Abstract
Nutritional immunology, immunometabolism, and identification of novel immunotherapeutic targets, are areas of active investigation in parasitology. There is a well-documented crosstalk among immune cells and cells in metabolically active tissues that is important for homeostasis. The numbers and function of these cells are altered by obesity leading to inflammation. A variety of helminths spend some part of their life cycle in the gastrointestinal tract and even entirely enteral nematode infections exert beneficial effects on glucose and lipid metabolism. The foundation of this review is the ability of enteric nematode infections to improve obesity-induced type 2 diabetes and the metabolic syndrome, which are significant health issues in developed areas. It considers the impact of nutrition and specific nutritional deficiencies, which are occur in both undeveloped and developed areas, on the host’s ability mount a protective immune response against parasitic nematodes. There are a number of proposed mechanisms by which parasitic nematodes can impact metabolism including effects gastrointestinal hormones, altering epithelial function, and changing the number and/or phenotype of immune cells in metabolic tissues. Nematodes can also exert their beneficial effects through Th2 cytokines that activate the transcription factor STAT6, which upregulates genes that regulate glucose and lipid metabolism.
Helminth Infection and Metabolic Diseases
It is estimated that one third of the world’s population is infected with parasitic helminths with the greatest burden in underdeveloped nations particularly Nigeria and the Congo (1). Nutrients are cofactors and activators for the developing immune system (2) and malnutrition as well as bacterial co-infections are frequent in these developing areas and promote the chronicity of helminth infection. There is also increasing recognition that specific deficiencies in vitamins and/or minerals can contribute to the severity of parasitic infections in endemic areas. Alternately, well developed urban areas with the lowest worm burden have a much greater incidence of metabolic diseases including obesity-induced type 2 diabetes (T2D) and the metabolic syndrome. Increasing evidence suggests that helminth infection regulates food intake and appetite, reduces body weight, and improves the symptoms of the metabolic syndrome and T2D (3).
There is a well-documented crosstalk among immune cells and cells in metabolically active tissues that is important for homeostasis. Parasitic nematodes or their products can impact cellular metabolism by a number of mechanisms including direct effects on hematopoietic and non-hematopoietic cell function or indirect effects mediated by downstream activation of genes that regulate production of metabolically active factors. There are a variety of helminths, including parasitic nematodes, which spend a large portion of their life cycle in the gastrointestinal (GI) tract. Their presence in the lumen initiates, extends, or amplifies signals that are critical to host defense against parasites. The GI tract provides a starting point for this review focused on the known and proposed mechanisms by which the nutritional status impacts host defense against parasitic nematodes and by which worm infection impacts host nutritional status and metabolism.
THE IMPACT OF NUTRITION ON HOST DEFENSE
For most of human history, malnutrition was common, and the effect of malnutrition on immunity, especially cellular immunity, has been studied extensively (4). A systemic review of the effects of malnutrition in children reported reduced gut barrier function, atrophied lymphatic tissue, and polarized cytokine production toward a Th2 response (2). The skewing of cytokine production toward a Th2 response; however, does not necessarily translate into improved resistance to nematode infections. Mice fed diets with reduced protein content showed delayed expulsion of primary Nippostrongylus brasiliensis (N. brasiliensis), Trichinella spiralis (T. spiralis) and Trichuris muris (T. muris) infections (5) and the Th2 response to a secondary Heligmosomoides polygyrus bakeri (H. polygyrus bakeri) infection was impaired resulting in increased worm burden (6). Similarly, mice infected with H. polygyrus bakeri and fed a diet with adequate protein and nutrient levels, but reduced caloric content, showed impaired lymphocyte proliferation, reduced Th2 cytokine production with lower levels of IgE, parasite-specific IgG1, and eosinophils, resulting in higher worm burdens and fecundity (7). In a recent study using multiple small (trickle) infections with H. polygyrus bakeri to mimic natural infections, the tolerance to infection, as measured by intestinal barrier function, was decreased by protein malnutrition (8). These results indicate that both sufficient protein and calories are required for optimal resistance to parasitic nematode infections.
In the twentieth and twenty-first centuries, consumption of “Western diets” has led to excessive caloric intake, increased consumption of highly refined foods, and decreased consumption of fruits and vegetables that may lead to deficiencies in at-risk populations including the elderly, the economically disadvantaged, or those with diseases that contribute to impaired absorption including Crohn’s disease, ulcerative colitis, and parasitic infections (9, 10). In particular, both gastrointestinal diseases and parasitic infections have been shown to impair micronutrient absorption. Several of these micronutrients, including vitamin A, selenium and zinc, play critical roles in immune function and resistance to parasitic infections.
The Role of Vitamin A in Resistance to Parasitic Infections
The role of vitamin A in immunity is highly pleiotropic. The effects are dose-, receptor form-, cell type-, and environmentally-dependent (reviewed in (11)). Dietary vitamin A or retinol is converted to retinaldehyde by ubiquitous alcohol dehydrogenases and then irreversibly acted on by cell-specific retinaldehyde dehydrogenases to generate its active metabolite, retinoic acid (RA), which binds to the RAR and RXR nuclear receptor families and function as transcription factors (11). RA can be produced locally by migratory CD103+ dendritic cells and macrophages in the lamina propria, and by stromal cells in the mesenteric lymph nodes and bone marrow (12, 13). In addition, RA is elaborated by intestinal epithelial cells that, in turn, promote gut-homing of IgA secreting B-cells (14), CD4+-, and CD8+ T cells (15, 16), a process that is impaired in vitamin A deficient mice (15, 17). B-cell development and antibody production are also vitamin A dependent [reviewed in (18)].
RA can act as a suppressor or activator of an inflammatory response depending on the circumstances. RA provides a critical signal for iTreg cell differentiation and iTreg cells can inhibit Th1- and Th17-type inflammatory responses (19–21). iTreg cells are decreased in vitamin A deficient mice leading to impaired oral tolerance (22). Differentiation of CD4+ T-cells is dependent on both vitamin A and RARα. Production of IFN-γ and IL-17A is decreased in T-cells lacking RA signaling and Th17 cells are severally reduced in vitamin A deficient mice (23). RA is important for maintenance of polarized Th1 cells and preventing conversion of Th1 cells to dual IFN-γ/IL-17-expressing Th17 cells (24). In contrast, RAR signals favor Th2 differentiation in naïve T-cells (25), is mediated via cytokine production by APC (25), and can impact resistance to parasitic infections which are classic inducers of Th2 immunity. This is important as the WHO showed that regions where soil-transmitted helminthiasis is most prevalent, Central America, especially Mexico, Central Africa, and Southeast Asia, are also areas of endemic vitamin A deficiency. Both low and high doses of RA increased localized Th1, Th2, Treg, and inflammatory responses in the liver and lung of Ascaris suum-infected pigs as well as increased BAL eosinophilia that may be related to enhanced induction of eosinophil chemokine activity by alveolar macrophages (26). The increase in type 2 innate lymphoid cells (ILC2) cells in vitamin A deficient mice was associated with increased resistance to a T. muris infection (23) that was dependent on fatty acid oxidation (27). This finding extends earlier work where egg excretion decreased more rapidly in Trichuris suis-infected, vitamin A deficient pigs than in vitamin A sufficient pigs (28), but contrasts with the increased parasite burden in Litomosoides carini-infected, vitamin A deficient cotton rats (29). Although worm expulsion was only slightly reduced in T. spiralis infected vitamin A deficient mice, differences in the immune response between sufficient and deficient mice were observed including higher IFN-γ and lower IL-4 production in MLN of infected deficient mice (30). Mice with a chronic infection of T. muris have reduced enzyme activity of and cell percentage staining for retinal dehydrogenase in lamina propria-derived dendritic cells and macrophages that did not rebound until the infection was cleared, indicating that chronicity may be related to decreased local RA levels (31) and impaired immune responses. The cause of this reduction was not identified, but may be a regulatory mechanism used by the parasite, or may result from reduced vitamin A absorption (32). These studies demonstrate that the ability of vitamin A to enhance or impair immunity to parasitic infections is at least partially parasite specific and additional studies are required to further clarify this dependency.
Selenium and Zinc are Key Minerals Required for Immune Function and Resistance to Infection
Selenium (Se), via its incorporation into selenocysteine-containing proteins (Sels), has substantial effects on immune function. There are 25 Sels identified in humans and 24 in mice with only partially characterized function. Selenium is important for both humoral and cell-mediated responses including cytotoxic T-lymphocytes and natural killer cells (33), chemokine and cytokine responses to viral infections (34, 35), respiratory burst (36), and for protection against LPS-induced oxidative stress (37). Many of the immune modulating effects of Se are due to its role in regulating activation of important transcription factors including NF-κB (38, 39), p38 MAPK (39), ERK (40), JNK (41) and AP-1, at least in part, by modulating redox status (42).
Specific Sels have been implicated in immune function. Both glutathione peroxidase 1 (GPx1) and glutathione peroxidase 2 (GPx2) are important for controlling Th2-dependent allergen-induced airway inflammation (43) with knockout of GPx1 shifting the Th cell bias toward Th1 and suppressing development of Th17 cells (44). Thioredoxin reductase maintains thioredoxin in its reduced state and thioredoxin is important for immune function and cell survival (45). Selenoprotein K KO mice exhibit aberrant calcium signaling in immune cells and an impaired immune response (46). Selenoprotein S is linked to regulation of inflammation (47). Selenoprotein P is also important for intercellular transport of Se, especially to the brain, and in controlling inflammation, and colitis-induced tumorigenesis (48–50).
Se status affects the immune response to parasitic infections. Selenium deficiency resulted in delayed expulsion of H. polygyrus bakeri (51, 52) due at least, in part, to decreased Th2 responses and production of Relm-β, a goblet cell protein critical for worm expulsion (53). Similarly, Se deficiency impaired clearance and reduced the Th2 response to N. brasiliensis infection in mice, an effect also observed in mice with conditional knockout of selenoprotein expression in macrophages (54). This effect of N. brasiliensis infection was attributed to the reduction in the transcription factor proliferator-activated receptor-γ (PPAR-γ)-mediated switch from a classically activated (M1) to an alternatively activated (M2) macrophage phenotype (55). This change was dependent on prostaglandin D2 synthase and 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) (55–57), highlighting a role for Se in regulating prostaglandin synthesis (56) and promoting the development of M2.
Many aspects of immunity are dependent on zinc. Zinc deficiency leads to atrophy of the thymus, a reduction in leukocytes, as well as in antibody-mediated, cell-mediated, and delayed-type hypersensitivity responses (58). In addition, NK cell activity is decreased in neutrophils, and macrophages have reduced levels of phagocytosis and respiratory burst in zinc deficiency (59). Production of the Th1 cytokines IL-2 and IFN-γ is attenuated by zinc deficiency resulting in a shift toward a Th2 response (60). Basal levels of pro-inflammatory cytokines are elevated in zinc deficiency, but production is ablated upon stimulation (61). Decreased cytokine production may result from decreased NF-κB activation in zinc deficiency (62, 63). Both immature and mature B-cells are reduced by zinc deficiency (64) as is antibody production (65). Zinc was found to increase Treg cell numbers in allergen-stimulated cells from atopic subjects and in mice with experimental autoimmune encephalitis (66, 67). Significantly, moderate zinc deficiency (3 mg/kg diet) in rats delayed expulsion and increased worm burden of T. spiralis, egg excretion, but not worm burden of N. brasiliensis, and delayed clearance of Strongyloides ratti (68). Moderate and severe (0.75 mg/kg), but not marginal (5 mg/kg) zinc deficiency, impaired the Th2 response to H. polygyrus bakeri and prolonged worm survival in primary H. polygrus bakeri-infected mice while in a challenge infection, only severely deficient mice had an impaired Th2 response and increased worm burdens (69). These data indicate that zinc deficiency impairs the Th2 response to parasitic infections and that zinc is important for Th2 immunity to parasitic infections. Supplementation may be indicated for at-risk or infected populations where inadequate dietary intake or malabsorption is present.
The impact of malnutrition and micronutrient deficiencies was focused for many years on developing nations. In areas of endemic infection, parasitic helminths that have evolved strategies that favor chronic infection are common (1) and the adverse effects of nutritional deficiencies to host defense further compound chronicity. Despite the epidemic of obesity and the metabolic syndrome in the United States and across the world, obese patients are often malnourished and exhibit similar deficiencies in micronutrients (70). The World Health Organization (WHO) estimates that 39% of adults aged 18 years and over were overweight in 2014, and 13% were obese in the world. The nutritional status of obese populations in developed nations merits equal attention as these conditions increase mortality, morbidity, and the economic costs of health care.
THE IMPACT OF PARASITIC NEMATODES ON HOST METABOLISM
There is little information on the impact of obesity on type 2 immune responses. Obesity prone mouse strains however, are more susceptible, while lean mouse strains were more resistant, to parasitic nematode infection (71). Obesity induces a wide variety of inflammatory and stress responses in metabolic tissues and higher concentrations of circulating inflammatory markers. This results in chronic, low grade inflammation termed “metaflammation” (72) which is central to insulin resistance and disruption of insulin receptor signaling (73), and requires the participation of both immune and non-immune cells. This has fostered the emerging field of immunometabolism that is focused on investigating the pro-inflammatory cytokines and mediators of obesity, the metabolic syndrome, and T2D (74). Parasitic infections, even those restricted to the intestine, increase circulating levels of IL-4, IL-5, and IL-13 which may act to blunt or reverse the Th1-induced inflammation in metabolic tissues.
Nematode Infection Alters Intestinal Barrier Function and the Intestinal Microenvironment
The surface epithelial cells that line the GI tract form the first line of defense in the gut and include the absorptive enterocytes, the mucus-producing goblet cells, and the hormone-secreting enteroendocrine cells (EEC). Along with immune cells, epithelial cells transduce specific pathogen-derived signals into effector functions; however, the mechanisms by which a wide variety of helminths induce a Th2 response remain to be elucidated. A confounding issue is that helminths elaborate antigens and excrete and secrete (E/S) a variety of products that may be involved in the initiation and maintenance of the type 2 immune response. How the cells respond to E/S products is also unclear, but highly implicated are pattern recognition receptors (PRR) and membrane-associated toll like receptors (TLR) that recognize conserved features of pathogens. There is also evidence that enteric parasitic nematodes elaborate trypsin-like serine proteases that activate protease activated receptors (PAR) such as PAR-2 on epithelial cells (75).
Worm-derived proteases may play a role in transducing the density and location of nematodes in the intestinal lumen (76). PAR-2 expression is ubiquitous along the GI tract and is expressed by epithelial cells, enteric nerves, and smooth muscle cells as well as by a variety of immune cells, including mast cells, macrophages, and T cells (77). Activation of PAR-2 on enterocytes increases epithelial permeability and fluid secretion from enterocytes and also enhances the nerve sensitivity of visceral afferent nerves (76), effects that are important for worm expulsion (78–81). Of interest is that in functional GI disorders, such as IBS, these effects are amplified and/or unresolved (82, 83). The reduced barrier function also facilitates the passage of E/S products across the intestinal barrier where they interact with resident immune cells to initiate and maintain the type 2 immune response. Activation of PAR-2 on macrophages also promotes development of the M2 phenotype (84).
The magnitude and duration of the effect of proteases is determined by the level of proteases and the number and availability of PARs on the cell surface (85, 86). PARs are “one shot” G protein-coupled receptors that must be continuously replenished from intracellular stores. Of interest is that the exposure to nematode proteases results in loss of surface PAR-2 on enterocytes thereby limiting the duration of their direct effects on epithelial permeability (76). With the loss of protease-mediated permeability, changes in barrier function are maintained during nematode infection by IL-25/IL-13/STAT6-dependent mechanisms (78, 79, 87).
The GI epithelium produces IL-25 and recent studies confirm that doublecortin-like kinase 1 (Dclk1)-expressing tuft cells are the sole epithelial source (88). Tuft cells are a distinct lineage that arise from stem cells in the located in the crypts and comprise approximately 0.4% of epithelial cells (89). The receptor for IL-25 (IL-25R) is a heterodimer consisting of IL-17RB and IL-17RA (90), which is expressed by various tissues/cells, including epithelial cells and immune cells including macrophages (91, 92) and ILC2. IL-25 plays a major role in the promotion and initiation of type 2 immunity, down-regulating pro-inflammatory cytokines, and facilitating development of M2 (91–93). IL-25 increases mucosal permeability through release of IL-13 (92) from resident mast cells and ILC2. Thus, the enhanced permeability during nematode infection is initiated by both worm-derived products and immune-mediated processes (76). Worm proteases and epithelial release of IL-25 facilitate the early passage of intraluminal products that promote the type 2 immune response. The increased permeability is sustained by IL-4, IL-13 working through STAT6-dependent mechanisms, including the influx of mast cells (79, 80, 94).
Parasitic Nematode Infection Regulates Glucose Transport
Obesity and T2D are associated with poor glycemic control as a result of dysregulated control of glucose sensing hormones and insulin resistance. Enteric nematode infection is associated with hypophagia and weight loss with improvement of the metabolic syndrome and T2D (3, 95). The mechanisms for the weight loss and decreased food intake remain unclear, but may be linked to local GI events including changes in intestinal glucose handling (78) or the immune cell phenotypes and the cytokine profile associated with infection (96).
Glucose is absorbed in the small intestine by transcellular pathways utilizing transporters as well as by paracellular pathways through solvent drag, a process that is modulated by changes in intestinal permeability. Enteric nematode infection slows enterocyte glucose absorption by inhibiting the activity of insulin-independent sodium-linked glucose transporter 1 (SGLT1) (97). This high affinity transporter can absorb glucose against a concentration gradient and is considered to be the major mechanism for postprandial glucose absorption in the small intestine (98). The nematode-induced effect on glucose absorption was dependent on M2, as depletion of macrophages during nematode infection restored SGLT1 activity (97). Given the prominent role of macrophages in insulin resistance, manipulation of macrophage phenotype may be a potential therapeutic strategy. Enteric nematode infection also decreased the expression of the insulin-dependent transporter GLUT2 by a mechanism that is independent of STAT6 (97). This is a facilitative transporter located on the basolateral membrane that is also trafficked to the apical side at high luminal glucose concentrations. The inhibited SGLT1 activity and reduced expression of GLUT2 during parasitic nematode infection lower enterocyte intracellular glucose (97, 99). This results in a metabolic stress that induces HIF-1α, leading to STAT6-dependent upregulation of GLUT1, a constitutive insulin independent transporter (97), thereby providing glucose for cellular metabolism.
The enhanced permeability during nematode infection also results in a greater absorption of glucose by the paracellular route and provides nutrients to fuel the high metabolic demands required by activated CD4+ T cells (100). Signaling through the T cell receptor activated mTOR leads to upregulation of GLUT1 and HIF-1α (101). Nematode infection induces an upregulation of GLUT1 in both enterocytes and T cells. Of interest is that GLUT1 is expressed by M1, which preferentially use glucose as an energy substrate, while M2 use free fatty acids (102), showing a preference of immune cells for specific energy substrates. Thus, by shifting the major route of intestinal glucose absorption to the paracellular pathway, parasitic nematode infection effectively bypasses insulin-dependent glucose transporters on enterocytes and fuels activated CD4+ T cell and macrophage metabolism. The increased demands of immune cell metabolism may contribute also to weight loss during nematode infection.
Parasitic Nematode Infection Reduces Appetite/Food Intake
The GI tract is the largest endocrine organ in the body. Comprising 1% of the epithelium, EEC arise from intestinal stem cells, are rapidly turned over, and function to sense the composition of the luminal contents and to coordinate release of hormone based on the location and type of nutrients detected and play a major role in satiety (103, 104). The numbers of EEC are modulated by diet and respond to the intraluminal nutrient composition through taste/chemosensory receptors that are sensitive to bitter, sweet, and umami compounds (105). Sweet taste receptors play a key role in secretion of GI hormones involved in glucose metabolism as well as the activity and expression of SGLT1 and GLUT2 (105). EEC also respond to products released by commensal bacteria (106), so it is likely EEC “sense” the presence of enteric nematodes or their products. There is now strong evidence of communication between immune cells and EEC (107) with EEC functioning as innate immunity sensors (104).
Recent studies show that N. brasiliensis induces an IL-25/IL-13 mediated expansion of the secretory lineage of epithelial cells that includes IL-25-producing tuft cells (108), which also express taste receptors (108). There are changes in the EEC number in T. spiralis infection, with increased numbers of cholecystokinin (CCK) positive (+) EEC that are dependent on the presence of CD4+ T cells (109). CCK plays many roles in intestinal, pancreatic, and liver function including a role in glucose metabolism and satiety (110). Both T. spiralis and N. brasiliensis induced a transient decrease in food intake in mice that returned to normal levels during the course of the infection (95, 111), implying a role for satiety hormones. T. muris infection of the colon also increased the number of EEC (112). Thus, enteric nematodes may affect metabolism through changes in the numbers of tuft cells and EEC thereby increasing the expression of taste receptors or by increasing the release of GI hormones that regulate satiety and/or glucose metabolism.
The Metabolic Consequences of Type 2 Immune Response to a Parasitic Nematode Infection Are Mediated by STAT6-Dependent and Independent Effects
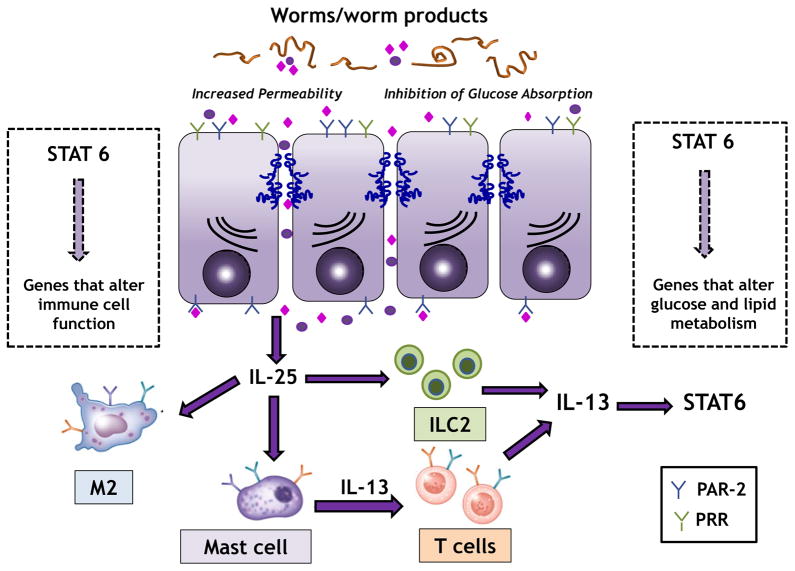
Evolution continually refines the interaction between host and parasites resulting in a sufficient response to clear worms while limiting immunopathology. For soil-based nematodes that spend all or part of their life cycle in the gut, worm expulsion is facilitated largely by IL-13-, STAT6- and M2-dependent changes in gut function (81, 113, 114). The presence of worms and their products induces the release of epithelial-derived cytokines such as TSLP, IL-25 and IL-33, which are associated with the transition of innate to adaptive immunity. In particular, binding of these cytokines to receptors on ILC2, macrophages, and mast cells induces release of IL-13, which plays a key role in the metabolic effects of enteric nematode infection (figure 1).
Figure 1.
Worms and worm products induce an increase in epithelial permeability, in part by activation of PAR-2, facilitating passage of these products across the mucosal barrier. Epithelial release of IL-25/IL-33 binds to mast cells and ILC2 leading to release of IL-13. IL-13 binds to the type 2 IL-4R and activates STAT6 on hematopoietic and non-hematopoietic cells. STAT6 u–pregulates genes for markers of alternatively activated macrophages (M2) and M2 play a key role in the STAT6-dependent inhibition of absorption of glucose in enterocytes. IL-13 also activates STAT6 on epithelial cells with upregulation of genes that maintain increased epithelial permeability. In addition, STAT6 activates genes in other cell types leading to alterations in glucose and lipid metabolism.
The metabolic benefits afforded by nematode infection have been attributed to their immunomodulatory effects including a shift from a Th1 to a Th2 response, promotion of the M2 phenotype, downregulation of the Th17 response, and development of ILC2 (115). ILC2 are a source of IL-13 and are the most recent cells proposed to regulate metabolic homeostasis in adipose tissue in both humans and mice (116–118). IL-13 binds to type 2 IL-4R located on non-hematopoietic cells and a few immune cells such as macrophages. This receptor is linked to the transcription factor, STAT6, with activation leading to upregulation of genes that control the phenotype and/or function of both hematopoietic and non-hematopoietic cells. Many of the enteric nematode infection-induced stereotypic STAT6-dependent changes in intestinal enterocyte function in the small intestine (78–81) are mimicked by exogenous administration of IL-13 as well as by IL-33 or IL-25 mediated release of IL-13 (78, 81).
There are several models of obesity-induced T2D and metabolic syndrome including the HFD-induced obesity, the ob/ob mouse, and the RIP2-OPa1 deficient mouse. Induction of obesity using a HFD is one of the most well-documented models of obesity and after 8–10 weeks on the diet, mice have elevated fasting blood glucose levels consistent with type 2 diabetes, insulin resistance, and hepatic steatosis (119). Infection of HFD-induced obese mice with N. brasiliensis resulted in weight loss, improved glucose metabolism, increased circulating insulin levels, and decreased adipose tissue masses (120). Of interest is that the weight loss effects of N. brasiliensis in HFD-induced obese mice were only partly dependent on STAT6, but fully dependent on IL-13 (95). In contrast, the ability of N. brasiliensis infection to reduce epididymal and brown fat was retained in STAT6−/− mice indicating some of the beneficial effects of nematode infection on metabolism are independent of IL-4 or IL-13 (120). Exogenous administration of IL-4 to mice fed HFD resulted in activation of STAT6 in the liver and attenuated adipose tissue inflammation which in turn lead to improvement of insulin action (121). HFD fed mice have reduced expression of IL-25 in the liver, and exogenous administration of IL-25 mimicked the beneficial effects of N. brasliensis infection on weight loss and hepatic steatosis in HFD fed mice (118). This effect was dependent on IL-13 and STAT6, as well as the development of alternatively activated Kupffer cells/macrophages. These data show the importance of IL-4, IL-13 and IL-25 in the ability of enteric nematode infection to improve the obesity-induced metabolic syndrome and T2D.
Specific STAT6 dependent genes regulating glucose metabolism
There is little information on the specific STAT6-dependent genes responsible for the beneficial effects of nematode infection on metabolism. M2 play a key role in these effects and up regulation of arginase-1, CD206, and other M2 markers are STAT6-dependent. In addition, there are several products of cells in the intestine, liver, or adipose tissue that have significant impact on glucose metabolism.
A family of four closely related cysteine-rich proteins, Resistin, and resistin-like molecules (RELM) α, β, and γ, (encoded by the genes Retnla, Retnlb, and Retnlg, respectively) have been identified in mice that share about 70% sequence homology, contain conserved C-terminal cysteine residues, and bind to unidentified receptors (122, 123). Two orthologs have been identified in humans, Resistin and RELM-β (124). RELM-α and γ have not been identified in humans, but the expression pattern of human Resistin is more similar to mouse RELM-α than mouse Resistin (125) and thus may share similar functions. Three of these genes, RELM-α, -β and -γ, are induced by parasite infections, including T. muris, H. polygyrus bakeri and N. brasiliensis, by a mechanism that is IL-4/IL-13 and STAT-6 dependent (126–129).
RELM-β is constitutively expressed in the colon, primarily in goblet cells, and is induced by colonization with commensal bacteria (130). Expression of RELM-β can be induced further by infection with pathogenic bacteria, parasitic nematodes, or dextran sodium sulfate suggesting that induction of RELM-β expression in the colon is a general response to mucosal insults. In contrast, RELM-β is not expressed constitutively in the small intestine, but is induced by parasite infections. RELM-β has been shown to bind to chemosensory organs on enteric parasitic nematodes resulting in impaired feeding and worm health. It is critical for expulsion of H. polygyrus bakeri (53) but may not be as important for expulsion of other parasites including T. muris (131) and N. brasiliensis (132).
In addition to regulation by commensal bacteria and infections, RELM-β expression is altered by diet and obesity. The circulating levels of RELM-β are increased in obese db/db mice and by feeding mice a high-fat diet (133). Furthermore, other dietary factors can alter RELM-α and -β expression. In the intestine, high-protein and high-carbohydrate diets suppressed gene expression of RELM-β while RELM-α expression was decreased in epididymal fat by a high-carbohydrate diet (134). Retnlb−/− mice are resistant to methionine-choline deficient, diet-induced non-alcoholic steatohepatitis (135). In this study, liver Kupffer cells were found to be a source of RELM-β, and expression in both colon and Kupffer cells was increased by the deficient diet and was necessary for full manifestation of the disease.
RELM-β also affects glucose metabolism. RELM-β inhibits SGLT-1 activity while increasing GLUT-2 dependent glucose transport (136). Mice infected with N. brasiliensis have increased Relm-β expression and decreased SGLT-1 activity; however, their GLUT2 expression also was decreased by infection (97). These data indicate the inhibitory effects of nematode infection on glucose absorption cannot be attributed fully to Relm-β. Rajala et al. demonstrated that increases in circulating RELM-β stimulated glucose production in the presence of fixed insulin levels (137). These changes were associated with increased activation of and flux through glucose-6-phosphatase. Injection of mice with RELM-β induced insulin resistance (137) and transgenic mice over-expressing RELM-β in the liver exhibit hyperglycemia, hyperlipidemia, fatty liver, and pancreatic islet enlargement when fed a high fat diet but not when fed a normal diet (138). Insulin resistance and glucose intolerance were associated with reduced protein expression of IRS-1 and IRS-2 as well as reduced insulin-induced activation of phosphatidylinositol 3-kinase and Akt. Additional in vitro studies with primary cultured hepatocytes demonstrated that RELM-β activated ERK and p38, and to a lesser extent, JNK.
RELM-α expression can also affect glucose metabolism. RELM-α mRNA was reported to be decreased in fasting or ob/ob mouse adipose tissue, and increased by hyperglycemia in rat adipose tissue (123). Interestingly, while fasting affected mRNA levels in adipose tissue, expression in lung tissue was unaffected suggesting that RELM-α may be differentially regulated depending on the cell or organ type. Retnla−/− mice have lower baseline levels of the satiety hormone, leptin, but no alterations in insulin levels were observed and mice exhibited similar weight gains on both normal and high-fat diet (139). Baseline glucose levels were also unaffected by normal or high-fat diet in Retnla−/− mice. In addition, when compared to WT mice, the kinetics of glucose clearance were unchanged in Retnla−/− mice. In another study, however, mice injected i.p with RELM-α for seven days had increased insulin resistance (140).
Recently, a tissue-resident CD301b mononuclear phagocyte population in adipose tissue was identified that secretes RELM-α and is required for positive energy balance under normal and high-fat metabolic conditions. Depletion of CD301b cells in mice caused hypoglycemia, increased insulin sensitivity, and weight loss in both lean and obese mice. Exogenous administration of RELM-α to CD301b-depleted mice fed a regular diet restored body weight and normoglycemia indicating that RELM-α was responsible for the altered glucose metabolism. Considering that both RELM-α and -β can decrease insulin sensitivity and increase glucose levels and improve the metabolic syndrome, the role of the high levels of both RELM-α and -β in enteric parasitic nematode infection merits further investigation.
STAT6 dependent genes regulating fat metabolism
Dyslipidemia and hepatic steatosis are common in obese individuals due to abnormalities in lipid metabolism. Hepatic steatosis is caused by lipid accumulation within hepatocytes, mainly due to excessive lipogenesis. There is evidence that enteric parasitic nematodes also induce a STAT6-dependent effect on genes that modulate fat metabolism. N. brasiliensis infection ameliorated the HFD-induced enlargement of the liver that was accompanied by increased levels of hepatic triglycerides (95). N. brasiliensis infection also downregulated genes encoding key lipogenic enzymes in the liver and epididymal fat, including Fasn, Acly, and Acaca, in both lean and HFD induced obese mice (99).
Cell death activator (CIDEA) is an important regulator of energy expenditure and lipid metabolism (141). CD36 in liver functions as a fatty acid plasma membrane transporter that takes up fatty acid into hepatocytes (142). Hepatic Cidea and Cd36gene expression were significantly upregulated in obese mice and N. brasiliensis infection normalized hepatic Cidea expression to levels in lean mice by a IL-13/STAT6 dependent mechanism (95). Exogenous administration of IL-25 also ameliorated HFD-induced hepatic steatosis, and decreased expression of the CIDEs in the livers of HFD-fed mice (118). In contrast, gene expression levels of major hepatic enzymes critical for lipolysis or FA oxidation, including hepatic lipase, carnitine palmitoyltransferase 1a, and hydroxyacyl-coenzyme A dehydrogenase, were not significantly altered by the HFD or infection (99). Thus, enteric nematodes improve hepatic steatosis through STAT6-dependent transcription of specific genes involved in the regulation of energy and lipid metabolism.
CONCLUSIONS
Throughout evolution, parasites have co-existed with humans resulting in an intricate interaction between the host and the parasite. For much of the twentieth century, parasite infections were only viewed as deleterious to the host, but as our understanding of the relationship between host and parasite has improved, it is evident our co-evolution has provided us with unappreciated benefits. It is clear that diet can impact resistance to parasitic infections and dietary interventions may be prudent in regions with endemic parasitic infections. Furthermore, ensuring adequate nutrition may improve any therapeutic interventions based on parasite products. It is also clear that parasite infections have significant effects on host metabolism and especially on energy metabolism, opening the door to new and novel approaches to treating obesity and T2D. In mice fed a HFD, N. brasiliensis infection attenuated body weight gain, improved glucose metabolism, decreased adiposity and hepatic steatosis, and increased M2 macrophages in adipose tissue (95). Further work is needed to determine if the benefits induce acute changes that persist only for as long as the parasite is present or chronic changes indicative of a new homeostasis.
There are inherent difficulties in obtaining regulatory approval for use of live parasites to treat otherwise healthy individuals and this has prompted exploration of alternative approaches. Experimental evidence demonstrated that the Th1 dominant C57Bl/6 mouse strain gains weight more rapidly on a HFD and has higher fasting glucose levels on both NCD and HFD than the Th2 dominant BALB/c mouse (143). Overexpression of IL-13 in fat tissue of C57Bl/6 mice blocked HFD-induced weight gain, improved glucose tolerance and insulin sensitivity and reduced inflammation in adipose tissue (144). Administration of IL-25 has beneficial effects on obesity-induced T2D and associated hepatic steatosis (117, 118). The effects of IL-25 are mediated by its ability to increase the numbers of ILC2, M2 macrophages and eosinophils in adipose tissue (116–118). Studies harnessing the therapeutic potential of parasite products or administration of cytokines that promote restoration of anti-inflammatory Th2 environment in metabolic tissues may prove more amenable to approval.
Acknowledgments
Funding: NIH R01-DK083418 (TSD) and USDA CRIS project 8040-51000-058 (AS)
References
- 1.Hotez PJ, Herricks JR. Helminth Elimination in the Pursuit of Sustainable Development Goals: A “Worm Index” for Human Development. PLoS Negl Trop Dis. 2015;9(4):e0003618. doi: 10.1371/journal.pntd.0003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rytter MJ, Kolte L, Briend A, Friis H, Christensen VB. The immune system in children with malnutrition--a systematic review. PLoS One. 2014;9(8):e105017. doi: 10.1371/journal.pone.0105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiria AE, Sartono E, Supali T, Yazdanbakhsh M. Helminth Infections, Type-2 Immune Response, and Metabolic Syndrome. PLoS Pathog. 2014;10(7):e1004140. doi: 10.1371/journal.ppat.1004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham-Rundles S, McNeeley DF, Moon A. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol. 2005;115(6):1119–28. doi: 10.1016/j.jaci.2005.04.036. quiz 29. [DOI] [PubMed] [Google Scholar]
- 5.Koski KG, Scott ME. Gastrointestinal nematodes, nutrition and immunity: breaking the negative spiral. Annu Rev Nutr. 2001;21:297–321. doi: 10.1146/annurev.nutr.21.1.297. [DOI] [PubMed] [Google Scholar]
- 6.Ing R, Su Z, Scott ME, Koski KG. Suppressed T helper 2 immunity and prolonged survival of a nematode parasite in protein-malnourished mice. Proc Natl Acad Sci U S A. 2000;97(13):707883. doi: 10.1073/pnas.97.13.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koski KG, Su Z, Scott ME. Energy deficits suppress both systemic and gut immunity during infection. Biochem Biophys Res Commun. 1999;264(3):796–801. doi: 10.1006/bbrc.1999.1596. [DOI] [PubMed] [Google Scholar]
- 8.Clough D, Prykhodko O, Raberg L. Effects of protein malnutrition on tolerance to helminth infection. Biol Lett. 2016;12(6) doi: 10.1098/rsbl.2016.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weisshof R, Chermesh I. Micronutrient deficiencies in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2015;18(6):576–81. doi: 10.1097/MCO.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 10.Hesham MS, Edariah AB, Norhayati M. Intestinal parasitic infections and micronutrient deficiency: a review. Med J Malaysia. 2004;59(2):284–93. [PubMed] [Google Scholar]
- 11.Larange A, Cheroutre H. Retinoic Acid and Retinoic Acid Receptors as Pleiotropic Modulators of the Immune System. Annu Rev Immunol. 2016;34:369–94. doi: 10.1146/annurev-immunol-041015-055427. [DOI] [PubMed] [Google Scholar]
- 12.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammerschmidt SI, Ahrendt M, Bode U, et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205(11):2483–90. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mora JR, von Andrian UH. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin Immunol. 2009;21(1):28–35. doi: 10.1016/j.smim.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21(4):527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Svensson M, Johansson-Lindbom B, Zapata F, et al. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol. 2008;1(1):38–48. doi: 10.1038/mi.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314(5802):1157–60. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 18.Ross AC, Chen Q, Ma Y. Vitamin A and retinoic acid in the regulation of B-cell development and antibody production. Vitam Horm. 2011;86:103–26. doi: 10.1016/B978-0-12-386960-9.00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204(8):1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 21.Xiao S, Jin H, Korn T, et al. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181(4):2277–84. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall JA, Cannons JL, Grainger JR, et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34(3):435–47. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spencer SP, Wilhelm C, Yang Q, et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343(6169):432–7. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown CC, Esterhazy D, Sarde A, et al. Retinoic acid is essential for Th1 cell lineage stability and prevents transition to a Th17 cell program. Immunity. 2015;42(3):499–511. doi: 10.1016/j.immuni.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross AC. Vitamin A and retinoic acid in T cell-related immunity. Am J Clin Nutr. 2012;96(5):1166S–72S. doi: 10.3945/ajcn.112.034637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson H, Solano-Aguilar G, Beal M, et al. Localized Th1-, Th2-, T regulatory cell-, and inflammation-associated hepatic and pulmonary immune responses in Ascaris suum-infected swine are increased by retinoic acid. Infect Immun. 2009;77(6):2576–87. doi: 10.1128/IAI.00827-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilhelm C, Harrison OJ, Schmitt V, et al. Critical role of fatty acid metabolism in ILC2-mediated barrier protection during malnutrition and helminth infection. J Exp Med. 2016;213(8):1409–18. doi: 10.1084/jem.20151448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedersen S, Saeed I, Jensen SK, Michaelsen KF, Friis H. Marginal vitamin A deficiency in pigs experimentally infected with Trichuris suis: a model for vitamin A inadequacy in children. Trans R Soc Trop Med Hyg. 2001;95(5):557–65. doi: 10.1016/s0035-9203(01)90040-9. [DOI] [PubMed] [Google Scholar]
- 29.Storey DM. Vitamin A deficiency and the development of Litomosoides carinii (Nematoda, Filarioidea) in cotton rats. Z Parasitenkd. 1982;67(3):309–15. doi: 10.1007/BF00927666. [DOI] [PubMed] [Google Scholar]
- 30.Carman JA, Pond L, Nashold F, Wassom DL, Hayes CE. Immunity to Trichinella spiralis infection in vitamin A-deficient mice. J Exp Med. 1992;175(1):111–20. doi: 10.1084/jem.175.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurst RJ, Else KJ. The retinoic acid-producing capacity of gut dendritic cells and macrophages is reduced during persistent T. muris infection. Parasite Immunol. 2013;35(7–8):229–33. doi: 10.1111/pim.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanumihardjo SA, Permaesih D, Muherdiyantiningsih, et al. Vitamin A status of Indonesian children infected with Ascaris lumbricoides after dosing with vitamin A supplements and albendazole. J Nutr. 1996;126(2):451–7. doi: 10.1093/jn/126.2.451. [DOI] [PubMed] [Google Scholar]
- 33.Kiremidjian-Schumacher L, Roy M, Wishe HI, Cohen MW, Stotzky G. Supplementation with selenium and human immune cell functions. II. Effect on cytotoxic lymphocytes and natural killer cells. Biol Trace Elem Res. 1994;41(1–2):115–27. doi: 10.1007/BF02917222. [DOI] [PubMed] [Google Scholar]
- 34.Beck MA, Levander OA. Host nutritional status and its effect on a viral pathogen. J Infect Dis. 2000;182(Suppl 1):S93–6. doi: 10.1086/315918. [DOI] [PubMed] [Google Scholar]
- 35.Sheridan PA, Zhong N, Carlson BA, Perella CM, Hatfield DL, Beck MA. Decreased selenoprotein expression alters the immune response during influenza virus infection in mice. J Nutr. 2007;137(6):1466–71. doi: 10.1093/jn/137.6.1466. [DOI] [PubMed] [Google Scholar]
- 36.Baker SS, Cohen HJ. Altered oxidative metabolism in selenium-deficient rat granulocytes. J Immunol. 1983;130(6):2856–60. [PubMed] [Google Scholar]
- 37.Sakaguchi S, Iizuka Y, Furusawa S, Tanaka Y, Takayanagi M, Takayanagi Y. Roles of selenium in endotoxin-induced lipid peroxidation in the rats liver and in nitric oxide production in J774A.1 cells. Toxicol Lett. 2000;118(1–2):69–77. doi: 10.1016/s0378-4274(00)00263-0. [DOI] [PubMed] [Google Scholar]
- 38.Youn HS, Lim HJ, Choi YJ, Lee JY, Lee MY, Ryu JH. Selenium suppresses the activation of transcription factor NF-kappa B and IRF3 induced by TLR3 or TLR4 agonists. Int Immunopharmacol. 2008;8(3):495–501. doi: 10.1016/j.intimp.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Kim SH, Johnson VJ, Shin TY, Sharma RP. Selenium attenuates lipopolysaccharide-induced oxidative stress responses through modulation of p38 MAPK and NF-kappaB signaling pathways. Exp Biol Med (Maywood) 2004;229(2):203–13. doi: 10.1177/153537020422900209. [DOI] [PubMed] [Google Scholar]
- 40.Liu H, Bian W, Liu S, Huang K. Selenium protects bone marrow stromal cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation by suppressing oxidative stress and ERK signaling pathway. Biol Trace Elem Res. 2012;150(1–3):441–50. doi: 10.1007/s12011-012-9488-4. [DOI] [PubMed] [Google Scholar]
- 41.Yoon SO, Kim MM, Park SJ, Kim D, Chung J, Chung AS. Selenite suppresses hydrogen peroxide-induced cell apoptosis through inhibition of ASK1/JNK and activation of PI3-K/Akt pathways. FASEB J. 2002;16(1):111–3. doi: 10.1096/fj.01-0398fje. [DOI] [PubMed] [Google Scholar]
- 42.Jozsef L, Filep JG. Selenium-containing compounds attenuate peroxynitrite-mediated NF-kappaB and AP-1 activation and interleukin-8 gene and protein expression in human leukocytes. Free Radic Biol Med. 2003;35(9):1018–27. doi: 10.1016/s0891-5849(03)00439-8. [DOI] [PubMed] [Google Scholar]
- 43.Dittrich AM, Meyer HA, Krokowski M, et al. Glutathione peroxidase-2 protects from allergen-induced airway inflammation in mice. Eur Respir J. 2010;35(5):1148–54. doi: 10.1183/09031936.00026108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Won HY, Sohn JH, Min HJ, et al. Glutathione peroxidase 1 deficiency attenuates allergen-induced airway inflammation by suppressing Th2 and Th17 cell development. Antioxid Redox Signal. 2010;13(5):575–87. doi: 10.1089/ars.2009.2989. [DOI] [PubMed] [Google Scholar]
- 45.Mahmood DF, Abderrazak A, Khadija EH, Simmet T, Rouis M. The Thioredoxin System as a Therapeutic Target in Human Health and Disease. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2012.4757. [DOI] [PubMed] [Google Scholar]
- 46.Verma S, Hoffmann FW, Kumar M, et al. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J Immunol. 2011;186(4):2127–37. doi: 10.4049/jimmunol.1002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seiderer J, Dambacher J, Kuhnlein B, et al. The role of the selenoprotein S (SELS) gene −105G>A promoter polymorphism in inflammatory bowel disease and regulation of SELS gene expression in intestinal inflammation. Tissue Antigens. 2007;70(3):238–46. doi: 10.1111/j.1399-0039.2007.00888.x. [DOI] [PubMed] [Google Scholar]
- 48.Burk RF, Hill KE, Motley AK, et al. Selenoprotein P and apolipoprotein E receptor-2 interact at the blood-brain barrier and also within the brain to maintain an essential selenium pool that protects against neurodegeneration. FASEB J. 2014;28(8):3579–88. doi: 10.1096/fj.14-252874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Chen X. Reducing selenoprotein P expression suppresses adipocyte differentiation as a result of increased preadipocyte inflammation. Am J Physiol Endocrinol Metab. 2011;300(1):E77–85. doi: 10.1152/ajpendo.00380.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barrett CW, Reddy VK, Short SP, et al. Selenoprotein P influences colitis-induced tumorigenesis by mediating stemness and oxidative damage. J Clin Invest. 2015;125(7):2646–60. doi: 10.1172/JCI76099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Au Yeung KJ, Smith A, Zhao A, et al. Impact of vitamin E or selenium deficiency on nematode-induced alterations in murine intestinal function. Exp Parasitol. 2005;109(4):201–8. doi: 10.1016/j.exppara.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 52.Smith AD, Cheung L, Beshah E, Shea-Donohue T, Urban JF., Jr Selenium status alters the immune response and expulsion of adult Heligmosomoides bakeri worms in mice. Infect Immun. 2013;81(7):2546–53. doi: 10.1128/IAI.01047-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herbert DR, Yang JQ, Hogan SP, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206(13):2947–57. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson SM, Shay AE, James JL, Carlson BA, Urban JF, Jr, Prabhu KS. Selenoprotein Expression in Macrophages Is Critical for Optimal Clearance of Parasitic Helminth Nippostrongylus brasiliensis. J Biol Chem. 2016;291(6):2787–98. doi: 10.1074/jbc.M115.684738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson SM, Lei X, Prabhu KS. Selenium levels affect the IL-4-induced expression of alternative activation markers in murine macrophages. J Nutr. 2011;141(9):1754–61. doi: 10.3945/jn.111.141176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gandhi UH, Kaushal N, Ravindra KC, et al. Selenoprotein-dependent up-regulation of hematopoietic prostaglandin D2 synthase in macrophages is mediated through the activation of peroxisome proliferator-activated receptor (PPAR) gamma. J Biol Chem. 2011;286(31):27471–82. doi: 10.1074/jbc.M111.260547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vunta H, Davis F, Palempalli UD, et al. The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12,14-prostaglandin J2 in macrophages. J Biol Chem. 2007;282(25):17964–73. doi: 10.1074/jbc.M703075200. [DOI] [PubMed] [Google Scholar]
- 58.Fraker PJ, Jardieu P, Cook J. Zinc deficiency and immune function. Arch Dermatol. 1987;123(12):1699–701. [PubMed] [Google Scholar]
- 59.Rink L, Haase H. Zinc homeostasis and immunity. Trends Immunol. 2007;28(1):1–4. doi: 10.1016/j.it.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Beck FW, Prasad AS, Kaplan J, Fitzgerald JT, Brewer GJ. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am J Physiol. 1997;272(6 Pt 1):E1002–7. doi: 10.1152/ajpendo.1997.272.6.E1002. [DOI] [PubMed] [Google Scholar]
- 61.Gruber K, Maywald M, Rosenkranz E, Haase H, Plumakers B, Rink L. Zinc deficiency adversely influences interleukin-4 and interleukin-6 signaling. J Biol Regul Homeost Agents. 2013;27(3):661–71. [PubMed] [Google Scholar]
- 62.Haase H, Ober-Blobaum JL, Engelhardt G, et al. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J Immunol. 2008;181(9):6491–502. doi: 10.4049/jimmunol.181.9.6491. [DOI] [PubMed] [Google Scholar]
- 63.Bao B, Prasad A, Beck FW, Suneja A, Sarkar F. Toxic effect of zinc on NF-kappaB, IL-2, IL-2 receptor alpha, and TNF-alpha in HUT-78 (Th(0)) cells. Toxicol Lett. 2006;166(3):222–8. doi: 10.1016/j.toxlet.2006.07.306. [DOI] [PubMed] [Google Scholar]
- 64.King LE, Osati-Ashtiani F, Fraker PJ. Depletion of cells of the B lineage in the bone marrow of zinc-deficient mice. Immunology. 1995;85(1):69–73. [PMC free article] [PubMed] [Google Scholar]
- 65.Luecke RW, Simonel CE, Fraker PJ. The effect of restricted dietary intake on the antibody mediated response of the zinc deficient A/J mouse. J Nutr. 1978;108(5):881–7. doi: 10.1093/jn/108.5.881. [DOI] [PubMed] [Google Scholar]
- 66.Rosenkranz E, Hilgers RD, Uciechowski P, Petersen A, Plumakers B, Rink L. Zinc enhances the number of regulatory T cells in allergen-stimulated cells from atopic subjects. Eur J Nutr. 2015 doi: 10.1007/s00394-015-1100-1. [DOI] [PubMed] [Google Scholar]
- 67.Rosenkranz E, Maywald M, Hilgers RD, et al. Induction of regulatory T cells in Th1-/Th17-driven experimental autoimmune encephalomyelitis by zinc administration. J Nutr Biochem. 2016;29:116–23. doi: 10.1016/j.jnutbio.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 68.Koski KG, Scott ME. GASTROINTESTINAL NEMATODES, NUTRITION AND IMMUNITY: Breaking the Negative Spiral. Annual Review of Nutrition. 2001;21(1):297–321. doi: 10.1146/annurev.nutr.21.1.297. [DOI] [PubMed] [Google Scholar]
- 69.Scott ME, Koski KG. Zinc deficiency impairs immune responses against parasitic nematode infections at intestinal and systemic sites. J Nutr. 2000;130(5S Suppl):1412S–20S. doi: 10.1093/jn/130.5.1412S. [DOI] [PubMed] [Google Scholar]
- 70.Via M. The Malnutrition of Obesity: Micronutrient Deficiencies That Promote Diabetes. ISRN Endocrinology. 2012;2012:103472. doi: 10.5402/2012/103472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong T, Hildebrandt M, Thrasher SM, Appleton JA, Ahima RS, Wu GD. Divergent Metabolic Adaptations to Intestinal Parasitic Nematode Infection in Mice Susceptible or Resistant to Obesity. Gastroenterology. 2007;133(6):1979–88. doi: 10.1053/j.gastro.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 73.Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, DeSouza CA. Influence of Metabolic Syndrome on Biomarkers of Oxidative Stress and Inflammation in Obese Adults. Obesity. 2006;14(12):2127–31. doi: 10.1038/oby.2006.248. [DOI] [PubMed] [Google Scholar]
- 74.Odegaard JI, Chawla A. Pleiotropic Actions of Insulin Resistance and Inflammation in Metabolic Homeostasis. Science. 2013;339(6116):172–7. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shea-donohue T, Notari L, Stiltz J, et al. Role of enteric nerves in immune-mediated changes in protease-activated receptor 2 effects on gut function. Neurogastroenterology & Motility. 2010;22(10):1138–e291. doi: 10.1111/j.1365-2982.2010.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shea-Donohue T, Notari L, Stiltz J, et al. Role of enteric nerves in immune-mediated changes in protease-activated receptor 2 effects on gut function. Neurogastroenterol Motil. 2010;22(10):1138–e291. doi: 10.1111/j.1365-2982.2010.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Colognato R, Slupsky JR, Jendrach M, Burysek L, Syrovets T, Simmet T. Differential expression and regulation of protease-activated receptors in human peripheral monocytes and monocyte-derived antigen-presenting cells. Blood. 2003;102(7):2645–52. doi: 10.1182/blood-2002-08-2497. [DOI] [PubMed] [Google Scholar]
- 78.Madden KB, Whitman L, Sullivan C, et al. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol. 2002;169(8):4417–22. doi: 10.4049/jimmunol.169.8.4417. [DOI] [PubMed] [Google Scholar]
- 79.Madden KB, Yeung KA, Zhao A, Gause WC, Finkelman FD, Katona IM, et al. Enteric nematodes induce stereotypic STAT6-dependent alterations in intestinal epithelial cell function. J Immunol. 2004;172(9):5616–21. doi: 10.4049/jimmunol.172.9.5616. [DOI] [PubMed] [Google Scholar]
- 80.Shea-Donohue T, Sullivan C, Finkelman FD, et al. The Role of IL-4 in Heligmosomoides polygyrus-Induced Alterations in Murine Intestinal Epithelial Cell Function. The Journal of Immunology. 2001;167(4):2234–9. doi: 10.4049/jimmunol.167.4.2234. [DOI] [PubMed] [Google Scholar]
- 81.Zhao A, McDermott J, Urban JF, Jr, et al. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol. 2003;171(2):948–54. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 82.Cenac N, Andrews CN, Holzhausen M, et al. Role for protease activity in visceral pain in irritable bowel syndrome. Journal of Clinical Investigation. 2007;117(3):636–47. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vergnolle N. Protease inhibition as new therapeutic strategy for GI diseases. Gut. 2016;65(7):1215–24. doi: 10.1136/gutjnl-2015-309147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nhu QM, Shirey KA, Pennini M, Stiltz J, Vogel SN. Proteinase-activated Receptor 2 Activation Promotes an Anti-inflammatory and Alternatively Activated Phenotype in LPS-stimulated Murine Macrophages. Innate immunity. 2012;18(2):193–203. doi: 10.1177/1753425910395044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amadesi S, Bunnett N. Protease-activated receptors: protease signaling in the gastrointestinal tract. Current Opinion in Pharmacology. 2004;4(6):551–6. doi: 10.1016/j.coph.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 86.Vergnolle Review article: proteinase-activated receptors — novel signals for gastrointestinal pathophysiology. Alimentary Pharmacology & Therapeutics. 2000;14(3):257–66. doi: 10.1046/j.1365-2036.2000.00690.x. [DOI] [PubMed] [Google Scholar]
- 87.Sun R, Urban JF, Jr, Notari L, et al. Interleukin-13 Receptor alpha1-Dependent Responses in the Intestine Are Critical to Parasite Clearance. Infect Immun. 2016;84(4):1032–44. doi: 10.1128/IAI.00990-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gerbe F, van Es JH, Makrini L, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. The Journal of Cell Biology. 2011;192(5):767–80. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gerbe F, Sidot E, Smyth DJ, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529(7585):226–30. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rickel EA, Siegel LA, Yoon B-RP, et al. Identification of Functional Roles for Both IL-17RB and IL-17RA in Mediating IL-25-Induced Activities. The Journal of Immunology. 2008;181(6):4299–310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 91.Yang Z, Grinchuk V, Urban JF, Jr, et al. Macrophages as IL-25/IL-33-responsive cells play an important role in the induction of type 2 immunity. PLoS One. 2013;8(3):e59441. doi: 10.1371/journal.pone.0059441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao A, Urban JF, Jr, Sun R, et al. Critical role of IL-25 in nematode infection-induced alterations in intestinal function. J Immunol. 2010;185(11):6921–9. doi: 10.4049/jimmunol.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008;226:57–79. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 94.Shea-Donohue T, Fasano A, Smith A, Zhao A. Enteric pathogens and gut function: Role of cytokines and STATs. Gut Microbes. 2010;1(5):316–24. doi: 10.4161/gmic.1.5.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang Z, Grinchuk V, Smith A, Qin B, Bohl JA, Sun R. Parasitic nematode-induced modulation of body weight and associated metabolic dysfunction in mouse models of obesity. Infect Immun. 2013:81. doi: 10.1128/IAI.00053-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Worthington JJ, Samuelson LC, Grencis RK, McLaughlin JT. Adaptive Immunity Alters Distinct Host Feeding Pathways during Nematode Induced Inflammation, a Novel Mechanism in Parasite Expulsion. PLoS Pathog. 2013;9(1):e1003122. doi: 10.1371/journal.ppat.1003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Notari L, Riera DC, Sun R, et al. Role of Macrophages in the Altered Epithelial Function during a Type 2 Immune Response Induced by Enteric Nematode Infection. PLoS ONE. 2014;9(1):e84763. doi: 10.1371/journal.pone.0084763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lehmann A, Hornby PJ. Intestinal SGLT1 in metabolic health and disease. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2016;310(11):G887–G98. doi: 10.1152/ajpgi.00068.2016. [DOI] [PubMed] [Google Scholar]
- 99.Yang Z, Grinchuk V, Smith A, et al. Parasitic nematode-induced modulation of body weight and associated metabolic dysfunction in mouse models of obesity. Infect Immun. 2013;81(6):1905–14. doi: 10.1128/IAI.00053-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. The Journal of Experimental Medicine. 2015;212(9):1345–60. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chang C-H, Pearce EL. Emerging concepts in immunotherapy – T cell metabolism as a therapeutic target. Nature immunology. 2016;17(4):364–8. doi: 10.1038/ni.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Freemerman AJ, Johnson AR, Sacks GN, et al. Metabolic Reprogramming of Macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. Journal of Biological Chemistry. 2014;289(11):7884–96. doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Engelstoft MS, Egerod KL, Lund ML, Schwartz TW. Enteroendocrine cell types revisited. Current Opinion in Pharmacology. 2013;13(6):912–21. doi: 10.1016/j.coph.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 104.Moran GW, Leslie FC, Levison SE, McLaughlin JT. Enteroendocrine Cells: Neglected Players in Gastrointestinal Disorders? Therapeutic Advances in Gastroenterology. 2008;1(1):51–60. doi: 10.1177/1756283X08093943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Depoortere I. Taste receptors of the gut: emerging roles in health and disease. Gut. 2014;63(1):179–90. doi: 10.1136/gutjnl-2013-305112. [DOI] [PubMed] [Google Scholar]
- 106.Cani PD, Everard A, Duparc T. Gut microbiota, enteroendocrine functions and metabolism. Current Opinion in Pharmacology. 2013;13(6):935–40. doi: 10.1016/j.coph.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 107.Worthington John J. The intestinal immunoendocrine axis: novel cross-talk between enteroendocrine cells and the immune system during infection and inflammatory disease. Biochemical Society Transactions. 2015;43(4):727–33. doi: 10.1042/BST20150090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Howitt MR, Lavoie S, Michaud M, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351(6279):1329–33. doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Worthington JJ, Samuelson LC, Grencis RK, McLaughlin JT. Adaptive Immunity Alters Distinct Host Feeding Pathways during Nematode Induced Inflammation, a Novel Mechanism in Parasite Expulsion. PLoS Pathogens. 2013;9(1):e1003122. doi: 10.1371/journal.ppat.1003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hopkins M, Blundell John E. Energy balance, body composition, sedentariness and appetite regulation: pathways to obesity. Clinical Science. 2016;130(18):1615–28. doi: 10.1042/CS20160006. [DOI] [PubMed] [Google Scholar]
- 111.McDermott JR, Leslie FC, D’Amato M, Thompson DG, Grencis RK, McLaughlin JT. Immune control of food intake: enteroendocrine cells are regulated by CD4+ T lymphocytes during small intestinal inflammation. Gut. 2006;55(4):492–7. doi: 10.1136/gut.2005.081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion 1. Science. 2005;308(5727):1463–5. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- 113.Shea-Donohue T, Urban JF., Jr Gastrointestinal parasite and host interactions. Curr Opin Gastroenterol. 2004;20(1):3–9. doi: 10.1097/00001574-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 114.Zhao A, Urban JF, Jr, Anthony RM, et al. Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology. 2008;135(1):217–25. e1. doi: 10.1053/j.gastro.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Harnett W. Secretory products of helminth parasites as immunomodulators. Molecular and Biochemical Parasitology. 2014;195(2):130–6. doi: 10.1016/j.molbiopara.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 116.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519(7542):242–6. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hams E, Locksley RM, McKenzie ANJ, Fallon PG. Cutting Edge: IL-25 Elicits Innate Lymphoid Type 2 and Type II NKT Cells That Regulate Obesity in Mice. The Journal of Immunology. 2013;191(11):5349–53. doi: 10.4049/jimmunol.1301176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang AJ, Yang Z, Grinchuk V, et al. IL-25 or IL-17E Protects against High-Fat Diet-Induced Hepatic Steatosis in Mice Dependent upon IL-13 Activation of STAT6. J Immunol. 2015;195(10):4771–80. doi: 10.4049/jimmunol.1500337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heydemann A. An Overview of Murine High Fat Diet as a Model for Type 2 Diabetes Mellitus. Journal of Diabetes Research. 2016;2016:14. doi: 10.1155/2016/2902351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang Z, Grinchuk V, Smith A, et al. Parasitic Nematode-Induced Modulation of Body Weight and Associated Metabolic Dysfunction in Mouse Models of Obesity. Infection and Immunity. 2013;81(6):1905–14. doi: 10.1128/IAI.00053-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proceedings of the National Academy of Sciences. 2010;107(52):22617–22. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Holcomb IN, Kabakoff RC, Chan B, et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19(15):4046–55. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rajala MW, Lin Y, Ranalletta M, et al. Cell type-specific expression and coregulation of murine resistin and resistin-like molecule-alpha in adipose tissue. Mol Endocrinol. 2002;16(8):1920–30. doi: 10.1210/me.2002-0048. [DOI] [PubMed] [Google Scholar]
- 124.Yang RZ, Huang Q, Xu A, et al. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun. 2003;310(3):927–35. doi: 10.1016/j.bbrc.2003.09.093. [DOI] [PubMed] [Google Scholar]
- 125.Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. J Immunol. 2006;177(3):1393–9. doi: 10.4049/jimmunol.177.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pesce JT, Ramalingam TR, Wilson MS, et al. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog. 2009;5(4):e1000393. doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stutz AM, Pickart LA, Trifilieff A, Baumruker T, Prieschl-Strassmayr E, Woisetschlager M. The Th2 cell cytokines IL-4 and IL-13 regulate found in inflammatory zone 1/resistin-like molecule alpha gene expression by a STAT6 and CCAAT/enhancer-binding protein-dependent mechanism. J Immunol. 2003;170(4):1789–96. doi: 10.4049/jimmunol.170.4.1789. [DOI] [PubMed] [Google Scholar]
- 128.Wang ML, Shin ME, Knight PA, et al. Regulation of RELM/FIZZ isoform expression by Cdx2 in response to innate and adaptive immune stimulation in the intestine. Am J Physiol Gastrointest Liver Physiol. 2005;288(5):G1074–83. doi: 10.1152/ajpgi.00442.2004. [DOI] [PubMed] [Google Scholar]
- 129.Artis D, Wang ML, Keilbaugh SA, et al. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci U S A. 2004;101(37):13596–600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.He W, Wang ML, Jiang HQ, et al. Bacterial colonization leads to the colonic secretion of RELMbeta/FIZZ2, a novel goblet cell-specific protein. Gastroenterology. 2003;125(5):1388–97. doi: 10.1016/j.gastro.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 131.Nair MG, Guild KJ, Du Y, et al. Goblet cell-derived resistin-like molecule beta augments CD4+ T cell production of IFN-gamma and infection-induced intestinal inflammation. J Immunol. 2008;181(7):4709–15. doi: 10.4049/jimmunol.181.7.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen G, Wang SH, Jang JC, Odegaard JI, Nair MG. Comparison of RELMalpha and RELMbeta Single- and Double-Gene-Deficient Mice Reveals that RELMalpha Expression Dictates Inflammation and Worm Expulsion in Hookworm Infection. Infect Immun. 2016;84(4):1100–11. doi: 10.1128/IAI.01479-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shojima N, Ogihara T, Inukai K, et al. Serum concentrations of resistin-like molecules beta and gamma are elevated in high-fat-fed and obese db/db mice, with increased production in the intestinal tract and bone marrow. Diabetologia. 2005;48(5):984–92. doi: 10.1007/s00125-005-1735-1. [DOI] [PubMed] [Google Scholar]
- 134.Fujio J, Kushiyama A, Sakoda H, et al. Regulation of gut-derived resistin-like molecule beta expression by nutrients. Diabetes Res Clin Pract. 2008;79(1):2–10. doi: 10.1016/j.diabres.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 135.Okubo H, Kushiyama A, Sakoda H, et al. Involvement of resistin-like molecule beta in the development of methionine-choline deficient diet-induced non-alcoholic steatohepatitis in mice. Sci Rep. 2016;6:20157. doi: 10.1038/srep20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Krimi RB, Letteron P, Chedid P, Nazaret C, Ducroc R, Marie JC. Resistin-like molecule-beta inhibits SGLT-1 activity and enhances GLUT2-dependent jejunal glucose transport. Diabetes. 2009;58(9):2032–8. doi: 10.2337/db08-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rajala MW, Obici S, Scherer PE, Rossetti L. Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J Clin Invest. 2003;111(2):225–30. doi: 10.1172/JCI16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kushiyama A, Shojima N, Ogihara T, et al. Resistin-like molecule beta activates MAPKs, suppresses insulin signaling in hepatocytes, and induces diabetes, hyperlipidemia, and fatty liver in transgenic mice on a high fat diet. J Biol Chem. 2005;280(51):42016–25. doi: 10.1074/jbc.M503065200. [DOI] [PubMed] [Google Scholar]
- 139.Munitz A, Seidu L, Cole ET, Ahrens R, Hogan SP, Rothenberg ME. Resistin-like molecule alpha decreases glucose tolerance during intestinal inflammation. J Immunol. 2009;182(4):2357–63. doi: 10.4049/jimmunol.0803130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Al-Azzawi HH, Mathur A, Lu D, Swartz-Basile DA, Nakeeb A, Pitt HA. Resistin-like molecule alpha reduces gallbladder optimal tension. J Gastrointest Surg. 2007;11(1):95–100. doi: 10.1007/s11605-006-0039-1. [DOI] [PubMed] [Google Scholar]
- 141.Gong J, Sun Z, Li P. CIDE proteins and metabolic disorders. Curr Opin Lipidol. 2009;20(2):121–6. doi: 10.1097/MOL.0b013e328328d0bb. [DOI] [PubMed] [Google Scholar]
- 142.He J, Lee JH, Febbraio M, Xie W. The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Exp Biol Med (Maywood) 2011;236(10):1116–21. doi: 10.1258/ebm.2011.011128. [DOI] [PubMed] [Google Scholar]
- 143.Jovicic N, Jeftic I, Jovanovic I, et al. Differential Immunometabolic Phenotype in Th1 and Th2 Dominant Mouse Strains in Response to High-Fat Feeding. PLoS ONE. 2015;10(7):e0134089. doi: 10.1371/journal.pone.0134089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Darkhal P, Gao M, Ma Y, Liu D. Blocking High Fat Diet-induced Obesity, Insulin Resistance and Fatty Liver by Overexpression of Il-13 Gene in Mice. International journal of obesity. 2015;39(8):1292–9. doi: 10.1038/ijo.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]