Abstract
In this paper, we examined whether meditation practice influences the epigenetic clock, a strong and reproducible biomarker of biological aging, which is accelerated by cumulative lifetime stress and with age-related chronic diseases. Using the Illumina 450 K array platform, we analyzed the DNA methylome from blood cells of long-term meditators and meditation-naïve controls to estimate their Intrinsic Epigenetic Age Acceleration (IEAA), using Horvath's calculator. IEAA was similar in both groups. However, controls showed a different IEAA trajectory with aging than meditators: older controls (age ≥ 52) had significantly higher IEAAs compared with younger controls (age < 52), while meditators were protected from this epigenetic aging effect. Notably, in the meditation group, we found a significant negative correlation between IEAA and the number of years of regular meditation practice. From our results, we hypothesize that the cumulative effects of a regular meditation practice may, in the long-term, help to slow the epigenetic clock and could represent a useful preventive strategy for age-related chronic diseases. Longitudinal randomized controlled trials in larger cohorts are warranted to confirm and further characterize these findings.
Keywords: Stress, Mindfulness, Epigenetics, Methylation, Biological aging, Blood
1. Introduction
Recently, DNA methylation has emerged as a robust biomarker of biological aging. The methylation level of a large number of genomic sites are highly associated with age, to such a point that methods based on a small number of these sites can predict the chronological age with an average error of only 4 years (Hannum et al., 2013; Horvath, 2013). More precisely, the DNA methylation (DNAm) age can be estimated from the methylation levels of 353 CpG sites of the genome and has been found to highly correlate to chronological age and to be stable across most tissues and cell types (i.e. whole blood, peripheral mononuclear cells, brain cells, breast, kidney, liver, lung and saliva) (Horvath, 2013). The deviation between the DNAm age and the chronological age of an individual provides information regarding his epigenetic aging rate (Chen et al., 2016). Faster-running epigenetic clocks have been negatively associated with longevity (Horvath et al., 2015), and positively associated with chronic diseases (Horvath et al., 2014; Horvath and Ritz, 2015; Levine et al., 2015a, 2015b; Perna et al., 2016), frailty (Breitling et al., 2016), cognitive and physical fitness in the elderly (Marioni et al., 2015b) and all cause mortality even after adjusting for chronological age and a variety of known risk factors (Christiansen et al., 2016; Marioni et al., 2015a; Perna et al., 2016). The epigenetic clock therefore may represent an accurate tool to measure the effectiveness of lifestyle-based interventions for the prevention of age-related diseases. This is a health priority area today as revealed by data from the Centers for Disease Control and Prevention indicating that 78% of adults aged 55 years and over present one or more chronic diseases (www.cdc.gov).
Consistent with previous work describing the noxious impact of psychological stress on health span (Juster et al., 2010), it has recently been shown that cumulative lifetime chronic stress (Zannas et al., 2015) and trauma (Boks et al., 2015) predict accelerated epigenetic aging. Meditation-based stress reduction interventions have increasingly become a focus of scientific interest to promote healthy aging. Recent research suggests that meditation has beneficial effects in stress and age-related neuroplastic changes, and mood and cognitive disorders (Davidson and McEwen, 2012; Acevedo et al., 2016; Luders, 2014). Molecular mechanisms involved in the aging process, such as inflammation, immune and epigenetic pathways (Black and Slavich, 2016; Kaliman et al., 2014), as well as telomere maintenance (Epel et al., 2009; Alda et al., 2016), are also sensitive to contemplative practices. However, it remains unexplored whether meditation experience modulates the rate of the epigenetic aging. Given the well-characterized effect of meditation practice on stress reduction (McEwen, 2016), we tested the hypothesis that long-term practitioners would show slower rates of epigenetic aging than age and sex matched controls. To do so, we analyzed DNA methylome data from blood cells of long-term meditators and meditation-naïve controls.
2. Material and methods
2.1. Participants
Long-term meditators (n = 18) and meditation-naïve controls (n = 20) were studied. The recruitment strategy, and inclusion and exclusion criteria, are described in detail in supplementary information S1. The two groups had similar group distribution of age, sex, body mass index and ethnicity (S2). Participant's written informed consent was approved by the UW-Madison. Meditators had more than 3 years of practice (with a minimum of 30 min of daily sitting meditation and at least 3 intensive retreats), including both mindfulness and compassion-related meditations. The control group comprised of individuals with no prior meditation experience.
These participants were studied in the context of our previous work (Kaliman et al., 2014), before (8 am, Time 1) and after (4 pm, Time 2) an intensive day of mindfulness meditation or leisure time in the same environment for meditators and controls, respectively. Detailed information about the interventions is provided in S3. As no impact of daytime or interventions was found on epigenetic aging measures (S3), results presented in this paper correspond to Time 1 samples.
2.2. Isolation of peripheral blood mononuclear cells (PBMC)
Blood samples were obtained from each participant and PBMCs were isolated, as described (Kaliman et al., 2014). DNA was isolated using QIAamp DNA Blood Mini Kit, and stored at −80 °C until processing. Sample processing is described in detail in the supplementary information (S1).
2.3. Genome-wide DNA methylation profiling
Methylation analysis was performed using Illumina Infinium HumanMethylation450 BeadChip. DNA methylation data from all participants were generated at the same time. DNA samples from both groups were randomized across the chips. For each participant, DNA methylation data at more than 485,512 sites was obtained. Four participants (3 controls and 1 meditator) with poor methylome quality were removed, leaving a data set of 17 meditators and 17 controls.
2.4. DNA methylation (DNAm) age and intrinsic epigenetic age acceleration (IEAA)
DNAm age was calculated from raw β-values using an elastic net regression model based on 353 CpGs. IEAA is defined as the residual resulting from a multivariate regression model of DNAm age on chronological age and blood cell counts (Chen et al., 2016). DNAm age and IEAA were estimated using the DNAm age calculator (Horvath, 2013). All values for DNAm age and IEAA are provided in S4.
We tested whether IEAA was significantly different between meditators and controls using a linear model including age and sex as covariates. To explore IEAA trajectory as a function of age in meditators and controls, we generated a total of four subgroups including 9 subjects with ages < 52 and 8 subjects with ages ≥ 52, both for meditators and controls. All tests were corrected for sex. The cut-off age was set to 52 following two criteria: (i) to generate balanced subgroups in terms of sample size (ii) that the older group includes ages at which most individuals present one or several chronic diseases (www.cdc.gov). We checked that BMI, ethnicity and sex showed similar distributions between the subgroups (t-test p-value for BMI and chi-square p-values with Yates’s correction for sex and ethnicity > 0.5 when comparing control subgroups and p-values =0.85, 0.08 and 0.25 respectively for these tests when comparing meditator subgroups). In addition, the two subgroups of expert meditators showed similar distribution of years of meditation experience (p-value = 0.59).
Regression analysis between IEAA and the number of years of practice, taking into account age and sex as covariates, were carried out within the meditators group without including controls, and in the two age subgroups including controls as basal (“zero years meditation experience”). We confirmed that IEAA and years of meditation followed a normal distribution with the Shapiro-Wilk test (p-value=0.59 and 0.17 respectively), and we did not identify any outlier in terms of IEAA (p-value=0.46) or years of meditation (p-value=0.09) using the Grubbs test.
3. Results
3.1. DNA methylation (DNAm) age and Intrinsic epigenetic age acceleration (IEAA) estimation
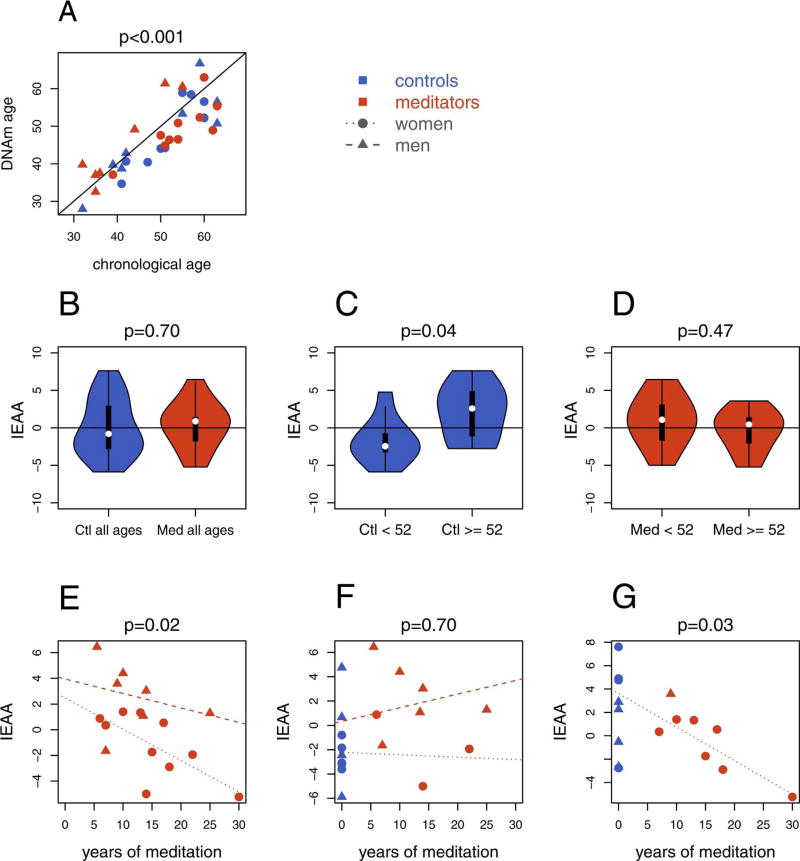
We measured the DNA methylation (DNAm) ages in long-term meditators and meditation-naïve controls. DNAm and chronological ages were highly correlated to each other (Pearson r = 0.88 in controls, r = 0.79 in meditators, r = 0.83 overall, p-value < 10−3 in all cases) (Fig. 1A), confirming the accuracy of the epigenetic clock model (Horvath, 2013).
Fig. 1.
(A) DNA methylation age (DNAm) correlation with chronological age by group and sex (B–D) Violin plots showing Intrinsic epigenetic age acceleration (IEAA) measured in (C) meditators and controls, (D) controls aged less than 52 and controls aged 52 and over and (E) meditators aged less than 52 and meditators aged 52 and over. (E–G) IEAA regressed on years of meditation practice in (F) the meditators’ group, (G) subjects aged less than 52, (H) subjects aged 52 and over. P-value for figure A indicates the significance level of the correlation and p-values for figures B–D are extracted from the linear models presented in Table 1. In Figs. 1G and H, the years of meditation practice was set to zero for controls. In Fig. 1H, the regression line for men is not traced because of their limited number. In the violin plots (Figures B–D), the white dot is the median, the thick vertical line corresponds to the interquartile range and the thin vertical line to the 95% confidence interval. The bulge corresponds to the kernel density estimation to show the distribution shape of the data. Wider sections of the violin plot represent a higher probability that the samples will have a given IEAA value, the skinnier sections represent a lower probability.
We calculated the Intrinsic Epigenetic Age Aceleration (IEAA) which adjusts the epigenetic aging rate for blood cell count estimates, leading to a measure unaffected by both variation in chronological age and blood cell composition (Chen et al., 2016). Correcting for age and sex, we found that meditators and controls exhibited similar IEAAs (p-value = 0.70) (Fig. 1B, Table 1A), indicating no difference in the epigenetic aging rate between the two groups.
Table 1.
Linear models fitted to test A) if IEAA differs between i) controls and meditators, ii) controls aged less than 52 and controls aged 52 and over, iii) meditators aged less than 52 and meditators aged 52 and over, and B) to test is IEAA depends on years of meditation practice (years_med) i) within the meditators group, ii) in all subjects younger than 52 years old and iii) in all subjects 52 years old and over. These models integrate age of participants in years and sex (0 females, 1 males) as covariates. In the two latter models, years_med was set to zero for controls. Beta coefficients, their 95% confidence intervals (95% CI) and P-values are listed for each explanatory variable. P-values below the 5% cutoff are in bold. Table S5 (in Supplementary information) provides the standardized beta coefficients from these linear models.
| A – Difference in IEAA between groups | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Controls versus meditators, all ages (Fig. 1B) | Controls < 52 versus Controls ≥ 52 (Fig. 1C) | Meditators < 52 versus Meditators ≥ 52 (Fig. 1D) | ||||
|
|
|
|
||||
| Beta [95% CI] | P-val | Beta [95% CI] | P-val | Beta [95% CI] | P-val | |
| Group | 0.47 [−1.88,2.82] | 0.7 | 3.8 [0.46,7.14] | 0.04 | 1.13 [−1.83,4.1] | 0.47 |
| Sex | 2.06 [−0.51,4.62] | 0.13 | −0.55 [−3.89,2.79] | 0.75 | 4.46 [1.45,7.47] | 0.01 |
| Age | 0.04 [−0.09,0.18] | 0.52 | – | – | – | – |
| B – Regression of IEAA on years of meditation practice | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Meditators, all ages (Fig. 1E) | All subjects < 52 (Fig. 1F) | All subjects ≥ 52 (Fig. 1G) | ||||
|
|
|
|
||||
| Beta [95% CI] | P-val | Beta [95% CI] | P-val | Beta [95% CI] | P-val | |
| Years_med | −0.24 [−0.42,−0.06] | 0.02 | 0.04 [−0.16,0.24] | 0.7 | −0.22 [−0.39,−0.05] | 0.03 |
| Sex | 4.25 [1.37,7.13] | 0.01 | 3.82 [−0.08,7.72] | 0.08 | −1.04 [−4.33,2.26] | 0.55 |
| Age | 0.09 [−0.07,0.25] | 0.28 | 0.05 [−0.25,0.36] | 0.73 | −0.35 [−0.74,0.04] | 0.11 |
3.2. Age-related IEAA trajectories in controls and meditators
We next examined how aging influenced IEAA in controls and long term meditators. We compared IEAA between ages < 52 and ≥ 52 in the control group, and we performed the same analysis in long term meditators.
We found that controls presented a different IEAA trajectory of aging compared with meditators: while controls aged 52 and over showed significantly higher IEAAs than younger controls (p-value = 0.04, Fig. 1C and Table 1A), meditators were protected from this effect, showing similar IEAAs in both age subgroups (p-value = 0.47, Fig. 1D and Table 1A).
3.3. IEAA and years of meditation practice
To further explore the influence of meditation practice on IEAA, we regressed IEAA on years of regular meditation practice including age and sex as covariates. Expert meditators showed a mean decrease in IEAA of 0.24 [0.06, 0.42] (p-value = 0.02) for each additional year of regular meditation practice (Fig. 1E and Table 1B). We also performed this analysis considering the control group as “zero meditation years” (Fig. 1F and G, Table 1B). We found a significant negative correlation between meditation years of practice and IEAA in subjects aged ≥ 52 (p-value = 0.03) while such an effect was not found in younger subjects (ages < 52) (p-value =0.7).
4. Discussion
The current study was designed to determine if the rate of epigenetic aging in long-term meditators was different than in meditationnaïve controls (the meditators recruited for this study had between 5 and 30 years of continuous regular practice). We found no difference in the intrinsic epigenetic aging acceleration (IEAA) between long-term meditators and meditation-naïve controls. However, we found a different IEAA trajectory in controls and meditators as a function of age, with an increase of IEAA with age in controls but not in meditators, suggesting that integrating meditation practice into daily routine may have a protecting effect in terms of epigenetic aging in the long run. Cumulative lifetime stress is one of the factors described to accelerate epigenetic aging (Zannas et al., 2015). Our study suggests that psychological stress reduction in meditators as a result of their regular practice may contribute to the stability of their IEAA across ages compared with controls. Consistent with this hypothesis, we found that in long-term meditators the epigenetic aging rate decreased significantly with the number of years of regular meditation practice. When controls were included in this analysis as “zero meditation experience”, the significant effect of years of meditation on IEAA was only found in subjects aged ≥ 52, indicating that this protective effect of meditation practice is observed mainly in older subjects. As a whole, these findings suggest that the protective effect of meditation on epigenetic age acceleration may be progressive and cumulative.
Data presented here extend previous evidence showing the influence of meditation training on aging biomarkers, notably the presence of longer telomeres in long-term meditators (Alda et al., 2016), the increase in telomerase activity after a 3 months meditation retreat (Jacobs et al., 2011), a yogic meditation training (Lavretsky et al., 2013) and a mindfulness-based stress reduction program (Lengacher et al., 2014). Telomere length and epigenetic aging rate, however, have been shown to be mutually uncorrelated and independently linked to disease and mortality risk, probably through different aging signaling pathways (Marioni et al., 2016). Previous reports on telomere biology, together with our findings on epigenetic aging, suggest that meditation practice may modulate different molecular mechanisms involved in cell aging and may represent a useful preventive strategy for age-related chronic diseases.
Our data may contribute to reveal the benefits of long-term meditation on healthy aging although they present several limitations that warrant further investigation. In particular, longitudinal randomized and controlled trials involving larger samples are required to help disentangle the effect of meditation from pre-existing individual differences and/or from other meditation-associated lifestyle factors on epigenetic aging. It is also critical to explore in future studies the type of practices (i.e. mindfulness-related meditations, compassion-related meditations, breath work, visualizations, body scanning or other mind-body techniques) and the frequency and duration of the trainings that could efficiently decrease the epigenetic aging rate in otherwise meditation-naïve healthy subjects or clinical populations.
Supplementary Material
Acknowledgments
Funding
This work was supported by CNRS PEPS INEE 2015 (RC), NCCAM (NIH) (P01-AT004952 (RJD and AL), Fetzer Institute, John Templeton Foundation, anonymous donor (RJD), LABEX CORTEX ANR-11- LABX-0042, Université de Lyon ANR-11-IDEX-0007 and ERC–Consolidator 617739-BRAINandMINDFULNESS (AL). We thank Claire Dandine, Hervé Perdry and Alice Urvoy for helpful discussions.
Footnotes
Authors’ contributions
RC, RJD, AL and PK designed the study; MJA and PK generated the samples; BR and LL produced the methylation data; RC, MF and BR analyzed the data; RC and PK wrote the manuscript.
All authors declare that they have no conflicts of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2017.08.016.
References
- Acevedo BP, Pospos S, Lavretsky H. The neural mechanisms of meditative practices: novel approaches for healthy aging. Curr. Behav. Neurosci. Rep. 2016;3:328–339. doi: 10.1007/s40473-016-0098-x. http://dx.doi.org/10.1007/s40473-016-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alda M, Puebla-Guedea M, Rodero B, Demarzo M, Montero-Marin J, Roca M, Garcia-Campayo J. Zen meditation, length of telomeres, and the role of experiential avoidance and compassion. Mindfulness (N. Y.) 2016;7:651–659. doi: 10.1007/s12671-016-0500-5. http://dx.doi.org/10.1007/s12671-016-0500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DS, Slavich GM. Mindfulness meditation and the immune system: a systematic review of randomized controlled trials. Ann. N. Y. Acad. Sci. 2016;1373:13–24. doi: 10.1111/nyas.12998. http://dx.doi.org/10.1111/nyas.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boks MP, Mierlo HC, Rutten van Radstake BPF, De Witte TRDJ, Geuze L, Horvath E, Schalkwyk S, Vinkers LC, Broen CH, Vermetten JCA. Longitudinal changes of telomere length and epigenetic age related to traumatic stress and post-traumatic stress disorder. Psychoneuroendocrinology. 2015;51:506–512. doi: 10.1016/j.psyneuen.2014.07.011. http://dx.doi.org/10.1016/j.psyneuen.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Breitling LP, Saum K-U, Perna L, Schöttker B, Holleczek B, Brenner H. Frailty is associated with the epigenetic clock but not with telomere length in a German cohort. Clin. Epigenet. 2016;8:21. doi: 10.1186/s13148-016-0186-5. http://dx.doi.org/10.1186/s13148-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BH, Marioni RE, Colicino E, Peters MJ, Ward-Caviness CK, Tsai PC, Roetker NS, Just AC, Demerath EW, Guan W, Bressler J, Fornage M, Studenski S, Vandiver AR, Moore AZ, Tanaka T, Kiel DP, Liang L, Vokonas P, Schwartz J, Lunetta KL, Murabito JM, Bandinelli S, Hernandez DG, Melzer D, Nalls M, Pilling LC, Price TR, Singleton AB, Gieger C, Holle R, Kretschmer A, Kronenberg F, Kunze S, Linseisen J, Meisinger C, Rathmann W, Waldenberger M, Visscher PM, Shah S, Wray NR, McRae AF, Franco OH, Hofman A, Uitterlinden AG, Absher D, Assimes T, Levine ME, Lu AT, Tsao PS, Hou L, Manson JAE, Carty CL, LaCroix AZ, Reiner AP, Spector TD, Feinberg AP, Levy D, Baccarelli A, Meurs J, van Bell JT, Peters A, Deary IJ, Pankow JS, Ferrucci L, Horvath S. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany. NY) 2016;8:1844–1865. doi: 10.18632/aging.101020. http://dx.doi.org/10.18632/aging.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen L, Lenart A, Tan Q, Vaupel JW, Aviv A, Mcgue M, Christensen K. DNA methylation age is associated with mortality in a longitudinal Danish twin study. Aging Cell. 2016;15:149–154. doi: 10.1111/acel.12421. http://dx.doi.org/10.1111/acel.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan JB, Gao Y, Deconde R, Chen M, Rajapakse I, Friend S, Ideker T, Zhang K. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016. http://dx.doi.org/10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Ritz BR. Increased epigenetic age and granulocyte counts in the blood of Parkinson ’ s disease patients. Aging (Albany NY) 2015;7:1–13. doi: 10.18632/aging.100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Erhart W, Brosch M, Ammerpohl O, von Schönfels W, Ahrens M, Heits N, Bell JT, Tsai P-C, Spector TD, Deloukas P, Siebert R, Sipos B, Becker T, Röcken C, Schafmayer C, Hampe J. Obesity accelerates epigenetic aging of human liver. Proc. Natl. Acad. Sci. U. S. A. 2014;111:15538–15543. doi: 10.1073/pnas.1412759111. http://dx.doi.org/10.1073/pnas.1412759111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Pirazzini C, Bacalini M, Gentilini D, Di Blasio A, Delledonne M, Mari D, Arosio B, Monti D, Passarino G, De Rango F, D’Aquila P, Giuliani C, Marasco E, Collino S, Descombes P, Garagnani P, Franceschi C. Decreased epigenetic age of PBMCs from Italian semi-supercentenarians and their offspring. Aging (Albany. NY) 2015;7:159–170. doi: 10.18632/aging.100861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. http://dx.doi.org/10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs TL, Epel ES, Lin J, Blackburn EH, Wolkowitz OM, Bridwell Da, Zanesco AP, Aichele SR, Sahdra BK, MacLean Ka, King BG, Shaver PR, Rosenberg EL, Ferrer E, Wallace BA, Saron CD. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36:664–681. doi: 10.1016/j.psyneuen.2010.09.010. http://dx.doi.org/10.1016/j.psyneuen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. http://dx.doi.org/10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kaliman P, Álvarez-López MJ, Cosín-Tomás M, Rosenkranz MA, Lutz A, Davidson RJ. Rapid changes in histone deacetylases and inflammatory gene expression in expert meditators. Psychoneuroendocrinology. 2014;40:96–107. doi: 10.1016/j.psyneuen.2013.11.004. http://dx.doi.org/10.1016/j.psyneuen.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H, Epel ES, Siddarth P, Nazarian N, Cyr NS, Khalsa DS, Lin J, Blackburn E, Irwin MR. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int. J. Geriatr. Psychiatry. 2013;28:57–65. doi: 10.1002/gps.3790. http://dx.doi.org/10.1002/gps.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengacher CA, Reich RR, Kip KE, Barta M, Ramesar S, Paterson CL, Moscoso MS, Carranza I, Budhrani PH, Kim SJ, Park HY, Jacobsen PB, Schell MJ, Jim HSL, Post-White J, Farias JR, Park JY. Influence of mindfulness-based stress reduction (MBSR) on telomerase activity in women with breast cancer (BC) Biol. Res. Nurs. 2014;16:438–447. doi: 10.1177/1099800413519495. http://dx.doi.org/10.1177/1099800413519495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Hosgood HD, Chen B, Absher D, Assimes T, Horvath S. DNA methylation age of blood predicts future onset of lung cancer in the women’s health initiative. Aging (Albany. NY) 2015a;7:690–700. doi: 10.18632/aging.100809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Lu AT, Bennett DA, Horvath S. Epigenetic age of the pre-frontal cortex is associated with neuritic plaques, amyloid load, and Alzheimer’s disease related cognitive functioning. Aging (Albany. NY) 2015b;7:1198–1211. doi: 10.18632/aging.100864. http://dx.doi.org/10.18632/aging.100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E. Exploring age-related brain degeneration in meditation practitioners. Ann. N. Y. Acad. Sci. 2014;1307:82–88. doi: 10.1111/nyas.12217. http://dx.doi.org/10.1111/nyas.12217. [DOI] [PubMed] [Google Scholar]
- Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, Gibson J, Henders AK, Redmond P, Cox SR, Pattie A, Corley J, Murphy L, Martin NG, Montgomery GW, Feinberg AP, Fallin MD, Multhaup ML, Jaffe AE, Joehanes R, Schwartz J, Just AC, Lunetta KL, Murabito JM, Starr JM, Horvath S, Baccarelli Aa, Levy D, Visscher PM, Wray NR, Deary IJ. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015a;16:25. doi: 10.1186/s13059-015-0584-6. http://dx.doi.org/10.1186/s13059-015-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Shah S, McRae AF, Ritchie SJ, Muniz-Terrera G, Harris SE, Gibson J, Redmond P, Cox SR, Pattie A, Corley J, Taylor A, Murphy L, Starr JM, Horvath S, Visscher PM, Wray NR, Deary IJ. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int. J. Epidemiol. 2015b;44:1388–1396. doi: 10.1093/ije/dyu277. http://dx.doi.org/10.1093/ije/dyu277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Harris SE, Shah S, McRae AF, von Zglinicki T, Martin-Ruiz C, Wray NR, Visscher PM, Deary IJ. The epigenetic clock and telomere length are independently associated with chronological age and mortality. Int. J. Epidemiol. 2016;45:424–432. doi: 10.1093/ije/dyw041. http://dx.doi.org/10.1093/ije/dyw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. In pursuit of resilience: stress, epigenetics, and brain plasticity. Ann. N. Y. Acad. Sci. 2016;1373:56–64. doi: 10.1111/nyas.13020. http://dx.doi.org/10.1111/nyas.13020. [DOI] [PubMed] [Google Scholar]
- Perna L, Zhang Y, Mons U, Holleczek B, Saum K-U, Brenner H. Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort. Clin. Epigenet. 2016;8:64. doi: 10.1186/s13148-016-0228-z. http://dx.doi.org/10.1186/s13148-016-0228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas AS, Arloth J, Carrillo-Roa T, Iurato S, Röh S, Ressler KJ, Nemeroff CB, Smith AK, Bradley B, Heim C, Menke A, Lange JF, Brückl T, Ising M, Wray NR, Erhardt A, Binder EB, Mehta D. Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol. 2015;16:266. doi: 10.1186/s13059-015-0828-5. http://dx.doi.org/10.1186/s13059-015-0828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.