Abstract
Analysis of multiplexed assays is highly important for clinical diagnostics and other analytical applications. Mass cytometry enables multi-dimensional, single-cell analysis of cell type and state. In mass cytometry, the rare earth metals used as reporters on antibodies allow determination of marker expression in individual cells. Barcode-based bioassays for CyTOF are able to encode and decode for different experimental conditions or samples within the same experiment, facilitating progress in producing straightforward and consistent results. Herein, an integrated protocol for automated sample preparation for barcoding used in conjunction with mass cytometry for clinical bioanalysis samples is described; we offer results of our work with barcoding protocol optimization. In addition, we present some points to be considered in order to minimize the variability of quantitative mass cytometry measurements. For example, we discuss the importance of having multiple populations during titration of the antibodies and effect of storage and shipping of labelled samples on the stability of staining for purposes of CyTOF analysis. Data quality is not affected when labelled samples are stored either frozen or at 4 °C and used within 10 days; we observed that cell loss is greater if cells are washed with deionized water prior to shipment or are shipped in lower concentration. Once the labelled samples for CyTOF are suspended in deionized water, the analysis should be performed expeditiously, preferably within the first hour. Damage can be minimized if the cells are resuspended in phosphate-buffered saline (PBS) rather than deionized water while waiting for data acquisition.
Keywords: Multiplexing, Barcoding, CyTOF, Mass cytometry
Introduction
Cytometry by Time-Of-Flight (CyTOF)
Cytometry by Time-Of-Flight (CyTOF or mass cytometry) is a relatively new and promising technology for real-time analysis of single cells using inductively coupled plasma time-of-flight mass spectrometry [1–8]. Researchers from a variety of disciplines, such as cancer research, cardiovascular research, embryonic stem cells and development, gene expression profiling, hematopoietic stem cells and progenitors, immunity/infectious disease, induced pluripotent stem cells, neural research, and RNA sequencing are expressing strong interest in single-cell experimentation methods [9–13]. Mass cytometry provides an important tool for single-cell biology and is currently being incorporated into studies in many medical fields including immunology, hematology, and oncology; while research in drug companies is focused on the mechanism of drug-target interactions and its resulting pharmacology, CyTOF allows a deeper analysis of cell phenotypes as compared to flow cytometry [1–6]. For example, CyTOF provides the ability to phenotypically and functionally profile cells from normal and diseased states. Single-cell technologies have allowed researchers to measure the effects of a drug at the single-cell level and better understand its mechanism of action.
The current system mass resolving power is between 400 and 600 and defined as R = M/ΔM, where M designates the mass (TOF at 159 Tb) and ΔM is the full width at half maximum (2×FWHM) at the concentration of Tb of 0.5 ppb. There is a trade-off between resolution and sensitivity; as resolution is increased the sensitivity decreases so a compromise often must be sought. It is important for CyTOF analysis to have optimum resolution. CyTOF resolves and detects multiple metal conjugated probes per cell with minimal signal overlap, which maximizes the information obtained from each individual sample. The CyTOF instrument allows the detection of more than 40 parameters at the single-cell level; this capacity will increase as more isotopes become available. Recent innovations in CyTOF bring the capability for the simultaneous detection of a major, and steadily growing, number of proteins at the single-cell level and facilitate greater understanding of both cell phenotype and function.
The importance of automated sample preparation for barcoding technique cannot be overstated. Careful sample preparation/cleanup is essential because it can affect the analyte ionization on mass spectrometry and subsequently the concentration of the analyte. The analytical techniques cannot correct problems generated by sample preparation errors. It is important to note that optimization protocols based on each application are necessary because no one protocol can fit all applications. Herein, in addition to automated sample preparation for barcoding, we will address other sources of variability in mass cytometry such as shipping and storage of labelled samples with stable heavy metal isotopes for CyTOF analysis, as well as the stability of staining in deionized water as a critical step in the analysis.
Experimental
Apparatus
Mass cytometry
Mass cytometry measurements were performed on a CyTOF 2 instrument (Fluidigm, Sunnyvale, CA). The CyTOF 2 instrument was started, tuned, and cleaned as per manufacturer’s instructions (Fluidigm, Sunnyvale, CA) [14]. Samples were injected into the sample loop in portions of 500 µl and run for 10 min at flow rate 45 µl/min. Cells were injected as a single-cell suspension in water supplemented with EQ™ Four element calibration beads (Fluidigm, Sunnyvale, CA) after filtration through a 35-µm nylon mesh (cell strainer cap tubes, BD, San Jose, CA) immediately prior to acquisition. Calibration bead signals were used to monitor the detector performance over the runtime. Before sample loading, QC was performed based on collecting information of the automated tuning procedure and data from EQ™ Four element calibration beads (Fluidigm), and successfully passed for all experiments. Data were acquired in Dual data calibration mode, with noise reduction turned off and lower and upper cell length parameter values set to 10 and 150, respectively. FCS files were generated by CyTOF instrument control software v6.0.622 (Fluidigm), which also served to control the instrument. The normalization software is based on the concept of a Bead Passport. The Bead Passport is a global standard generated by the manufacturer for a specific lot of EQ beads. This Passport is universal across all instruments of the same type and cannot be changed by individual users. Using a global standard allows normalization of data within and across experiments as well as across instruments [14].
Materials and human subjects
Ir-intercalator stock solution 125 M cat # 201192A, metal conjugated antibodies and Cell-ID™ 20-Plex Pd Barcoding Kit cat #201060, EQ™ Four Element Calibration Beads contain natural abundance cerium (140/142Ce), europium (151/153Eu), holmium (165Ho), and lutetium (175/176Lu), Catalog#: 201078 were obtained from (Fluidigm, Sunnyvale, CA), cisplatin cat # 15663-27-1 and RPMI-1640, cat # R0883 (Sigma-Aldrich, St. Louis, MO), and Foxp3 / Transcription Factor Staining Buffer Set cat # 00-5523-00 (eBioscience, Inc. San Diego, CA), BD GolgiStop™ (BD Biosciences, catalog number: 554715). For human subjects, heparinized blood from healthy volunteers was obtained after written informed consent under the guidelines and approval of the Human Investigations Committee of Yale University School of Medicine. Donors had no acute illness and took no antibiotics or non-steroidal anti-inflammatory drugs within 1 month of enrollment.
Sample preparation using barcoding for CyTOF
Sample collection, preparation, and storage
Fresh blood was collected and placed on lithium heparin; after PBMC isolation cells were washed in x-vivo medium, counted and the concentration adjusted to 10 million/ml (2 million cells total). One-half (1 million) of the cells were incubated with GolgiStop alone. The other half was incubated with GolgiStop and PMA + ionomycin (250/50 ng/ml) for 4 h. After 4 h cells were washed twice with PBS (centrifuged at 1000×g for 4 min) and incubated for 1 min with a solution of cis-Diammineplatinum(II) dichloride (cisPt, Sigma-Aldrich, CAS Number 15663-27-1, St. Louis, MO 63103) 150 µl/sample (50 µM, 1 min). Incubation with cisPt was stopped by adding equal volume of staining buffer (SB) containing BSA and centrifugation at 350×g 5 min. The cell pellet was resuspended in 300 µl SB and the washing step was repeated twice. Samples were immediately fixed in 200 µl fixation buffer, FB (Foxp3/Transcription Factor Staining Buffer Set eBioscience, Inc. San Diego, CA cat # 00-5523-00) and stored at −80 °C until use (up to 18 months).
Cell staining for CyTOF mass spectrometry
Samples were processed in batches of 20 samples using an automated Biomek robotic platform (Beckman Coulter) for cell barcoding and labeling for CyTOF analysis. Samples were transferred from −80 °C to a hood and allowed to thaw slowly on ice. Two volumes of PBS were added to each sample, then centrifuged for 4 min at 1000×g. The pellet was resuspended in 250 µl of PBS, and the 20 samples were transferred to two 10 rows of a V bottom 96 well plate. The plate was centrifuged at 1000×g for 4 min, supernatant discarded and the pellet resuspended in 250 µl of PBS. This step was repeated twice. Five microliters of Fc block was added to each well followed by adding 25 µl of a cocktail of metal conjugated antibodies for the following surface markers (CXCR3, CCR4, CXCR5, CCR6, CCR7, CD45RO, CD127 obtained from Fluidigm, Sunnyvale, CA) in SB; this mixture was incubated before barcoding for 30 min. Two hundred twenty-five microliters of SB was added to each well. The plate was centrifuged at 1000×g, the supernatants removed, and samples were washed once with 250 µl of MCB (mass-tag cellular barcoding, Fluidigm, Sunnyvale, CA) permeabilization buffer. The pellet was resuspended in 200 µl of MCB permeabilization buffer (PB). 100× combinatorial MCB tubes (20 uL) were thawed as needed (2 rows of tubes for 20 samples of barcodes 1 to 20) at 37 °C. Then, 100 µl of PB was added to each MCB tube and mixed. 30 µl of MCB were transferred to each well followed by mixing well with the samples and incubation at RT for 30 min. The remaining MCB tubes were capped and stored for up to 1 week at 4 °C. The samples were centrifuged and washed three times with 250 µl SB, then the 20 samples were combined in one eppendorf tube by successively washing all the wells with 200 µl SB four times.
The barcoded samples were counted using Guava cell counter (up to 10 million cells), resuspended in 100 µl of the cocktail mixture of metal conjugated antibodies of interest and incubated for 30 min at room temperature. At the end of the incubation, 900 µl of SB were added to the samples and centrifuged at 1000×g for 4 min. The samples were washed once with 1 mL FB, then incubated overnight at 4 °C with 1 ml of FB containing 1 µl/ml of 191 and 193 Iridium DNA intercalator. Samples were washed twice with 1 ml PBS, then the cells were counted. Cells were washed with 500 µL deionized water, then centrifuged at 800×g for 10 min at 4 °C and supernatant discarded. An aliquot for cell count was taken prior to wash to remove debris. As an example of a point to be considered in preparation, as well as interpretation of the results, the amount of debris must be taken into account, as this affects adjustment of the final concentration for running on CyTOF. Cells were left pelleted until ready to run on CyTOF. The cell concentration was adjusted to 106/mL with deionized water. Immediately prior to CyTOF data acquisition, the pellet was resuspended in a volume of EQ Four element calibration beads (mixed well and diluted 1:10) needed to bring cells to a final concentration of 106/mL; cells were then filtered into cell strainer cap tubes.
Results and discussion
Comparison of mass cytometry and flow cytometry
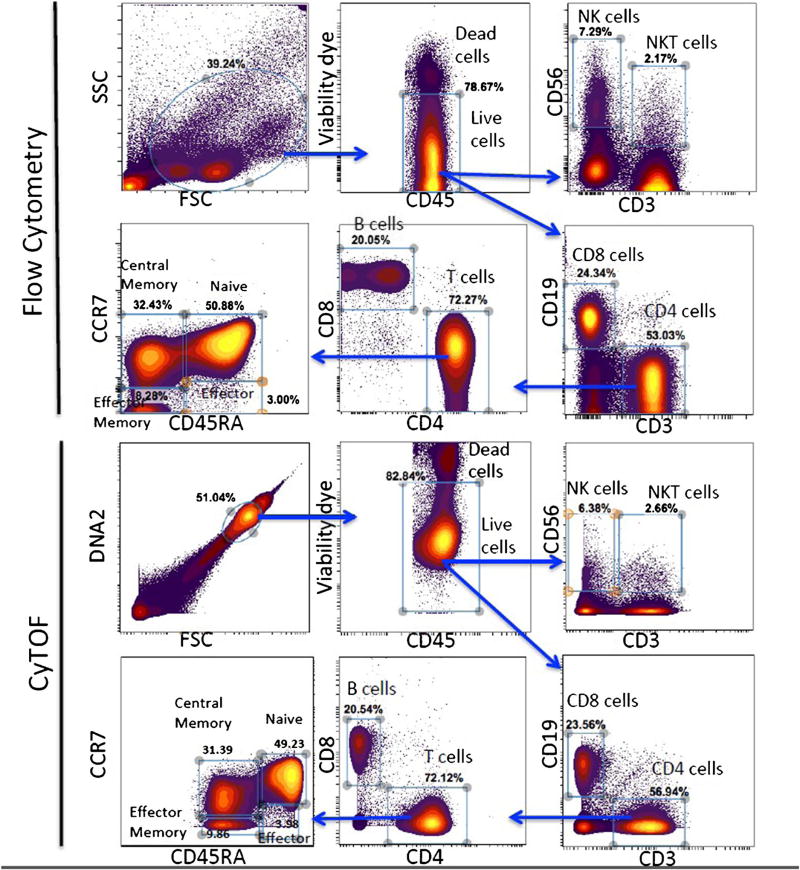
Flow Cytometry is a laser-based technology employed in cell counting, cell sorting, biomarker detection, and protein engineering, by suspending cells in a stream of fluid and passing them by an electronic detection apparatus. Mass cytometry is similar to flow cytometry in that antibodies are labelled with heavy metal ion tags rather than fluorochromes [2, 3, 15]. Mass cytometry can be applied extensively for use in a variety of experiments and analyses for clinical research and drug development. However, mass cytometers require qualified personnel for operation and maintenance of the instrument in order to ensure high-quality results. Although the protocols for sample preparation and staining are similar to those used in flow cytometry assays, further optimizations are required to ensure that the reagents’ stability and reproducibility are maintained. Figure 1 shows a comparison between CyTOF and flow cytometry using the same sample and comparing some of the most used markers in immune monitoring. Both the profile and the proportion of the different immune populations are similar using either method; the choice of using one over the other depends on the aim pursued. While flow cytometry has proven to be fairly reproducible and the reagent in general well controlled, it is very limited as to deep profiling and discovery of smaller, rare, or unique cell subsets. Table 1 provides a detailed comparison side by side of the two methods and the pros and cons for each method. High throughput in quantitative bioanalysis applies to steps such as assay development, sample collection and sorting, sample preparation, sample analysis, and data processing and reporting. The CYTOF2 instrument can measure ~500 cells/s, the upgrade system or Helios can measure up to 1000 cells/s, and flow cytometry ~80,000 cells/s [15].
Fig. 1.
Comparison between CyTOF and flow cytometry data: PBMCs (peripheral blood mononuclear cells) were prepared from cryopreserved peripheral blood of a healthy donor and split in two tubes, one for flow cytometry staining and one for CyTOF staining. After gating on live cells, different markers are compared between flow cytometry (upper panel) and CyTOF (lower panel)
Table 1.
Comparison between mass cytometry and flow cytometry
| Technology | Mass Cytometry | Flow Cytometry |
|---|---|---|
| Measurement | Stable mass isotope probes | Fluorescent probes |
| Application | Biomarkers, immunophenotyping, function, pharmacodynamic (PD) | Biomarkers, immunophenotyping, function, PD |
| Workflow |
|
|
| Multiplex Capacity | ~40 | ~20 |
| It can be up to120 if more isotopes become available | ||
| Sample Barcoding | Yes | Yes |
| Detection limit | ~300 molecules/single cell | 100–300 molecules/single cell (varies by fluorophor, without compensation) |
| Data analysis Complexity | Moderate-high | Low-high (Deconvolution for high- parameter flow) |
| Information Content / Sample | High | Moderate |
| Single-cell resolution | Yes | Yes |
| Throughput | ~500 cells/s | ~80,000 cells / s |
| Analytical efficiency | ~30% | ~90% |
| Live cell Analysis/Cell Sorting | Not possible | Possible |
| Cost/Sample | ~$150 | ~$5 |
| Instrument cost | ~$650,000 | ~$500,000 |
| Vendor(s) | Fluidigm | Multiple |
| Impact | Multiplex Capacity, Specificity, Small Sample Size | Sensitivity, Downstream Live Cell Analysis |
| Maintenance | 1 h daily | 15 min daily |
| 4 h weekly | 30 min weekly |
Sources of technical variability in quantitative mass cytometry
In order to design a robust quantitative mass cytometry study, we need to be aware of the inherent heterogeneity of the biological samples as well as the technical variability of the mass cytometry instrument. The storage conditions between sampling and analysis must be controlled to ensure that the samples do not degrade. Given that it is preferable to run samples within 1 h, such operator-related delays are likely to be very costly and wasteful. Other sources of variabilities involved sample processing, contamination, sample stability, instrument stability, and data processing. Also sample collection is important for any quantitative analysis to ensure that a representative and sufficiently homogeneous sample is taken for analysis. Correct data processing is a fundamental step in generating good quality quantitative data. Salts in samples will reduce the sensitivity but this can be minimized when cells are washed with water before analysis. If particular attention is not paid to all of the above, sample integrity may be sacrificed and the analysis data affected, compromised, or rendered invalid. Below, we will address some of these issues and offer suggestions to minimize labor, time, and effort, and reduce overall errors, through use of automated equipment.
Shipping of labelled samples for CyTOF analysis
Collection, temperature-controlled stability, packaging, and safe, timely delivery of labelled samples for CyTOF analysis are crucial throughout. Results from overnight shipment of stained samples showed when samples are washed with 2% PFA in PBS pre-shipment, processing resulted in 79% cell recoveries [16], while for samples washed with 2% PFA in deionized water pre-shipment processing resulted in 48% cell recoveries [16]. This suggested that cell loss is greater if cells are washed with deionized water prior to shipment. Also, we found out that the cell loss is greater when shipping lower numbers of cells [16]. It was suggested that three critical factors in determining cell recovery from shipped samples are (1) cells should be washed and fixed in PBS buffer prior to shipping, (2) cells should be shipped in bulk and serial dilutions prepared at analysis site, if possible, (3) samples should remain cold during shipping and sample preparation [16].
Storage of labelled samples with stable heavy metal isotopes for CyTOF analysis
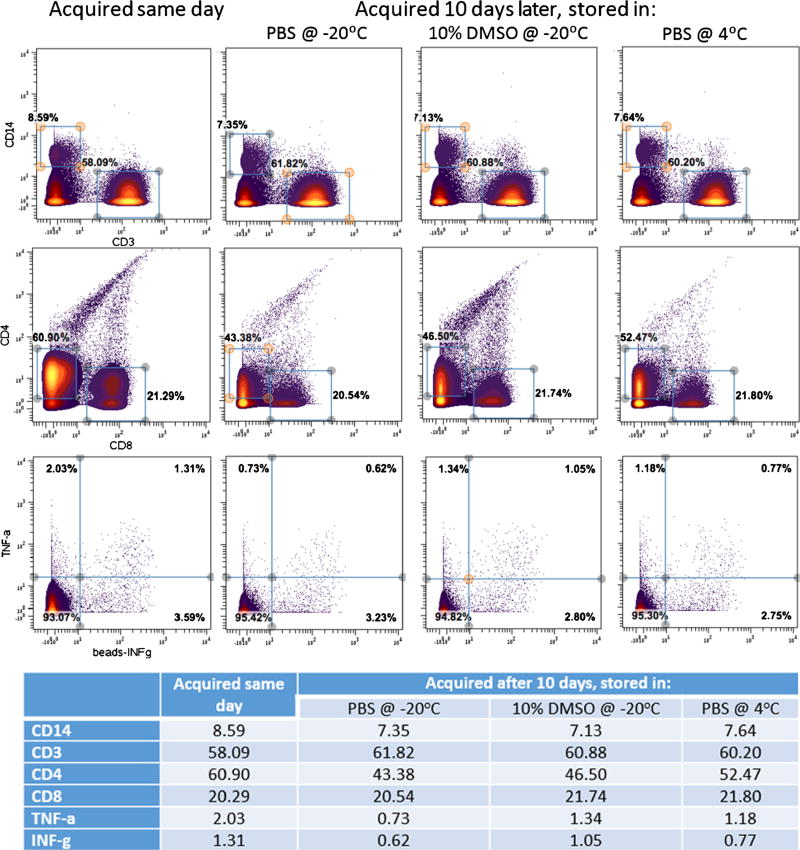
Storing samples that are already labelled with stable heavy metal isotope and ready for acquisition is an important issue when the CyTOF system is not available within a few days of sample staining. Recently, we reported that storage of labelled CyTOF samples in water can affect some of the markers [15]. In this article, we have studied the effect of storage of the samples over 10 days. Figure 2 represents a comparison between acquiring the same sample on the same day and storing it to be acquired 10 days later. Storage of labelled CyTOF samples: PBMC were isolated from a healthy donor’s peripheral blood and stained for CyTOF. The sample was divided in four equal aliquots; one aliquot of the sample was acquired the same day on the CyTOF. The three remaining aliquots were centrifuged and re-suspended in PBS + 10% DMSO and stored at −20 °C, or in PBS and stored at either −20 or 4 °C. After 10 days, the samples were brought to room temperature, washed with deionized water and acquired. The figure shows a comparison of some of the biomarkers used for all the four conditions; there is no significant difference between them. This shows that the samples can be stored either frozen or at 4 °C and acquired within 10 days without affecting the quality of the data.
Fig. 2.
Illustration of a comparison between acquiring the same sample on the same day and storing it to be acquired 10 days later
Stability of staining in deionized water
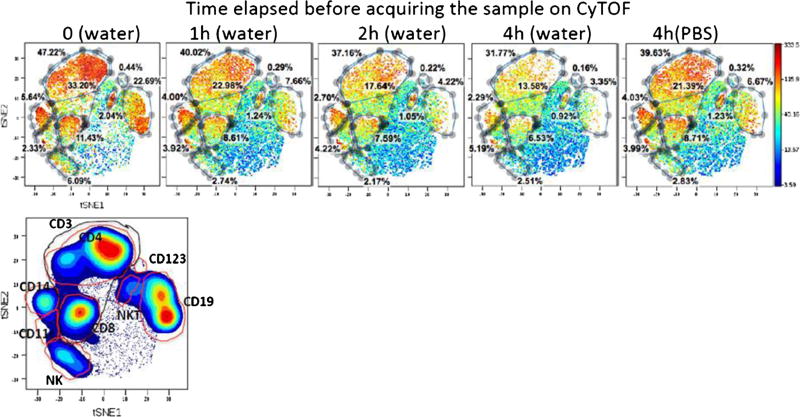
Another important consideration is the sample stability in deionized water. Sample preparation for CyTOF requires that the last step consist of washing the sample in deionized water and resuspending it in deionized water before acquisition. Due to the limited number of events that can be acquired on CyTOF per minute, several consecutive runs of the same sample are often necessary in order to collect enough events for a meaningful analysis, especially for rare cell populations. viSNE is a new high-dimensional cytometry analysis tool based on the t-distributed stochastic neighbor embedding (t-SNE) algorithm [17]. Figure 3 is a viSNE comparison of the staining pattern of different markers over time when stored in deionized water. CyTOF antibodies are rather unstable in deionized water: the biomarker pattern is significantly disrupted after 1 h and even more after 4 h. Most cell markers seem to fade; the biggest loss involves B and NK cells. This suggests that, once the cells are suspended in deionized water, the analysis should be performed expeditiously, preferably within the first hour. The damage can be minimized if the cells are resuspended in PBS rather than deionized water while waiting for data acquisition (Fig. 3). For terms longer than 10 days, cell storage in DMSO is preferred.
Fig. 3.
Representative antibody surface-staining results after the sample has been stored in deionized water for 0, 1, 2, and 4 h, or resuspended in PBS for 4 h
Discriminating live and dead cells in CyTOF
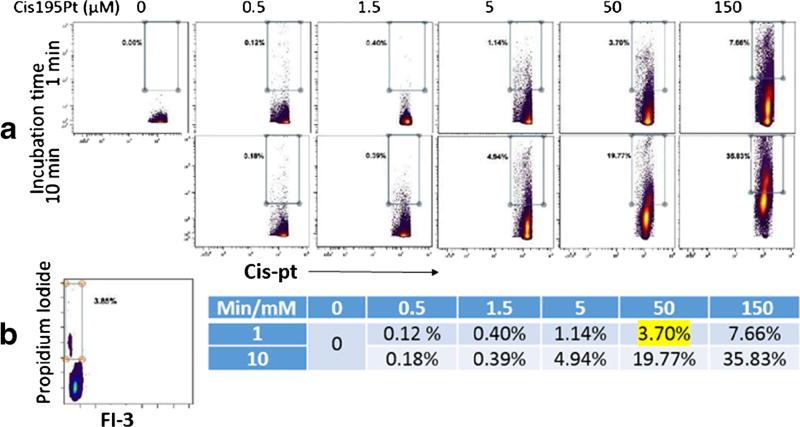
Discriminating and excluding dead cells decreases non-specific binding of antibodies to the cells and provides better data. Many viability dyes are currently used in flow cytometry, such as propidium iodide. There was a lack of such products for CyTOF until several years ago when cisplatin (195Pt or 198Pt) was adopted to stain dead cells for CyTOF. However, some considerations should be taken while using cisplatin in the protocol; for example, cell degradation and/or death occur at higher concentrations or longer incubation times. The proper concentration level (50 M) is critical to achieve the best results. When the cells are incubated for a very short time (1 min) with cisplatin at this concentration, there is 3.7% dead cells; this result is comparable to viability measurements produced by flow cytometry after staining with propidium iodide (3.85% dead cells) (Fig. 4). Another factor is that cisplatin should be fresh. Its chemical properties are altered by lengthy storage at room temperature or repeated freeze-thaw cycles. Such conditions cause the reagent to display increased potential for non-specific binding, which in turn may interfere with discrimination between live and dead cells.
Fig. 4.
Use of cis195Pt as a viability dye for CyTOF: a Freshly isolated PBMCs were incubated with different doses of cis195Pt for 1 or 10 min, then they were washed and labelled with DNA intercalator and washed again before injection to CyTOF. b the same PBMC were labelled with propidium iodide and analyzed on a flow cytometer
Titration of antibodies for CyTOF analysis
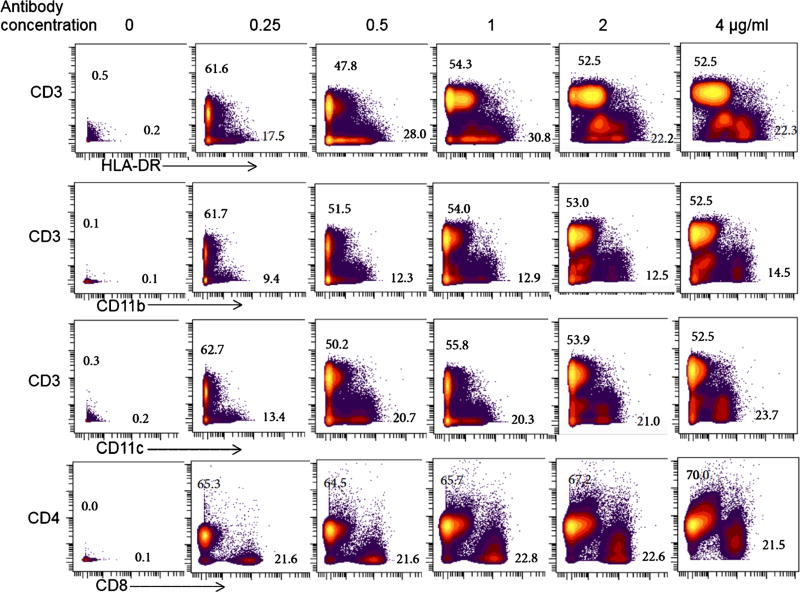
This is done in order to optimize the separation of positive and negative populations. If the antibody conjugate is bright and there are plenty of cells in the target population, this may not be as much of an issue. But for many surface and intracellular markers, the positive peak will not be clearly distinct from the negative peak. Titrating antibodies can significantly improve this. Not all traditional antibodies work well in all fixation realization buffer conditions. It is critical to have multiple populations during titration; the ideal is to find the best separation between known positive and negative reference samples. It is also important to titrate the antibodies in the conditions matching those of the particular study: fresh, frozen, or fixed. By cleaning data and gating out the dead cells, debris, and doublets, the populations look well defined and easy to identify. Figure 5 is freshly isolated PBMCs from peripheral blood which were labelled with different concentrations of CyTOF antibodies against 40 different markers. The master mix of the antibody cocktail started at 4 µg/ml of each antibody. The PBMC sample containing 12 × 106 cells was split equally in 6 wells of a 96-well titer plate. Each well received a dilution of the antibody cocktail (4, 2, 1, 0.5, 0.25 µg/ml) or no antibodies (0); the staining volume was kept constant (100 µl). After 20 min the cells were washed, fixed and labelled with 191 and 193 Ir for DNA, 1.0 µl of 125 µM Ir-intercalator overnight at 4 °C. The samples were acquired on a CyTOF and analyzed using Cytobank software. The results shown in the figure were obtained after first gating on singlets, then live CD45 positive cells, and finally plotting some of the PBMC population markers using two-dimensional density plots. The best resolutions of population clusters and signals were generally seen with antibody concentrations of 1 µg/ml or lower. At high antibody concentrations, the signals from different channels showed more noise and spilled into other channels, generating false positive and double (multiple) stained populations.
Fig. 5.
Freshly isolated PBMCs from peripheral blood were labelled with different concentrations of antibody cocktail (4, 2, 1, 0.5, 0.25 µg/ml) or no antibodies (0) against 40 different markers
Automated sample preparation
Sample preparation is an important and critical step in the entire workflow [14, 18] and represents the largest bottleneck in the modern research lab. Most sample preparation techniques for CyTOF are still non-automated and require substantial labor-intensive and time-consuming operations, which leads to unwanted levels of variation in bioanalytical measurements. Because of the effect of sample preparation on analysis time and error generation, automation of the procedure can minimize the time, labor, and error-producing aspects of a typical bioanalytical method. Automated sample preparation methods consistently, and rapidly, process all samples in the same way. Our robotic platform uses automated methods which have been validated on the Biomek FXp [19–21] and is capable of batch processing a range of between 24 and 96 coded samples. By combining barcoding and this higher level of throughput, standardization, accuracy, precision, flexibility, and operational logistics are significantly improved.
Important considerations for sample preparation
If particular attention is not paid to all of sources of error such as sample processing, contamination, sample stability, instrument stability, storage conditions, and correct data processing, the sample integrity may be sacrificed and the analysis data affected, compromised, or rendered invalid [14, 18]. Sample preparation and the number of cells per sample are critical factors in determining cell recovery from shipped samples. Recovery rates for most of the current protocols are limited to 50% of cells; much of this is due to the number of washing steps required [22]. Cell loss is greater if cells are washed with deionized water prior to shipment [16]. Cell loss is greater when shipping lower numbers of cells. Strategies for shipping staining samples: wash and fix cells in PBS buffer prior to shipping; ship cells in bulk and prepare serial dilutions at analysis site if possible; ensure that samples remain cold during shipping. The automated robotic platform is essential for sample preparation and provides more reliable results [23].
Mass cytometry signal drift during the analysis
Suppression ionization, resulting from easily ionized elements in the reagent/sample mix, is a significant source of interference; it can harm both sensitivity and reproducibility of quantitative data. Signal drift could result from the following:
Matrix effects attributed to the space charge effect in the ion beam in the ICP-MS.
Matrix effects originating in the plasma where the easily ionized matrix elements increase the electron density in the central channel of the plasma, thus decreasing the analyte ion signal.
Sample settling during long run, gently vortex every 10 min
Stability in water, avoid leaving the sample in water for more than 1 h
Space charge effects exist in an ion beam with excess positive charge, where the charges on the ions repel each other to form space-charge-limited flow. There are three important effects caused by the space charge force in such an ion beam: depression of the potential in the beam; beam spreading; and limitation to the maximum current. Heavier elements with higher kinetic energies have higher transmission through the ion optics under the space-charge-limited flow, while lighter elements with lower kinetic energies are more susceptible to space charge repulsion and thus will deviate from the center of the ion beam. Hence, light ions are transmitted less efficiently than heavy ions.
Matrix effects are characterized as a function of analyte ion mass, matrix ion mass, matrix concentration, lens voltages, and nebulizer gas flow rate and can be divided into two types: signal drift due to the deposition of solids on the sampling apertures and/or signal suppression or enhancement by the presence of the dissolved salts. Polyatomic interferences result from the combination of two or more isotopes from different elements, which usually occur in the plasma. The elements that form the polyatomic interferences usually result from the sample matrix, sample diluent, and argon itself. The dissolved salts, especially refractory oxides, tend to deposit on the cool tip of the sampling cone. The clogging of the orifices reduces the ion flow into the ICP-MS, lowers the pressure in the first stage of ICP-MS, and enhances the level of metal oxide ions. Because the extent of the clogging increases over time, the signal drifts down. Perhaps the most satisfactory method to eliminate matrix effects is to remove the matrix elements altogether. Suggestions for minimizing matrix interferences in ICP-MS due to high concentrations of concomitant elements present in the solutions are as follows:
The degree of the matrix effect is strongly dependent on the nebulizer flow-rate and tends to be less severe at low nebulizer flow-rates than at high nebulizer flow-rates.
Minimize acid interferences for elements of interest
Internal calibration is also included to correct for instrumental drift, using multiple internal standard elements so that an internal standard could be used to correct for matrix effects on signals from analyte ions with similar masses. This correction works best when a known amount of the internal standard is added to the sample, and if the element being added is near the same mass as the analyte element.
Use the standard additions method for analyte element to correct for sensitivity interferences.
Use the isotope dilution method to avoid all sensitivity interferences and sample recovery issues during processing. Dilution is necessary for ICP-MS analysis of biological samples because large amounts of proteins and salts can cause an irreversible reduction of the analyte signal intensity due to clogging of the nebulizer, torch, sampling, and skimmer orifices. Besides preventing clogging and reducing the matrix effect, dilution is also necessary to improve the accuracy of internal calibration.
Given that CyTOF has a cell transmission rate of 30% when 0.5 M cells inject at 45 µl per min, a large quantity of cells is required to achieve meaningful data. This can be a severe constraint when dealing with limited clinical samples. This low recovery makes it a challenge to measure rare populations and may produce inaccurate quantitation results for those populations. Also, where flow cytometry can process about 80,000 events/s, CyTOF can process about 1000 events/s. This limited capacity may develop bias when a larger data set is analyzed, due to drift of the signal intensity over time. Also, this bias further constrains the high throughput capability of CyTOF. Cell fixation and permeabilization are required for the present barcoding method; however, some surface markers, such as CCR6, CXCR3, CCR7, CD45RO, CD127, CD11b, and CD56, show poor performance upon barcoding.
Our modified barcoding protocol addresses this by staining the samples for these fixation-sensitive markers prior to starting the barcoding procedure. When possible, use of antibodies that can recognize their epitopes after fixation is another solution. It is important to label some markers (e.g., CXCR3, CCR4, CXCR5, CCR6, CCR7, CD45RO, CD127) before barcoding, so as to avoid potential damage during preparation.
Washing steps are important to minimize cross talk between barcoding and to remove the potential interfering elements. Once barcoded, the samples must be thoroughly mixed; it is advised to gently vortex the sample or pipette up and down several times to ensure complete mixing. Incomplete mixing is manifested by populations that have irregular shapes and broader distributions. Both yield and separation are improved by gating out debris and doublets according to DNA intercalator staining prior to application of the debarcoding software.
Conclusions
In this article, we have compared flow cytometry and CyTOF with reference to a wide variety of parameters, with an eye toward their relative strengths and weaknesses. We address points to be considered during sample preparation for barcoding that allow for a detailed examination of the variability of quantitative mass cytometry measurements. Also, several considerations such as cisplatin concentration, sample stability, and storage were presented to optimize our sample preparation protocol for barcoding CyTOF in support of clinical research. Manual sample preparation can be inconsistent and time-consuming. On the other hand, automated sample preparation for barcoding has been very useful in that the steps are performed the same way every time. Multiplexing large numbers of samples produces consistent results in terms of standardization, accuracy, and operational logistics by eliminating variations such as sample-specific staining and in data collection; additionally, there is a significant reduction in waste created by preparation errors.
Acknowledgments
This work is supported in part by a grant from Centers for Disease Control and Prevention/The National Institute for Occupational Safety and Health (OH110941). The authors thank the members of the Yale CyTOF user’s group for helpful discussions.
Abbreviations
- CyTOF
Cytometry by Time-Of-Flight
- FB
Fixation buffer
- ICP-MS
Inductively coupled plasma-mass spectrometry
- MCB
Mass-tag cellular barcoding
- PBMC
Peripheral blood mononuclear cell
- PMA
Phorbol 12-myristate 13-acetate, also known as 12-O-tetradecanoylphorbol-13-acetate (TPA)
- SB
Maxpar Cell Staining Buffer
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Spitzer MH, Nolan GP. Mass cytometry: single cells, many features. Cell. 2016;165(4):780–91. doi: 10.1016/j.cell.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendall SC, Simonds EF, Qiu P, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332(6030):687–96. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao Y, Liu R, Shin MS, Trentalange M, Allore H, Nassar A, et al. CyTOF supports efficient detection of immune cell subsets from small samples. J Immunol Methods. 2014;415:1–5. doi: 10.1016/j.jim.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandura DR, Baranov VI, Ornatsky OI, et al. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal Chem. 2009;81(16):6813–22. doi: 10.1021/ac901049w. [DOI] [PubMed] [Google Scholar]
- 5.Cheung RK, Utz PJ. Screening: CyTOF-the next generation of cell detection. Nat Rev Rheumatol. 2011;7(9):502–3. doi: 10.1038/nrrheum.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosma A, Le Grand R. Brief introduction to mass cytometry. Med Sci (Paris) 2011;27(12):1072–4. doi: 10.1051/medsci/20112712012. [DOI] [PubMed] [Google Scholar]
- 7.Doerr A. A flow cytometry revolution. Nat Methods. 2011;8(7):531. doi: 10.1038/nmeth0711-531. [DOI] [PubMed] [Google Scholar]
- 8.Giesen C, Wang HA, Schapiro D, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods. 2014;4:417–22. doi: 10.1038/nmeth.2869. [DOI] [PubMed] [Google Scholar]
- 9.Saadatpour A, Guo G, Orkin SH, et al. Characterizing heterogeneity in leukemic cells using single-cell gene expression analysis. Genome Biol. 2014;15(12):525. doi: 10.1186/s13059-014-0525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piccirillo S, Colman S, Potter NE, et al. Genetic and functional diversity of propagating cells in glioblastoma. Stem Cell Reports. 2015;13;4(1):7–15. doi: 10.1016/j.stemcr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu J, Stone NR, Liu L, et al. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Rep. 2013;1(3):235–47. doi: 10.1016/j.stemcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Victor M, Richner M, Hermanstyne TO, et al. Generation of human striatal neurons by microRNA-dependent direct conversion of fibroblasts. Neuron. 2014;84(2):311–23. doi: 10.1016/j.neuron.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saliba AE, Westermann AJ, Gorski SA, et al. Single-cell RNA-seq: advances and future challenges. Nucleic Acids Res. 2014;42(14):8–845. 60. doi: 10.1093/nar/gku555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leipold MD, Maecker HT. Mass cytometry: protocol for daily tuning and running cell samples on a CyTOF mass cytometer. J Vis Exp. 2012;2(69) doi: 10.3791/4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nassar AF, Ogura H, Wisnewski AV. Impact of recent innovations in the use of mass cytometry in support of drug development. Drug Discov Today. 2015;20(10):1169–75. doi: 10.1016/j.drudis.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nassar AE, Carter B, Lannigan J, et al. The first multi-center comparative study using a novel technology mass cytometry Time-of-Flight mass spectrometer (CyTOF) for high-speed acquisition of highly multi-parametric single cell data: a status report; CYTO 2015 30th Congress of the International Society for Advancement of Cytometry Scottish Exhibition and Conference Centre; Glasgow, Scotland. 2015. [Google Scholar]
- 17.Van der Maaten L, Hinton G. Visualizing data using t-SNE. J Mach Learn Res. 2008;9:2579–605. [Google Scholar]
- 18.Kleinsteuber K, Corleis B, Rashidi N, Nchinda N, Lisanti A, Cho JL, et al. Standardization and quality control for high-dimensional mass cytometry studies of human samples. Cytometry A. 2016;89(10):903–13. doi: 10.1002/cyto.a.22935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian F, Goel G, Meng H, Wang X, You F. Systems immunology reveals markers of susceptibility to West Nile virus infection. Clin Vaccine Immunol. 2015;22(1):6–16. doi: 10.1128/CVI.00508-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohanty S, Joshi SR, Ueda I, Wilson J, Blevins TP. Prolonged proinflammatory cytokine production in monocytes modulated by interleukin 10 after influenza vaccination in older adults. J Infect Dis. 2015;1;211(7):1174–84. doi: 10.1093/infdis/jiu573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindow JC, Wunder EA, Jr, Popper SJ, Min JN, et al. Cathelicidin insufficiency in patients with fatal leptospirosis. PLoS Pathog. 2016;12(11) doi: 10.1371/journal.ppat.1005943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nassar AF, Wisnewski AV, Raddassi K. Mass cytometry moving forward in support of clinical research: advantages and considerations. Bioanalysis. 2016;8(4):255–7. doi: 10.4155/bio.15.257. [DOI] [PubMed] [Google Scholar]
- 23.Nassar AF, Wisnewski AV, Raddassi K. Progress in automation of mass cytometry barcoding for drug development. Bioanalysis. 2016;8(14):1429–35. doi: 10.4155/bio-2016-0135. [DOI] [PubMed] [Google Scholar]