Putko BN, Tien J, et al. Hydroxychloroquine-induced cardiomyopathy: case report, pathophysiology, diagnosis, and treatment. Can J Cardiol 2014;30:1706-15. 10.1016/j.cjca.2014.08.016 [DOI] [PubMed] [Google Scholar]
- 7.Azimian M, Gultekin SH, Hata JL, et al. Fatal antimalarial-induced cardiomyopathy: report of 2 cases. J Clin Rheumatol 2012;18:363-6. 10.1097/RHU.0b013e31826852db [DOI] [PubMed] [Google Scholar]
- 8.Saussine A, Loriot MA, Picard C, et al. Chloroquine cardiotoxicity in long-term lupus therapy in two patients. Ann Dermatol Venereol 2009;136:530-5. 10.1016/j.annder.2009.01.016 [DOI] [PubMed] [Google Scholar]