Abstract
OBJECTIVE
There are no evidence-based guidelines on the preferred approach to treating early-life epilepsy. We examined initial therapy selection in a contemporary US cohort of children with newly diagnosed, nonsyndromic, early-life epilepsy (onset before age three years).
METHODS
Seventeen pediatric epilepsy centers participated in a prospective cohort study of children with newly diagnosed epilepsy with onset under 36 months of age. Details regarding demographics, seizure types, and initial medication selections were obtained from medical records.
RESULTS
About half of the 495 enrolled children with new-onset, nonsyndromic epilepsy were less than 12 months old at the time of diagnosis (n = 263, 53%) and about half (n = 260, 52%) had epilepsy with focal features. Of 464 who were treated with monotherapy, 95% received one of five drugs: levetiracetam (n = 291, 63%), oxcarbazepine (n = 67, 14%), phenobarbital (n = 57, 12%), topiramate (n = 16, 3.4%), and zonisamide (n = 13, 2.8%). Phenobarbital was prescribed first for 50 of 163 (31%) infants less than six months old versus seven of 300 (2.3%) of children six months or older (P < 0.0001). Although the first treatment varied across study centers (P < 0.0001), levetiracetam was the most commonly prescribed medication regardless of epilepsy presentation (focal, generalized, mixed/uncertain). Between the first and second treatment choices, 367 (74%) of children received levetiracetam within the first year after diagnosis.
CONCLUSIONS
Without any specific effort, the pediatric epilepsy community has developed an unexpectedly consistent approach to initial treatment selection for early-life epilepsy. This suggests that a standard practice is emerging and could be utilized as a widely acceptable basis of comparison in future drug studies.
Keywords: epilepsy, antiepileptic drugs, generalized seizures, focal seizures, levetiracetam, oxcarbazepine, phenobarbital
Introduction
Over the last twenty years, many new antiseizure medications have become available. Medications typically receive US Food and Drug Administration approval for use based upon add-on trials in adults with pharmacoresistant focal epilepsy. Once approved, however, medications may also be prescribed for patients of all ages.1 With the exception of infantile spasms,2,3 there are no evidence-based treatment guidelines or published opinion-based recommendations regarding the preferred approach for prescribing antiseizure medications for the optimal treatment of early-life epilepsy. A working group of the International League Against Epilepsy (ILAE) was tasked with the development of such guidelines but was unable to do so because of the lack of high-quality published evidence.4
Nonsyndromic early-life epilepsies, forms of epilepsy that do not fit clinical criteria for West syndrome or other well-recognized electroclinical syndromes, affect about 8000 children under three years of age each year in the United States.5,6 Although there are legitimate concerns about the effect of antiseizure medications on the developmental trajectory of the young child’s brain,7–9 failure to control early-life seizures may be associated with adverse neurodevelopmental outcomes.10 More than 20 antiseizure medications are now available, but there are few data to suggest that one antiseizure medication is more effective than another.
In light of the seriousness of the outcomes in early-life epilepsies, the absence of evidence-based guidelines or even opinion-based recommendations on the preferred approach to treating these epilepsies represents an important gap in providing optimal care. We examined the selection of initial medications in children with nonsyndromic early-life epilepsy in an effort to identify opportunities for rational standardization of practice.
Methods
From January 2013 to March 2015, 17 US pediatric epilepsy centers participated in a prospective observational cohort study of infants and toddlers with newly diagnosed epilepsy with onset under 3 years of age. The centers were all members of the Pediatric Epilepsy Research Consortium, a nonprofit organization of pediatric epilepsy centers whose mission is to facilitate collaborative clinical research designed to answer practical questions related to the care of children with epilepsy. The institutional review board at each participating hospital approved this study, and a parent or a guardian of every enrolled child provided written informed consent.
Children were eligible if they were less than 36 months old at the onset of epilepsy and no older than 42 months when newly diagnosed with epilepsy at one of the participating centers. Children were considered to have new-onset epilepsy if they had unprovoked seizures on two or more separate days. To reflect recent recommendations11 we also included children who presented with a single seizure or multiple seizures on a single day if, based on the underlying cause or electrographic features, the children were judged by the treating physician to be at very high risk of recurrence and epilepsy treatment was initiated. Only children who could not be diagnosed at their initial evaluation with a specific epilepsy syndrome that might influence treatment selection were included in this analysis. Genetic test results that became available after treatment initiation for children whose initial presentation did not fit a specific epilepsy syndrome did not lead to exclusion because clinicians had made their initial treatment decisions without those results. Infants with West syndrome or infantile spasms and other specific electroclinical syndromes (e.g., Dravet, Ohtahara and Lennox-Gastaut syndromes, myoclonic-atonic epilepsy, early-onset absence epilepsy, and benign familial infantile epilepsy) were excluded. Recommendations already exist for West syndrome/infantile spasms; the most appropriate treatments are adrenocorticotropic hormone (ACTH), prednisolone, or vigabatrin.2,3 For the other excluded syndromes, especially Dravet syndrome, there are opinion pieces12,13 and some evidence from either observational or randomized trials to support certain treatment preferences.14
Data were abstracted from standardized medical chart reviews. Trained research assistants extracted the information, which was reviewed by the site principal investigator (PI) (a pediatric epileptologist) who oversaw the coding of data according to a structured code manual and manual of operations provided by the study. All data were then entered into a central REDCap15 database housed at Northwestern University. All data were centrally reviewed by the lead study coordinators, with final review of each case by the principal investigator (ATB). Questions were returned to the sites until all questions had been satisfactorily addressed. Demographic data (sex, race, ethnicity, insurance type) were directly extracted from the electronic medical record. Distance from site was based on home address provided in the record and, when necessary, an internet search to determine the distance from home to the hospital. A history of prior provoked seizures (febrile, acute neonatal, etc.) was taken from the history recorded in the clinician’s electronic medical record notes. For this study, age at onset was based on date of birth without correction for gestational age. The descriptors “focal” and “generalized” for type of epilepsy and seizure onset were taken as used in the medical records. When interpretation was needed, “focal” was used for findings that were completely lateralized or markedly asymmetric. “Generalized” was used for findings that were bilaterally symmetric. When information indicated both clear focal and generalized features or was insufficient to interpret, the term “mixed/uncertain” was applied.
Selection of epilepsy treatments was according to the clinicians’ best judgment and was not dictated by study participation. Specific rationale for individual clinical decision making or medication selection was not systematically queried. Although consensus-based dosing strategies were suggested to the participating centers, there was no effort to enforce any specific medication selection, dosing, or escalation plan. For children not on medication at the time of their diagnostic electroencephalography, the first medication was considered to be the one started immediately after diagnosis. Some children were already on an antiseizure medication at the time of epilepsy diagnosis. If that medication was continued as his or her epilepsy therapy, it was considered the first medication, but if it was discontinued and a new medication started, the new medication was considered to be the first epilepsy treatment. In a few instances, a child came to diagnosis with two medications already started, or a second medication was added to one started earlier. In these instances we considered that the initial treatment consisted of polytherapy.
Data analysis: All descriptive and bivariate analyses were conducted using SAS (SAS Institute, Inc., Cary, NC). Bivariate associations were tested with an appropriate chi-square test. A P-value <0.05 was considered the minimal criterion for statistical significance.
Results
Between January 2013 and March 2015, 495 children with new-onset nonsyndromic epilepsy were enrolled (252, 51%, male; 262). Just over half of these children (n = 263, 53%) were less than one year old at the time of epilepsy diagnosis, and about half (n = 260, 52%) had epilepsy with focal features. Demographic and clinical profiles are presented in Table 1.
TABLE 1.
Demographic and Clinical Details of N = 495 Children With New-Onset, Nonsyndromic Early-Life Epilepsy*
| Total sample | 495 |
| Sex | 252 (50.9%) male |
| Age at onset | |
| <6 months | 177 (35.8%) |
| 6 to <12 months | 85 (17.2%) |
| 12 to 23 months | 132 (26.7%) |
| ≥24 months | 101 (20.4%) |
| Presentation of epilepsy | |
| Focal | 260 (52.5%) |
| Generalized | 149 (30.1%) |
| Mixed/uncertain | 86 (17.4%) |
| History of neonatal seizures | 23 (4.6%) |
| History of febrile seizures | 72 (14.6%) |
| History of prematurity | 108 (21.8%) |
| <30 weeks gestation | 12 (2.5%) |
| 30 to 36 weeks gestation | 96 (19.7%) |
| ≥37 weeks gestation | 376 (77.2%) |
| Insurance | |
| Public | 235 (47.5%) |
| Private | 213 (43.0%) |
| Self-pay, uninsured, or unknown | 47 (9.1%) |
| Race | |
| Caucasian | 295 (59.6%) |
| Black/African American | 60 (12.1%) |
| Asian | 20 (4.0%) |
| Other | 64 (12.9%) |
| Unknown | 56 (11.3%) |
| Ethnicity | |
| Hispanic | 76 (15.4%) |
| Non-Hispanic | 299 (60.4%) |
| Unknown | 120 (24.2%) |
| Distance from epilepsy center | |
| Same city | 87 (17.6%) |
| Within 100 miles | 345 (69.7%) |
| 101 to 499 miles | 57 (11.5%) |
| >500 miles | 6 (1.2%) |
Data are presented as n (%).
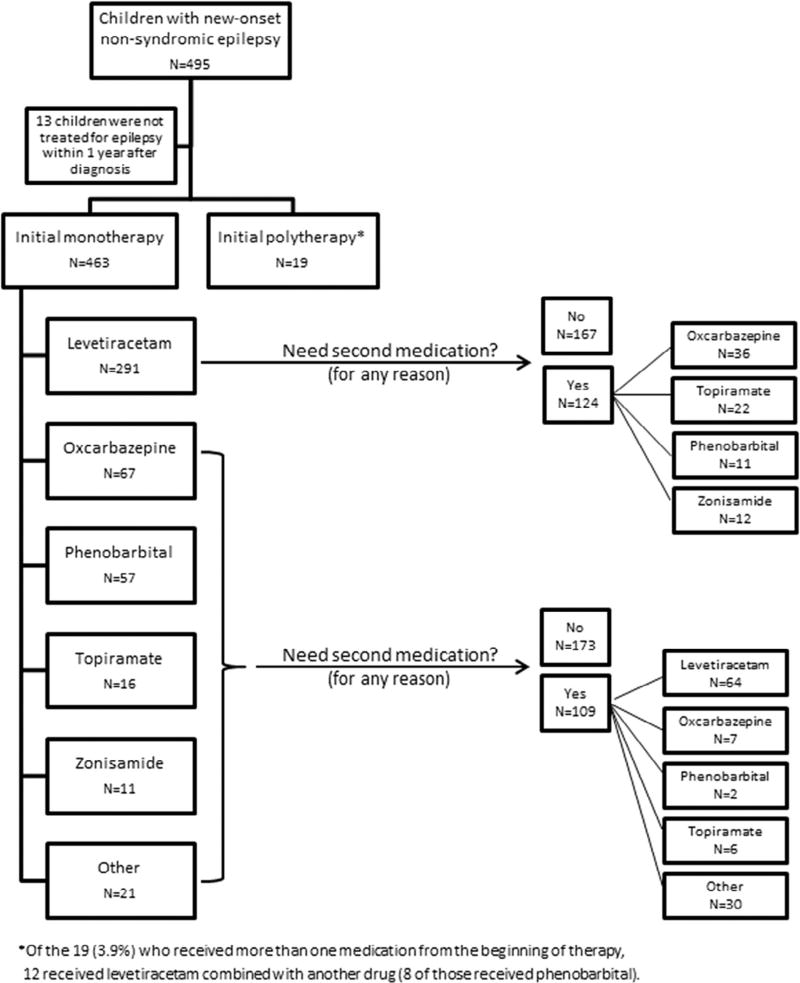
Thirteen (2.6%) children were not treated during the year after diagnosis. Of the 483 children who were treated with medication, 19 (3.9%) received polytherapy from the outset. Of the 463 treated with monotherapy, the initial choice was levetiracetam (n = 291, 62.7%), oxcarbazepine (n = 67, 14.4%), phenobarbital (n = 57, 12.3%), topiramate (n = 16, 3.4%), or zonisamide (n = 11, 2.4%) (Fig 1). The ten other drugs used as initial treatment in this cohort were each prescribed to fewer than five individual children. No child was started on the ketogenic diet as first-line therapy in this cohort.
FIGURE 1.
First and second medication choices for 495 children with early-life epilepsy.
Seventy-two children (14.5%) were already on a medication at the time they received their initial diagnosis of epilepsy (n = 44 levetiracetam, n = 19 phenobarbital, n = 6 oxcarbazepine, n = 2 topiramate, n = 1 phenytoin, n = 1 lorazepam, n = 1 gabapentin). Most of these were children who had a medication initiated in the emergency department just before their diagnostic visit (n = 55) or who were on treatment for prior provoked seizures (n = 10 neonatal, n = 4 febrile, and n = 12 other).
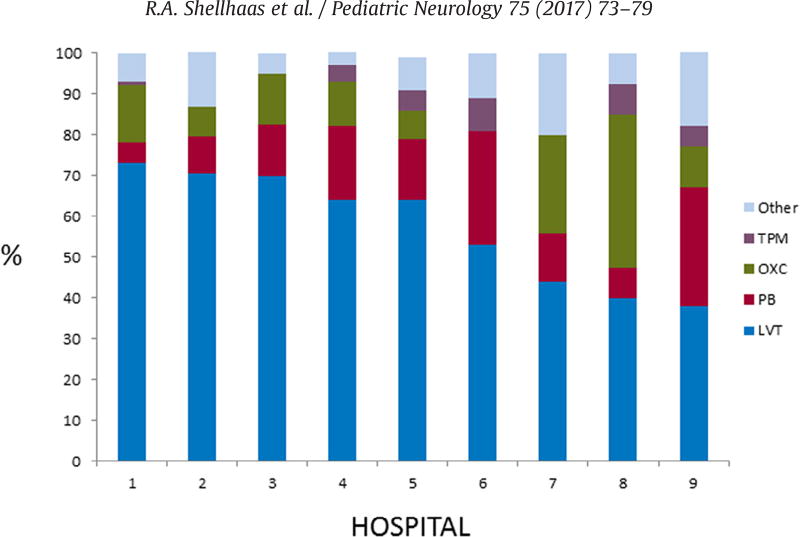
There was no difference in initial medication selection by sex, ethnicity, insurance (private versus public), or the distance the children lived from their pediatric epilepsy center. However, the probability that levetiracetam was selected as first-line therapy varied significantly by site (chi-square test, P < 0.001). Indeed, the percentage of patients receiving levetiracetam as first-line therapy within a site ranged from 29% (four of 14) to 75% (three of four). Among the nine study centers that enrolled at least 20 children with nonsyndromic epilepsy, levetiracetam was the single most commonly selected first medication at each site (Fig 2). Phenobarbital and oxcarbazepine were the next most commonly prescribed medications.
FIGURE 2.
Distribution of first anti-seizure medication selected in nine pediatric epilepsy centers. Among the nine study centers that enrolled at least 20 children with nonsyndromic epilepsy, levetiracetam was the most commonly selected first medication at each hospital; however, the proportion of children prescribed levetiracetam at the sites ranged from 29% to 75% (P < 0.0001). TPM, topiramate; OXC, oxcarbazepine; PB, phenobarbital; LVT, levetiracetam.
Several clinical features were associated with the choice of the first medication. In particular, the choice in first medication varied strongly by age; phenobarbital was almost exclusively prescribed for infants less than six months old (50 of 163 [31%] of infants less than six months old versus seven of 300 [2.3%] of children ≥6 months old received phenobarbital, P < 0.0001, Table 2). In addition, initial treatment selection varied by epilepsy presentation (focal, generalized, and mixed/uncertain). Overall, levetiracetam remained the most commonly prescribed medication regardless of the epilepsy presentation. Use of oxcarbazepine was more common for focal than generalized or mixed presentation (Table 3). Medication selection also varied based on seizure semiologies and seizure burden at the time of epilepsy diagnosis (Table 3).
TABLE 2.
Initial Medications Varied by Age at Epilepsy Onset
| Age | n | Levetiracetam (n = 291) |
Oxcarbazepine (n = 67) |
Phenobarbital (n = 57) |
Topiramate (n = 16) |
Other† (n = 32) |
None (n = 12) |
|---|---|---|---|---|---|---|---|
| First medication* | |||||||
| <6 months | 163 | 90 (55.2%) | 10 (6.1%) | 50 (30.7%) | 6 (3.7%) | 7 (4.3%) | 1 (0.6%) |
| 6 to <12 months | 81 | 59 (72.8%) | 9 (11.1%) | 4 (4.9%) | 3 (3.7%) | 6 (7.4%) | 3 (3.7%) |
| 12 to 23 months | 125 | 86 (68.8%) | 22 (17.6%) | 3 (2.4%) | 6 (4.8%) | 8 (6.4%) | 4 (3.2%) |
| ≥24 months | 94 | 56 (59.6%) | 26 (27.7%) | 0 (0%) | 1 (1.1%) | 11 (11.7%) | 4 (4.3%) |
19 children received more than one medication at the time of epilepsy onset; these individuals were excluded from the above table. Of these 19, levetiracetam was used in 12, phenobarbital in 11, oxcarbazepine in six, and other drugs in the rest.
The 32 children who received other medications were prescribed: zonisamide (11), valproic acid (4), lamotrigine (4), ethosuximide (3), vigabatrin (3), clobazam (2), phenytoin (2), carbamazepine (1), clonazepam (1), and immediate resective epilepsy surgery (1).
TABLE 3.
Initial Medications by Epilepsy Type, Seizure Semiologies, and Baseline Seizure Burden*
| n | Levetiracetam | Oxcarbazepine | Phenobarbital | Topiramate | Other | |
|---|---|---|---|---|---|---|
| Epilepsy presentation | ||||||
| Focal | 248 | 137 (55.2%) | 53 (21.4%) | 35 (14.1%) | 8 (3.2%) | 15 (5.9%) |
| Generalized | 136 | 104 (76.5%) | 5 (3.7%) | 11 (8.0%) | 5 (3.7%) | 11 (8.0%) |
| Mixed/unclear | 79 | 50 (63.3%) | 9 (11.4%) | 11 (13.9%) | 3 (3.8%) | 6 (7.6%) |
| Seizure semiology | ||||||
| Convulsion† | 316 | 213 (67.4%) | 35 (11.1%) | 36 (11.4%) | 13 (4.1%) | 19 (6.0%) |
| Focal seizures‡ | 65 | 35 (53.9%) | 17 (26.2%) | 11 (16.9%) | 1 (1.5%) | 1 (1.5%) |
| Behavioral arrest | 128 | 75 (58.6%) | 32 (25.0%) | 11 (8.6%) | 5 (3.9%) | 5 (3.9%) |
| Absence | 11 | 7 (63.6%) | 1 (9.1%) | 0 (0%) | 0 (0%) | 3 (27.3%) |
| Myoclonic§ | 38 | 28 (73.7%) | 1 (2.6%) | 2 (5.3%) | 0 (0%) | 7 (18.4%) |
| Baseline seizure frequency | ||||||
| <1/month | 48 | 36 (75.0%) | 9 (18.8%) | 0 (0%) | 1 (2.0%) | 2 (4.2%) |
| 1 to 3 per month | 64 | 46 (71.9%) | 11 (17.2%) | 3 (4.7%) | 2 (3.1%) | 3 (4.7%) |
| 1 to 6 per week | 72 | 47 (65.3%) | 11 (15.3%) | 8 (11.1%) | 3 (4.2%) | 3 (4.2%) |
| 1 per day | 48 | 27 (56.3%) | 5 (10.4%) | 8 (16.7%) | 0 (0%) | 8 (16.7%) |
| >1 per day | 188 | 108 (57.5%) | 27 (14.4%) | 29 (15.4%) | 10 (5.3%) | 14 (7.5%) |
| Not determined | 43 | 27 (62.8%) | 4 (9%) | 10 (23.3%) | 0 (0%) | 2 (4.7%) |
19 children received more than one medication at the time of epilepsy onset; these individuals are excluded from the data presented in this table.
Convulsions include tonic, clonic, and tonic-clonic (includes focal seizures with secondary generalization).
Focal seizures include focal motor, sensory, and auras.
Myoclonic seizures include myoclonic, myoclonic-atonic, myoclonic-tonic, atonic.
The first medication provided complete freedom from seizures without significant side effects for 165 children. For 224 children the medication was inefficacious in controlling seizures, and 30 children discontinued the first medication because of side effects only. The medication was stopped for a variety of other reasons for 44 children. Most (n = 28) in this last group did not start a second medication.
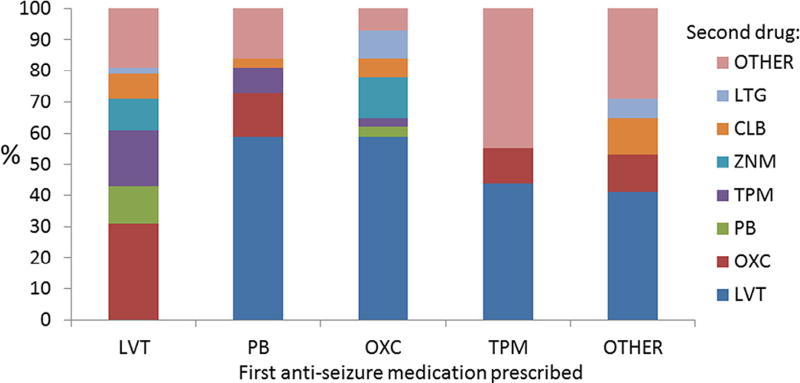
A total of 233 children were prescribed a second medication during the first year after epilepsy diagnosis. The pattern of second drug selection strongly mirrored the pattern of the first drug selection. Among children who received a drug other than levetiracetam as their initial medication, 64 of 109 (58.7%) received levetiracetam as their second medication. The 124 children who received levetiracetam as the initial medication and required a second treatment were prescribed oxcarbazepine (n = 36, 29%), topiramate (n = 22, 18%), phenobarbital (n = 11, 9%), or zonisamide (n = 12, 10%) as their second medication (Fig 3). In all, 367 (74%) of the children treated for nonsyndromic epilepsy received levetiracetam during the first year after diagnosis (303 as the first and 64 as the second choice). Oxcarbazepine was, overall, the second most commonly prescribed medication during the first year (n = 68 as first treatment and n = 43 as second), followed by phenobarbital (n = 65 first and n = 13 second) and topiramate (n = 16 first and n = 28 second).
FIGURE 3.
Second anti-seizure medication selected for 233 children with early-life epilepsy. For 233 children a second anti-seizure medication was prescribed because of lack of seizure control (n = 188) or side effects (n = 29). Among 109 children who received a drug other than levetiracetam as their initial medication, 68 (62%) received levetiracetam as their second medication. LTG, lamotrigine; CLB, clobazam; ZNM, zonisamide; TPM, topiramate; OXC, oxcarbazepine; PB, phenobarbital; LVT, levetiracetam.
Discussion
Although there are many available medications for early-life epilepsy treatment, and little evidence that any individual medication has superior efficacy compared with the others, our data suggest that the pediatric neurology community in the United States appears to have reached an informal consensus on the initial treatment of early-life epilepsies. Levetiracetam was, by far, the most commonly prescribed initial antiseizure medication for young children with newly diagnosed epilepsy (62% versus 14% prescribed oxcarbazepine and 13% phenobarbital). Even more strikingly, 62% of children who were not treated with levetiracetam initially but required a second medication because of inadequate efficacy or unacceptable side -effects received levetiracetam as their second drug. Thus, despite the availability of more than 20 antiseizure medications, 74% of all children were prescribed levetiracetam as either their first or their second epilepsy drug.
A consensus in treatment selection for early-life epilepsy is not equivalent to an evidence-based standard of care. Although levetiracetam is frequently prescribed, it is unknown whether treatment with this medication results in improved seizure control, quality of life, or developmental outcomes for children with early-life epilepsy. This drug is widely used today as it is available in a liquid formulation, has no drug interactions, and also can be started intravenously. Among the 291 children prescribed levetiracetam monotherapy as their first treatment, 43 (15%) were also prescribed pyridoxine specifically to control behavioral side effects. This is a practice that has come into being with minimal evidence to support its value.16
Compared with published data from the 1990s, our findings demonstrate a shift in medications prescribed for early-onset epilepsy. In a community-based study of 613 children aged one month to 15 years with newly diagnosed epilepsy, carbamazepine and valproic acid were the most commonly prescribed initial medications.17 By contrast, only one child in the present study was treated with carbamazepine and just four were prescribed valproic acid as an initial treatment. Since the 1990s, numerous new agents have become available. Despite a dearth of data regarding their use in infants and children, some of the newer medications have been suggested as initial treatments for children.18 In comparison with practice from the 1990s, it is noteworthy that new agents have largely supplanted the older drugs as first-line therapies.
Although this study provides real-world clinical data regarding management strategies for children treated at tertiary, academic epilepsy centers in the United States, treatment preferences vary internationally. A survey of 733 individuals from 96 countries requested information about preferred treatments for infants with a range of epilepsies and seizure types.19 Respondents outside of North America indicated that they were most likely to treat focal seizures with carbamazepine or oxcarbazepine and generalized seizures (including myoclonic seizures) with valproic acid. In contrast, clinicians in North America were most likely to indicate a preference for levetiracetam to treat both focal and generalized onset seizures.
It is not clear what underlies North American clinicians’ indicated preference for levetiracetam in the international survey19 and actual prescription of this medication for most young children in our study. Among the four most commonly prescribed antiseizure medications in our study, topiramate and oxcarbazepine have a US Food and Drug Administration indication for monotherapy in children (≥4 years of age); these drugs also have indications as adjunctive therapies for younger children (levetiracetam for ≥1 month; oxcarbazepine ≥2 years; topiramate ≥2 years). All four of the most commonly prescribed medications in this study have readily available generic formulations that are typically covered by American medical insurance plans. Levetiracetam, however, may be more difficult to access in other countries.19 Given the trend in the United States toward strict policies that limit contact between academic clinicians and pharmaceutical company representatives, we do not have any reason to believe that medication selection during this study was related to any specific drug company influence.
There is increasing concern about potentially adverse effects of anticonvulsant medications on the developing brain. This is particularly true for phenobarbital, for which animal data demonstrate abnormal neuronal apoptosis and human clinical data suggest potential for long-term developmental consequences of early-life exposure.8,9,20 It is possible that selecting an alternative to phenobarbital might result in improved developmental outcomes. Data from animal models suggest that other medications, including topiramate and levetiracetam, may not induce such deleterious effects and may, therefore, be preferable for use in early-life epilepsies.21–24 However, there are no published data from human trials to suggest that any one medication provides improved efficacy, tolerability, or long-term outcomes for children with early-life epilepsy. Comparative effectiveness studies of commonly prescribed antiseizure medications, especially the four most commonly prescribed agents in our study, are urgently needed.
Our data reflect current prescribing practices but have some limitations. This is a chart review study. Seizure semiologies and epilepsy syndromes were extracted from medical records from pediatric epilepsy centers but were not independently verified. Clinicians’ rationale for medication selection for individual children is not consistently recorded in medical records. Although consensus-based dosing strategies were suggested to the participating centers, no systematic guidance was provided to the study centers regarding which medications to prescribe, and specific medication doses or escalation plans were not required. Neither exact medication doses, nor serum anticonvulsant levels, are available for this cohort. Additionally, the decision to change from the initial treatment choice to another drug was based on clinical judgment and not on any study protocol.
Evidence-based treatment recommendations for the early-life epilepsies remain elusive.4 Despite the absence of data, the lack of any specific effort during this study to standardize care, and the availability of more than 20 antiseizure medications to choose from, pediatric neurologists at these US epilepsy centers appear to have come to an informal consensus regarding the initial approaches for pharmacologic treatment of nonsyndromic early-life epilepsies. This has happened with relatively little evidence regarding the pharmacokinetics, efficacy, or long-term implications of treatment with levetiracetam during the first years of life.25 Although it remains unclear if a shift toward treatment with this broad-spectrum medication will result in improved outcomes, it appears that the stage is ready for comparative effectiveness studies that could lead to standardization and optimization of the use of current drugs and prepare the way for future trials of novel therapies.
Acknowledgments
Funding: This work was supported by a grant from the Pediatric Epilepsy Research Foundation. The foundation had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Dr. Millichap and Dr. Saneto report previous grants from UCB Pharma, the company that makes Keppra (levetiracetam).
The authors thank the study coordinators, especially Stephanie Rau and Sierra Lord-Halvorson, for their tireless efforts on this project.
Footnotes
Conflicts of interest: All the other authors report no potential conflicts of interest.
References
- 1.Franco V, Canevini MP, Capovilla G, et al. Off-label prescribing of antiepileptic drugs in pharmacoresistant epilepsy: a cross-sectional drug utilization study of tertiary care centers in Italy. CNS Drugs. 2014;28:939–949. doi: 10.1007/s40263-014-0189-8. [DOI] [PubMed] [Google Scholar]
- 2.Knupp KG, Coryell J, Nickels KC, et al. Response to treatment in a prospective national infantile spasms cohort. Ann Neurol. 2016;79:475–484. doi: 10.1002/ana.24594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go CY, Mackay MT, Weiss SK, et al. Evidence-based guideline update: medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2012;78:1974–1980. doi: 10.1212/WNL.0b013e318259e2cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilmshurst JM, Gaillard WD, Vinayan KP, et al. Summary of recommendations for the management of infantile seizures: task force report for the ILAE Commission of Pediatrics. Epilepsia. 2015;56:1185–1197. doi: 10.1111/epi.13057. [DOI] [PubMed] [Google Scholar]
- 5.Camfield CS, Camfield PR, Gordon K, Wirrell E, Dooley JM. Incidence of epilepsy in childhood and asolescence: a population-based study in Nova Scotia from 1977 to 1985. Epilepsia. 1996;37:19–23. doi: 10.1111/j.1528-1157.1996.tb00506.x. [DOI] [PubMed] [Google Scholar]
- 6.Camfield P, Camfield C. Incidence, prevalence and aetiology of seizures and epilepsy in children. Epileptic Disord. 2015;17:117–123. doi: 10.1684/epd.2015.0736. [DOI] [PubMed] [Google Scholar]
- 7.Sulzbacher S, Farwell JR, Temkin N, Lu AS, Hirtz DG. Late cognitive effects of realy treatment with phenobarbital. Clin Pediatr. 1999;38:387–394. doi: 10.1177/000992289903800702. [DOI] [PubMed] [Google Scholar]
- 8.Farwell JR, Lee YJ, Hirtz DG, Sulzbacher SI, Ellenberg JH, Nelson KB. Phenobarbital for febrile seizures—effects on intelligence and on seizure recurrence. NEJM. 1990;322 doi: 10.1056/NEJM199002083220604. [DOI] [PubMed] [Google Scholar]
- 9.Meador KJ, Loring DW. Developmental effects of antiepileptic drugs and the need for improved regulations. Neurology. 2016;86:297–306. doi: 10.1212/WNL.0000000000002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmes GL. Effects of early seizures on later behavior and epileptogenicity. Ment Retard Dev Disord. 2004;10:101–105. doi: 10.1002/mrdd.20019. [DOI] [PubMed] [Google Scholar]
- 11.Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 12.Wirrell EC. Treatment of Dravet syndrome. Can J Neurol Sci. 2015;43:S13–S18. doi: 10.1017/cjn.2016.249. [DOI] [PubMed] [Google Scholar]
- 13.Aras LM, Isla J, Mingorance-Le Meur A. The European patient with Dravet syndrome: results from a parent-reported survey on antiepileptic drug use in the European population with Dravet syndrome. Epilepsy Behav. 2015;44:104–109. doi: 10.1016/j.yebeh.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Brigo F, Igwe SC. Antiepileptic drgus for the treatment of infants with severe myoclonic epilepsy. Cochrane Database Syst Rev. 2015;(10):CD010483. doi: 10.1002/14651858.CD010483.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42 doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Major P, Greenberg E, Khan A, Thiele EA. Pyridoxine supplementation for the treatment of levetiracetam-induced behavior side effects in children: preliminary results. Epilepsy Behav. 2008;13:557–559. doi: 10.1016/j.yebeh.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Berg AT, Levy SR, Testa FT, Shinnar S. Treatment of newly diagnosed epilepsy: a community-based study. Arch Pediatr Adol Med. 1999;153:1267–1271. doi: 10.1001/archpedi.153.12.1267. [DOI] [PubMed] [Google Scholar]
- 18.Glauser T, Ben-Menachem E, Bourgeois B, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54:551–563. doi: 10.1111/epi.12074. [DOI] [PubMed] [Google Scholar]
- 19.Wilmshurst JM, Burman R, Gaillard WD, Cross JH. Treatment of infants with epilepsy: common practices around the world. Epilepsia. 2015;56:1033–1046. doi: 10.1111/epi.13003. [DOI] [PubMed] [Google Scholar]
- 20.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaushal S, Tamer Z, Opoku F, Forcelli PA. Anticonvulsant drug-induced cell death in the developing white matter of the rodent brain. Epilepsia. 2016;57:727–734. doi: 10.1111/epi.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park HJ, Kim HJ, Park HJ, et al. Protective effect of topiramate on kainic acid-induced cell death in mice hippocampus. Epilepsia. 2008;49:163–167. doi: 10.1111/j.1528-1167.2007.01308.x. [DOI] [PubMed] [Google Scholar]
- 23.Kilicdag H, Daglioglu K, Erdogan S, et al. The effect of levetiracetam on neuronal apoptosis in neonatal rat model of hypoxic ischemic brain injury. Early Hum Dev. 2013;89:355–360. doi: 10.1016/j.earlhumdev.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Kim J-S, Kondratyev A, Tomita Y, Gale K. Neurodevelopmental impact of antiepileptic drugs and seizures in the immature brain. Epilepsia. 2007;48(suppl 5):19–26. doi: 10.1111/j.1528-1167.2007.01285.x. [DOI] [PubMed] [Google Scholar]
- 25.Cormier J, Chu CJ. Safety and efficacy of levetiracetam for the treatment of partial onset seizures in children from one month of age. Neuropsychiatr Dis Treat. 2013;9:295–306. doi: 10.2147/NDT.S30224. [DOI] [PMC free article] [PubMed] [Google Scholar]