Abstract
Animal studies reveal that early deprivation impairs regulation of the hypothalamic–pituitary–adrenocortical (HPA) axis, potentially increasing vulnerability to stressors throughout life. To examine early deprivation effects on basal HPA axis activity in humans, basal cortisol levels were examined in 164 internationally adopted children who had experienced varying degrees of preadoption deprivation. Duration of institutional care, age at adoption, and parent ratings of preadoption neglect indexed a latent factor of Deprived Care. Adoption measures of height and weight standardized to World Health Organisation norms indexed a latent factor of Growth Delay that was viewed as another reflection of deprivation. Cortisol samples were collected 3.3–11.6 years postadoption (Md = 7.3 years) at home on 3 days approximately 30 min after wakeup and before bedtime. Both early a.m. levels and the decrease in cortisol across the day were examined. A structural equation model revealed that preadoption Deprived Care predicted Growth Delay at adoption and Growth Delay predicted higher morning cortisol levels and a larger diurnal cortisol decrease.
Children who are neglected and abused early in life are at heightened risk for physical and mental disorders (Cicchetti & Toth, 1995). There is currently considerable interest in understanding how adverse early care influences brain development and contributes to individual differences in vulnerability (e.g., Cicchetti & Tucker, 1994). Animal studies of the impact of early adverse experiences on the hypothalamic–pituitary–adrenocortical (HPA) system provide a compelling explanatory model (e.g., see Graham, Heim, Goodman, Miller, & Nemeroff, 1999; Heim, Owen, Plotsky, & Nemeroff, 1997). However, their applicability to human development remains uncertain. Glucocorticoids (cortisol in primates, corticosterone in rodents) are hormones produced by the HPA axis that are essential both for maintaining homeostasis and adapting to physical and psychological stressors (de Kloet, Rots, & Cools, 1996; Sapolsky, Romero, & Munck, 2000). Although increases in glucocorticoids above basal levels are typically examined in research on stress, basal levels also contribute importantly to stress vulnerability and resilience (Sapolsky et al., 2000). According to the allostatic load model (McEwen, 1998), the HPA system supports adaptation to stress through increasing or decreasing its basal set points and responsiveness. These changes, although permitting individuals to continue to function, carry a risk or allostatic load that increases vulnerability to physical and mental disorders. Indeed, both chronically elevated and chronically suppressed basal glucocorticoid levels have been associated with physical and psychological disorders (de Kloet, Vreugdenhil, Oitzl, & Joels, 1998; Heim, Ehlert, & Hellhammer, 2000; Rosen & Schulkin, 1998; Yehuda, 2000).
Animal studies suggest that during early development allostatic processes that permit immature organisms to adapt to adverse care may have especially prolonged effects because these adjustments are produced by and imposed on organisms undergoing rapid maturation (de Kloet, Rosenfeld, Van EeKelen, Sutanto, & Levine, 1988). Much of this early experience work has been conducted using rat models (Levine, 2005). Low levels of maternal care produces a permanent silencing of a gene that regulates the HPA response to stressors (Weaver et al., 2001). As a result, HPA responses to stressors in adulthood are larger and more prolonged. Deprivation, in the form of repeated, daily removal of the dam from her pups for several hours produces similar effects on offspring (Meaney & Szyf, 2005; Plotsky & Meaney, 1993). Typically, the degree of deprivation imposed in rat studies does not reduce or stunt physical growth, nor does it alter basal levels of HPA axis activity. Nonetheless, when conditions of deprivation are made severe enough, stunting of physical growth and elevated basal HPA activity have been observed (Avishai-Eliner, Gilles, Eghbal-Ahmadi, & Baram, 2001; Gilles, Schultz, & Baram, 1996).
Although it has been suggested that rodent models may explain the impact of early adversity in humans (De Bellis, 2005; Graham et al., 1999; Heim et al., 1997; Kaufman & Charney, 1999; Teicher, Andersen, Polcarri, Anderson, & Navalta, 2002), the translation to human development is uncertain on several counts. The first challenge is that it is not clear how well the rat model translates even to nonhuman primate development. There is little evidence that completely depriving infant monkeys of parental care through isolation rearing has long-term effects on the HPA axis (Levine, 2005; Sanchez, Ladd, & Plotsky, 2001). Less severe disturbances in early life parental care (e.g., repeated separations, peer-only rearing, and variable foraging paradigms), however, have been associated with disturbances in the diurnal rhythm of the HPA axis among juvenile monkeys and in elevated production of corticotropin-releasing hormone among mature monkeys (Coplan et al., 1996; Suomi, 1997). Thus, rather than a lack or low amount of parental stimulation observed to affect rat offspring, the monkey data implicates unpredictable or uncontrollable (e.g., unresponsive) parental care in altering activity of this stress-sensitive neuroendocrine system. In addition, translation of the rodent findings to primates is challenged by evidence that early disruptions in parental care in primates may produce lower than expected HPA activity, rather than the elevated activity typical observed in rodent paradigms (Boyce, Champoux, Suomi, & Gunnar, 1995; Dettling, Feldon, & Pryce, 2002; Sanchez et al., 2005).
The second challenge to translation is that, whereas animal studies have focused on neglect or deprivation, most attempts to address issues of translation in research on children have focused on abuse (for discussion, see De Bellis, 2005). Studies of severely physically and sexually maltreated children with posttraumatic stress disorder (PTSD) have revealed elevated basal cortisol levels several years postrescue (Carrion et al., 2002; De Bellis et al., 1999). Several researchers have suggested that, with time, hyperactivity of the HPA axis will produce a downregulation of the HPA system, resulting in a suppressed pattern of cortisol production typically noted in adults with PTSD (De Bellis, 2001; Yehuda, Halligan, & Grossman, 2001). However, adult studies of women sexually abused as children (before puberty) indicate normal basal cortisol activity but elevated HPA axis reactivity to psychosocial challenge (Heim, Newport et al., 2000). Cicchetti and Rogosch (2001a, 2001b) observed elevated basal levels only among children who had experienced severe, multiple, and prolonged forms of abuse or those who had significant internalizing problems. In their work, children who were neglected but not otherwise maltreated did not show altered basal cortisol activity. One major difficulty in interpreting this literature is that it is not clear whether altered cortisol levels in these studies were due to early maltreatment, to ongoing adversity in the children’s lives, and/or to the children’s concurrent emotional and behavioral problems. Indeed, in one study it was noted that only the maltreated children whose families continued to experience major life stressors exhibited disturbances in HPA axis activity (Kaufman et al., 1997).
In summary, rodent models of early deprivation or reduced species-typical parental stimulation clearly demonstrate impacts on the developing HPA system. Low levels of maternal stimulation result in hyperresponsiveness of the axis that persists into adulthood. In rat studies, when deprivation is severe enough to stunt physical growth, elevated basal cortisol levels also have been observed. In nonhuman primates, radically depriving the animal of normal maternal care does not have any clear effect on the developing HPA system. In contrast, paradigms that do not involve such radical deprivation, but instead involve unpredictable or uncontrollable disruptions in care, do seem to heighten reactivity of the HPA system, although perhaps in combination with abnormally low basal cortisol levels. Finally, the studies in humans are difficult to summarize. Although some long-term impacts on basal cortisol levels and HPA axis reactivity have been noted, the patterns observed may depend on the individual’s psychiatric diagnosis and/or on whether there is ongoing adversity in the individual’s life. There is very little evidence that early deprivation or neglect, per se, has any long-term impacts on activity of this stress-sensitive neuroendocrine system in humans.
The one exception to this latter conclusion comes from a small study of children adopted in the early 1990s from Romanian orphanages (Gunnar, Morison, Chisholm, & Schuder, 2001). As noted by Rutter (1972), children adopted from orphanages or other institutional settings provide valuable information about the long-term impact of early neglect or deprivation, because the period of deprivation can be delimited. Infants and young children living in institutions experience chronic neglect in multiple forms, including little human contact and sensory deprivation (Frank, Klass, Earls, & Eisenberg, 1996; Rutter, 1998). Nonetheless, the degree of deprivation is not uniform across all institutional settings, varying in severity among institutions and even from one room to the next in the same institution (Johnson, 2001). Although some children in institutions likely experience physical and sexual maltreatment, most experts who have observed the care environment in these institutions describe conditions of benign neglect (Ames, 1990; Smyke, Dumitrescu, & Zeanah, 2002). That is, the neglect these children experience appears to be because of too many children combined with too few social and physical resources for their care.
Families in the United States and other Western nations are adopting increasing numbers of children from orphanages around the world. In 2005, according to the US Department of State, over 22,700 children were adopted internationally by families in the United States alone (US Department of State, 2006), with over 85% adopted from countries that predominantly use institutions, as opposed to foster care, to provide for abandoned or orphaned children (see also Johnson, 2001). Children adopted internationally range widely in age at adoption. Although some are adopted within a few months of birth, others do not reach their adoptive families until they are 2 or more years old. Age at adoption is a significant factor in predicting child outcomes (Gunnar & van Dulmen, 2008). However, this is likely because adoption age is a proxy for multiple forms of neglect and maltreatment. In an epidemiological study of children adopted internationally into The Netherlands, Verhulst Althaus and Versluisden Bieman (1992) noted that they were unable to disentangle effects of age at adoption from preadoption experiences of neglect and abuse. Regardless of whether children were in institutions or not, the longer they lived without a permanent family, the more adverse experiences they reportedly suffered prior to adoption.
One reason to focus on internationally adopted children to address questions about early deprivation is that adoption typically marks a dramatic improvement in children’s care. Nearly all of these children are adopted into families with adequate monetary and educational resources. In a recent survey of over 2,000 internationally adopted children (Gunnar & van Dulmen, 2008), adoptive family incomes were high (Md income > $75,000), parents were well educated (≥70% college educated), and most families (>95%) included two adults in the home. Fewer than 2% of these families described major life stressors (separation, divorce, death of a family member) in the years since the child joined the family. Observational studies of parent–child interaction tend to reveal generally positive patterns of parenting (Croft, O’Connor, Keavene, Groothues, & Rutter, 2001).
As noted, there has been one study of cortisol levels in children adopted from institutional care settings (Gunnar et al., 2001). This study was conducted on a small sample of children adopted in 1990–1991 by families in British Columbia, Canada, after living for 8 months or more in Romanian institutions. Although children still living in Romanian orphanages have been shown to lack a diurnal rhythm in cortisol production over the daytime hours (Carlson & Earls, 1997), an average of 6.5 years after adoption all of the Romanian postinstitutionalized children in the study by Gunnar et al. (2001) exhibited the expected decrease in cortisol from wakeup to bedtime. However, cortisol levels averaged across the day were elevated when compared to Romanian children adopted early (up to 4 months of age) and children reared in their families of origin in Canada. Although suggestive of long-term elevations in basal cortisol, this study was far from conclusive because of several substantial limitations that are addressed by the present study.
The first limitation was that the sample was quite small, and included children adopted from some of the most globally depriving conditions imaginable (e.g., Ames, 1990; Frank et al., 1996). Studies of Romanian orphanage children adopted into British Columbia (Ames & Burnaby, 1997) and the United Kingdom (Rutter, 1998) in the early 1990s revealed patterns of severe physical, social, and cognitive delays at adoption. Although effects of the most severe kind of deprivation still would be noteworthy, it is important to know whether impacts on the HPA axis vary with the degree and duration of deprivation among a large, representative sample of children exposed to a wide range of preadoption conditions.
A second limitation that is addressed in the present study involves the impact of severe deprivation on physical growth and the possibility that growth delay rather than institutional care, per se, might predict altered HPA axis activity. Rutter (1998) reported that the average growth percentile for Romanian children adopted into the United Kingdom in the early 1990s was more than 2.0 SD below normal. This is consistent with evidence that children in orphanages lose approximately 1 month of linear growth for every 2–3 months in the institution (Miller & Hendrie, 2000). Following adoption, children grow 1.5–2 times their expected rate (Johnson, 2001). This pattern of growth delay mirrors what has been termed psychosocial short stature (Mason & Narad, 2005). Psychosocial short stature (Types I and II) is characterized by weight remaining proportional to height and reversible growth hormone abnormalities (Gohlke, Frazer, & Stanhope, 2004). That is, without a change in nutrition, improving the child’s psychosocial environment increases growth hormone production and tissue sensitive to growth factors. Hyperactivity of the HPA axis, particularly elevated levels of corticotropin-releasing hormone and cortisol, are believed to mediate inhibition of growth hormone and growth factors under conditions of psychosocial deprivation (Cianfarani, Geremia, Scott, & Germani, 2002; Cianfarani et al., 1998). Linear growth delay, especially if accompanied by weight that is proportional to height, may reflect chronic HPA drive on the growth hormone system. Children with growth delay associated with prolonged or intense deprivation may be particularly likely to have experienced chronic stress effects on the HPA axis. Of course, growth delays documented at adoption may to some extent also reflect malnutrition induced by intestinal parasites, poor nutrition, illness endemic to many orphanages and prenatal growth restriction (Johnson, 2001).
A third limitation of the Gunnar et al. (2001) findings that is addressed by the present study is that they were based only on children from Eastern Europe. In Romania, Russia, and other Eastern European countries prenatal alcohol exposure is common among children residing in institutions (Johnson, 2001). Prenatal alcohol exposure can alter postnatal activity of the HPA axis (Ogilvie & Rivier, 1997; Schneider, Moore, Kraemer, Roberts, & DeJesus, 2002). Thus, extending examination of cortisol levels in postinstitutionalized children beyond the study of Russian/Eastern European children will help reduce the possibility that results will be because of prenatal alcohol exposure rather than postnatal care.
The present study was designed to examine the long-term impact of varying amounts of early deprived care on basal cortisol levels among children adopted internationally from many different countries and preadoption conditions. All of the children had lived with their adoptive families for at least 3 years prior to sampling, were between 7 and 11 years of age at testing and were not on any forms of steroidal or psychotropic medications. Basal cortisol levels were examined at two times of day, approximately 30 min after wakeup in the morning and 30 min before bedtime. Multiple measures of deprivation were examined, including duration of institutional care, age at adoption, and parent-reported preadoption neglect and abuse. Growth delay at adoption included measures of linear growth (i.e., height) and weight for height at adoption. We hypothesized that preadoption deprivation would be positively associated with basal cortisol concentrations, that greater preadoption deprivation would be associated with greater growth delays at adoption, and that growth delay might mediate the effects of deprivation on basal cortisol levels.
Methods and Materials
Participants
The participants were 164 children (45% boys) recruited from a registry of over 3,000 internationally adopted children in the northern Midwest whose parents indicated interest in research participation. The children were selected to be 7–11 years of age at the time of testing and to have been with their adoptive families for at least three years. Approximately 29% of the participant registry met these selection criteria. To provide a broad range of preadoption experiences and broad representation of birth regions, children on the registry who met the criteria for age and time in the family were stratified according to birth region (Russia/Eastern Europe, Asia, Latin America/Caribbean), gender (male/female), age at adoption (pre/post 24 months), and whether the child was adopted from an institution. Two hundred forty children were randomly selected (i.e., 20 within each strata) to create a diverse sample.
Parents of children who were randomly selected were contacted by phone. After describing the study, a brief screen for medications was performed. Children who were on steroidal or psychotropic medications were excluded; 12% of those screened were eliminated based on psychotropic medications. Of those passing the medication screen, 93% agreed to take part in the study and completed the protocol. Thirty-five families had more than one biological sibling in the age range for testing. When more than one biological sibling was available, one was randomly selected for inclusion in the analysis. The sample described here (N = 164) was the sample after the medication screen, random selection of one biological sibling, and parent agreement to participate.
Preadoption living arrangements and countries of origin
The children had been adopted from 27 different countries (32.9% from Russia/Eastern Europe, 37.8% Asia, and 29.3% from Latin America/Caribbean). Most (66%) of the children had experienced two or more preadoption care arrangements, including some time with birth parents or relatives (35%), in foster care (46%), and in institutions (hospitals, baby homes, or orphanages; 70%). There were a few children who were described as only experiencing foster care (16%) or institutional care (19%), but very few described as only experiencing birth family care (2%) or adoption at birth (2%). Age at adoption varied from birth to 98 months (M = 13 months; see also Table 1). Information was lacking for 15% of the children for at least some periods of their preadoption lives (M = 4 months), although duration of institutional care was always available.
Table 1.
Descriptive statistics
| Variable | Mean | SD |
|---|---|---|
| a.m. cortisol (μg/dl) | ||
| Day 1 | 0.68 | 0.34 |
| Day 2 | 0.62 | 0.28 |
| Day 3 | 0.54 | 0.25 |
| p.m. cortisol (μg/dl) | ||
| Day 1 | 0.15 | 0.28 |
| Day 2 | 0.11 | 0.11 |
| Day 3 | 0.12 | 0.25 |
| Age at adoption (months) | 22.04 | 20.37 |
| Institutional duration (months) | 11.27 | 15.37 |
| Preadoption neglect/abuse | 1.3 | 0.58 |
| Adoption Z | ||
| Height for age | −1.63 | 2.26 |
| Weight for height | 0.81 | 1.3 |
| Testing Z | ||
| Height for age | −0.34 | 1.4 |
| Weight for height | 0.35 | 1.4 |
| Age at testing (years) | 9.27a | 1.34 |
| Family income | 4.27b | 2 |
| Parent education (averaged) | 4.92c | 1.13 |
Not included in the structural equation.
In units of $25,000; median between 3 and 4 or $75,000–$100,000 and $100,000–$125,000.
4 = Associate’s degree, 5 = Bachelor’s degree.
Instruments and measures
Preadoption care
Parents were asked to describe whether the child experienced any of the following types of care prior to adoption: birth family, relatives, hospital, baby home or orphanage, or foster care. They were asked to indicate the number of months in each type of care if known. They noted if any time in the child’s preadoption life was unaccounted for and if so, how many months. They reported on the child’s age when the child came into their full-time care. In addition, they also rated the quality of preadoption care. These ratings, of course, reflect unmeasured biases either on the part of the parents or on the part of those describing the child’s preadoption care to the families. Ratings of physical neglect (e.g., deficits in food, clothing, medical care), social neglect (lack of meeting the child’s needs for affection and interaction with adults) and physical abuse were made on 4-point scales from 1 (none) to 4 (severe). Sexual abuse was reported as yes, no, or suspected. For purposes of this analysis, known and suspected abuse were both assigned the value of 4, whereas no responses were assigned the value of 1 to place this measure on the same scale as the other preadoption neglect and abuse items. Nearly 31% of the families reported no neglect or abuse prior to adoption. Those who believed their children had suffered no neglect or abuse had adopted their children quite young (M = 6 months, including the three families adopting at birth) following no or only brief periods of institutional care. Very few parents reported moderate or severe physical abuse prior to adoption (n = 8) or any known or suspected sexual abuse (n = 9), with a total of 14 children or 9% of the sample receiving these scores. Thus, for the majority of children, parents described varying degrees of social and physical neglect. Three measures derived from this questionnaire were used to collectively index the latent construct deprived care prior to adoption. Age at adoption was the age when the child came into the family’s full-time care. Duration of institutional care was the sum of months spent in hospitals, baby homes, or orphanages. Parent-rated neglect and abuse was the average of physical neglect, social neglect, physical abuse, and sexual abuse (scale reliability was Cronbach α = .76).
To rule out effects because of major current child–family stressors the Child Life Events Scale (Boyce, Chesney, et al., 1995) was administered. The scale obtains occurrence of 37 life events that might impact children, ranging from items like changing to a new school, parent’s job change, or addition of a sibling to parental separation/divorce or death of a family member. The inventory has good test–retest reliability. Parents completed the inventory for the 6-month period prior to cortisol sampling. Life events during this time frame were exceptionally low averaging one out of 37 with 79% of the sample scoring two events or fewer. Examination of the events endorsed indicated that the most common one was birth or adoption of a sibling or move to a new home. Because of the low number and range of life events, this variable was not examined further.
Behavior problems
Because we screened children for use of psychotropic medications, we did not anticipate that many of the children in the sample would have significant behavior problems. However, to describe behavior problems parents completed the version of the Child Behavior Checklist/6–18 (CBCL) published in 2004 (Achenbach, 2006). The t scores were calculated and a cutoff of 65 was used to identify children with clinical levels of internalizing and externalizing problems. Internalizing problems (6.7%; 11/164) were more common than externalizing problems (3.7%; 6/164). There was a suggestion of an interaction between internalizing problems and trials (a.m./p.m.), F (1, 159) = 6.42, p <.05, with internalizing children having higher a.m. cortisol levels than noninternalizing children (M = 0.79 mg/dl, SD = 0.40 versus 0.60 mg/dl, SD = 0.22. However, given the small number of children with significant internalizing problems and the absence of a significant correlation between internalizing scores (raw) and cortisol levels (a.m., r = .13, N = 164, ns; p.m., r = .02, N = 164, ns) we did not include internalizing problems in the statistical model described below.
Anthropometric data
Parents reported their child’s weight and height data provided at their first physician’s check up in the United States, along with the child’s age in months at that physician’s visit. Data provided in inches/pounds, metric units, or percentiles were converted to Z scores using World Health Organisation (WHO) norms. Nearly all (88%) parents provided these measures. We, of course, have no reliability information on these data and must assume some error in measurement and/or recording. Parents also measured the height and weight of their child at the time of assessment, again with unknown reliability, and these values were also converted to WHO standard scores. Two indices were used to describe Growth Delay at the time of adoption: WHO standardized height (length) for age at adoption and WHO standardized weight for height at adoption (hereafter referred to as height for age at adoption and weight for height at adoption). Based on research on growth retardation because of neglect, shorter stature and greater (not lesser) weight for height were anticipated. Indeed, children who were shorter for age at adoption (more linear growth delay) were heavier for their height, r (142) = −.60, p < .01. Thus height for age at adoption was reverse scored such that higher scores on both measures would be consistent with psychosocial growth delay.
A demographic questionnaire requested child’s current age, family income in the last year before taxes (in $25,000 increments), parent education (less than high school, high school diploma, some college, associate’s degree, bachelor’s degree, postbaccalaureate degree), and family composition. Time in the family was computed by subtracting current age from age at adoption. Because age at adoption and years since adoption were highly correlated, r (162) = −.78, p < .001, to provide an estimate of time since adoption uninfluenced by adoption age, years since adoption was regressed on age at adoption and the residuals were retained denoted as residualized time since adoption. Preliminary analyses indicated that this residualized variable was not correlated with any of the latent indicators or with the cortisol data and all the correlation coefficients were very small (rs < .09). Therefore, this variable was not considered further. However, child gender, parent education (averaged), and family income were used as covariates as they exhibited correlations with indicators of the latent constructs.
Procedures for salivary cortisol collection
Once parental consent was obtained, saliva collection kits and parent questionnaires were mailed along with parent consent and child assent forms. Saliva was sampled on three school days (to assure stable day to day wake/sleep schedules). We requested that samples be taken 30 min after wakeup and 30 min before bedtime or between 8 and 9 p.m. if bedtime was after 9:30 p.m. Sampling was avoided, whenever possible, on days when children had scheduled evening activities, especially sport activities as these activities may elevate bedtime cortisol levels in children (Kertes & Gunnar, 2004). Caffeine consumption was precluded 2 hr prior to sampling and days representing a significant departure from the typical routine were avoided. Parents maintained a diary for each sampling day supplying requested information on the child’s schedule (wakeup, meals, bedtime), activities, and health (medications taken, health ratings). Regular contact with families was maintained to guide sampling day selection and to answer questions. Daily diaries were examined for procedural compliance. Mean sampling times were within the requested ranges. For the 3 days of sampling, a.m. sampling time means ranged from 7:20 to 7:25, SDs from 34 to 37 min, rs (162) > .80, ps < .001; whereas p.m. sampling time means ranged from 8:20 to 8:25, SDs from 39 to 43 min, rs (162) > .70, ps < .001.
Saliva was collected by having the children chew a piece of original flavor Trident gum for 1 min and then spit through a 3-in. straw into a vial (Eppendorf, Westbury, NY) based on procedures tested by Schwartz, Granger, Susman, Gunnar, and Laird (1998). The sealed vials were time/date labeled and stored in a ziplocked bag in the family’s refrigerator until collection was completed. They were then mailed to the laboratory and stored at −20°C until assayed. These storing and mailing procedures do not affect cortisol levels (Clements & Parker, 1998). Samples were assayed in duplicate using a time-resolved fluorescence immunoassay with acceptable intraassay (5.4%) and interassay (8.1%) coefficients of variance based on control samples inserted into the assay batches.
Missing and out of range values
Nearly 98% of the requested saliva samples were provided. Two children refused the sampling procedure (both adopted under 6 months; one postinstitutionalized, one not). Cortisol values were examined for biologically implausible values defined as >4.0 μg/dl. Three such values were noted, each from different subjects, and were deleted.
Analysis plan
Analyses were conducted in three stages. First, descriptive statistics were examined, including measures of children’s current height and weight to determine whether growth parameters had normalized. Second, the correlations among the indicators were examined for consistency with the measurement models for the main analysis. Third, structural equation modeling was used to test models of a.m. cortisol level and diurnal change as predicted by deprivation and growth delay at adoption and to determine whether growth delay at adoption mediated the impact of deprivation. To accomplish these goals, two structural models were fit: a full model specifying deprived care and growth delay as predictors of initial a.m. cortisol status and cortisol change (a.m. to p.m.), and a reduced model omitting the growth delay paths with all other variables.
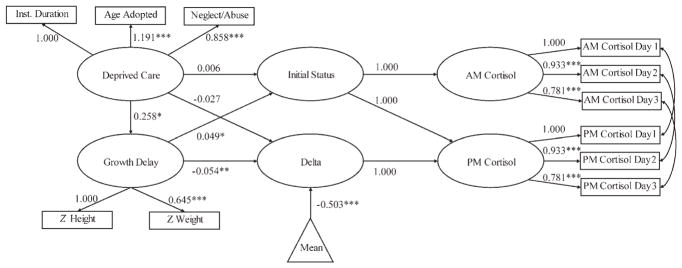
The full model can be seen in the results Figure 1. We have not included the control variables to simplify the drawing. The a.m. cortisol and p.m. cortisol were defined as latent variables with corresponding indicators measured over 3 days. Residuals of cortisol indicators measured on the same day were allowed to correlate. The right-hand side of the figure shows an embedded latent difference score model of the type discussed by McArdle and Hamagami (2001). The constant is the initial status of a.m. cortisol, and delta is the latent change score. The definition of delta can be seen from the regression equations implied by the path model. Ignoring residual terms, the path model indicates a.m. cortisol = (1) constant, and p.m. cortisol =(1) constant + (1) delta. By substitution, delta = p.m. cortisol – a.m. cortisol, which is the latent change score. The mean variable (enclosed in a triangle) indicates the mean of the latent difference score was estimated (averaged over participants) and its path coefficient reflects the unstandardized change in daily cortisol. Interpretation of the latent difference scores required an unstandardized solution. To yield more interpretable comparisons of predictors of change, all variables except for cortisol and gender were standardized in the analysis. In the full model, paths for the control variables gender, parent education, and family income predicting initial status, delta, and the deprived care and growth delay variables were estimated. In the reduced model, all paths were retained from the full model except for the paths between growth delay and all other variables (control variables predicting growth delay, deprived care predicting growth delay, and growth delay predicting initial status and delta).
Figure 1.
The full structural equation model with unstandardized path coefficients. The control variables gender, parent education, and family income are not depicted for clarity of presentation. The control variables have paths pointing to all of the latent variables in the figure except a.m. cortisol and p.m. cortisol. Deprived Care, deprived care with indicators; Inst Duration, duration of institutional care; Age Adoption, age (months) child came into adoptive parents’ care; Neglect/Abuse, parent-rated neglect and abuse; Growth Delay, growth delay at adoption with indicators: Z Height, standardized height for age at adoption (reverse scored), Z Weight, standardized weight for height at adoption. Initial Status, predicted a.m. cortisol; Delta, latent difference score (p.m. cortisol – a.m. cortisol). *p < .05, **p < .01, ***p < .001.
All models were estimated using Mplus 3.13 (Muthén & Muthén, 2001). Maximum likelihood estimation was used and absolute model fit was assessed with the chi-squared statistic, the comparative fit index (CFI), the Tucker–Lewis index (TLI), and the root mean square error of approximation (RMSEA). Based on results of simulation studies (e.g., Hu & Bentler, 1999) the criterion of acceptable fit was ≥.95 for the CFI and TLI and ≤.06 for RMSEA. Relative fit of the full and reduced models was assessed by the difference in chi-squared values evaluated against the referent distribution with df equal to the difference in free parameters between the models.
Results
Descriptive statistics
Descriptive statistics (means and standard deviations) are presented in Table 1. As expected, early a.m. cortisol levels were higher than p.m. cortisol levels on each day of testing. On closer inspection of the data, only 10 children exhibited an increase in cortisol from a.m. to p.m. on any of the sampling days. For all but one of these children this atypical diurnal pattern was noted on only one of the days. Age at adoption and duration of institutional care exhibited large standard deviations, indicating that we had obtained a diverse sample with regard to these indices of deprivation. Parent-rated neglect and abuse, however, had a relatively small standard deviation and a low mean of slightly over 1 on a 4-point scale, suggesting that most parents believed that their children had been subjected to little or no neglect. As noted earlier, very few of these parents (14 of 164) reported any preadoption abuse. As expected, growth parameters at adoption indicated linear growth delay was common. Children’s linear growth (height for age) at adoption averaged over 1.5 SD below the mean, but their weight for height at adoption averaged a bit over 0.05 SD above the mean. Reduced height coupled with weight that is normal or above normal for height is consistent with patterns of growth associated with psychosocial neglect. The standard deviation for height at adoption was large, indicating that, like the other measures of deprivation, we had obtained a diverse sample. Consistent with this, closer inspection of the height data revealed that although 26% of the sample was more than 2.5 SD below the mean indicating extreme growth delay, 26% were at or above the WHO mean, indicating adequate linear growth. Notably, approximately 80% of the extremely growth delayed children had lived in an institution prior to adoption, and the average duration of institutional care for these children was 21 months (range = 1–68 months).
By the time the cortisol measures were obtained several years after adoption, the children had largely caught up in growth averaging within 0.5 SD of the WHO mean on height and averaging close to the mean (i.e., zero) on weight for height. To determine whether current anthropometric variables needed to be included as control variables, we correlated the standardized height for age at testing and weight for height at testing with the six cortisol indicators. Of the 12 correlations, only one was significant, with the rest ranging from r =−.15 to r =+ .11. The one significant correlation occurred between weight for height at testing and p.m. cortisol on the first day of testing, r (162) = .28, p < .01. Inspection of a scatterplot of these data suggested that the significant correlation was because of one outlying case. Removing that case reduced the correlation to r (161) =−.02, ns. Therefore, growth measures at the time of testing were not considered further in the analyses.
Intercorrelations among indicators
The intercorrelations among the indicators of the key latent constructs (i.e., not the covariates) are shown in Table 2. Of note, in this table it is apparent that a.m. and p.m. cortisol levels were positively correlated. In addition, the within-cluster correlations were higher than the between-cluster correlations.
Table 2.
Simple correlations among indicators
| Indicators | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. a.m. cortisol Day 1 | 1.0 | |||||||||
| 2. a.m. cortisol Day 2 | .48** | 1.0 | ||||||||
| 3. a.m. cortisol Day 3 | .54** | .49** | 1.0 | |||||||
| 4. p.m. cortisol Day 1 | .33** | .26** | .33** | 1.0 | ||||||
| 5. p.m. cortisol Day 2 | .26** | .16* | .19* | .38** | 1.0 | |||||
| 6. p.m. cortisol Day 3 | .25** | .18* | .32** | .37** | .36** | 1.0 | ||||
| 7. Age at adoption | .00 | .04 | .05 | −.16* | −.11 | −.09 | 1.0 | |||
| 8. Institutional duration | .02 | .01 | .06 | −.15* | −.10 | −.07 | .71** | 1.0 | ||
| 9. Preadoption neglect/abuse | .16* | .08 | .16* | −.04 | .02 | −.02 | .63** | .54** | 1.0 | |
| 10. Adoption Z height for age | .17* | .18* | .15* | −.09 | −.08 | .06 | .20* | .24** | .12 | 1.0 |
| 11. Adoption Z weight for height | .18* | .12 | .18* | .02 | .04 | .18* | .01 | .07 | −.05 | .49** |
p <.05.
p <.01.
Structural equation models
Full model
The first row of Table 3 shows the absolute fit of the full model. The values indicated a good fit of the model to the data. Figure 1 shows the path model with unstandardized path coefficients and corresponding p values (recall all variables were standardized before the analysis except for the cortisol variables). Regarding the measurement models, the figure shows all indicators with estimated loadings were significant. The estimated latent difference score mean was significant and indicated a decline of approximately 0.50 μg/dl over the day. Growth delay was a significant predictor of both the initial status and the difference score. The positive relationship with the initial status indicates those children with higher latent growth delay scores tended to have higher levels of a.m. cortisol and those with lower latent growth delay scores tended to have lower levels of a.m. cortisol. Regarding the difference score prediction, the negative coefficient indicated that higher latent growth delay scores were associated with a greater decline in cortisol over the day, whereas lower latent growth delay scores were associated with lesser decline. Growth delay was also significantly predicted by deprived care with higher deprivation being associated with greater growth delay at adoption.
Table 3.
Model fit indices for the full and reduced structural equation models
| Model | Absolute Fit | Relative Fit | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| χ 2 | df | p Value | CFI | TLI | RMSEA | Δχ 2 | Δdf p | Value | |
| Full | 71.870 | 61 | .161 | 0.975 | 0.965 | 0.033 | |||
| Reduced | 91.439 | 67 | .025 | 0.945 | 0.937 | 0.047 | 19.569 | 6 | .003 |
Note: CFI, comparative fit index; TLI, Tucker–Lewis index; RMSEA, root mean square error of approximation.
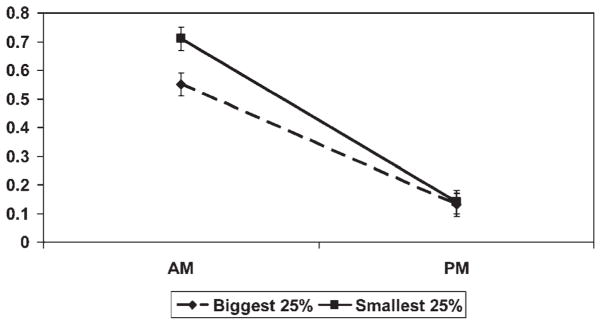
For clarity of presentation, Figure 1 does not depict the control variable effects. The initial status (reflecting a.m. cortisol) was significantly related to gender (b = .092, p < .05) and parent education (b = .041, p < .05). Delta (latent difference score) was also significantly related to gender (b = −.081, p < .05) and parent’s education (b = −.042, p < .05). The estimated coefficients indicated that females and adopted children of parents with higher education had higher a.m. cortisol and a greater drop in a.m. to p.m. cortisol. Finally, growth delay was significantly predicted by family income (b = −.181, p < .05), such that families with lower income on average adopted children with greater growth delay. The effect of growth delay at adoption has been displayed visually in Figure 2, which depicts the a.m. and p.m. values for the top 25% of most growth delayed children at adoption (smallest) and the bottom 25% or least growth delayed at adoption (biggest). The group labeled biggest was at the mean (zero) or larger in height for age at adoption than other children of their age by WHO norms. The top 25% of growth delayed children at adoption were 2.5 SD or more below normal linear growth parameters for their age. As shown in the figure, the most growth delayed children had higher a.m. levels and a steeper decline from a.m. to p.m., but this was because of higher a.m. values and not lower p.m. values.
Figure 2.
The a.m. and p.m. cortisol levels in micrograms per deciliter (μg/dl) for children in the lowest (smallest) and highest (biggest) quartiles of standardized height for age at adoption. Error bars reflect the standard error of the mean.
Reduced model
To test the extent to which growth delay at adoption mediated deprived care effects, a reduced model was also fit. The reduced model omitted all paths emanating from and leading to growth delay (six paths total). The full model results showed that growth delay significantly predicted cortisol initial status and change (difference score) but deprived care did not. As discussed above, it was hypothesized that growth delay may mediate the effects of deprived care. Thus, omitting the growth delay paths in the model might yield significant path coefficients for deprived care. The second row of Table 3 shows the absolute fit indices for the reduced model and the relative fit test. As the table indicates, absolute fit was lower than for the full model and the relative fit test was significant, suggesting the growth delay paths were justified in the full model. The results for the measurement models, cortisol change, and the control variables (not presented) were very similar to the full model. The coefficient of deprived care predicting initial status (b = .019, p = .488) and the coefficient of deprived care predicting delta (b = −.041, p = .122) were both stronger in absolute value in the reduced than in the full model, although still not statistically significant. Thus, there was no evidence in the absence of growth delay that deprived care predicted basal cortisol levels.
Discussion
The purpose of this study was to assess the long-term effects of early deprivation on basal activity of the HPA axis by sampling internationally adopted children who varied in their experiences of preadoption deprivation. Preadoption deprived care was examined as a latent construct that included children’s age at adoption, how long the children had been in institutional care, and parental reports of the extent to which they believed the children had experienced neglect (physical and social) and abuse (physical and sexual) prior to adoption. Because few parents reported abuse, this variable primarily described variations in parent-reported preadoption neglect. Growth delay was also examined as a latent construct that included height for age at adoption, reverse scored to reflect delayed linear growth, and weight for height at adoption. Growth delay at adoption was viewed as indexing predominantly psychosocial short stature, although we cannot rule out contributions of prenatal growth or postnatal poor nutrition and illness. Controlling for adoptive parent education and income and child gender, significant associations were obtained between these latent constructs of preadoption adversity and basal cortisol levels several years postadoption; but the effects were subtle. Deprived care was not directly associated with basal cortisol levels. However, deprived care predicted growth delay, which in turn, predicted higher early morning cortisol levels and a steeper a.m. to p.m. diurnal decrease several years after adoption.
Before discussing these results, it is important to address one of the major limitations of the present study. Specifically, we did not include a comparison group of nondeprived children who had been reared with their parents from birth. Previous reports, however, have described a.m. and p.m. levels for such children, including at least two studies using the same sampling and assay procedures used in the present investigation. In a study of p.m. cortisol levels in a low-risk sample of children ages 7–11 years, the mean p.m. cortisol levels reported were within the standard error of the mean of those found in the present study (Kertes & Gunnar, 2004). Likewise, when a.m. and p.m. cortisol levels were obtained from slightly older children (10–12 years) in a large study (N = 1,768; Rosmalen et al., 2005), again means were within the standard error of the mean reported in the present study. Thus, overall, the present results are comparable to previous reports on typically developing, family-reared children. This comparability suggests that being internationally adopted, in and of itself, does not mean that children’s basal cortisol levels will be substantially different than children reared in their families since birth. This also suggests that effects of preadoption deprivation on basal cortisol levels will not be large; indeed, the statistically significant effects we obtained were small in magnitude.
A second limitation is that we did not examine children on psychotropic medications. We chose to examine medication-free children to avoid needing to request a drug washout period. However, by eliminating those children with psychological problems requiring medication, we also likely eliminated some children who had been among the most adversely affected by their preadoption experiences. Indeed, in a previous survey of internationally adopted children, those who had received mental health treatment had experienced more preadoption deprivation than children not needing mental health services (Gunnar & van Dulmen, 2008). Very few children in the present study exhibited substantial behavioral and emotional problems as indicated by CBCL scores falling in the clinical range. It is certainly possible that we underestimated the impact of preadoption adversity by eliminating children on psychotropic medications, therefore eliminating most of the children with behavioral and emotional problems. There was some evidence that the few (6%) of children who exhibited significant internalizing problems may have had higher early morning cortisol levels. Given that the number of affected children was small and identified only with the CBCL, we need to be cautious in drawing any conclusions about internalizing problems and basal cortisol levels among children adopted internationally. Future studies will need to include children who have been prescribed psychotropic medications, but will need to address possible confounding effects of drugs or assess such children following a period of drug washout.
With these limitations in mind, we turn now to the results for deprived care. Participants in this study varied extensively in how likely it was that they experienced substantial deprivation prior to adoption. Some (7%) were adopted within 2 months of birth without any institutional care experience, whereas some (26%) were adopted after having lived for 2 or more years in institutional care. Although very few adoptive parents reported preadoption physical or sexual abuse, over 30% reported moderate to severe preadoption physical or social neglect. Duration of institutional care, parent-rated neglect prior to adoption, and age at adoption all contributed significantly to the latent construct, deprived care. As expected, this construct predicted the latent construct of growth delay. Indeed, nearly 80% of the children who were considered extremely growth delayed at adoption (>2.5 SD below the WHO mean in height for age) were adopted after an average of 21 months of institutional care. These findings were consistent with prior evidence that children lose approximately 1 month of linear growth for every 2 to 3 months in institutional care (Johnson, 2001; Miller & Hendrie, 2000). Nonetheless, not all of the severely growth delayed children had been in institutional care and some of the children who were growing adequately had been in institutions for many months prior to adoption. The variation in adoption growth parameters for these children likely reflects variations in the quality of care provided by different institutions as well as differences in children’s susceptibility to delayed growth under conditions of neglect.
Notably, the latent variable deprived care had no direct effects on basal cortisol values. This was true whether or not we included growth delay in the model. Based on these data, there is no reason to conclude that prolonged periods of early neglect or deprivation will directly affect children’s basal cortisol levels several years after being removed from deprived living conditions. This stands in contrast to evidence indicating that although young children are living in institutions they clearly show suppressed a.m. levels (Carlson & Earls, 1997; Kroupina, Gunnar & Johnson, 1997, also described in Gunnar & Vazquez, 2001). Combined with the present data, these findings suggest that the institutional suppression of the diurnal cortisol rhythm is transitory. After adoption the diurnal cortisol rhythm is again observed.
The lack of an effect of early deprived care on basal cortisol levels several years after adoption is consistent with most of the early experience animal models (e.g., Sanchez et al., 2001). Although low levels of maternal care and/or deprivation of maternal care in animal studies affects the HPA stress response, in nearly all studies basal cortisol levels have been unaffected. The typical early deprivation animal paradigm also does not produce animals that are physically smaller than nondeprived controls. As noted, this is only observed with extreme deprivation models that also produce elevated basal cortisol levels (Avishai-Eliner et al., 2001; Gilles et al., 1996). Thus, it may take deprivation that is severe enough to significantly disturb the growth system before one observes long-term impacts on basal activity of the HPA axis.
The present study appears to provide some support for this hypothesis. Children who were shorter for age but normal in weight for height at adoption (the pattern associated with psychosocial short stature) had higher a.m. cortisol levels and a more marked diurnal a.m. to p.m. decrease several years after adoption. These results were comparable in some respects to those obtained for the Romanian children adopted in 1990 (Gunnar et al., 2001). Specifically, the Romanian-adopted children also exhibited higher a.m. cortisol levels. However, unlike in the present study, their p.m. values were also higher than those of birth children and Romanian children adopted within a few months of birth. The Gunnar et al. (2001) study, however, involved a much smaller sample of children than the present study, and thus the results were less reliable. In addition, children in that study, unlike those in the present study, were not restricted from engaging in evening group activities (i.e., sports) on sampling days. Evening activities have been found to produce small increases in bedtime cortisol levels (Kertes & Gunnar, 2004). Given these differences, it seems likely that the present results provide a more accurate picture of the long-term impact of deprivation-induced growth delay.
Certainly, other factors associated with growth delay prior to adoption might have contributed to the present findings. Of particular concern would be the possibility that prenatal alcohol exposure influenced both growth delay and activity of the HPA axis. Prenatal alcohol exposure is believed to be common among children adopted from Russia/Eastern Europe (Johnson, 2001) and is associated both with linear growth retardation and altered HPA axis development (Ogilvie & Rivier, 1997; Schneider et al., 2002). Although we cannot rule out prenatal alcohol exposure as a common factor linking growth delays to elevated cortisol, one critical aspect of the present data argues against this explanation. Specifically, children who are growth delayed because of fetal alcohol syndrome do not typically exhibit rapid catchup growth (Hannigan & Armant, 2000). Nonetheless, although children in the present study were well below average in stature at the time of adoption, they were near the 50th percentile for height and weight in middle childhood.
From the present data, we cannot determine the physiological changes in the HPA system that may account for the observed effects. Early experience effects in rodents have been ascribed, in part, to glucocorticoid receptor changes (for a review see Sanchez et al., 2001). Basal cortisol activity is influenced by both mineralocorticoid (MR) and glucocorticoid (GR) receptors. At the peak of the diurnal cycle, GR as well as MR are occupied, whereas MR but not GR are occupied as basal cortisol levels decline across the day (de Kloet et al., 1998). MR blockade results in elevated bedtime cortisol levels, but has little effect on cortisol levels around wakeup (Young, Lopez, Murphy-Weinberg, Watson, & Akil, 1998). In contrast, the use of mifepristone, a GR antagonist, increases cortisol levels around wakeup, but does not affect bedtime cortisol levels (Wiedemann, Lauer, Hirschmann, Knaudt, & Holsboer, 1998). The present results, thus, would be consistent with a reduction in GR more so than effects on MR. Notably, this is the pattern typically reported in rodent studies of early deprivation (e.g., Sanchez et al., 2001). Alternatively, the present results could reflect differences in the magnitude of the cortisol awakening response (CAR) as a function of postnatal growth delay. We asked families to sample cortisol approximately 30 min after wakeup. This was done to capture the peak daily basal cortisol level, as cortisol rises about 40–60% in the first 30 min after awakening (Schmidt-Reinwald et al., 1999). The magnitude of the CAR is positively correlated with the adrenal response to adrenocorticotropin hormone (ACTH; Schmidt-Reinwald et al., 1999). Thus, an alternative mechanism might be alterations in the sensitivity of the adrenal cortex to increases in ACTH in response to morning awakening. Pharmacological challenge tests and examinations of the magnitude and time course of cortisol responses to stressor tasks will be needed to differentiate among these alternative mechanisms.
Several additional aspects of the present findings are noteworthy. First, as reported in previous samples (e.g., Essex, Klein, Cho, & Kalin, 2002; Halligan, Herbert, Goodyer, & Murray, 2004; Netherton, Goodyer, Tamplin, & Herbert, 2004), a small gender effect was observed, with girls showing slightly higher basal a.m. cortisol levels and thus more of a diurnal decrease than boys. Second, children adopted by parents with higher levels of education, but not income, also exhibited higher a.m. cortisol and steeper cortisol decreases. To our knowledge, an association of parent education but not income with children’s basal cortisol activity has not been previously reported. In fact, negative associations between socioeconomic status (SES) and children’s cortisol levels have been reported (Lupien, King, Meaney, & McEwen, 2000), even when SES has been defined as the average of parent education and income (Essex et al., 2002). Inconsistency with previous results suggests that the positive association with parent education may be a chance finding. Third, parents reported very few family and child stressors in the 6 months prior to cortisol sampling. This was relevant because current life stress may either amplify (Essex et al., 2002) or confound (Kaufman et al., 1997) the effects of early deprivation or maltreatment on the HPA axis. Thus, it was important to verify that the children were not currently living in stressful family circumstances. The median current life event score in the present study was 1 out of 37 possible events. This level of life stress was actually somewhat lower than that noted in other samples of low-risk children reared in their families of origin (e.g., Kertes & Gunnar, 2004). Of course, it is certainly possible that the lives of some of the children may have been fraught with unmeasured emotional stressors that were not included in the 37 events sampled in the life event questionnaire.
In summary, children who experienced more deprived care (duration and degree) prior to adoption exhibited greater physical growth delays at adoption. The pattern of growth delay was consistent with psychosocial short stature or a delay in linear growth because of chronic psychosocial stress. By the time we assessed children’s salivary cortisol levels in middle childhood, the children had largely caught up in physical growth, with both height and weight distributions being close to WHO norms. Concurrent growth parameters were not associated with basal cortisol levels. Deprived care prior to adoption (reflecting age at adoption, duration of institutional care, and parental reports of preadoption neglect) was not significantly associated with children’s basal cortisol levels. Thus, the present study provided no evidence that early neglect has long-term, direct impact on basal cortisol levels. Deprived care, however, significantly predicted growth delay assessed at the time of adoption, while greater growth delay at adoption predicted higher early a.m. cortisol levels and, consequently, a larger diurnal decrease in cortisol levels over the day. These results are consistent with the majority of the animal studies of early deprivation that yield no evidence of long-term effects on cortisol basal set points except among extreme deprivation models that also produce stunted growth. Animal studies do yield evidence of effects on the magnitude and duration of the HPA response to stressors. What is not known at this point is whether experiences prior to adoption have any significant impact on HPA stress reactivity either as a function of deprived care or for those children who suffer severe physical growth retardation as a result of preadoption experiences.
Acknowledgments
This research was supported by a National Institute of Mental Health Grant (MH59848-03S1), a National Institute of Mental Health Senior Scientist Award MH66208 to Megan Gunnar, and a National Science Foundation Fellowship to Darlene Kertes. The authors thank the families who participated in this research. We also thank Margaret Bale and Elizabeth Steele for help in conducting the study, Maria Kroupina for guidance in calculating anthropometrics, Andrea Gierens for assaying cortisol, and members of the Minnesota International Adoption Project Team (D. Johnson, H. Grotevant, W. Hellerstedt, R. Lee, S. Iverson, and K. Dole) for their support of this work.
References
- Achenbach T. Child Behavior Checklist for Children ages 6–18 (CBCL/6–18) ASEBA: Achenbach System of Empirically Based Assessment Website. 2006 Retrieved July 15, 2006, from http://www.aseba.org/products/cbcl6-18.html.
- Ames EW. Spitz revised: A trip to Romanian “orphanages. Canadian Psychological Association Developmental Psychology Section Newsletter. 1990;9:8–11. [Google Scholar]
- Ames EW, Burnaby BC. The development of Romanian orphanage children adopted to Canada. Ottawa: National Welfare Grants Program; 1997. [Google Scholar]
- Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Baram TZ. Altered regulation of gene and protein expression of hypothalamic–pituitary–adrenal axis components in an immature rat model of chronic stress. Journal of Neuroendocrinology. 2001;13:799–807. doi: 10.1046/j.1365-2826.2001.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Champoux M, Suomi SJ, Gunnar M. Salivary cortisol in nursery-reared rhesus monkeys: Reactivity to peer interactions and altered circadian activity. Developmental Psychobiology. 1995;28:257–267. doi: 10.1002/dev.420280502. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Chesney M, Alkon A, Tschann JM, Adams S, Chesterman B, et al. Psychobiologic reactivity to stress and childhood respiratory illnesses: Results of two prospective studies. Psychosomatic Medicine. 1995;57:411–422. doi: 10.1097/00006842-199509000-00001. [DOI] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biological Psychiatry. 2002;51:575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Cianfarani S, Geremia C, Scott CD, Germani D. Growth, IGF system, and cortisol in children with intrauterine growth retardation: Is catch-up growth affected by reprogramming of the hypothalamic–pituitary–adrenal axis? Pediatric Research. 2002;51:94–99. doi: 10.1203/00006450-200201000-00017. [DOI] [PubMed] [Google Scholar]
- Cianfarani S, Germani D, Rossi L, Argiro G, Boenmi S, Lemon M, et al. IGF-I and IGF-binding protein-1 are related to cortisol in human cord blood. European Journal of Endocrinology. 1998;138:524–529. doi: 10.1530/eje.0.1380524. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001a;13:783–804. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001b;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. A developmental psychopathology perspective on child abuse and neglect. Journal of the American Academy of Child & Adolescent Psychiatry. 1995;34:541–565. doi: 10.1097/00004583-199505000-00008. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Tucker D. Development and self-regulatory structures of the mind. Development and Psychopathology. 1994;6:533–549. [Google Scholar]
- Clements AD, Parker RC. The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, et al. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: Implications for the pathophysiology of mood and anxiety disorders. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft C, O’Connor TG, Keaveney L, Groothues C, Rutter M. Longitudinal change in parenting associated with developmental delay and catch-up. Journal of Child Psychology and Psychiatry. 2001;42:649–659. [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: The psychobiological development of maltreated children and its implications for research, treatment, and policy. Development and Psychopathology. 2001;13:539–564. doi: 10.1017/s0954579401003078. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. The psychobiology of neglect. Child Maltreatment. 2005;10:150–172. doi: 10.1177/1077559505275116. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JB, Boring AM, et al. Developmental traumatology, Part 2: Brain development. Biological Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Rosenfeld P, Van EeKelen JA, Sutanto W, Levine S. Stress, glucocorticoids and development. Progress in Brain Research. 1988;73:101–120. doi: 10.1016/S0079-6123(08)60500-2. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Rots NY, Cools AR. Brain-corticosteroid hormone dialogue: Slow and persistent. Cellular and Molecular Neurobiology. 1996;16:345–356. doi: 10.1007/BF02088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Vreugdenhil E, Oitzl M, Joels A. Brain corticosteroid receptor balance in health and disease. Endocrine Reviews. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Dettling A, Feldon J, Pryce CR. Repeated parental deprivation in the infant common marmoset (Callithrix jacchus, Primates) and analysis of its effects on early development. Biological Psychiatry. 2002;52:1037–1046. doi: 10.1016/s0006-3223(02)01460-9. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein M, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biological Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Frank DA, Klass PE, Earls F, Eisenberg L. Infants and young children in orphanages: One view from pediatrics and child psychiatry. Pediatrics. 1996;97:569–578. [PubMed] [Google Scholar]
- Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatric Neurology. 1996;15:114–119. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohlke BC, Frazer FL, Stanhope R. Growth hormone secretion and long-term growth data in children with psychosocial short stature treated by different changes in environment. Journal of Pediatric Endocrinology and Metabolism. 2004;17:637–643. doi: 10.1515/jpem.2004.17.4.637. [DOI] [PubMed] [Google Scholar]
- Graham YP, Heim C, Goodman SH, Miller AH, Nemeroff CB. The effects of neonatal stress on brain development: Implications for psychopathology. Development and Psychopathology. 1999;11:545–565. doi: 10.1017/s0954579499002205. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Development and Psychopathology. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar M, van Dulmen M. Behavior problems in post-institutionalized internationally-adopted children. 2008. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Vazquez D. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer IM, Murray L. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biological Psychiatry. 2004;55:376–381. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Armant DR. Alcohol in pregnancy and neonatal outcome. Seminars in Neonatology. 2000;5:243–254. doi: 10.1053/siny.2000.0027. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DK. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox MBR, Miller AH, et al. Pituitary–adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Journal of the American Medical Association. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heim C, Owen MJ, Plotsky PM, Nemeroff CB. The role of early adverse life events in the etiology of depression and posttraumatic stress disorder: Focus on corticotropin-releasing factor. Annals of the New York Academy of Sciences. 1997;821:194–207. doi: 10.1111/j.1749-6632.1997.tb48279.x. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Johnson DE. The impact of orphanage rearing on growth and development. In: Nelson CA, editor. The effects of adversity on neurobehavioral development: Minnesota Symposia on Child Psychology. Vol. 31. Mahwah, NJ: Erlbaum; 2001. pp. 113–162. [Google Scholar]
- Kaufman J, Birmaher B, Perel J, Dahl RE, Moreci P, Nelson B, et al. The corticotropin-releasing hormone challenge in depressed abused, depressed nonabused, and normal control children. Biological Psychiatry. 1997;42:669–679. doi: 10.1016/s0006-3223(96)00470-2. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Charney DS. Neurobiological correlates of child abuse. Biological Psychiatry. 1999;45:1235–1236. doi: 10.1016/s0006-3223(99)00064-5. [DOI] [PubMed] [Google Scholar]
- Kertes D, Gunnar M. Evening activities as a potential confound in research on the adrenocortical system in children. Child Development. 2004;75:193–204. doi: 10.1111/j.1467-8624.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- Kroupina M, Gunnar M, Johnson D. Report on salivary cortisol levels in a Russian baby home. Minneapolis, MN: University of Minnesota, Institute of Child Development; 1997. [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biological Psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Mason P, Narad C. Long-term growth and puberty concerns in international adoptees. Pediatric Clinics of North America. 2005;52:1351–1368. doi: 10.1016/j.pcl.2005.06.016. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F. Latent difference score structural models for linear dynamic analysis with incomplete longitudinal data. In: Collins LM, Sayer AG, editors. New methods for the analysis of change. Washington, DC: American Psychological Association; 2001. pp. 137–176. [Google Scholar]
- McEwen B. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Science. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Meaney M, Szyf M. Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LC, Hendrie NW. Health of children adopted from China. Pediatrics. 2000;105:1–6. doi: 10.1542/peds.105.6.e76. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus: User’s guide. Los Angeles: Author; 2001. [Google Scholar]
- Netherton C, Goodyer I, Tamplin A, Herbert J. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology. 2004;29:125–140. doi: 10.1016/s0306-4530(02)00150-6. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Rivier C. Prenatal alcohol exposure results in hyperactivity of the hypothalamic–pituitary–adrenal axis of the offspring: Modulation by fostering at birth and postnatal handling. Alcoholism: Clinical and Experimental Research. 1997;21:424–429. doi: 10.1111/j.1530-0277.1997.tb03786.x. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Molecular Brain Research. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychological Review. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Rosmalen JG, Oldehinkel AJ, Ormel J, de Winter AF, Buitelaar JK, Verhulst FC, et al. Determinants of salivary cortisol levels in 10–12 year old children; A population-based study of individual differences. Psychoneuroendocrinology. 2005;30:483–495. doi: 10.1016/j.psyneuen.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Rutter M. Maternal deprivation reconsidered. Journal of Psychosomatic Research. 1972;16:241–250. doi: 10.1016/0022-3999(72)90005-0. [DOI] [PubMed] [Google Scholar]
- Rutter M. Developmental catch-up, and deficit, following adoption after severe global early privation. English and Romanian Adoptees (ERA) Study Team. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1998;39:465–476. [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–450. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, et al. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biological Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schurmeyer TH, et al. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sciences. 1999;64:1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT. The impact of prenatal stress, fetal alcohol exposure, or both on development: Perspectives from a primate model. Psychoneuroendocrinology. 2002;27:285–298. doi: 10.1016/s0306-4530(01)00050-6. [DOI] [PubMed] [Google Scholar]
- Schwartz EP, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Development. 1998;69:1503–1513. [PubMed] [Google Scholar]
- Smyke AT, Dumitrescu A, Zeanah CH. Attachment disturbances in young children. I: The continuum of caretaking casualty. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:972–982. doi: 10.1097/00004583-200208000-00016. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Early determinants of behaviour: Evidence from primate studies. British Medical Bulletin. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcarri A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatric Clinics of North America. 2002;25:397–426. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- US Department of State. Immigrant visas issued to orphans coming to the U.S. 2006 Retrieved July 15, 2006, from http://travel.state.gov/family/adoption/stats/stats_451.html.
- Verhulst FC, Althaus M, Versluis-den Bieman HJ. Damaging backgrounds: Later adjustment of international adoptees. Journal of the American Academy of Child & Adolescent Psychiatry. 1992;31:518–524. doi: 10.1097/00004583-199205000-00020. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, La Plante P, Weaver S, Parent A, Sharma S, Diorio J, et al. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: Characterization of intracellular mediators and potential genomic target sites. Molecular and Cellular Endocrinology. 2001;185:205–218. doi: 10.1016/s0303-7207(01)00635-9. [DOI] [PubMed] [Google Scholar]
- Wiedemann K, Lauer CJ, Hirschmann M, Knaudt K, Holsboer F. Sleep endocrine effects of mifepristone and megestrol acetate in healthy men. American Journal of Physiology. 1998;274:139–145. doi: 10.1152/ajpendo.1998.274.1.E139. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. Journal of Clinical Psychiatry. 2000;61(Suppl 7):15–21. [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Grossman R. Childhood trauma and risk for posttraumatic stress disorder: Relationship to intergenerational effects of trauma, parental posttraumatic stress disorder, and cortisol excretion. Development and Psychopathology. 2001;13:733–753. doi: 10.1017/s0954579401003170. [DOI] [PubMed] [Google Scholar]
- Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. The role of mineralocorticoid receptors in hypothalamic–pituitary–adrenal axis regulation in humans. Journal of Clinical Endocrinology and Metabolism. 1998;83:3339–3345. doi: 10.1210/jcem.83.9.5077. [DOI] [PubMed] [Google Scholar]