Abstract
Strong evidence implicates intracellular signaling cascades dysfunction in the pathophysiology of Bipolar Disorder (BD). Regulation of AKT/mTOR pathway is a critical signaling pathway in synaptic neurotransmission and plasticity, also modulating cell proliferation and migration. Gene expression of the AKT/mTOR pathway was assessed in 25 BD (DSM-IV-TR criteria) unmedicated depressed individuals at baseline and after 6 weeks of lithium therapy and 31 matched healthy controls. Decreases in blood AKT1 and mTOR mRNA expression, as well as in BAD/BCL-2 expression ratio were observed in short-term BD patients during depressive episodes in comparison to healthy controls. There was no significant change in the expression of AKT1, mTOR, BCL-2, BAD and NDUFA6 after lithium therapy in the total group of BD subjects. However, the changes in AKT1 expression after lithium treatment were positively correlated with depression improvement. An integrated activity within this pathway was observed at both baseline and post-treatment. The present results support an integrated AKT/mTOR signaling pathway activity in a similar fashion to the described in previous human postmortem and rodents brain studies. Overall, the results reinforce a role for AKT1 and mTOR in the pathophysiology of BD and support the relevance of blood mRNA expression as a valid surrogate biological source to study brain intracellular signaling cascades changes and convergent molecular pathways in psychiatric disorders.
Keywords: Bipolar disorder, AKT, mTOR, Lithium, Treatment, Depression
1. Introduction
Strong evidence supports the presence of intracellular signaling cascades dysfunction in Bipolar Disorder (BD) (Machado-Vieira et al., 2014). These cascades comprise interconnected pathways involved in the pathophysiology of psychiatric disorders such as the AKT/mTOR. Regulation of AKT/mTOR pathway represents a key signaling target in synaptic neurotransmission, also directly affecting cell migration, proliferation and plasticity (Kitagishi et al., 2012). The serine/threonine kinase AKT is highly expressed in the brain and regulates cellular metabolism and growth, being critically involved in cell survival. When activated, AKT moves to the cytoplasm and nucleus, modulating several downstream targets, including the activation of the mammalian target of rapamycin (mTOR) (Kitagishi et al., 2012, Hashimoto, 2011). Reduced AKT activity is associated with impaired synaptic plasticity, protein synthesis and neurotransmission (Zheng et al., 2012), whereas AKT1 activation can suppress apoptosis in a transcription-independent manner (Thiselton et al., 2008). AKT1 genetic variants have been associated with both BD and schizophrenia (Karege et al., 2012).
Activation of mTOR is also a central regulator of protein synthesis required for long-term potentiation and new synaptic connections (Hashimoto, 2011). Reduced mTOR activity has been implicated in depression pathogenesis while mTOR activation has been associated with the rapid antidepressant effects of the N-methyl-D-aspartate (NMDA) antagonist ketamine (Li et al., 2010). These striking effects seem to be due to a rapid mTOR activation of synaptogenesis and increase in synaptic signaling proteins (Li et al., 2010).
The AKT/mTOR pathway also controls several downstream proteins such as the apoptosis regulator BCL-2 (B-cell lymphoma 2) and the pro-apoptotic BAD (BCL-2 associated death promoter). Regarding apoptosis, evidence has shown that apoptotic factors are altered in BD, based on post-mortem brain studies (Benes et al., 2006) and peripheral cells (Machado-Vieira et al., 2009). Reduced BCL-2 expression has been found in postmortem brain studies of BD (Jarskog et al., 2000; Kim et al., 2010). These downstream proteins have a central role in energy metabolism and mitochondrial function, which also includes NDUFA6 (NADH dehydrogenase [ubiquinone] 1 alpha 6). Decreased NDUFA expression in lymphocytes of BD subjects has been reported by two independent studies evaluating markers of mitochondrial oxidative phosphorylation (Washizuka et al., 2005; Naydenov et al., 2007).
The importance of these cascades in BD is also highlighted by lithium’s ability to inhibit these critical loci in second messenger/signal transduction pathways (Machado-Vieira et al., 2009; Soeiro-de-Souza et al., 2012). Lithium has been shown to activate AKT1 – through the suppression of glutamate-induced inhibition of AKT1 – and also to increase BCL-2 expression in neurons (Chalecka-Franaszek, Chuang, 1999; Nciri et al., 2013).
In the present investigation, we employed a longitudinal approach to investigate the pathophysiology and potential targets for lithium response in BD. Previous investigations have studied medicated BD patients at variable mood phases using cross-sectional approaches, with chronic illness and multiple treatments, which limit conclusions. Here we report findings on the expression of intracellular signaling cascade proteins pertaining to a network related to neuroplasticity and cellular resilience during acute bipolar depression episodes and after 6 weeks of treatment with lithium. These subjects, who were early in the illness course and are here referred as “short-term BD”, were compared to healthy controls. We hypothesized that (1) acutely depressed BD patients would exhibit reduced AKT1, mTOR, BCL-2, BAD and NDUFA6 mRNA expression relative to healthy controls and that (2) expression of these intracellular enzymes/ proteins would increase after 6-weeks of treatment with lithium.
2. Experimental procedures
2.1. Participants and design
All subjects (men/women, 18–45 years) were recruited and followed-up at the Outpatient Clinic, Mood Disorders Program LIM27, Institute of Psychiatry, University of Sao Paulo, Brazil. Inclusion criteria consisted of patients with BD subtypes I or II in a current depressive episode according to the DSM-IV-TR criteria, a baseline Hamilton Depression Rating Scale (HAM-D) total score ≥18 and within 5 years from first mood episode. Subjects with neurological disorders or any medical disorder were excluded as well as current substance abuse or dependence and intellectual disability. Also, a current Axis I psychiatric disorder other than BD was considered exclusion criteria.
Lithium was flexible dosed for 6 weeks (up to 900 mg/ day). In this report, we only included completers with baseline and endpoint blood samples. Response was defined as 50% or more decrease in HAM-D scores from baseline and remission as a HAM-D <8 at endpoint. The local institutional ethics committee approved the study and all subjects provided written consent.
2.2. Total RNA extraction and cDNA synthesis
Blood samples were collected in PAXgene RNA tubes (Pre-AnalytiX GmbH, 8634 Hombrechtikon, CH, Switzerland). RNA extraction and purification was performed manually utilizing the PAXgene Blood RNA kit (PreAnalytiX GmbH, 8634 Hombrechtikon, CH, Switzerland) according to the manufacturer’s protocol. Evaluation of RNA quantification and purification was carried out by measuring absorbance and A260/A280 ratios, a range of 1.8–2.0 was considered satisfactory for purity standards. Denaturing agarose gel electrophoresis was used to assess the quality of the samples. Synthesis of cDNA was performed by reverse transcription from 1 μg total RNA, previously treated with 1 unit of DNase I (FPLC-pure, GE Healthcare, Piscataway, NJ), using random and oligo(dT) primers, RNase inhibitor and SuperScript III (Invitrogen Inc, Carlsbad, CA), following the manufacturer’s recommendations. The resulting cDNA was then treated with 1 unit of RNase H (GE Healthcare). For 200–500 ng of total RNA the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) was used. 10 μL of total RNA was reverse transcribed using MultiScribe™ Reverse Transcriptase according to the manufacturer’s instructions. All cDNA samples were diluted with TE buffer, and stored at −20 °C until later use.
2.3. Quantitative real time (qRT-PCR)
The AKT1, mTOR, BCL-2, BAD and NDUFA6 mRNA expression were determined by qRT-PCR using the SYBR Green approach, in duplicate. Quantitative data was normalized relative to internal housekeeping control genes: hypoxanthine guanine phosphoribosyltransferase gene (HPRT), beta-glucuronidase gene (GUSB) and TATA-box binding protein (TBP). The geometric mean of the three genes was used for relative expression analysis. Sybr Green I amplification mixtures (10 μl) contained 2.5 μl of cDNA, 5 μl of 2 × Maxima® SYBR Green qPCR Master Mix (Fermentas Life Sciences, Burlington Ontario, Canada), and forward and reverse primers. Reactions were run on an ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA). The cycle conditions comprised of incubation at 50 °C for 2 min to activate UNG, initial denaturation at 95 °C for 10 min, and 40 cycles at 95 °C for 15 s, and 60 °C for 1 min. The minimum concentration of primers was determined by the lowest threshold cycle (Ct) and maximum amplification efficiency while minimizing non-specific amplification (at final concentration 100 nM for mTOR, BCL-2, AKT1, BAD, GUSB, TBP and 400 nM for HPRT and NDUFA6). Analysis of DNA melting curves demonstrated a single peak for the whole set of primers. Standard curves were analyzed for all genes to check the efficiency of amplification of each gene. In addition, agarose gel electrophoresis was employed to check the size of PCR product amplified. The equation 2−ΔCt was applied to calculate the absolute expression of patients and controls sample tissues where ΔCt=mean Ct gene–geometric mean Ct of housekeeping genes (17).
2.4. Statistical analysis
T test was used for parametric data when comparing baseline markers in bipolar depression versus controls and paired t test (two-tailed) for comparison between pre- and post-lithium treatment. Mann–Whitney test was used to compare nonparametric data. Kruskal–Wallis and Dunn tests for the non-parametric data and ANOVA with post-hoc Tukey’s test for parametric variables were used when comparing three groups. Correlation between relative gene expression values and potential association with clinical outcomes were assessed using the non-parametric Spearman-rho correlation test and the parametric Pearson’s correlation test when appropriate. A linear regression model was applied to evaluate the association between improvement with lithium and changes in biomarkers expression, also controlling for potential confounders (e.g. demographics). Differences were considered statistically significant when p<0.05, two-tailed. Data are presented as mean (±standard deviation). Calculations were performed using SPSS, version 15.0 (IBM, USA) and GraphPad 6.0.
3. Results
3.1. Demographic and clinical data
Twenty-five BD I (n=9) or BD II (n=16) patients in a major depressive episode (18F, age=28.44±5.60) according to the DSM-IV-TR were age-matched to 31 healthy controls (14F, age=27.6±6.04). Eighteen patients (72%) were drug-naïve and 22 (88%) were medication-free. Mean duration of illness was 35.66±20.45 months. The mean HAM-D score at baseline was 22.72±3.70 and at endpoint, 7.16±5.88. Demographic and clinical information are presented in Table 1. In the total sample, 16 (64%) patients achieved remission and 22 (88%) achieved clinical response. At the endpoint, mean oral dose of lithium was 727.5±111 mg and mean lithium plasma level was 0.50±0.21 mEq/L. Lithium plasma levels at endpoint were not associated with any biological measure at baseline, endpoint or changes over time (data not shown).
Table 1.
Demographic and clinical information of patients with bipolar disorder during depressive episodes (BD) and healthy controls (HC).
| BD (n=25) | HC (n=31) | Statistical tests | |
|---|---|---|---|
| Age (mean±SD) | 28.44±5.6 | 27.65±6.0 | t= −0.50, df=54, p=0.615 |
| Gender- n females(%) | 18 (72.0%) | 16 (51.6%) | χ2=2.41, df=1, p=0.120 |
| BD subtype- n Bipolar II (%) | 16 (64.0%) | – | – |
| Duration of illness (months; mean±SD) | 35.66±20.4 | – | – |
| Medication-free N (%) | 22 (88.0%) | – | – |
| Treatment-naïve N (%) | 18 (72.0%) | – | – |
| Previous psychosis | 3 (12%) | – | – |
| Family history mood disorders | 9 (36.0%) | – | – |
| Tobacco use | 4 (16%) | 4 (16%) | n.s. |
| BMI baseline | 29.3±5.41 | 27.45±4.01 | n.s. |
3.2. BD subjects with bipolar depression showed decreased expression of AKT1 and mTOR compared to healthy controls
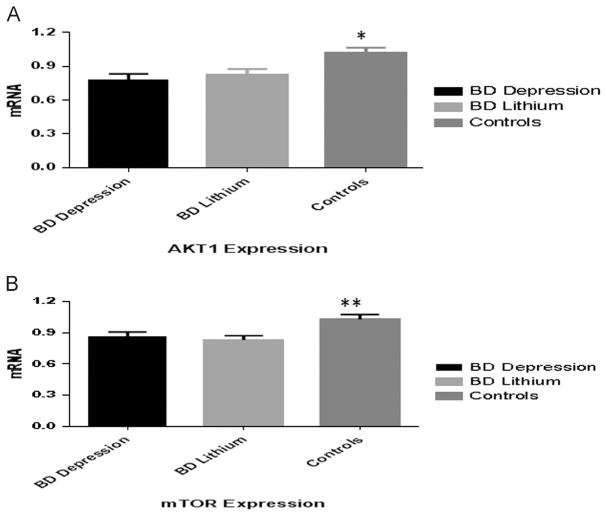
At baseline, AKT1 expression was significantly lower in bipolar depression (0.780±0.264) compared to healthy controls (1.024±0.235) (t=3.63, df=54, p<0.001) (Figure 1), with no significant increase observed after 6 weeks of lithium treatment (0.824±0.261) (t=1.149, df=24, p=0.261. Similarly, mTOR expression was decreased in bipolar depression (0.861±0.225) compared to controls (1.028±0.255) at baseline (t=2.55, df=54, p=0.013), with no changes in mTOR expression after lithium treatment (0.831±0.201) versus baseline (t=0.66, df=24, p=0.5) (Figure 1). The BAD/BCL-2 expression ratio was decreased during depressive episodes (0.608±0.247) compared to the control group (1.223±0.769), (t=3.82, df=54, p<0.001), with a mild change after lithium treatment (0.847±0.667), (t=2.11, df=24, p=0.045). No significant difference in NDUFA6 was observed in bipolar depression (1.232±0.731) compared to post-lithium (1.175± 0.987), p=0.58, or in comparison to healthy controls (1.081± 0.480), p=0.56, Mann–Whitney test. Patients with BD type I and type II subtypes showed similar expression of AKT1, mTOR, BCL-2, BAD and NDUFA6 both at baseline and after lithium treatment (all p=n.s., data not shown).
Figure 1.
AKT1 (A) and mTOR (B) expression in unmedicated, short-term bipolar disorder during acute depressive episode (BD Depression), after 6-weeks of lithium treatment (BD Lithium) and healthy subjects (Controls); *p≤0.001, **p≤0.01.
3.3. Interconnected network among AKT1, mTOR, BCL-2, and NDUFA6 mRNA expression
Regarding associations between biomarkers, mTOR expression in bipolar depression at baseline showed a robust positive correlation with AKT1 (p<0.001, r=0.672) and less significantly with BCL-2 (p=0.03, r=0.418). After lithium treatment, the strong positive correlation between mTOR and AKT1 was still present (p<0.001, r=0.739) as well as between mTOR and BAD/BCL-2 ratio (p=0.025, r=−0.445). Similar findings were observed when evaluating the expression change from baseline to endpoint (mTOR correlated with AKT1 (p=0.001, r=0.624) and BCL-2 (p<0.001, r=0.601). Changes in AKT1 expression were also positively associated with changes in BCL-2 (p=0.039, r=0.415) over time. Post-treatment BAD/BCL-2 ratio also showed a consistent positive association with NDUFA6 expression (p<0.001, r=0.745) as well as when considering changes in expression over time (p<0.001, r=0.708). When including the total sample (patients and healthy controls), AKT1 expression also showed significant correlation with mTOR expression (r =0.638, p<0.001).
3.4. Association between AKT1 and BCL-2 expression and clinical improvement
A linear regression showed a significant association between changes in HAM-D scores with alteration in AKT-1 (p=0.017, F=6.56) and BCL-2 (p=0.016, F=6.65) expression after lithium treatment. No association with other biomarker was observed (even when considering response and remission). This analysis also excluded a role for demographic variables as potential confounders (p>0.1 for all variables). Regarding predictors of response, baseline BCL-2 mRNA expression predicted overall improvement of depressive symptoms after lithium therapy (p=0.026, r=0.454).
4. Discussion
This is the first study to assess AKT1, mTOR, BCL-2, BAD and NDUFA6 mRNA expression in bipolar depression before and after 6 weeks of lithium treatment. Reduced blood mRNA expression of AKT1 and mTOR in unmedicated BD during a depressive episode was observed. These findings support a role for AKT1/mTOR signaling pathway in the pathophysiology of BD. AKT1, mTOR and BCL-2 mRNA expression did not significantly changed after 6 weeks of lithium therapy. A possible explanation for the negative results with lithium here is that the PI3K/AKT pathway is not only important in apoptosis, but it also has been involved in the regulation of diverse downstream proteins (Zhang et al. 2012).
mTOR has been identified as one of the targets most likely associated with the rapid antidepressant response obtained with glutamatergic agents such as ketamine (Liu et al., 2013; Niciu et al., 2014). A preclinical study suggested that increased mTOR signaling activity in the prefrontal cortex enhances cellular resilience following chronic mild stress in rodents (Suo et al., 2013). The exact mechanism by which AKT1/ mTOR signaling pathway may modulate mood is unknown. One plausible hypothesis is that the down-regulation of AKT1/ mTOR signaling pathway observed in BD impacts cellular energy production through its action in the mitochondria. Cunningham et al. (2007) demonstrated that the inhibition of mTOR decreases the genetic expression of mitochondrial transcriptional regulators, resulting in a decrease in mitochondrial gene expression and oxygen consumption. Another important action of AKT is to decrease glycogen synthase kinase-3β (Gsk3β) activity; Gsk3β is responsible for the phosphorylation of several metabolic, signaling, and structural proteins (Grimes and Jope, 2001). Through its action on different proteins, Gsk3β modulates several neuronal functions and regulates mood in preclinical models (Jope and Bijur, 2002).
In our study we found that changes in BCL-2 expression was associated with clinical improvement. Consistent with an involvement of the anti-apoptotic BCL-2 in the pathophysiology of BD found here, gene and protein expression of BCL-2 were found downregulated in the cerebral cortex of patients with BD. Also, a polymorphism of the BCL-2 gene (rs956572) was associated with enhanced levels of glutamate in the anterior cingulate cortex of BD patients (Soeiro-de-Souza et al., 2013). In line with a role of pro-apoptotic activation in BD pathophysiology, lymphocyte BAX/BCL-2 ratio was increased in manic and depressed patients with BD compared with controls (Moutsatsou et al., 2014). Moreover, supporting the therapeutic action of lithium in BD, this agent was found to increase BCL-2 protein levels in rat frontal cortex (Chen et al., 1999) and hippocampus (Son et al., 2003), and in lymphoblasts of patients with BD (Machado-Vieira et al., 2011).
Strengths of the present study are a sample comprising young patients with a short duration of illness, mostly drug-free and treated with lithium monotherapy. Limitations include the small sample size and the short-term follow-up period, which may have influenced the results.
The present findings of integrated alterations in the AKT/ mTOR/BCL-2 signaling pathway in whole blood of patients with BD depression correspond to that observed in brain samples, thus supporting the relevance of blood as a reliable surrogate biological source to study intracellular signaling cascades associated with neuroprotection in psychiatric disorders (Figure 2). The AKT/mTOR pathway may also represent core target for shared action of psychoactive drugs. Specifically, it may represent a promising target for BD pharmacotherapy, since activation of this pathway induces synaptic plasticity, cellular resilience and regulation of stress and mood changes. Further studies in psychiatry on upstream pathways and association with downstream targets and therapeutic response in blood are warranted.
Figure 2.
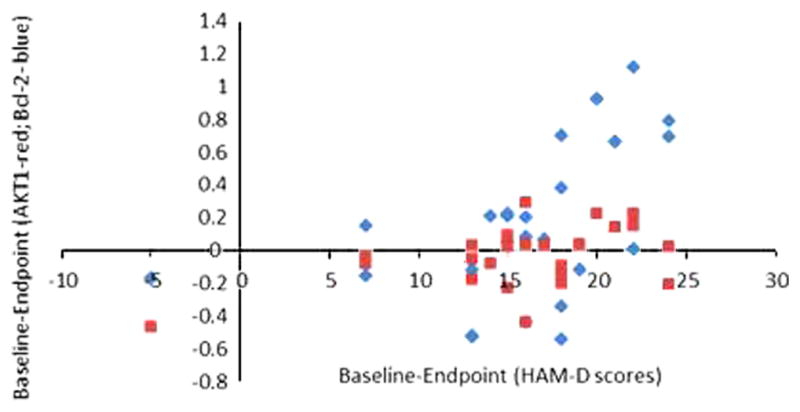
Association between changes in AKT-1 and Bcl-2 with improvement in depression symptoms with lithium.
Acknowledgments
Role of funding source
This study was sponsored by Sao Paulo Research Foundation (Fapesp, Brazil, 2009/14891-9, RM-V).
This study was sponsored by Sao Paulo Research Foundation (Fapesp, Brazil, 2009/14891-9, RM-V). The Laboratory of Neuroscience, LIM27 is also supported by the Associação Beneficente Alzira Denise Hertzog da Silva (ABADHS).
Footnotes
Contributors
Rodrigo Machado-Vieira conceived and led the study, analyzed the data, wrote the paper and managed communications between the different centers. Marcus Zanetti, Rafael de Sousa, Leandro Valiengo followed-up patients and reviewed the manuscript. Wagner Gattaz, Antonio Teixeira, Marcio Soeiro-de-Souza and Carlos Zarate provided materials, shared their knowledge on this discussed topic and reviewed the paper. Suely K Marie defined the technique and targets, led the experiments and contributed to the revised version. Miyuki Uno, Sueli Oba-Shinjo performed the experiments and revised the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest
Dr Zarate is listed as a coinventor on a patent for the use of ketamine and its metabolites in major depression. Dr Zarate has assigned his rights in the patent to the US government but will share a percentage of any royalties that may be received by the government. The other authors declare no biomedical financial interests or potential conflicts of interest.
References
- Benes FM, et al. The expression of proapoptosis genes is increased in bipolar disorder, but not in schizophrenia. Mol Psychiatry. 2006;11:241–251. doi: 10.1038/sj.mp.4001758. [DOI] [PubMed] [Google Scholar]
- Chalecka-Franaszek E, Chuang DM. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc Natl Acad Sci USA. 1999;96:8745–8750. doi: 10.1073/pnas.96.15.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JT, et al. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- Chen G, et al. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem. 1999;72:879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Role of the mTOR signaling pathway in the rapid antidepressant action of ketamine. Expert Rev Neurother. 2011;11:33–36. doi: 10.1586/ern.10.176. [DOI] [PubMed] [Google Scholar]
- Jarskog LF, et al. Cortical bcl-2 protein expression and apoptotic regulation in schizophrenia. Biol Psychiatry. 2000;48:641–650. doi: 10.1016/s0006-3223(00)00988-4. [DOI] [PubMed] [Google Scholar]
- Jope RS, Bijur GN. Mood stabilizers, glycogen synthase kinase-3beta and cell survival. Mol Psychiatry. 2002;7(Suppl 1):S35–S45. doi: 10.1038/sj.mp.4001017. [DOI] [PubMed] [Google Scholar]
- Kitagishi Y, et al. Roles of PI3K/AKT/GSK3/mTOR pathway in cell signaling of mental illnesses. Depress Res Treat. 2012;2012:752563. doi: 10.1155/2012/752563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, et al. Genetic overlap between schizophrenia and bipolar disorder: a study with AKT1 gene variants and clinical phenotypes. Schizophr Res. 2012;135:8–14. doi: 10.1016/j.schres.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Kim HW, et al. Altered expression of apoptotic factors and synaptic markers in postmortem brain from bipolar disorder patients. Neurobiol Dis. 2010;37:596–603. doi: 10.1016/j.nbd.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, et al. GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology. 2013;38:2268–2277. doi: 10.1038/npp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, et al. Multiple levels of impaired neural plasticity and cellular resilience in bipolar disorder: developing treatments using an integrated translational approach. World J Biol Psychiatry. 2014;15:84–95. doi: 10.3109/15622975.2013.830775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, et al. The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 2009;11(Suppl 2):92–109. doi: 10.1111/j.1399-5618.2009.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, et al. Bcl-2 gene polymorphism rs956572AA increases inositol 1,4,5-trisphosphate receptor-mediated endoplasmic reticulum calcium release in subjects with bipolar disorder. Biol Psychiatry. 2011;69:344–352. doi: 10.1016/j.biopsych.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsatsou P, et al. Peripheral blood lymphocytes from patients with bipolar disorder demonstrate apoptosis and differential regulation of advanced glycation end products and S100B. Clin Chem Lab Med. 2014;52:999–1007. doi: 10.1515/cclm-2013-0978. [DOI] [PubMed] [Google Scholar]
- Naydenov AV, et al. Differences in lymphocyte electron transport gene expression levels between subjects with bipolar disorder and normal controls in response to glucose deprivation stress. Arch Gen Psychiatry. 2007;64:555–564. doi: 10.1001/archpsyc.64.5.555. [DOI] [PubMed] [Google Scholar]
- Nciri R, et al. Neuroprotective effects of chronic exposure of SH-SY5Y to low lithium concentration involve glycolysis stimulation, extracellular pyruvate accumulation and resistance to oxidative stress. Int J Neuropsychopharmacol. 2013;16:365–376. doi: 10.1017/S1461145712000132. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, et al. Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Annu Rev Pharmacol Toxicol. 2014;54:119–139. doi: 10.1146/annurev-pharmtox-011613-135950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro-de-Souza MG, et al. Translating neurotrophic and cellular plasticity: from pathophysiology to improved therapeutics for bipolar disorder. Acta Psychiatr Scand. 2012;126:332–341. doi: 10.1111/j.1600-0447.2012.01889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro-de-Souza MG, et al. Bcl-2 rs956572 polymorphism is associated with increased anterior cingulate cortical glutamate in euthymic bipolar I disorder. Neuropsychopharmacology. 2013;38:468–475. doi: 10.1038/npp.2012.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo L, et al. Predictable chronic mild stress in adolescence increases resilience in adulthood. Neuropsychopharmacology. 2013;38:1387–1400. doi: 10.1038/npp.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son H, et al. Lithium enhances long-term potentiation independently of hippocampal neurogenesis in the rat dentate gyrus. J Neurochem. 2003;85:872–881. doi: 10.1046/j.1471-4159.2003.01725.x. [DOI] [PubMed] [Google Scholar]
- Thiselton DL, et al. AKT1 is associated with schizophrenia across multiple symptom dimensions in the Irish study of high density schizophrenia families. Biol Psychiatry. 2008;63:449–457. doi: 10.1016/j.biopsych.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washizuka S, et al. Expression of mitochondria-related genes in lymphoblastoid cells from patients with bipolar disorder. Bipolar Disord. 2005;7:146–152. doi: 10.1111/j.1399-5618.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- Zheng W, et al. The possible role of the Akt signaling pathway in schizophrenia. Brain Res. 2012;1470:145–158. doi: 10.1016/j.brainres.2012.06.032. [DOI] [PubMed] [Google Scholar]