Abstract
Objective
Infantile spasms (IS) represent a severe epileptic encephalopathy presenting in the first 2 years of life. Recommended first-line therapies (hormonal therapy or vigabatrin) often fail. We evaluated response to second treatment for IS in children in whom the initial therapy failed to produce both clinical remission and electrographic resolution of hypsarhythmia and whether time to treatment was related to outcome.
Methods
The National Infantile Spasms Consortium established a multicenter, prospective database enrolling infants with new diagnosis of IS. Children were considered nonresponders to first treatment if there was no clinical remission or persistence of hypsarhythmia. Treatment was evaluated as hormonal therapy (adrenocorticotropic hormone [ACTH] or oral corticosteroids), vigabatrin, or “other.” Standard treatments (hormonal and vigabatrin) were compared to all other nonstandard treatments. We compared response rates using chi-square tests and multivariable logistic regression models.
Results
One hundred eighteen infants were included from 19 centers. Overall response rate to a second treatment was 37% (n = 44). Children who received standard medications with differing mechanisms for first and second treatment had higher response rates than other sequences (27/49 [55%] vs. 17/69 [25%], p < 0.001). Children receiving first treatment within 4 weeks of IS onset had a higher response rate to second treatment than those initially treated later (36/82 [44%] vs. 8/34 [24%], p = 0.040).
Significance
Greater than one third of children with IS will respond to a second medication. Choosing a standard medication (ACTH, oral corticosteroids, or vigabatrin) that has a different mechanism of action appears to be more effective. Rapid initial treatment increases the likelihood of response to the second treatment.
Keywords: Infantile spasms, Adrenocorticotropic hormone, Vigabatrin, Second-line treatment
Infantile spasms (IS) are an age-specific seizure type that occurs in the first 2 years of life. IS are associated with severe epileptic encephalopathy with an incidence of 2–5 per 10,000 live births.1–4 Treatment is recommended urgently; delays in diagnosis and treatment are associated with subsequent intellectual impairment.5 Sixty percent of children with IS will develop other seizure types,6 and 75–87% will develop intellectual impairment.6,7 There has been little improvement in outcome of these children over the past 30 years.8 Despite this, there is continued debate regarding initial treatment and there are limited data addressing treatment following failure of initial treatment.
Steroid treatment with adrenocorticotropic hormone (ACTH) and oral corticosteroids (OCS) have demonstrated efficacy since 1958,9,10 with more recent studies showing a response rate between 55%11 and 73%.12 The United Kingdom Infantile Spasms study observed similar response rates between OCS and tetracosactide, the synthetic form of ACTH, and these were considered superior to vigabatrin.12 Vigabatrin is effective in 38–48% of children without tuberous sclerosis complex.13,14 Evidence-based guidelines developed in 2004 state that “ACTH is probably effective for the short-term management of IS,” and an update in 2012 adds that vigabatrin “may be useful for short term treatment of IS with ACTH considered preferentially over vigabatrin.”15,16 Despite guidelines, there is little uniformity among providers’ practices.17,18 This could be due in part to variation in outcome measures among the studies, with clinical cessation of IS as the most oft-used primary outcome measure, but relapse rates and electroencephalography (EEG) improvement must also be considered in assessing efficacy. Nonetheless, relapse rates and failure rates remain high, with all standard treatments leaving a large percentage of children without successful treatment.
Many studies report the use of nonstandard therapies for IS in infants for whom traditional medications have been ineffective. In a single study comparing topiramate and levetiracetam as second therapy after failure of oral steroids, there was a poor response to either medication given sequentially.19 Long-term use of high-dose topiramate has been reported, but again after there had been failure of several medications.20 Felbamate,21 lamotrigine,22,23 and zonisamide24 responses have been reported in similar small studies as well as the use of the ketogenic diet.25 Recent guidelines suggest several alternative treatments based on expert opinion.26
This study evaluates treatment response after failure of initial medication in a large national prospective database. We hypothesized that children prescribed standard second treatments would have higher response rates than children prescribed nonstandard second treatments, given the superiority of standard treatments (ACTH, OCS, and vigabatrin) as first-line therapy and the poor response rate of IS to anything else. We also hypothesized that a second standard treatment with a mechanism of action different from that of the failed first treatment would result in higher cumulative response rates due to evidence that medications with different mechanisms of action are often effective for epilepsy.27,28
Methods
Standard protocol approvals, registrations, and patient consents
The study was approved by all participating site institutional review boards (IRBs). The parents or guardians provided written informed consent for participation via center-specific IRB requirements.
In 2012, The Pediatric Epilepsy Research Consortium (PERC) developed the National Infantile Spasms Consortium (NISC) database. NISC is a multicenter database enrolling children in a prospective manner. Children with new-onset infantile spasms between 2 months and 2 years of age were eligible for the study. Clinical information was collected at time of diagnosis and 3 months after diagnosis. Medication dosing was standardized based on published experience and guidelines for ACTH, OCS, and vigabatrin, as reported previously,11 although compliance with these recommendations was not necessary for inclusion. Treatment decisions for individual children were deferred to the treating clinicians.
Data collected from June 2012 to July 2014 were used for this study. These children’s demographic profile and initial treatment responses have been reported elsewhere.11,29 Children with an early infantile epileptic encephalopathy (Ohtahara syndrome/early myoclonic encephalopathy) were excluded from the analysis, as this represents a different disease process. Records with missing treatment or response data due to loss to follow-up or incomplete data entry were also excluded, as outcome could not be determined.
Data collected for each child included age at onset of IS, gestational age at birth, sex, presence of seizures prior to spasms, etiology, height, weight, magnetic resonance imaging (MRI), genetic and metabolic testing, developmental assessment, presence of hypsarhythmia at onset, IS medication, and dosage. Hypsarhythmia was assessed at individual institutions and defined as multifocal spikes, disorganization, and >200 µV (trough-to-peak) in any epoch on a bipolar longitudinal montage, and included modified hypsarhythmia variants.30 At 3 months after study enrollment, we collected new MRI findings, new genetic and metabolic testing, developmental assessment, response to medication(s), EEG findings and assessment of etiology. Clinical response was assessed at 2 weeks and at 3 months following treatment initiation using both electrical and clinical data.
Standard therapy was defined as ACTH, OCS, or vigabatrin. All other treatments were considered nonstandard therapy for the purposes of this study. Children initiated on simultaneous standard and nonstandard therapy (e.g., ACTH and levetiracetam) had response attributed to the standard medication. For primary statistical analyses, a treatment sequence variable was constructed looking at first and second treatments simultaneously. We grouped children into two categories: (1) those prescribed two standard treatments as first and second therapy, but with different mechanisms of action (e.g., first treatment ACTH, second treatment vigabatrin); and (2) all other treatment sequences (e.g., combination of standard and nonstandard therapies or OCS with ACTH).
Response to first spasms treatment (FST) was initially classified into one of two response categories: responders and nonresponders. Responders were defined as those who had resolution of both clinical spasms and hypsarhythmia/modified hypsarhythmia (if present at onset) within 2 weeks of IS treatment, which was sustained at the 3-month follow-up, and no second treatment for IS was introduced during this interval. Nonresponders included children who did not have resolution of clinical IS and/or hypsarhythmia, or who initially met response criteria and then had return of either clinical spasms or hypsarhythmia within the 3-month study period. Nonresponders to FST were the subjects of this analysis.
Response to second spasm treatment (SST) was classified into responders and nonresponders. Responders included those who had resolution of clinical spasms and hypsarhythmia (if present at diagnosis) within 2 weeks of initiation of the second medication without subsequent relapse of clinical IS or hypsarhythmia at the time of the 3-month data collection point; however, the true interval of follow-up after SST was variable. Nonresponders were all others.
Development was recorded as the clinician’s perception of overall development, motor, and cognitive status, with each defined as normal, mild or equivocal delay, or definite abnormality. These three domains were then used to create an overall assessment of development categorized as normal, mild, moderate, and severe delay. Children with no domain marked as abnormal were classified as having normal development. If one domain was marked as mild, the child was included in the mild developmental delay group. The moderate developmental delay group consisted of children with two or more domains marked as mild or one domain marked as a definite abnormality. Severe developmental delay included children with two or more domains marked as definite abnormality.
Etiology was classified into five primary etiologic classifications: genetic/metabolic, malformation of cortical development, prior acquired injury, other structural, and unknown. Tuberous sclerosis was classified as other structural according to International League Against Epilepsy (ILAE) guidelines.31 For data analysis, those with unknown etiology were further categorized into normal and abnormal development. Unknown etiology with normal development was analyzed as a separate category, whereas genetic/metabolic was combined with unknown etiology and abnormal development. The latter group likely represents presumed genetic causes, but without an identified etiology in the 3-month follow-up period (either due to late diagnosis, decreased utilization of testing, or genetic influences that are non-Mendelian). In addition, malformations of cortical development, prior injury, and other structural were categorized together as a structural cause of epilepsy.
Statistical analysis
We compared demographic and clinical characteristics by treatment group (ACTH, oral steroid, vigabatrin, or other) using chi-square tests for categorical covariates and Kruskal-Wallis tests for continuous covariates. To understand the association of demographic and clinical covariates with treatment response, we used chi-square tests to compare the proportions of responders in each group. Next, we fit multivariable logistic regression models to estimate crude and adjusted relative risk of responding to a specified treatment sequence using the method of Kleinman and Norton.32 All analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, U.S.A.).
Results
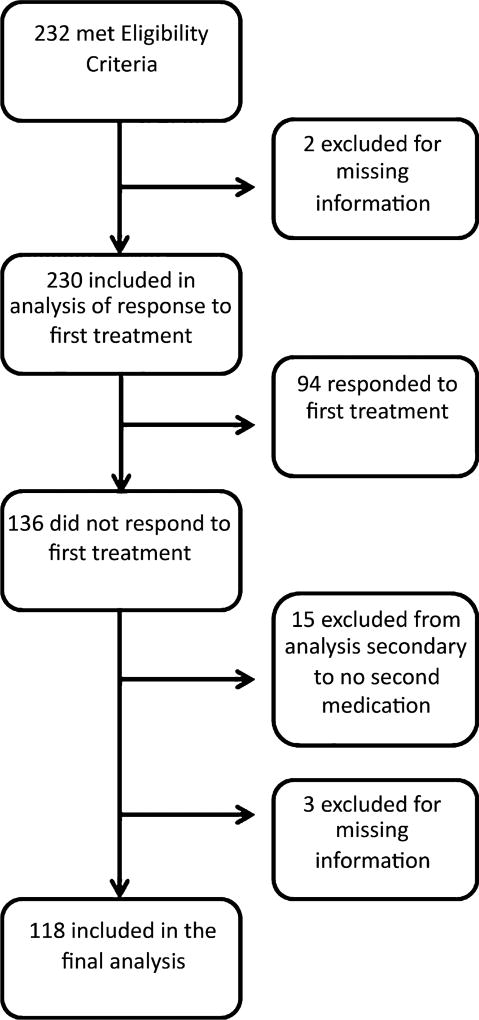
Figure 1 shows the flow diagram of participants included in our analyses. First spasms treatment failed in 136 (59%) of 230 children with infantile spasms. Of these, 18 were excluded, leaving 118 children in the cohort for our current analysis (see Table 1 for baseline demographics). We did not observe any significant differences in demographics, etiology, development, or treatment delay between children included in our analysis versus those excluded. Hypsarhythmia was present in 47% (48/103), modified hypsarhythmia in 28% (29/103), and 25% (26/103) had EEG findings that were abnormal but not considered hypsarhythmia. Hormonal therapy (ACTH and OCS) was used as a second medication in 41 children, vigabatrin in 38, and other treatments (topiramate, rufinamide, clonazepam, valproic acid, gabapentin, clobazam, oxcarbazepine, levetiracetam, zonisamide, pyridoxine, ketogenic diet, and phenobarbital) in 39 children. We did not observe differences in demographics based on second treatment choices, with the exception of development at onset of IS, with a higher percentage of infants exhibiting severe delay being more likely to be on a hormonal therapy or nonstandard therapy as their second treatment than vigabatrin, which may reflect bias of choice of FST. Clinicians followed NISC dosing recommendations in 23 (79%) of 29 ACTH-treated children, 11 (92%) of 12 OCS-treated children, and 24 (63%) of 38 of those treated with vigabatrin. Time to initiation of FST and time to initiation of SST were similar between the treatment groups (Table 1).
Figure 1.
Flow chart of participant eligibility and inclusion.
Epilepsia © ILAE
Table 1.
Baseline characteristics by type of second treatment for spasms
| Second treatment for spasms | |||||
|---|---|---|---|---|---|
|
|
|||||
| Characteristic | ACTH or oral steroid N = 41 |
Vigabatrin N = 38 |
Other N = 39 |
Total N = 118 |
p-Valuea |
| Sex | |||||
| Male | 24 (59) | 22 (58) | 18 (46) | 64 (54) | 0.46 |
| Race | |||||
| Black | 5(13) | 4(11) | 8 (22) | 17 (16) | 0.13 |
| White | 30 (79) | 28 (80) | 20 (56) | 78 (72) | |
| Other | 3(8) | 3(9) | 8 (22) | 14 (13) | |
| Ethnicity | |||||
| Hispanic | 4(12) | 3(9) | 6(18) | 13 (13) | 0.54 |
| Gestational age | |||||
| Weeks | 38 (34, 40) | 40 (38, 40) | 39 (37, 40) | 39 (37, 40) | 0.31 |
| At least 37 weeks | 28 (70) | 32 (84) | 31 (80) | 91 (78) | |
| Age at spasm onset | |||||
| Months | 6.5 (3.9, 8.2) | 5.0 (4.2, 7.0) | 5.7 (4.0, 9.0) | 5.6 (4.0, 7.8) | 0.93 |
| <12 months | 35 (88) | 34 (90) | 34 (87) | 103 (90) | |
| First spasm to treatment start | |||||
| Days | 12 (6, 25) | 9 (4, 29) | 18 (6, 61) | 14 (5, 36) | 0.28 |
| Within 4 weeks | 31 (78) | 27 (73) | 24 (62) | 82 (71) | |
| First treatment to second treatment | |||||
| Days | 28 (18, 41) | 25 (16, 35) | 24 (17, 34) | 25 (17, 36) | 0.50 |
| Within 4 weeks | 21 (51) | 24 (63) | 24 (62) | 69 (59) | |
| First spasm to second treatment | |||||
| Days | 49 (31, 68) | 43 (24, 70) | 48 (28, 104) | 44 (27, 79) | |
| Prior seizures | 19 (46) | 11 (29) | 16 (41) | 46 (39) | 0.27 |
| History of AED use | 21 (51) | 11 (29) | 17 (44) | 49 (42) | 0.13 |
| Etiologyb | |||||
| Genetic/metabolic | 10 (24) | 6(16) | 10 (26) | 26 (22) | 0.76 |
| Prior brain injury | 11 (27) | 9(24) | 7(18) | 27 (23) | |
| MCD/other structural | 9 (22) | 5(13) | 7(18) | 21 (18) | |
| Unknown abnormal | 8 (20) | 12 (32) | 10 (26) | 30 (25) | |
| Unknown normal | 3(7) | 6(16) | 5(13) | 14 (12) | |
| Developmental issues | |||||
| None/mild/moderate | 12 (30) | 25 (68) | 12 (31) | 49 (42) | <0.001 |
| Severe | 28 (70) | 12 (32) | 27 (69) | 67 (58) | |
Values are N (column %) or median (Q1, Q3).
Chi-square test.
MCD, malformations of cortical development. There were five participants with tuberous sclerosis complex (TSC) (included in the MCD/other structural etiology group).
The following variables had missing values: race (9), ethnicity (16), gestational age (1), age at spasm onset (4), time between first spasm and treatment start (2), time between first spasm and second treatment (2), and development (2).
Forty-four (37%; 95% confidence interval [CI] 29–46%) of the 118 children responded to their second treatment, 36 of 79 (46%; CI 35–57%) to a standard treatment, and 8 of 39 (21%; CI 8–33%) to a nonstandard treatment (p = 0.008, chi-square test). Table 2 shows the response rates to all observed treatment sequences. Three (21%) of 14 children who received repeated hormonal therapy responded. Children who were treated initially with a nonstandard treatment and were subsequently treated with a standard therapy had an overall response rate of 37% (6/16), whereas all of those treated with nonstandard treatments for both first and second therapy failed to respond to either treatment (0/7, Table 2).
Table 2.
Treatment sequence effect on response
| Response to second treatment |
|||
|---|---|---|---|
|
|
|||
| First treatment | Second treatment | Response N = 44 |
Nonrespons N = 74 |
| ACTH/oral steroid | ACTH/Oral steroid | 3(21) | 11(79) |
| Vigabatrin | 17(55) | 14 (45) | |
| Other | 6(23) | 20 (77) | |
| Vigabatrin | ACTH/Oral steroid | 10 (56) | 8 (44) |
| Other | 2(33) | 4(67) | |
| Other | ACTH/Oral steroid | 5(56) | 4 (44) |
| Vigabatrin | 1(14) | 6 (86) | |
| Other | 0(0) | 7 (100) | |
Values are N (row %).
When first and second spasms treatments were standard medications but with different mechanisms of action (e.g., hormonal therapy followed by vigabatrin, or vigabatrin followed by hormonal therapy), there was a response rate of 55% (27/49 CI 41–69%), which was superior to the 25% (17/69 CI 14–35%) overall response rate to all other treatment sequences (p < 0.001, chi-square test, Table 3). This result corresponds to an absolute risk reduction of 30% (95% CI 13–48%), and number needed to treat of 3.28 (95% CI 2.10–7.56). We observed a significantly higher response rate to SST in children who had initially been treated more rapidly, even though FST failed. Specifically, children who received FST within 4 weeks of their first clinical spasm had a 44% (36/82 CI 33–55%) response rate to SST, whereas children who were not initiated on FST until after 4 weeks had only a 24% (8/34 CI 9–38%) response rate to SST (p = 0.040, chi-square test, Table 3). The interval between IS onset and initiation of SST was not a significant predictor of response. We observed a lower response rate in children with severe developmental issues than in children with less severe developmental issues (30% [20/67 CI 19–41%] vs. 47% [23/49 CI 33–61%] p = 0.06, chi-square test) (Table 3), but this result was not statistically significant. The relative probability of response between groups, estimated via logistic regression modeling, is shown in Table 3. Even after adjustment for developmental category and time to treatment initiation, the treatment sequence remained a significant predictor of response. Children prescribed two standard treatments—the second with a different mechanism of action—had approximately twice the probability of responding as children prescribed other treatment sequences (Table 3).
Table 3.
Relative probability (relative risk) of response to treatment
| Characteristic | Total N |
Responders N(%) |
p-Valuea | Crude risk ratio (95% CI) |
Adjustedb risk ratio (95% CI) |
Adjustedc risk ratio (95% CI) |
|---|---|---|---|---|---|---|
| Treatment sequence | ||||||
| Standard-standard, mechanism change | 49 | 27 (55) | <0.001 | 2.26 (1.60, 3.22) | 2.01 (1.46, 3.08) | 2.25 (1.64, 3.35) |
| All other sequences | 69 | 17 (25) | REF | REF | REF | |
| Development | ||||||
| None/mild/moderate | 49 | 23 (47) | 0.06 | 1.59 (0.97, 2.78) | 1.31 (0.79, 2.10) | - |
| Severe | 67 | 20 (30) | REF | REF | - | |
| Time to first treatment | ||||||
| Within 4 weeks | 82 | 36 (44) | 0.040 | 1.90 (1.05, 4.62) | - | 1.82 (1.08, 4.10) |
| >4 weeks | 34 | 8(24) | REF | - | REF |
REF, reference group.
Relative risks estimated via logistic regression models using the method of Kleinman and Norton.32
Chi-square test.
Model including treatment sequence and development as covariates.
Model including treatment sequence and time to first treatment as covariates.
We did not observe significant differences in response to second treatment based on the child’s sex, race, ethnicity, gestational age, age at spasm onset, etiology, or prior seizures (Table 4). Of five children with tuberous sclerosis who failed first treatment, two responded to a second therapy (vigabatrin and topiramate).
Table 4.
Characteristics by response to second spasm treatment
| Response to second treatment | |||
|---|---|---|---|
|
|
|||
| Characteristic | Response N = 44 |
Non-response N = 74 |
p-Valuea |
| Sex | |||
| Female | 18 (33) | 36 (67) | 0.42 |
| Male | 26(41) | 38 (59) | |
| Race | |||
| Black | 5(29) | 12 (71) | 0.58 |
| White | 31 (40) | 47 (60) | |
| Other | 4(29) | 10 (71) | |
| Ethnicity | |||
| Hispanic | 3(23) | 10 (77) | 0.20 |
| Non-Hispanic | 37(42) | 52 (58) | |
| Gestational age | |||
| <37 weeks | 9(35) | 17 (65) | 0.80 |
| At least 37 weeks | 34 (37) | 57 (63) | |
| Age at spasm onset | |||
| <12 months | 40 (39) | 63 (61) | 0.87 |
| At least 12 months | 4(36) | 7(64) | |
| First treatment to second treatment | |||
| Within 4 weeks | 21 (30) | 48 (70) | 0.12 |
| 4–8 weeks | 17(52) | 16 (49) | |
| >8 weeks | 6(38) | 10 (63) | |
| First spasm to second treatment | |||
| Within 3 weeks | 5(29) | 12 (71) | 0.17 |
| 3–6 weeks | 19 (50) | 19 (50) | |
| >6 weeks | 20 (33) | 41 (67) | |
| Prior seizures | |||
| Yes | 18 (39) | 28 (61) | 0.74 |
| No | 26 (36) | 46 (64) | |
| History of AED use | |||
| Yes | 18 (37) | 31 (63) | 0.92 |
| No | 26 (38) | 43 (62) | |
| Etiology | |||
| Genetic/metabolic/unknown abnormal | 17(30) | 39 (70) | 0.16 |
| Prior brain injury/MCD/other structural | 19 (40) | 29 (60) | |
| Unknown normal | 8 (57) | 6(43) | |
MCD, malformation of cortical development.
Values are N (row %).
Chi-square test.
The following variables had missing values: race (9), ethnicity (16), gestational age (1), age at spasm onset (4), time between first spasm and treatment start (2), time between first spasm and second treatment start (2), and development (2).
Discussion
This is the largest prospective study that evaluates response to second treatment for IS. Our data demonstrate that 37% of children for whom a first IS treatment fails will subsequently respond to a second medication. Response rates to standard medications (ACTH, oral steroids, and vigabatrin) were greater than that to nonstandard medications. Timing of SST did not significantly affect outcome, whereas initiation of FST within 4 weeks of IS onset did. Characteristics of the child such as development, etiology, and prior seizures did not have an impact on response to SST, and therefore perhaps should not be considered in making treatment choices. Etiology has been associated with long-term cognitive outcomes,7 which were not measured in this study.
Similar to prior studies, the use of standard medications demonstrated a greater response rate. ACTH, vigabatrin, and OCS have been well studied in the treatment of IS as initial treatment, but have not been studied in children for whom initial medication is ineffective. This study supports the view that standard therapies are also more successful for second-line treatment, regardless of whether the initial therapy was standard or nonstandard. A prior smaller study similarly demonstrated a low response to nonstandard medication after failure of initial treatment with oral steroids, with only 2 of 18 children responding.19 An additional study demonstrated that a protocol with sequential standard medications led to improved outcomes compared to patients who were treated with a nonstandard medication (52% vs. 25%), although all subjects were initially treated with vigabatrin.33 Fedak et al. demonstrated an overall improvement in response rates to initial medications when a protocol was instituted using standard therapies for IS. These data further support the ongoing use of clinical care guidelines encouraging the use of standard therapies, although the response rates in our standardized treatment group are not as high as the 78% reported by that group.34 Other factors may have played a role in the higher response rate in the Fedak study, such as all patients received standardized care and early changes in ineffective treatment.
Timing of initiation of first spasms treatment did not significantly predict outcome after FST,11 but was related to response to SST. Of interest, timing of second medication (either related to spasm onset or duration between first and second medication) was not associated with a change in 3-month outcome. Other studies have demonstrated improved outcomes with initiation of treatment within 4 weeks of spasms present as well as early response to treatment.6,35–39 Cohen et al.37 have reported improved seizure and cognitive outcome with early initiation of ACTH. The majority of these studies have cohorts that are exclusively “cryptogenic children,” who have no prior developmental delay and no identifiable etiology. Koo et al.40 demonstrated that lag in treatment was related to a poor cognitive outcome, but not seizure outcome. Our study design did not allow for assessment of developmental outcome.
The highest response rate was achieved when the SST was switched from a steroid therapy to vigabatrin or vice versa. This may be attributable to presenting a treatment with a different mechanism of action. Further investigation is required to determine if different responses are attributable to complementary or even additive mechanisms of action, or alternatively, this may reflect individualized responses to single treatments due to a myriad of pharmacogenomic and epigenetic factors. If the former is true, this would suggest that combination therapy at initiation may lead to overall improved response rates. A better understanding of these factors may help to further new drug development (novel mechanisms are sought for those have not responded to currently available seizure medications) as well as rational polypharmacy.
Previous studies have evaluated the importance of early spasm resolution to improved neurodevelopmental outcome. While recognizing that prognosis, in part, is heavily linked to the underlying etiology, resolution of an epileptic encephalopathy likely plays a role. This study was not designed to evaluate longitudinal development; however, the results indicate that there is a high percentage of infants with spasms who will respond to a second treatment, and it is important to identify if this subgroup similarly shows improved development relative to the refractory population and if this benefit is seen independent of etiology.
One limitation of this project is a nonrandomized study design. As such, bias in the initial medication choice based on baseline developmental status as well as etiology at the time of medication initiation is present. We attempted to minimize the impact of prescribing bias by fitting multivariable logistic regression models. However, we were limited by our sample size in the number of variables for which we were able to adjust in a single model, and our study is likely underpowered to detect differences in response by certain clinical characteristics. In addition, the developmental measure used in this study is a subjective measure. Given that this was not a randomized trial, the dosing regimens and intervals between medication changes were not uniform. A high utilization of NISC dosing guidelines among subjects helped to minimize this variability. Furthermore, for the purposes of this study, nonstandard treatments were grouped together, as there were insufficient numbers to analyze each individually. Treatments with more published evidence such as ketogenic diet, valproate, and topiramate may have had superior benefit as second therapies theoretically than others (e.g., oxcarbazepine, pyridoxine, and phenobarbital). Larger cohorts are needed to evaluate efficacy of these specific nonstandard treatment options.
More than one third of children who require a second medication for treatment of IS will achieve resolution of clinical spasms and hypsarhythmia. Use of a standard medication improves outcome, and the evidence of benefit for nonstandard treatments, as a group, is relatively weak. Although rapid initiation of medications did not affect response to first medication, response to second therapy is improved in those who were treated early. Other factors such as development and etiology did not appear to influence overall resolution of IS.
Key Points.
More than one third of children with IS will respond to a second medication
Rapid initiation of first treatment for IS increases the likelihood of response to a second treatment
Standard medications are more effective than nonstandard medications for IS
Acknowledgments
Funding for this study was received from the American Epilepsy Society (KGK and EW) and the Pediatric Epilepsy Research Foundation (ATB and DRN). The authors would like to acknowledge and thank the numerous research assistants at all the institutions who gathered and entered data for making this study possible. The authors appreciate the contribution of SAS code by Dr. Lawrence C. Kleinman and Professor Edward C. Norton to assist with the regression risk analysis.
Author SH received grant support from Lundbeck, which owns rights to vigabatrin, and served on the scientific advisory board of Questcor/Mallinckrodt, which owns rights to ACTH as well as grant support from Insys therapeutics and GW Pharma. Author TL reports grants from Lundbeck, Eisai, Upsher-Smith, Acorda, and Pfizer; personal fees from Zogenix, Lundbeck, Upsher Smith, and Takeda outside the submitted work. Author JM reports grants from UCB Pharma and nonfinancial support from Mallinkrodt, outside the submitted work. Author JRM reports personal fees from Eisai, outside the submitted work. Author EHK reports grants from Nutricia and from Vitaflo, outside the submitted work. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Biography

Dr. Kelly Knupp is an associate professor of pediatrics and neurology at the University of Colorado.
Appendix
Additional Contributors
The named authors participated in the conception and design of the study, and in the data analysis and editing. The members of the Pediatric Epilepsy Research Consortium contributed data and participated in editing of the manuscript. They and their academic affiliations are included in the table.
| Author | Institution | Role |
|---|---|---|
| Amy Brooks-Kayal | Departments of Pediatrics and Neurology, University of Colorado School of Medicine and Children’s Hospital Colorado | Data collection, edit manuscript |
| Cynthia Stack | Ann & Robert H. Lurie Children’s Hospital of Chicago; and Departments of Pediatrics and Neurology, Northwestern University Feinberg School of Medicine | Data collection, edit manuscript |
| Lawrence Brown | Division of Neurology, The Children’s Hospital of Philadelphia and Perelman School of Medicine at the University of Pennsylvania, Philadelphia | Data collection, edit manuscript |
| Cynthia Keator | Jane and John Justin Neurosciences Department, Cook Children’s Hospital | Data collection, edit manuscript |
| Wendy G. Mitchell | Children’s Hospital Los Angeles Keck School of Medicine, University of Southern California Department of Neurology | Data collection, edit manuscript |
| Laura A. Jansen | University of Virginia | Data collection, edit manuscript |
| Shilpi Kumar | Department of Pediatrics Wright State University | Data collection, edit manuscript |
| Gogi Kumar | Department of Pediatrics Wright State University | Data collection, edit manuscript |
| Elizabeth Theile | Massachusetts General Hospital | Data collection, edit manuscript |
| Catherine Chu | Massachusetts General Hospital | Data collection, edit manuscript |
| Sarah A. Kelley | Departments of Neurology and Pediatrics Johns Hopkins Hospital | Data collection, edit manuscript |
| Elissa Yozawitz | Departments of Neurology and Pediatrics Montefiore Medical Center Albert Einstein College of Medicine | Data collection, edit manuscript |
| Charuta N. Joshi | Division of Pediatric Neurology, Children’s Hospital- Iowa. | Data collection, edit manuscript |
| Ignacio Valencia | St. Christopher’s Hospital for Children, Drexel University College of Medicine | Data collection, edit manuscript |
| Courtney J. Wusthoff | Department of Neurology & Neurological Sciences and by courtesy, Pediatrics-Neonatal and Developmental Medicine Stanford Division of Child Neurology | Data collection, edit manuscript |
| Edward J. Novotny | Departments of Neurology, Pediatrics, Neurosurgery and Radiology University of Washington Seattle Children’s Hospital | Data collection, edit manuscript |
| Russell P. Saneto | Seattle Children’s/University of Washington Division of Pediatric Neurology | Data collection, edit manuscript |
| Shaun A. Hussain | Division of Pediatric Neurology David Geffen School of Medicine Mattel Children’s Hospital UCLA | Data collection, edit manuscript |
Footnotes
Disclosure of Conflict of Interest
No additional authors had conflicts of interest to disclose.
References
- 1.Riikonen R, Donner M. Incidence and aetiology of infantile spasms from 1960 to 1976: a population study in Finland. Dev Med Child Neurol. 1979;21:333–343. doi: 10.1111/j.1469-8749.1979.tb01625.x. [DOI] [PubMed] [Google Scholar]
- 2.Cowan LD, Hudson LS. The epidemiology and natural history of infantile spasms. J Child Neurol. 1991;6:355–364. doi: 10.1177/088307389100600412. [DOI] [PubMed] [Google Scholar]
- 3.Luthvigsson P, Olafsson E, Sigurthardottir S, et al. Epidemiologic features of infantile spasms in Iceland. Epilepsia. 1994;35:802–805. doi: 10.1111/j.1528-1157.1994.tb02514.x. [DOI] [PubMed] [Google Scholar]
- 4.Sidenvall R, Eeg-Olofsson O. Epidemiology of infantile spasms in Sweden. Epilepsia. 1995;36:572–574. doi: 10.1111/j.1528-1157.1995.tb02569.x. [DOI] [PubMed] [Google Scholar]
- 5.O’Callaghan FJ, Lux AL, Darke K, et al. The effect of lead time to treatment and of age of onset on developmental outcome at 4 years in infantile spasms: evidence from the United Kingdom Infantile Spasms Study. Epilepsia. 2011;52:1359–1364. doi: 10.1111/j.1528-1167.2011.03127.x. [DOI] [PubMed] [Google Scholar]
- 6.Lagae L, Verhelst H, Ceulemans B, et al. Treatment and long term outcome in West syndrome: the clinical reality. A multicentre follow up study. Seizure. 2010;19:159–164. doi: 10.1016/j.seizure.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Lee JH, Yu HJ, et al. Prognostic factors of infantile spasms: role of treatment options including a ketogenic diet. Brain Dev. 2013;35:821–826. doi: 10.1016/j.braindev.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Widjaja E, Go C, McCoy B, et al. Neurodevelopmental outcome of infantile spasms: a systematic review and meta-analysis. Epilepsy Res. 2015;109:155–162. doi: 10.1016/j.eplepsyres.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Sorel L, Dusaucy-Bauloye A. Findings in 21 cases of Gibbs’ hypsarrhythmia; spectacular effectiveness of ACTH. Acta Neurol Psychiatr Belg. 1958;58:130–141. [PubMed] [Google Scholar]
- 10.Low NL. Infantile spasms with mental retardation. II. Treatment with cortisone and adrenocorticotropin. Pediatrics. 1958;22:1165–1169. [PubMed] [Google Scholar]
- 11.Knupp KG, Coryell J, Nickels KC, et al. Response to treatment in a prospective national infantile spasms cohort. Ann Neurol. 2016;79:475–484. doi: 10.1002/ana.24594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lux AL, Edwards SW, Hancock E, et al. The United Kingdom Infantile Spasms Study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomised controlled trial. Lancet. 2004;364:1773–1778. doi: 10.1016/S0140-6736(04)17400-X. [DOI] [PubMed] [Google Scholar]
- 13.Vigevano F, Cilio MR. Vigabatrin versus ACTH as first-line treatment for infantile spasms: a randomized, prospective study. Epilepsia. 1997;38:1270–1274. doi: 10.1111/j.1528-1157.1997.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 14.Appleton RE, Peters AC, Mumford JP, et al. Randomised, placebo-controlled study of vigabatrin as first-line treatment of infantile spasms. Epilepsia. 1999;40:1627–1633. doi: 10.1111/j.1528-1157.1999.tb02049.x. [DOI] [PubMed] [Google Scholar]
- 15.Mackay MT, Weiss SK, Adams-Webber T, et al. Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004;62:1668–1681. doi: 10.1212/01.wnl.0000127773.72699.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Go CY, Mackay MT, Weiss SK, et al. Evidence-based guideline update: medical treatment of infantile spasms: report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2012;78:1974–1980. doi: 10.1212/WNL.0b013e318259e2cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mytinger JR, Joshi S. The current evaluation and treatment of infantile spasms among members of the child neurology society. J Child Neurol. 2012;27:1289–1294. doi: 10.1177/0883073812455692. [DOI] [PubMed] [Google Scholar]
- 18.Wheless JW, Clarke DF, Carpenter D. Treatment of pediatric epilepsy: expert opinion, 2005. J Child Neurol. 2005;20(Suppl. 1):S1–S56. doi: 10.1177/088307380502000101. quiz S59-60. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoud AA, Rizk TM, Mansy AA, et al. Ineffectiveness of topiramate and levetiracetam in infantile spasms non-responsive to steroids. Open labeled randomized prospective study. Neurosciences (Riyadh) 2013;18:143–146. [PubMed] [Google Scholar]
- 20.Glauser TA, Clark PO, McGee K. Long-term response to topiramate in patients with West syndrome. Epilepsia. 2000;41(Suppl 1):S91–S94. doi: 10.1111/j.1528-1157.2000.tb02180.x. [DOI] [PubMed] [Google Scholar]
- 21.Hurst DL, Rolan TD. The use of felbamate to treat infantile spasms. J Child Neurol. 1995;10:134–136. doi: 10.1177/088307389501000215. [DOI] [PubMed] [Google Scholar]
- 22.Cianchetti C, Pruna D, Coppola G, et al. Low-dose lamotrigine in West syndrome. Epilepsy Res. 2002;51:199–200. doi: 10.1016/s0920-1211(02)00100-6. [DOI] [PubMed] [Google Scholar]
- 23.Veggiotti P, Cieuta C, Rex E, et al. Lamotrigine in infantile spasms. Lancet. 1994;344:1375–1376. doi: 10.1016/s0140-6736(94)90741-2. [DOI] [PubMed] [Google Scholar]
- 24.Lotze TE, Wilfong AA. Zonisamide treatment for symptomatic infantile spasms. Neurology. 2004;62:296–298. doi: 10.1212/01.wnl.0000103284.73495.35. [DOI] [PubMed] [Google Scholar]
- 25.Hong AM, Turner Z, Hamdy RF, et al. Infantile spasms treated with the ketogenic diet: prospective single-center experience in 104 consecutive infants. Epilepsia. 2010;51:1403–1407. doi: 10.1111/j.1528-1167.2010.02586.x. [DOI] [PubMed] [Google Scholar]
- 26.Wilmshurst JM, Gaillard WD, Vinayan KP, et al. Summary of recommendations for the management of infantile seizures: Task Force Report for the ILAE Commission of Pediatrics. Epilepsia. 2015;56:1185–1197. doi: 10.1111/epi.13057. [DOI] [PubMed] [Google Scholar]
- 27.Brodie MJ, Yuen AW. Lamotrigine substitution study: evidence for synergism with sodium valproate? 105 study group. Epilepsy Res. 1997;26:423–432. doi: 10.1016/s0920-1211(96)01007-8. [DOI] [PubMed] [Google Scholar]
- 28.Margolis JM, Chu BC, Wang ZJ, et al. Effectiveness of antiepileptic drug combination therapy for partial-onset seizures based on mechanisms of action. JAMA Neurol. 2014;71:985–993. doi: 10.1001/jamaneurol.2014.808. [DOI] [PubMed] [Google Scholar]
- 29.Wirrell EC, Shellhaas RA, Joshi C, et al. How should children with West syndrome be efficiently and accurately investigated? Results from the National Infantile Spasms Consortium. Epilepsia. 2015;56:617–625. doi: 10.1111/epi.12951. [DOI] [PubMed] [Google Scholar]
- 30.Lux AL, Osborne JP. A proposal for case definitions and outcome measures in studies of infantile spasms and West syndrome: consensus statement of the West Delphi group. Epilepsia. 2004;45:1416–1428. doi: 10.1111/j.0013-9580.2004.02404.x. [DOI] [PubMed] [Google Scholar]
- 31.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 32.Kleinman LC, Norton EC. What’s the risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res. 2009;44:288–302. doi: 10.1111/j.1475-6773.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granstrom ML, Gaily E, Liukkonen E. Treatment of infantile spasms: results of a population-based study with vigabatrin as the first drug for spasms. Epilepsia. 1999;40:950–957. doi: 10.1111/j.1528-1157.1999.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 34.Fedak EM, Patel AD, Heyer GL, et al. Optimizing care with a standardized management protocol for patients with infantile spasms. J Child Neurol. 2015;30:1340–1342. doi: 10.1177/0883073814562251. [DOI] [PubMed] [Google Scholar]
- 35.Djuric M, Kravljanac R, Tadic B, et al. Long-term outcome in children with infantile spasms treated with vigabatrin: a cohort of 180 patients. Epilepsia. 2014;55:1918–1925. doi: 10.1111/epi.12847. [DOI] [PubMed] [Google Scholar]
- 36.Kivity S, Lerman P, Ariel R, et al. Long-term cognitive outcomes of a cohort of children with cryptogenic infantile spasms treated with high-dose adrenocorticotropic hormone. Epilepsia. 2004;45:255–262. doi: 10.1111/j.0013-9580.2004.30503.x. [DOI] [PubMed] [Google Scholar]
- 37.Cohen-Sadan S, Kramer U, Ben-Zeev B, et al. Multicenter long-term follow-up of children with idiopathic West syndrome: ACTH versus vigabatrin. Eur J Neurol. 2009;16:482–487. doi: 10.1111/j.1468-1331.2008.02498.x. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto A, Watanabe K, Negoro T, et al. Prognostic factors of infantile spasms from the etiological viewpoint. Brain Dev. 1981;3:361–364. doi: 10.1016/s0387-7604(81)80064-2. [DOI] [PubMed] [Google Scholar]
- 39.Riikonen R. A long-term follow-up study of 214 children with the syndrome of infantile spasms. Neuropediatrics. 1982;13:14–23. doi: 10.1055/s-2008-1059590. [DOI] [PubMed] [Google Scholar]
- 40.Koo B, Hwang PA, Logan WJ. Infantile spasms: outcome and prognostic factors of cryptogenic and symptomatic groups. Neurology. 1993;43:2322–2327. doi: 10.1212/wnl.43.11.2322. [DOI] [PubMed] [Google Scholar]