Abstract
The World Health Organization (WHO) has estimated that there are about 8 million new cases annually of active Tuberculosis (TB). Despite its irregular effectiveness (0–89%), the Bacillus Calmette-Guérin) BCG is the only vaccine available worldwide for prevention of TB; thus, the design is important of novel and more efficient vaccination strategies. Considering that β-defensin-2 is an antimicrobial peptide that induces dendritic cell maturation through the TLR-4 receptor and that both ESAT-6 and Ag85B are immunodominant mycobacterial antigens and efficient activators of the protective immune response, we constructed two DNA vaccines by the fusion of the gene encoding β-defensin-2 and antigens ESAT6 (pDE) and 85B (pDA). After confirming efficient local antigen expression that induced high and stable Interferon gamma (IFN-γ) production in intramuscular (i.m.) vaccinated Balb/c mice, groups of mice were vaccinated with DNA vaccines in a prime-boost regimen with BCG and with BCG alone, and 2 months later were challenged with the mild virulence reference strain H37Rv and the highly virulent clinical isolate LAM 5186. The level of protection was evaluated by survival, lung bacilli burdens, and extension of tissue damage (pneumonia). Vaccination with both DNA vaccines showed similar protection to that of BCG. After the challenge with the highly virulent Mycobacterium tuberculosis strain, animals that were prime-boosted with BCG and then boosted with both DNA vaccines showed significant higher survival and less tissue damage than mice vaccinated only with BCG. These results suggest that improvement of BCG vaccination, such as the prime-boost DNA vaccine, represents a more efficient vaccination scheme against TB.
Keywords: Tuberculosis, DNA vaccine, BCG, Defensins, Ag85B, ESAT6
1. Introduction
Tuberculosis (TB) is an infection that produces 8.8 million new cases of active disease worldwide, 1.4 million deaths annually [1], and is considered a global emergency due to the increased appearance of new highly virulent [2] and drug-resistant strains [3,4]. Therefore, it is urgent to create new vaccines and/or vaccination schemes that can generate an efficient prophylactic effect or that can boost protective immunity in Bacillus Calmette-Guérin (BCG)-vaccinated individuals.
Mycobacterium bovis BCG, a live attenuated mycobacterial strain, is the sole vaccine available against TB to date. It has been used for nearly 100 years and its protection is extremely variable, from 0–89% [5–7]. Variability in BCG efficacy is associated with multiple factors [8–10]. Different approaches have been proposed to generate new and more effective vaccines, such as Mycobacterium tuberculosis (Mtb) mutants [11,12], recombinant BCG, which expresses highly immunogenic antigens [13,14], subunit vaccines based on the majority of immunogenic Mtb antigens [15,16], and DNA vaccines [17,18]. However, limited success has been achieved in this matter.
Recently, we have been working on the role that some antimicrobial peptides play in the innate immune response and activation of the immune responses acquired in experimental TB. This is the specific case of β-defensin-2, an antimicrobial peptide that induces dendritic cell maturation in a Toll-like (TLR) receptor 4-dependent manner [19–21]. Interestingly, genetic construct coding sequences of β-defensin-2 generate a polarized and antigen-specific Th1 immune response [22–24]. This is important because multiple reports indicate that a Th1/CD8+ cytotoxic cellular immune response is essential for Mtb growth control [25]. Several Mtb antigens induce a strong Th1 response, such as the Early Secretory Antigenic Target-6 kDa (ESAT-6) protein, which is a potent immunogen encoded by the RD1 gene complex of Mtb [26] that is absent in BCG [27]. Similarly, Ag85B is a protein related with the mycolyl transferase secreted by Mtb and is a highly immunogenic antigen that induces a cytotoxic immune response [28–31]. Thus, in this work we designed DNA vaccines based on β-defensin-2 fused with ESAT6 or Ag85B, which actually induce a polarized Th1 immune response, and their efficiency was evaluated in a murine model of pulmonary TB challenged with Mtb strains, which possess diverse virulence levels.
2. Materials and methods
2.1. Gene cloning, fusion, and plasmid constructions
DNA constructs were made by amplification and cloning of the gene-of-interest; the gene for mature murine β-defensin-2 (mBD2) was cloned from mouse skin treated with LPS (10 ng/ml) by RT-PCR from total RNA utilizing specific primers: β-defensin-2 (Defb2) F-5′-ACCATGGAACTTGACCACTGCCACACC-3′, R-5′-TGAATTCAAGATCTTTCATGTACTTG CAACAGGGGTTGTT, ESAT6 (esxA) F-5′-TATCTCGAGACCACC-3′, R-5′-CACCACCATCACCATCACTAAGGATCCCGG GTAA-3′, Ag85B (fbpB) F-5′-ATGGATCCTATGTCGACCACATGACAGACGT GAGCCGAAAGATT-3′, R-5′-ATCCCGGGAAGGGTCCTTAGTGATGGTGATG GTGGTGGCCGGCGCCTAACGAACTCTGCA-3′, and GAPDH F-5′-CTGGTGCTGAGTATGTCGTG-3′ R-5′-CAGTCTTCTGAGTGGCAGTG-3′. Amplification of the esxA and fbpB genes was performed from genomic DNA of Mtb H37Rv strain, isolated as reported elsewhere [32]; these sequences encode for ESAT6 or Ag85B antigens, respectively. The DNA constructs are based on a pCMV vector; the specific characteristics of the construct were reported previously by our group [23,33]. The following four constructs were generated: pCMV-mDF2B-esxA (pDE); pCMV-mDF2B-fbpB (pDA); pCMV-esxA (pE); pCMV-fbpB (pA), and the empty pCMV vector that was used as control.
The constructs were analyzed by PCR, enzyme digestion, and sequencing in order to confirm insertion and open reading frame (ORF). XL10 Gold bacteria (Invitrogen, Carlsbad, CA, USA) were transformed with each construct and grown in LB broth base medium (Invitrogen). Plasmid purification was performed with the Endofree Plasmid Maxi kit as referred by the supplier (QIAGEN, Hilden, Germany). The plasmids were eluted in sterile pyrogen-free phosphate buffer (SIGMA, Steinheim, Germany).
2.2. Vaccination
All animal studies were approved by the Institutional Ethics Committee in accordance with the guidelines of the Mexican National Regulations on Animal Care and Experimentation NOM 062-ZOO-1999. BALB/c mice aged between 6 and 8 weeks of age were anesthetized with sevoflurane (Abbott, Quebec, Canada). Then, the respective plasmid was administered intramuscularly (i.m.) in the right thigh, using 100 μl of sterile PBS as vehicle. In order to increase the efficiency of the DNA vaccination, an electric shock was applied at the injection site with the CYTOPULSE pulseAgile® Model PA-3000 electroporator system, according to the manufacturer’s suggestions. The amount of DNA vaccine to be employed was determined with a dose-response curve. Best expression profile was observed at 50 μg for DNA vaccine alone and for co-administration, this was 25 μg DNA of each vaccine. A second dose of the DNA vaccine was applied 8 days after the first vaccination as a boost. In the case of BCG vaccination, 8 × 103 viable bacterial cells BCG substrain Phipps were injected subcutaneously (s.c.) in the base of the tail. This BCG substrain was the most protective of 10 strains tested in the BALB/c mouse model of progressive pulmonary tuberculosis [34]. The immunization schedule is depicted in detail in Fig. 1.
Fig. 1.
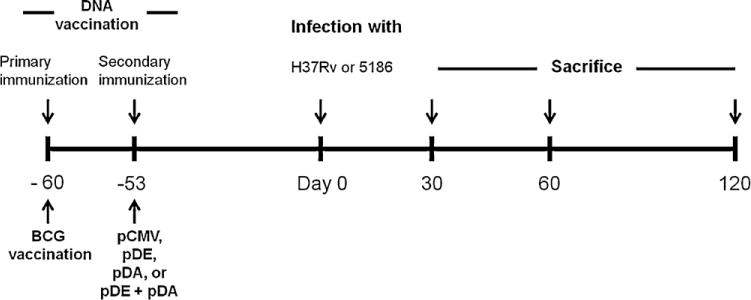
Timeline of the animal vaccination, infection and sacrifice.
Groups of at least 6 animals were immunized by administration of 50 μg of DNA vaccine in thigh muscles. Electroporation was applied at the vaccination site. Bacillus Calmette-Guérin (BCG) vaccination was performed with 8 × 103 viable bacilli at day 60 prior to infection as shown in the time-line graph. Sixty days after immunization with DNA vaccine, vehicle, BCG, or a combination of any of the latter, the mice were infected with either of the two Mtb strains: H37Rv, and LAM 5186. Infected mice were sacrificed at different time points after Mtb infection, depending on the virulence of each strain. This strategy was used for all of the experiments. At least three independent experiments were conducted.
2.3. RT-PCR for mRNA expression assessment of DNA constructs
One, 3, 8, and 14 days post-vaccination, the animals were euthanized and thigh muscles were immediately excised; one fragment was fixed by immersion in 10% formaldehyde dissolved in PBS for immunohistochemistry, and the remaining tissue fragment was preserved in TRIzol (Invitrogen) for gene analysis. For nucleic acid purification, tissue was homogenated in Ultra-Turrax® T-10 apparatus (IKA, Wilmington, NC, USA). The RNAeasy mini kit with DNAase (QIAGEN, Düsseldorf, Germany) was employed for RNA isolation according to the manufacturer’s instructions. One hundred nanograms of purified RNA was used for cDNA synthesis utilizing the Omniscript cDNA synthesis kit (QIAGEN) and submitted to PCR.
2.4. Immunohistochemistry
For immunohistochemistry, 5-μm-thick sections were deparaffinized and the endogenous peroxidase quenched with 0.03% H2O2. Then, the sections were blocked with PBS supplemented with 2% human pool serum. Muscle sections were incubated with rabbit polyclonal anti ESAT6 and rabbit polyclonal anti Ag85B (Abcam, Cambridge, U.K.) and subsequently incubated with a biotin-labeled anti rabbit IgG antibody. Bound antibodies were detected with avidin–biotin peroxidase (Biocare Medical, Concord, CA, USA) and counterstained with hematoxylin.
2.5. Immunogenicity of DNA vaccines
Groups of six BALB/c mice were vaccinated with either pDE, pDA, pE, pA, pCMV, or PBS and sacrificed at 14, 21, and 40 days post-vaccination. Cell suspensions from inguinal lymph nodes, spleen, and lungs were cultured and stimulated with mycobacterial Culture filtrate antigens (CFA), rESAT-6, or rAg85B, as previously reported [35]. Cultures for cytokine production (1 × 105 cells in 100 μl of culture medium) were performed in flat-bottom, 96-well plates with 5 μg CFA, rESAT6, or rAg85B. After 72 h, the supernatants were collected and utilized for Interferon gamma (IFN-γ) quantification by means of a commercial Enzyme-linked immunosorbent assay (ELISA) test kit (Pharmingen, San Diego, CA, USA).
2.6. Experimental model of progressive pulmonary TB
The experimental model of progressive pulmonary TB has been previously described in detail [14]. Groups of vaccinated mice were challenged 60 days after the first immunization with M. tuberculosis strains H37Rv or with the Latin-American Mediterranean (LAM) clinical isolate (5186 strain), which is highly virulent in this mouse model [2,36]. Each animal was anesthetized with sevoflurane and was intratracheally (i.t.) instillated with 2.5 × 105 viable bacterial cells suspended in 100 μl of sterile, pyrogen-free PBS. To determine vaccination effectiveness, groups of six mice in two independent experiments were euthanized after 30, 60, or 120 days of challenge; their lungs were employed to determine bacilli burdens by colony forming unit (CFU) quantification and histology/morphometry, thus determining the percentage of lung surface affected by pneumonia. Infected mice were sacrificed at different time points after Mtb infection, depending on the virulence of each strain. Another group of 10 mice was left untouched and mortality was recorded to construct survival curves.
2.7. Determination of colony-forming units and histopathological analysis of infected lungs
The right lungs of six mice at each time point in at least two independent experiments were utilized for CFU quantifications. Briefly, the lungs were homogenized with a polytron (Kinematica, Lucerne, Switzerland) in sterile 50-ml tubes containing 3 ml of isotonic saline. Four dilutions of each homogenate were spread onto duplicates containing Bacto Middlebrook 7H10 agar plates enriched with OADC. The plates were incubated at 37°C and the CFU were counted at day 21.
For histopathological analysis, lungs were perfused i.t. with absolute ethanol, immersed for 24 h, and embedded in paraffin. Five-μm-thick sections taken through the hilus were stained with H&E. In these slides, the area (μm2) occupied by the inflammatory infiltrate was determined using an image analyzer (Axiovert 200 M with Axiovision ver.4.3; Carl Zeiss, Jena, Germany).
2.8. Statistical analysis
Data normality was assessed through the Kolmogorov-Smirnov test. Normal distribution data were analyzed with one-way Analysis of variance (ANOVA) and Bonferroni’s post-test. For CFU nonparametric data, a Kruskal–Wallis multiple comparisons test was employed with Dunn’s post-test. Immunogenicity assays based in gamma interferon production data were analyzed with two-way ANOVA using Bonferroni’s post-test. Statistical analysis of Kaplan–Meier survival curves was performed using Log-rank test. P values of ≤0.05 were considered significant.
3. Results
3.1. DNA vaccine constructs are expressed and translated in the muscle cells of mice
In order to determine whether muscle cells were efficiently transfected by DNA vaccine electroporation, the expression in situ of mRNA and antigen protein were evaluated. The kinetics for ESAT6 or Ag85B mRNA are depicted in Fig. 2A and C, respectively. The expression of both constructs is stronger at day 1, exhibiting a progressive decrease until day 14, when lowest expression was observed. Similar protein expression kinetics determined by immunohistochemistry is illustrated in Fig. 2B and D; both proteins were specifically detected in the cytoplasm of striated muscle cells located at the vaccination site.
Fig. 2.
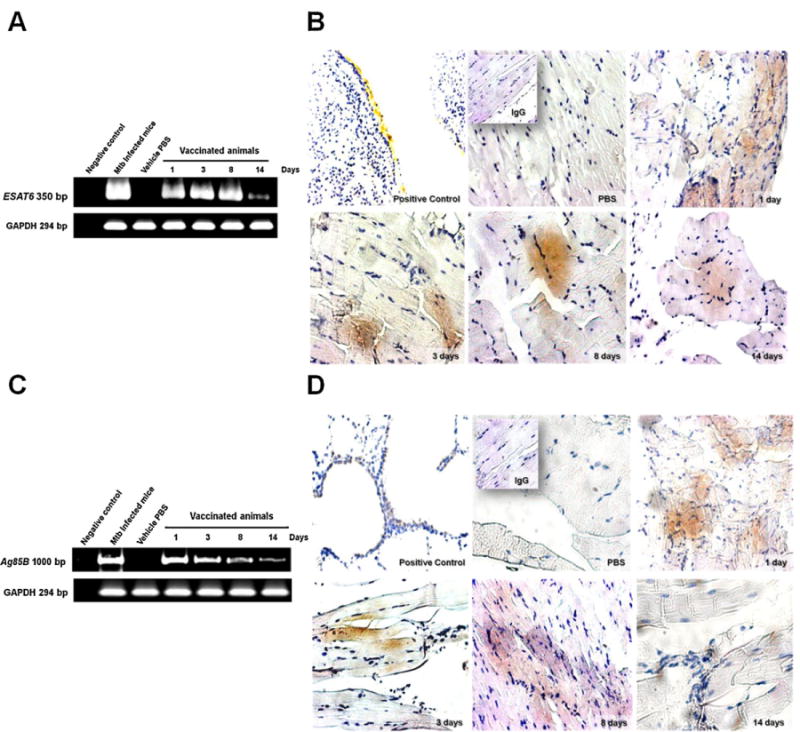
Kinetics of ESAT6 and Ag85B mRNA and protein expression in the muscle of immunized mice.
Two groups of six BALB/c mice per group were immunized with either pDE or pDA and sacrificed at 1, 3, 8, and 14 days. (A) RT-PCR for ESAT6 mRNA expression from thigh muscle samples extracted from vaccinated mice. GAPDH mRNA expression by RT-PCR was used for determination of basal expression; (B) Micrographs showing antigenic protein expression and immunohistochemistry for ESAT6 at 20× magnification. Positive control was from mice infected with H37Rv strain at a 10× magnification. Similarly, panels depict protein expression at 1, 3, 8, and 14 days after vaccination; (C) RT-PCR for expression of Ag85B mRNA extracted from the muscle of immunized mice. GAPDH mRNA expression by RT-PCR was utilized for determination of basal expression; (D) Ag85B antigenic protein expression analyzed by immunohistochemistry at 20× magnification. Positive control from mice infected with H37Rv at 10× magnification. Protein expression at 1, 3, 8, and 14 days after vaccination is illustrated. Representative results from three independent experiments are shown.
3.2. pDA and pDE dna vaccines induce a specific Th1 immune response against mycobacterial antigens
Subsequently, we tested the immunogenicity of vaccine constructs. Mice were DNA-immunized with pE, pA, pDE, pDA, and pDE + pDA constructs by assessing IFN-γ production from T-cells 14, 21, and 40 days after immunization, measuring cytokine levels in the supernatants of cell suspensions from inguinal lymph nodes, spleen, and lung after stimulation with rESAT6, rAg85B, or CFA. IFN-γ production from inguinal lymph node cells from animals vaccinated with either or both pDE and pDA DNA vaccines showed that there is a specific response from these cultured cells to these mycobacterial antigens. Significant differences are demonstrated in animals vaccinated with pCMV, pE, and pA compared with those vaccinated with pDE and pDA (p < 0.0001), confirming the efficient role of mBD2 as an adjuvant inducing a stronger immune response. Similar levels of IFN-γ were detected in the different organs along the experiment, indicating that there is a sustained effect on the production and elicitation of this cytokine (Fig. 3A–C).
Fig. 3.
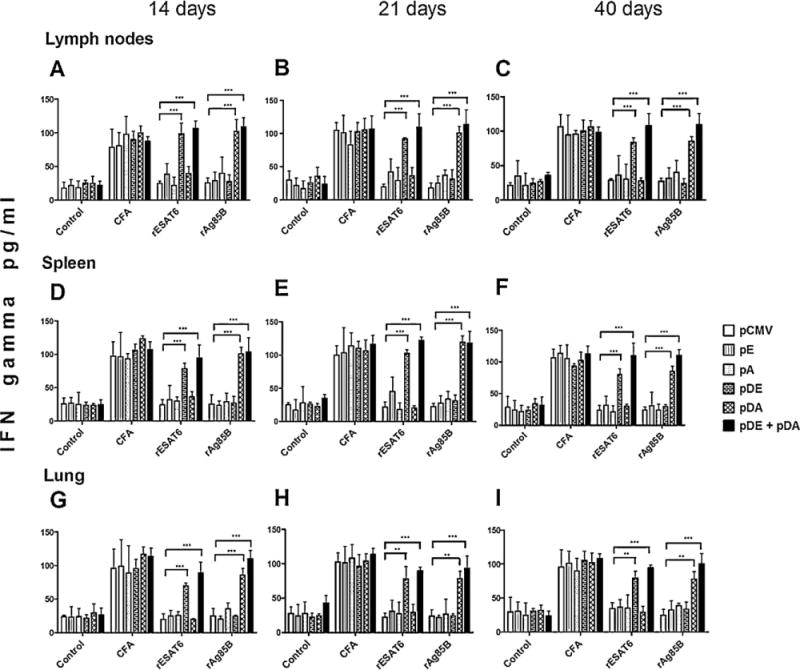
Interferon gamma (IFN-γ) quantification in culture supernatants of mononuclear cells from immunized mice stimulated with specific mycobacterial antigens.
Mice were immunized with pE, pA, pDE, pDA, or pCMV. Mononuclear cells extracted from immunized mice were cultured in 96-well plates and exposed to antigens such as culture filtrate antigen (CFA), rESAT6, or rAg85B proteins for 72 h. The cell supernatants were employed to determine IFN-γ production by ELISA. Cells were isolated from the inguinal lymph node at 14, 21, and 40 days post-vaccination (A, B, and C, respectively); spleen cells were isolated at days 14, 21, and 40 days post-vaccination (D, E, and F, respectively). The same procedure was performed for cells isolated from the lungs of vaccinated mice (G, H, and I). Each group of animals consisted of 6 mice in two independent experiments. Two-way ANOVA and Bonferroni’s post-test was performed in order to identify differences among groups; p values of <0.05 are considered statistically significant.
3.3. Protective effect of DNA vaccines
After confirming the immunogenicity efficiency of our DNA vaccines, pE and pA vaccines were not employed for further experiments because they had non-significant differences with the control (pCMV). Animals were vaccinated and challenged (Fig. 1) with strains of different virulence and genotype levels. Survival, lung bacilli burdens, and extensions of tissue damage (pneumonia) were analyzed at each sacrifice point.
Fig. 4 shows the survival curves: control animals that received only the empty vector and that were challenged with the H37Rv reference strain began to die after 2 months and 85% survived to the end of the experiment. Animals vaccinated with pDE exhibited similar responses to pCMV, while mice vaccinated with pDA or both pDE + pDA exhibited better survival (90%) than those of the control group. Mice vaccinated with BCG and boosted with both DNA vaccines showed 100% survival after 4 months of challenge and there was a statistical difference (p < 0.04) when these were compared with pCMV-vaccinated mice (Fig. 4A). Similar results were observed when mice were challenged with the highly virulent LAM 5186 strain; pCMV-vaccinated mice died after 30 days of i.t. infection, and similar survival trends were observed in mice vaccinated with pDE. The animals vaccinated with pDA, BCG, or both pDE + pDA died at 40 ± 5 days post-challenge. In contrast, significant survival was observed in animals vaccinated first with BCG and boosted with pDA and pDE, which demonstrated two-fold survival (80 days; p < 0.0001) compared with BCG alone.
Fig. 4.
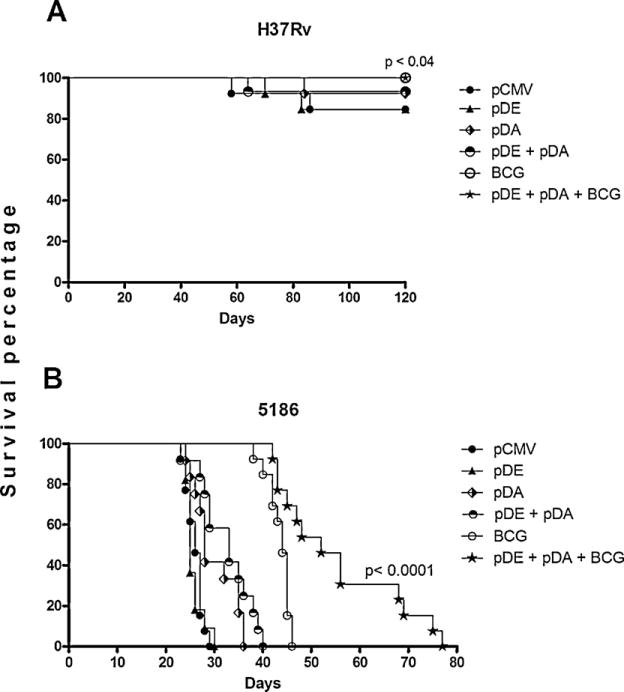
Survival rate of vaccinated animals challenged with different Mtb strains.
Several groups of animals were vaccinated with DNA constructs, controls, and BCG. The immunized animals were challenged with the H37Rv strain (A), and the 5186 strain (B). Death of animals was recorded daily. Twenty animals were included in each group in two independent experiments. Kaplan–Meier survival curves were performed, in addition to statistical analysis using the log-rank test; a p value of <0.05 was considered significant.
Survival curves were consistent with lung bacilli burdens, because mice immunized with any vaccine type and challenged with strain H37Rv showed significantly lower bacilli loads than the control group at both time points, with the lowest in the prime-boosted group (Fig. 5A and B).
Fig. 5.
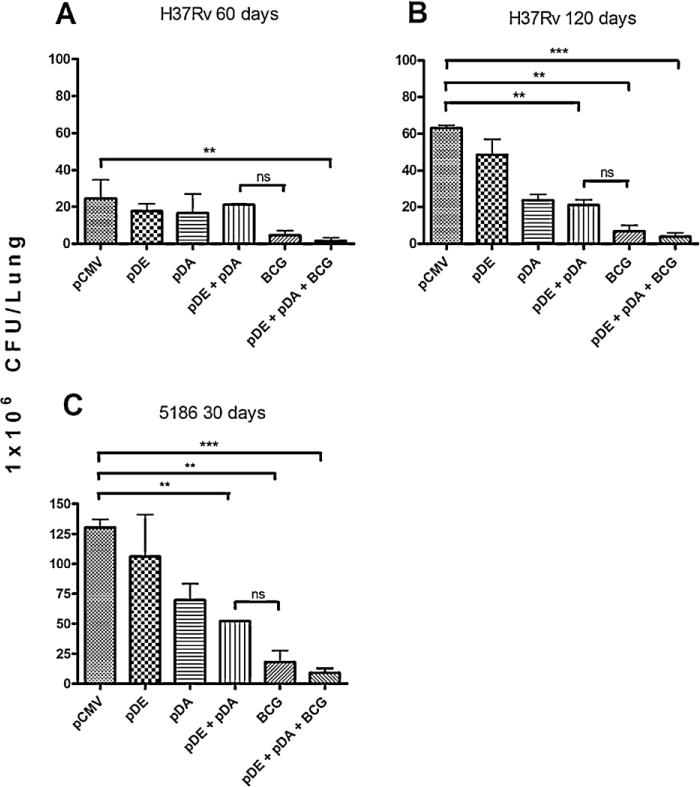
Determination of Colony-forming units (CFU) in mice immunized and challenged with two different Mycobacterium tuberculosis strains.
Groups of mice were immunized with DNA vaccines, BCG, and controls according to the schematic description in Fig. 1. Then, animals were challenged with several Mtb strains and CFU determination was performed in the lungs of infected animals at different time points. (A) Animals were challenged with the H37Rv strain and sacrificed at days 60 and (B) 120 post-infection; (C) Mice were immunized and later challenged with the 5186 strain and sacrificed at 30 days post-infection. Groups of six mice per group were sacrificed at the specified times, and the experiments were repeated three times independently. One-way ANOVA with Bonferroni’s multiple comparison test was performed to establish statistical significance. P values of <0.05 were considered statistically significant.
Similar results were observed in vaccinated animals challenged with the highly virulent LAM 5186 strain. The group of animals vaccinated with pDA + pDE showed higher, but non-significant, lung bacilli loads than the BCG-vaccinated group, while this latter group showed higher, but non-significant, bacilli burdens than the group previously vaccinated with BCG and boosted with both DNA vaccines (Fig. 5C).
All groups showed a considerable reduction in pneumonic area when compared with control mice, this more evident at day 120. Similar protection was observed between the groups vaccinated with both DNA vaccines and the BCG-vaccinated group, while this latter group showed a significant, five-fold higher pneumonic area than the group previously vaccinated with BCG and boosted with both DNA vaccines (Fig. 6A and B). Comparable results were observed in vaccinated mice challenged with the highly virulent strain (Fig. 6C). Thus, vaccination with both DNA vaccines and prime-boosted with BCG confers greater significant tissue damage protection than the BCG vaccination alone.
Fig. 6.
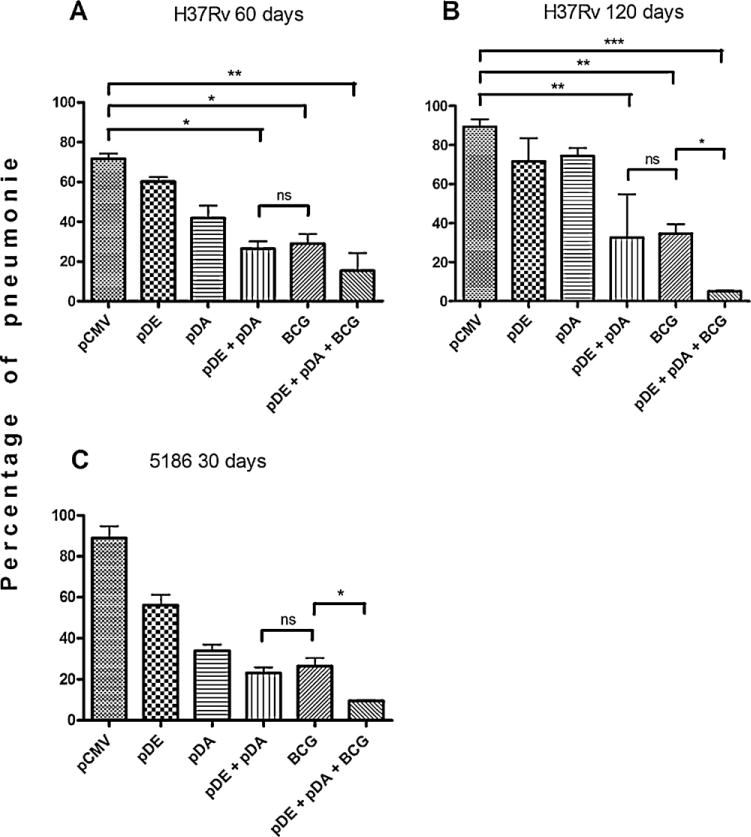
Pneumonic area in mice immunized and challenged with Mtb strains.
Mice were immunized with pDE, pDA, or pCMV DNA vaccines, employing the vehicle as control. Sixty days after vaccination, the animals were infected with either the H37Rv or the 5186 strain. Animals were then sacrificed and the left lungs were ethanol-fixated and paraffin-embedded. After Hematoxylin–eosin (H&E) staining, the pneumonic area was determined in imaging analysis software. (A) Shows pneumonic area analysis of mice challenged with the H37Rv strain and sacrificed at days 60 and (B) 120 post-infection; (C) After immunization, mice were challenged with the 5186 strain and sacrificed at 30 days post-infection. Each group consisted of six mice, and three experiments were conducted independently. One-way ANOVA with Bonferroni’s post-test was performed for assessment of differences. P values of p < 0.05 were considered statistically significant.
4. Discussion
In this work, a new vaccination strategy was used to induce a greater protective response against Mtb based on the use of a DNA vaccine conformed of mBD-2 and the immunodominant mycobacterial antigens ESAT-6 and Ag85B. Our immunogenicity results based on the release of IFN-γ from antigen-specific T-cells derived from inguinal lymph nodes, spleen, and lung confirm this property by demonstrating that in the immediate vicinity of the vaccination site (inguinal lymph node), in the systemic milieu (spleen), and in the lung, both of the pDE or pDA constructs induced a better immune response compared with constructs coding for Ag85B (pA) and ESAT6 (pE) antigens alone, indicating that β-defensin-2 increased the specific immune response when employed as an adjuvant in the DNA construct, similarly to data previously reported for other antigen targets, such as the HIV gp120 protein [22]. This effect is probably due to the more efficient maturation and activation of immature dendritic cells (iDC) mediated by mBD-2 in a TLR4-dependent manner [20]. These results are also consistent with published reports in cancer models, in which DNA vaccines expressing β-defensin-2 increased the immune response specifically against strong or poorly immunogenic neoplastic antigens in different cancer types [23,24,37].
It has been demonstrated in experimental models that BCG protection depends on the virulence of the infecting organism [38,39]. In agreement with this, both pneumonia and bacilli burdens determined from pDE- or pDA-vaccinated mice showed differences depending on the Mtb strain utilized for the challenge. These differences could probably also be due to differences in the expression of ESAT6 or Ag85B, considering that strains with higher expression of either ESAT6 or Ag85B antigens would be better recognized by animals vaccinated with pDE and pDA. However, more studies are needed for clarify concerning this issue.
When pDA and pDE were co-administrated, they showed a similar protective effect in animals compared with BCG-vaccinated mice, particularly when the animals were infected with the highly virulent 5186 strain. This is probably due to higher expression of ESAT-6 and Ag85B by this strain and perhaps by the role of both antigens in mycobacterial virulence [40,41]. Moreover, several reports indicate that one participating factor in the variability of the BCG efficacy is probably associated with the low expression and absence of Ag85B and ESAT6, respectively [9,42].
Because the majority of the human population in the developing world is already BCG-vaccinated, an attractive strategy would be to boost this existing immune response. Moreover, children in endemic countries where BCG vaccination is a generalized practice are highly sensitized due to the combination of BCG vaccination, environmental mycobacteria, and latent TB infection. In these settings, BCG revaccination did not increase protection and is generally not recommended [43]. This is in agreement with animal studies showing that revaccination with BCG lowers effectiveness [44] or can produce by Interleukin (IL)-17-mediated necrosis [45]. Thus, BCG, despite its variable effectiveness, can be employed as a priming agent for a booster vaccination scheme [46]. Therefore, we performed the co-administration of BCG plus pDE + pDA in order to complement the antigenic repertoire required to generate a greater immune response. Considering the genetic diversity among the different BCG sub-strains that have showed diverse levels of protection, choice of the BCG strain utilized for vaccination is a very important issue; we used substrain Phipps because it was the most efficient in conferring protection among 10 different BCG strains in our murine model [34]. Interestingly, mice vaccinated with BCG Phipps boosted with both DNA vaccines and challenged with the highly virulent 5186 strain showed significant higher protection than mice with a single vaccination of BCG, suggesting an improvement of the antigenic repertoire by the mBD2 Th-1 polarizing immune response to ESAT6, Ag85B, plus the BCG antigens.
5. Conclusions
Our data suggest that use of DNA vaccines containing coding sequences for β-defensin-2 induces a Th1 adaptive response against highly immunogenic antigens from Mtb, and when these DNA vaccines were used for booster immunization after BCG vaccination, a significant improvement of protection against Mtb strains was produced. This new strategy could greatly improve BCG vaccination efficacy against highly transmittable and virulent strains, such as the LAM 5186 strain, suggesting that improvement of BCG vaccination combined with DNA vaccines in a prime-boost scheme is a good choice for the rational design of a more efficient vaccine against TB.
Acknowledgments
This work was supported by Consejo Nacional de Ciencia y Tecnologia-México (CONACyT-México grant CB-2007-01-82424 to BR-S and 84456 to RH-P.
The authors acknowledge Dr. Felipe Villagrana-Félix for his help in animal anesthesia. ARC-V acknowledges CONACyT-México for scholarship number 203661/CVU 208395.
Footnotes
Conflicts of interest
The authors declare no conflict of interest
References
- 1.World Health Organization. Global tuberculosis control: WHO report 2011. Geneva: World Health Organization; 2011. p. viii, 246. [Google Scholar]
- 2.Marquina-Castillo B, Garcia-Garcia L, Ponce-de-Leon A, Jimenez-Corona ME, Bobadilla-Del VM, Cano-Arellano B, et al. Virulence, immunopathology and transmissibility of selected strains of Mycobacterium tuberculosis in a murine model. Immunology. 2009;128(1):33–123. doi: 10.1111/j.1365-2567.2008.03004.x. Epub 2009/02/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mlambo CK, Warren RM, Poswa X, Victor TC, Duse AG, Marais E. Genotypic diversity of extensively drug-resistant tuberculosis (XDR-TB) in South Africa. Int J Tuberc Lung Dis. 2008;12(1):99–104. Epub 2008/01/05. [PubMed] [Google Scholar]
- 4.Engstrom A, Morcillo N, Imperiale B, Hoffner SE, Jureen P. Detection of first-and second-line drug resistance in Mycobacterium tuberculosis clinical isolates using pyrosequencing. J Clin Microbiol. 2012 doi: 10.1128/JCM.06664-11. Epub 2012/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brewer TF. Preventing tuberculosis with bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin Infect Dis. 2000;31(Suppl. 3):S64–7. doi: 10.1086/314072. Epub 2000/09/30. [DOI] [PubMed] [Google Scholar]
- 6.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346(8986):45–1339. doi: 10.1016/s0140-6736(95)92348-9. Epub 1995/11/18. [DOI] [PubMed] [Google Scholar]
- 7.Aronson NE, Santosham M, Comstock GW, Howard RS, Moulton LH, Rhoades ER, et al. Long-term efficacy of BCG vaccine in american indians and alaska natives: a 60-year follow-up study. JAMA. 2004;291(17):91–2086. doi: 10.1001/jama.291.17.2086. Epub 2004/05/06. [DOI] [PubMed] [Google Scholar]
- 8.Martino A, Sacchi A, Sanarico N, Spadaro F, Ramoni C, Ciaramella A, et al. Dendritic cells derived from BCG-infected precursors induce Th2-like immune response. J Leukoc Biol. 2004;76(4):34–827. doi: 10.1189/jlb.0703313. Epub 2004/07/09. [DOI] [PubMed] [Google Scholar]
- 9.Berredo-Pinho M, Kalume DE, Correa PR, Gomes LH, Pereira MP, da Silva RF, et al. Proteomic profile of culture filtrate from the Brazilian vaccine strain Mycobacterium bovis BCG Moreau compared to M. bovis BCG Pasteur. BMC Microbiol. 2011;11:80. doi: 10.1186/1471-2180-11-80. Epub 2011/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hylkema MN, Timens W, Luinge M, Van Der Werf N, Hoekstra MO. The effect of bacillus Calmette-Guerin immunization depends on the genetic predisposition to Th2-type responsiveness. Am J Respir Cell Mol Biol. 2002;27(2):9–244. doi: 10.1165/ajrcmb.27.2.4735. Epub 2002/08/02. [DOI] [PubMed] [Google Scholar]
- 11.Sambandamurthy VK, Jacobs WR., Jr Live attenuated mutants of Mycobacterium tuberculosis as candidate vaccines against tuberculosis. Microbes Infect. 2005;7(5–6):61–955. doi: 10.1016/j.micinf.2005.04.001. Epub 2005/05/26. [DOI] [PubMed] [Google Scholar]
- 12.Williams A, Hatch GJ, Clark SO, Gooch KE, Hatch KA, Hall GA, et al. Evaluation of vaccines in the EU TB Vaccine Cluster using a guinea pig aerosol infection model of tuberculosis. Tuberculosis (Edinb) 2005;85(1–2):29–38. doi: 10.1016/j.tube.2004.09.009. Epub 2005/02/03. [DOI] [PubMed] [Google Scholar]
- 13.Hsu T, Hingley-Wilson SM, Chen B, Chen M, Dai AZ, Morin PM, et al. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci USA. 2003;100(21):5–12420. doi: 10.1073/pnas.1635213100. Epub 2003/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM, Chen B, et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J Clin Invest. 2007;117(8):88–2279. doi: 10.1172/JCI31947. Epub 2007/08/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pal PG, Horwitz MA. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992;60(11):92–4781. doi: 10.1128/iai.60.11.4781-4792.1992. Epub 1992/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinrich Olsen A, van Pinxteren LA, Meng Okkels L, Birk Rasmussen P, Andersen P. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85b and esat-6. Infect Immun. 2001;69(5):8–2773. doi: 10.1128/IAI.69.5.2773-2778.2001. Epub 2001/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denis O, Tanghe A, Palfliet K, Jurion F, van den Berg TP, Vanonckelen A, et al. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect Immun. 1998;66(4):33–1527. doi: 10.1128/iai.66.4.1527-1533.1998. Epub 1998/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brun P, Zumbo A, Castagliuolo I, Delogu G, Manfrin F, Sali M, et al. Intranasal delivery of DNA encoding antigens of Mycobacterium tuberculosis by nonpathogenic invasive Escherichia coli. Vaccine. 2008;26(16):41–1934. doi: 10.1016/j.vaccine.2008.02.023. Epub 2008/03/18. [DOI] [PubMed] [Google Scholar]
- 19.Torres AM, Kuchel PW. The beta-defensin-fold family of polypeptides. Toxicon. 2004;44(6):8–581. doi: 10.1016/j.toxicon.2004.07.011. Epub 2004/10/27. [DOI] [PubMed] [Google Scholar]
- 20.Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298(5595):9–1025. doi: 10.1126/science.1075565. Epub 2002/11/02. [DOI] [PubMed] [Google Scholar]
- 21.Rivas-Santiago B, Cervantes-Villagrana A, Sada E, Hernandez-Pando R. Expression of Beta defensin 2 in experimental pulmonary tuberculosis: tentative approach for vaccine development. Arch Med Res. 2012 doi: 10.1016/j.arcmed.2012.06.005. Epub 2012/06/19. [DOI] [PubMed] [Google Scholar]
- 22.Biragyn A, Belyakov IM, Chow YH, Dimitrov DS, Berzofsky JA, Kwak LW. DNA vaccines encoding human immunodeficiency virus-1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood. 2002;100(4):9–1153. doi: 10.1182/blood-2002-01-0086. Epub 2002/08/01. [DOI] [PubMed] [Google Scholar]
- 23.Biragyn A, Surenhu M, Yang D, Ruffini PA, Haines BA, Klyushnenkova E, et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167(11):53–6644. doi: 10.4049/jimmunol.167.11.6644. Epub 2001/11/21. [DOI] [PubMed] [Google Scholar]
- 24.Mei HF, Jin XB, Zhu JY, Zeng AH, Wu Q, Lu XM, et al. beta-defensin 2 as an adjuvant promotes anti-melanoma immune responses and inhibits the growth of implanted murine melanoma in vivo. PLoS ONE. 2012;7(2):e31328. doi: 10.1371/journal.pone.0031328. Epub 2012/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delogu G, Fadda G. The quest for a new vaccine against tuberculosis. J Infect Dev Ctries. 2009;3(1):5–15. doi: 10.3855/jidc.99. Epub 2009/09/15. [DOI] [PubMed] [Google Scholar]
- 26.Berthet FX, Rasmussen PB, Rosenkrands I, Andersen P, Gicquel B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10) Microbiology. 1998;144(Pt 11):203–3195. doi: 10.1099/00221287-144-11-3195. Epub 1998/12/10. [DOI] [PubMed] [Google Scholar]
- 27.Gordon SV, Brosch R, Billault A, Garnier T, Eiglmeier K, Cole ST. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol Microbiol. 1999;32(3):55–643. doi: 10.1046/j.1365-2958.1999.01383.x. Epub 1999/05/13. [DOI] [PubMed] [Google Scholar]
- 28.Feng CG, Palendira U, Demangel C, Spratt JM, Malin AS, Britton WJ. Priming by DNA immunization augments protective efficacy of Mycobacterium bovis Bacille Calmette-Guerin against tuberculosis. Infect Immun. 2001;69(6):6–4174. doi: 10.1128/IAI.69.6.4174-4176.2001. Epub 2001/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S. Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci USA. 2000;97(25):8–13853. doi: 10.1073/pnas.250480397. Epub 2000/11/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olsen AW, Williams A, Okkels LM, Hatch G, Andersen P. Protective effect of a tuberculosis subunit vaccine based on a fusion of antigen 85B and ESAT-6 in the aerosol guinea pig model. Infect Immun. 2004;72(10):50–6148. doi: 10.1128/IAI.72.10.6148-6150.2004. Epub 2004/09/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.You Q, Wu Y, Jiang D, Wang C, Wei W, Yu X, et al. Immune responses induced by heterologous boosting of recombinant Bacillus Calmette-Guerin with Ag85B-ESAT6 fusion protein in levamisole-based adjuvant. Immunol Invest. 2012;41(4):28–412. doi: 10.3109/08820139.2012.658940. Epub 2012/03/01. [DOI] [PubMed] [Google Scholar]
- 32.van Soolingen D, Hermans PW, de Haas PE, Soll DR, van Embden JD. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991;29(11):86–2578. doi: 10.1128/jcm.29.11.2578-2586.1991. Epub 1991/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biragyn A, Tani K, Grimm MC, Weeks S, Kwak LW. Genetic fusion of chemokines to a self tumor antigen induces protective T-cell dependent antitumor immunity. Nat Biotechnol. 1999;17(3):8–253. doi: 10.1038/6995. Epub 1999/03/30. [DOI] [PubMed] [Google Scholar]
- 34.Castillo-Rodal AI, Castanon-Arreola M, Hernandez-Pando R, Calva JJ, Sada-Diaz E, Lopez-Vidal Y. Mycobacterium bovis BCG substrains confer different levels of protection against Mycobacterium tuberculosis infection in a BALB/c model of progressive pulmonary tuberculosis. Infect Immun. 2006;74(3):24–1718. doi: 10.1128/IAI.74.3.1718-1724.2006. Epub 2006/02/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aguilar D, Infante E, Martin C, Gormley E, Gicquel B, Hernandez Pando R. Immunological responses and protective immunity against tuberculosis conferred by vaccination of Balb/C mice with the attenuated Mycobacterium tuberculosis (phoP) SO2 strain. Clin Exp Immunol. 2007;147(2):8–330. doi: 10.1111/j.1365-2249.2006.03284.x. Epub 2007/01/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez B, Aguilar D, Orozco H, Burger M, Espitia C, Ritacco V, et al. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin Exp Immunol. 2003;133(1):7–30. doi: 10.1046/j.1365-2249.2003.02171.x. Epub 2003/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang YS, Wang GQ, Wen YJ, Wang L, Chen XC, Chen P, et al. Immunity against tumor angiogenesis induced by a fusion vaccine with murine beta-defensin 2 and mFlk-1. Clin Cancer Res. 2007;13(22 Pt 1):87–6779. doi: 10.1158/1078-0432.CCR-07-1587. Epub 2007/11/17. [DOI] [PubMed] [Google Scholar]
- 38.Olivares N, Leon A, Lopez Y, Puig A, Cadiz A, Falero G, et al. The effect of the administration of human gamma globulins in a model of BCG infection in mice. Tuberculosis (Edinb) 2006;86(3–4):72–268. doi: 10.1016/j.tube.2006.01.006. Epub 2006/05/09. [DOI] [PubMed] [Google Scholar]
- 39.Medina E, Ryan L, LaCourse R, North RJ. Superior virulence of Mycobacterium bovis over Mycobacterium tuberculosis (Mtb) for Mtb-resistant and Mtb-susceptible mice is manifest as an ability to cause extrapulmonary disease. Tuberculosis (Edinb) 2006;86(1):7–20. doi: 10.1016/j.tube.2005.04.003. Epub 2005/10/29. [DOI] [PubMed] [Google Scholar]
- 40.Woodworth JS, Fortune SM, Behar SM. Bacterial protein secretion is required for priming of CD8+ T cells specific for the Mycobacterium tuberculosis antigen CFP10. Infect Immun. 2008;76(9):205–4199. doi: 10.1128/IAI.00307-08. Epub 2008/07/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armitige LY, Jagannath C, Wanger AR, Norris SJ. Disruption of the genes encoding antigen 85A and antigen 85B of Mycobacterium tuberculosis H37Rv: effect on growth in culture and in macrophages. Infect Immun. 2000;68(2):78–67. doi: 10.1128/iai.68.2.767-778.2000. Epub 2000/01/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao V, Dhar N, Tyagi AK. Modulation of host immune responses by overexpression of immunodominant antigens of Mycobacterium tuberculosis in bacille Calmette-Guerin. Scand J Immunol. 2003;58(4):61–449. doi: 10.1046/j.1365-3083.2003.01321.x. Epub 2003/09/26. [DOI] [PubMed] [Google Scholar]
- 43.Tala-Heikkila MM, Tuominen JE, Tala EO. Bacillus Calmette-Guerin revaccination questionable with low tuberculosis incidence. Am J Respir Crit Care Med. 1998;157(4 Pt 1):7–1324. doi: 10.1164/ajrccm.157.4.9706037. Epub 1998/05/01. [DOI] [PubMed] [Google Scholar]
- 44.Buddle BM, Wedlock DN, Parlane NA, Corner LA, De Lisle GW, Skinner MA. Revaccination of neonatal calves with Mycobacterium bovis BCG reduces the level of protection against bovine tuberculosis induced by a single vaccination. Infect Immun. 2003;71(11):9–6411. doi: 10.1128/IAI.71.11.6411-6419.2003. Epub 2003/10/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):77–369. doi: 10.1038/ni1449. Epub 2007/03/14. [DOI] [PubMed] [Google Scholar]
- 46.Agger EM, Andersen P. A novel TB vaccine; towards a strategy based on our understanding of BCG failure. Vaccine. 2002;21(1–2):7–14. doi: 10.1016/s0264-410x(02)00447-4. Epub 2002/11/22. [DOI] [PubMed] [Google Scholar]


