Abstract
Background and purpose
Mechanisms of fatigue reported during radiotherapy are poorly defined but may include inflammatory cytokines and/or sleep disturbances. This prospective, longitudinal, phase II study assessed fatigue, sleep, and serum cytokine levels during radiotherapy for early-stage prostate cancer (PCa).
Material and methods
Twenty-eight men undergoing radiotherapy for early-stage PCa wore an Actiwatch Score to record fatigue level, sleep time, onset latency, efficiency and wake after sleep onset. Serum levels of IL-1α, IL-1β, TNF-α, IL-6, IL-8, IL-10 and VEGF were measured weekly during radiotherapy. Patient reported quality of life (QOL) metrics were collected before and after treatment. Linear mixed effects models examined trajectories across treatment weeks.
Results
Fatigue increased across treatment weeks (P < .01), and fatigue was associated with decreased patient-reported QOL. Sleep efficiency increased across treatment weeks (rate of change over time = .29, P = .03), and sleep onset latency decreased (rate of change over time = .86, P = .06). IL-6 tended to increase during treatment (P = 0.09), but none of the cytokine levels or sleep variables were significantly related to fatigue trajectories.
Conclusions
Despite increased sleep efficiency across treatment weeks, fatigue significantly increased. Although IL-6 increased during the course of radiotherapy, cytokines levels were not associated with fatigue scores or sleep disturbance. Further studies are needed to define the mechanisms for fatigue during radiotherapy.
Keywords: Radiotherapy, Cancer treatment related fatigue, Prosate Cancer, Cytokines, Sleep disturbance
Patients with cancer can experience debilitating fatigue that significantly interferes with their quality of life (QOL). Fatigue is the most common symptom experienced by cancer patients with an estimated incidence range of 60–90% [1]. The National Comprehensive Cancer Network defines cancer-related fatigue as “a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” [2].
Patients undergoing surgery [3], chemotherapy [4], radiotherapy (RT) or chemoradiotherapy (CRT) [5] experience significant cancer treatment-related fatigue (CTRF) that begins during treatment and declines following treatment. A growing body of evidence suggests that fatigue is not only the most prevalent symptom associated with cancer and its treatment, it is also the most distressing, and one that has a profoundly negative effect on physical functioning and QOL [6]. Besides having a negative impact on QOL, CTRF can also impact a patient’s ability to complete the prescribed treatment [7] For patients undergoing potentially curative RT, such unscheduled breaks can also contribute to tumor re-growth and compromise outcome [8].
Despite much investigation, the molecular mechanisms underlying the initiation and perpetuation of CTRF are not well established [9], but fatigue intensity seems to increase over the course of treatment and gradually decline over time [10]. One proposed etiologic factor is increased inflammatory cytokine production which is a normal physiological response to treatment-induced tissue injury [11]. Cancer patients undergoing chemotherapy, RT or CRT produce high levels of IL-1β [12], TNF-α [13], and IL-6 [14].
Further complicating the elusive molecular mechanism of CTRF are the many other contributing factors including depression [15], sleep disturbance [16]. There also are treatment- and site-specific symptoms that can affect fatigue. Patients undergoing RT for prostate cancer (PCa), for example, can experience bowel and urinary symptoms during treatment including loose and/or frequent stools, dysuria, polyuria and nocturia [17] Androgen-deprivation therapy (ADT), which is often given prior to and during RT for PCa, has also been shown to increase fatigue [18]. The hot flashes and night sweats associated with ADT can also lead to sleep disturbance and CTRF [19].
In order to best diagnose and treat CTRF in patients with PCa, we first must better understand the contributing factors. Therefore, to better elucidate the mechanism of CTRF in men with early-stage PCa, we performed a prospective, longitudinal, phase II study to assess fatigue, sleep quality, QOL and serum cytokine levels during external beam RT (EBRT). Ultimately, we sought to gain better understanding of whether CTRF is initiated by the production of inflammatory cytokines with the future goal of developing new treatment strategies to improve patients’ physical functioning, QOL and ability to complete the prescribed treatment.
Methods
After approval by the Institutional Review Board, patients were screened for study eligibility at their initial RT consultation. The target population was men who were scheduled to undergo definitive EBRT for early-stage PCa. Exclusion criteria included: (1) patients who were receiving concurrent ADT; (2) patients who received brachytherapy; (3) patients who were unable to complete the surveys and/or operate the Actiwatch Score (AW-S) (Respironics Inc.); (4) current psychiatric disorders or major depression, based on a score of 31 or greater on the Beck depression scale; (5) baseline fatigue level as a 10 on a 1–10 scale where 1 = “no fatigue” and 10 = “very severe fatigue” and (6) patients who had received treatment for any cancer, except skin cancer, within the past 12 months. Informed consent was obtained. Each participant underwent depression screening using the Beck Depression Inventory (BDI) [20]. Any person who was ineligible based on his BDI score (total score > 31) or reported thoughts of suicide was referred to a mental health professional.
Demographics and clinical data
A demographic form was used to obtain information on each subject’s age, ethnicity, gender, education, marital status, occupation, living situation, income and medical comorbidities. Patients were categorized has having medical comorbidities if they took medication for any of the following medical diagnoses: diabetes, asthma/COPD, hyperlipidemia or hypertension, otherwise they were categorized as having no medical comorbidities. These medical comorbidities were chosen for evaluation in particular because of their potential to affect sleep. A medical record chart audit form was used to obtain data regarding type and stage of cancer, levels of prostate specific antigen (PSA), height and weight, comorbidities, smoking status, and history of previous cancer treatment(s).
Patient-reported metrics
Eligible participants completed baseline demographic and two patient reported outcomes (PRO) tools, the Short Form 36 (SF-36 v 1.0) and International Prostate Symptom Score (IPSS) questionnaires [21]. The multi-item SF-36 (distributed by Quality Metric, Lincoln, RI) was used to evaluate QOL prior to and at the end of treatment. Higher scores (possible 0–100) indicate higher QOL [22]. The IPSS was used to evaluate urinary symptoms prior to and at the end of treatment, and increasing scores (possible 0–35) indicate increasing urinary symptoms [21].
Real-time monitoring of fatigue and sleep quality
Patients were given the AW-S [23] to put on their non-dominant wrist each Monday when they arrived in the department for radiation therapy. The goal was to capture four full nights of sleep data and three whole days of fatigue data per week. They returned the AW-S to study staff each Friday after they completed treatment. This occurred during week 0 (before the start of treatment), and during each week of treatment (weeks 1–8). Each AW-S device had an electronic keypad that allowed for the rating of fatigue on a scale of 1–10 in real-time (1 = no fatigue, and 10 = worst fatigue defined as the highest fatigue ever experienced). Participants were instructed to enter their self-reported fatigue levels manually four times each day (after waking in the morning, at lunch time, at dinner time and before bed). For each week, the recorded fatigue scores were averaged.
Patients were also instructed to wear the AW-S during the night in order to capture sleep data. The AW-S contains very sensitive omnidirectional accelerometers that count wrist movements to estimate sleep duration [23], and wrist actigraphy has been validated against polysomnography and demonstrated a total sleep duration correlation of >0.9 [24]. Participants were also instructed to mark their sleep and their wake times by pressing an indicator on the AW-S. Patient-reported ‘onset of sleep’ was defined as when the participant is in bed and turns off their light and patient-reported ‘wake’ defined as when the participant wakes and before they step out of their bed. Patient-reported sleep and wake times as well as AW-S manufacturer software calculated sleep duration were used to determine sleep metrics such as sleep latency (time required for full transition from wakefulness to sleep), sleep efficiency (total time spent asleep after turning off the lights) and wake after sleep onset (WASO, time spent awake after falling asleep but before final awakening). Participants needed to meet the following criteria to have their AW-S data included in the final analysis: to wear the watch for at least three nights during each week of the study (i.e. week 0 through week 8) and to enter at least six fatigue scores during a three-day period.
Blood collection and serum cytokine measurement
Peripheral blood was collected five times throughout the study; immediately before the first radiation treatment as well as 1 h after the 5th, 15th (end of week 3), 25th (end of week 5), and 39th (final) radiation treatment. Serum levels of IL-1α, IL-1β, TNF-α, IL-6, IL-8, IL-10, and VEGF were measured using bead-based immunofluorescence assays commercially available from Millipore Inc. Assays were run in duplicate according to the manufacturers’ protocol. A five-parameter regression formula was used to calculate the sample concentrations from the standard curves. Data were collected and analyzed using the Luminex-100 system Version IS (Luminex, Austin, TX).
Statistical analysis
Paired t-tests were used to examine changes in SF-36 and IPSS subscores from pre-treatment to the last treatment day. A Bonferroni adjustment was performed due to the large number of variables tested. As 19 variables were tested, the P-value below which differences were considered statistically significant was 0.05 divided by 19, which is equal to 0.003. Actigraphy data were analyzed using the Actiware 5.0 software (Respironics Inc.). Average daily fatigue level was calculated for baseline (week 0) and each treatment week (weeks 1–8). Sleep efficiency, wake after sleep onset (WASO), total sleep time (at night) and number of sleep bouts were calculated for weeks 0–9. Cytokine values were log transformed to correct for skewness. Changes in outcomes were examined across the study time course with a linear mixed effects modeling approach implemented in the lme package for the R statistical computing environment (R Core Team, Vienna, Austria, 2015). Model testing was used to determine the random effects structure for each outcome (i.e., intercepts and slopes as random or fixed). Multivariate models were then fit to determine whether changes in cytokines and sleep variables predicted changes in fatigue across time. Body mass index (BMI), smoking status, age, and the presence or absence of medical comorbidities were included as time invariant covariates as appropriate. A final set of models was used to determine the relation between changes in fatigue and changes in the SF-36 subscale scores.
Results
Twenty-nine eligible men were approached to participate in the study and all consented. One decided to continue treatment elsewhere and as a result withdrew from the study. Of the 28 men who ultimately enrolled and participated in this prospective, longitudinal trial, the majority of patients were Caucasian (96%), married/partnered (64%), and retired or disabled (79%). The mean ± SD age of participants was 66.9 ± 5.6 years. All participants were diagnosed with stage T1 or T2 prostate cancer. Prior to treatment the mean Gleason score was 6.5 ± 0.5 and mean prostate specific antigen (PSA) level was 7.7 ± 4 ng/mL. Demographic and clinical characteristics of the cohort are presented in Table 1.
Table 1.
Demographic information and tumor characteristics.
| N = 28* | |
|---|---|
| Age; mean ± SD | 66.9 ± 5.6 |
| BMI; mean ± SD | 31.5 ± 5.8 |
| Smoking status; n (%) | |
| Current smoker | 7 (25%) |
| Past smoker | 14 (50%) |
| Never smoked | 7 (25%) |
| Medical comorbidities§; n (%) | |
| Yes | 19 (67.9%) |
| No | 7 (25%) |
| Missing | 2 (7.1%) |
| Marital status; n (%) | |
| Single (never married) | 1 (3%) |
| Separated or divorced | 8 (29%) |
| Widowed | 1 (3%) |
| Married | 18 (64%) |
| People living in household; mean (range) | 2 (1–4) |
| Education; n (%) | |
| High school or technical school | 13 (47%) |
| Some college or college graduate | 15 (54%) |
| Employment status; n (%) | |
| Retired or disabled | 22 (79%) |
| Full time or part time | 6 (21%) |
| Annual income; n (%) | |
| </=$25,000 | 9 (32%) |
| $25,000–75,000 | 18 (64%) |
| >$75,000 | 1 (3%) |
| Clinical stage; n (%) | |
| T1c | 16 (57%) |
| T2a | 10 (36%) |
| T2b | 2 (7%) |
| Gleason score; n (%) | |
| 6 | 15 (54%) |
| 7 | 13 (46%) |
| PSA at diagnosis; mean ± SD | 7.7 ± 4 |
SD = standard deviation, BMI = body mass index, PSA = prostate specific antigen in ng/mL.
Demographic data were obtained for all 28 participants except for two patients who did not provide information regarding their medical comorbidities.
Patients were categorized as having medical comorbidities if they took medication for any of the following medical diagnoses: diabetes, asthma/COPD, hyperlipidemia or hypertension.
Patient-reported metrics
QOL data were complete for all patients except for one who missed the end of treatment measurement. There were significant differences in the QOL and urinary symptom scores reported by participants before and after treatment, and the vitality and social functioning sub-scores both significantly decreased. Total IPSS score increased from 12.1 ± 9.0 to 20.7 ± 7.0 (P = .001) indicating increased bother, and incomplete emptying, urgency, weak stream, nocturia and IPSS QOL scores also increased. Pre- and post-EBRT scores are presented in Table 2.
Table 2.
Patient reported quality of life and urinary symptom scores before and after radiation therapy for prostate cancer.
| Pre-RT Mean ± SD |
Post-RT Mean ± SD |
P§ | |
|---|---|---|---|
| N = 28* | |||
| QOL (SF-36) (possible 0–100) | |||
| Physical function | 70.9 ± 24.5 | 68.1 ± 23.3 | .464 |
| Role limits physical | 65.3 ± 26.9 | 55.7 ± 29.1 | .146 |
| Role limits emotional | 84.8 ±19.1 | 78.4 ± 27.6 | .180 |
| Vitality | 62.5 ± 22.4 | 51.3 ± 24.7 | .005 |
| Emotional well being | 76.3 ± 16.9 | 78.5 ± 18.5 | .429 |
| Social functioning | 86.7 ± 16.5 | 73.8 ± 23.8 | .008 |
| Bodily pain | 68.1 ± 24.0 | 73.8 ± 20.4 | .117 |
| General health | 64.8 ± 19.9 | 70.6 ± 22.3 | .117 |
| PCS | 65.3 ± 19.0 | 65.6 ± 20.4 | .907 |
| MCS | 77.1 ± 15.4 | 70.0 ± 20.3 | .028 |
| Urinary symptoms (IPSS) (possible 0–35 for total, 0–5 for each subset) | |||
| IPSS total | 12.9 ± 9.2 | 20.7 ± 7.0 | .001 |
| Incomplete emptying | 1.6 ± 1.6 | 2.5 ± 1.3 | .040 |
| Frequency | 2.3 ± 2.0 | 3.3 ± 1.5 | .011 |
| Intermittency | 1.5 ± 1.7 | 2.0 ± 1.5 | .170 |
| Urgency | 1.6 ± 1.8 | 3.4 ± 1.4 | <.001 |
| Weak stream | 2.0 ± 1.8 | 2.8 ± 1.5 | .042 |
| Straining | 0.8 ± 1.4 | 1.4 ± 1.2 | .143 |
| Nocturia | 1.8 ± 1.3 | 2.7 ± 1.4 | <.001 |
| IPSS quality of life | 1.8 ± 1.4 | 3.2 ± 1.3 | <.001 |
Complete data were available for 27 participants. One patient failed to complete his end of treatment questionnaire.
A Bonferroni adjustment was performed due to the large number of variables tested. As 19 variables were tested, the P-value below which differences were considered statistically significant was 0.05 divided by 19, which is equal to 0.003. Bold type indicates a P-value <0.003. SD = standard deviation, QOL = quality of life, IPSS = International Prostate Symptom Score, PCS = Physical Component Score, MCS = Mental Component Score.
Change in fatigue and sleep quality over treatment weeks
Sixteen patients recorded at least six fatigue values per week for all nine weeks of the study. Six patients had one or two weeks where they did not record at least six fatigue values, and six patients did not record at least fatigue values per week for three or more weeks in the study and were therefore excluded from this analysis. For the 22 men included in the fatigue trajectory analysis, the mean ± SD number of fatigue entries per week was 11 ± 4.4 (median [range] was 11 [0–25]). Fatigue, significantly increased across treatment weeks (rate of change over time = .18, P < .01). Fig. 1 shows the trends for fatigue across time for each patient and the average trend with 95% CI’s.
Fig. 1.
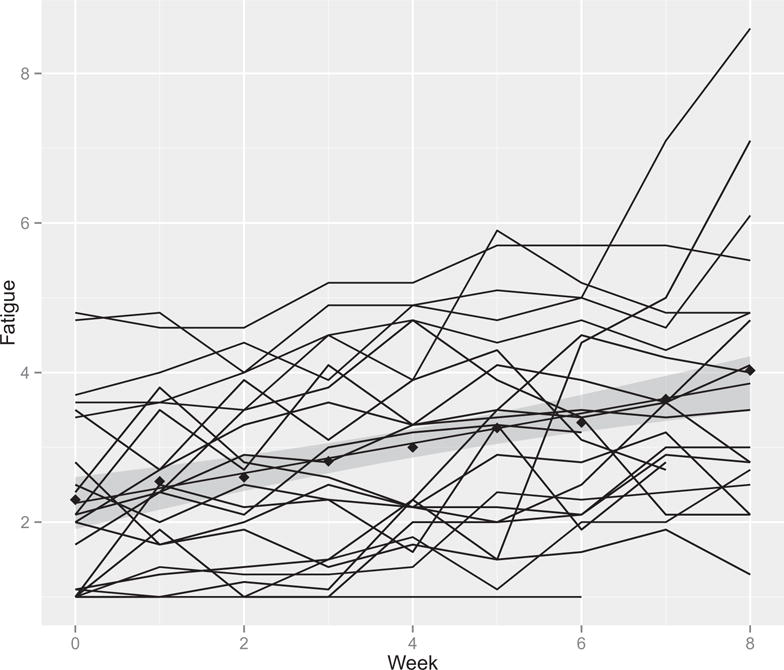
Fatigue and sleep quality measures across time for each subject. The shaded region around the slope represents the 95% confidence interval.
Four patients did not provide usable data for the sleep variables. There was a trend toward decreased sleep onset latency across treatment weeks (rate of change over time = −.86, P = .06), suggesting patients fell asleep faster as treatment progressed. However, there were two subjects with exceptionally high sleep latencies which may have influenced the results. When these two outliers were excluded, the P = .20. Sleep efficiency also increased across treatment weeks (rate of change over time = .29, P = .03), indicating patients spent a greater percentage of time in bed actually sleeping. There was no significant change found in sleep duration (rate of change over time = −.92, P = .43) or WASO (rate of change over time = −.72, P = .26) across treatment weeks.
Change in serum cytokine levels over treatment weeks
One patient provided cytokine measures at only two time points (weeks 1 and 3) and a second patient provided cytokine data at four of five time points. All other patients provided complete cytokine data. There were no significant changes in IL-1α, IL-1β, IL-8, IL-10, TNF-α or VEGF across treatment weeks (Table 3). However, IL-6 values tended to increase across treatment weeks (P = .09) (Fig. 2).
Table 3.
Levels of six cytokines during radiation therapy for prostate cancer.
| Analyte* | Pre-treatment Mean ± SD |
Day 5 Mean ± SD |
Day 15 Mean ± SD |
Day 25 Mean ± SD |
Last treatment Mean ± SD |
|---|---|---|---|---|---|
| IL-1α | 439.1 ± 707.4 | 441.5 ± 738.0 | 452.7 ± 734.0 | 480.0 ± 854.9 | 424.6 ± 637.5 |
| IL-1β | 30.1 ± 73.2 | 32.0 ± 65.9 | 36.2 ± 86.6 | 42.9 ± 96.1 | 24.1 ± 45.3 |
| TNF-α | 23.7 ± 25.4 | 25.2 ± 27.9 | 26.9 ± 33.2 | 41.0 ± 104.0 | 41.8 ± 84.5 |
| IL-6 | 188.5 ± 251.5 | 177.4 ± 221.9 | 221.3 ±279.4 | 228.6 ± 325.6 | 236.9 ± 315.8 |
| IL-8 | 82.4 ± 91.4 | 169.7 ±314.9 | 84.6 ± 93.5 | 150.6 ± 378.5 | 92.5 ± 103.6 |
| IL-10 | 63.3 ± 205.1 | 55.8 ± 179.0 | 60.4 ± 190.4 | 86.3 ± 231.3 | 68.6 ± 159.9 |
| VEGF | 690.1 ± 656.2 | 661.3 ± 653.0 | 749.7 ± 777.6 | 729.3 ± 802.4 | 795.3 ± 871.1 |
Cytokine levels in pictograms per milliliter.
SD = standard deviation, analyte levels in picograms per milliliter (pg/mL).
Fig. 2.
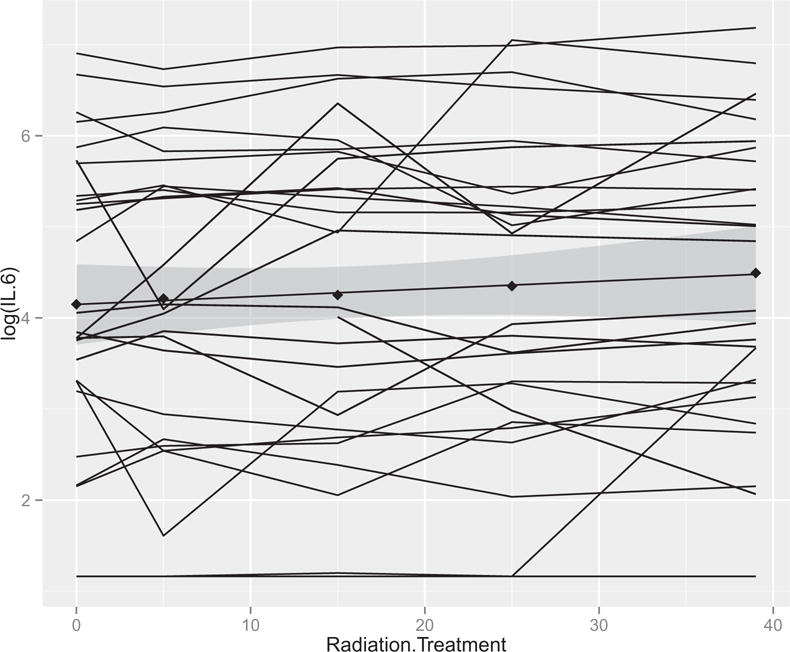
IL-6 measure across time for each subject. The shaded region around the slope represents the 95% confidence interval.
Predictors of fatigue trajectory
None of the cytokine variables or sleep variables were significantly related to fatigue trajectories with or without controlling for demographic characteristics (age, BMI, smoking, diabetes, respiratory medications, cholesterol medications, and hypertension medications) or treatment-related nocturia.
Relationship between fatigue and QOL
Increasing fatigue was significantly associated with a decreased patient-reported mental health sub-score on the SF-36 (rate of change over time = −6.62; P < .01). Increasing fatigue was also significantly associated with decreased emotional role limits (rate of change over time = −11.26; P < .01), decreased emotional well-being (rate of change over time = −5.39; P < .01) and decreased physical role limits (rate of change over time = −5.63; P = .05) sub-scores.
Discussion
The results of this prospective, longitudinal, phase II study show that, although fatigue levels do increase significantly throughout the course of EBRT for early-stage PCa, this could not be easily explained by changes in sleep metrics or cytokine levels. Patients had similar sleep duration, sleep latency, wake after sleep onset as the treatment weeks progressed and actually developed increased sleep efficiency. IL-6 was the only cytokine evaluated that tended to increase during the course of radiotherapy, and none of the cytokines studied were significantly associated with fatigue scores or sleep disturbance.
In our cohort, QOL scores were lower at the end of EBRT compared to pretreatment baseline. There is some evidence to suggest EBRT for early stage PCa is not associated with a decrement in global QOL [25], but several studies have described and quantified the urinary, bowel, and sexual function symptoms that affect men after EBRT for PCa [26]. Fatigue also increased across treatment weeks in our cohort, and increasing fatigue was significantly associated with decreased QOL in several physical, emotional and mental health categories. The effect of CTRF on QOL is widely accepted, and several multidisciplinary programs including nutrition, psychosocial support, and physical activity have been developed to improve both fatigue and QOL for cancer patients [27].
Although sleepiness and fatigue are distinct entities and the NCCN definition of fatigue requires that the symptom be out of proportion to recent activity [2], the effect of cancer-treatment-related sleep disturbance has been shown to contribute to CTRF [28]. Additionally, sleep disturbance in cancer patients have also independently been shown to negatively impact QOL [29]. In our cohort, we did not see an association with increasing sleep disturbance across EBRT treatment weeks. In fact, though most sleep metrics did not change during EBRT, sleep efficiency actually increased across treatment weeks. One potential reason for increased sleep efficiency as the course of EBRT progressed is that patients did have increasing fatigue, and therefore slept a greater percentage of the time they were in bed. Another contributing factor could be that patients adjust and adapt to their schedule of daily radiotherapy and frequent medical visits and can sleep more efficiently as the treatment course progresses.
The physiologic mechanism of CTRF is unclear, but many have hypothesized that increased cytokine projection may play a role [30]. Several lines of evidence support this hypothesis. First, in addition to fatigue, cancer patients undergoing treatment often experience several other symptoms including anorexia, cachexia, pain, sleep disturbance, depression, and anemia, which can impact the subjective sensation of fatigue [7]. Considerable evidence generated in animal models and in clinical populations implicate inflammatory cytokines like IL-1β, TNF-α, and IL-6 in the etiology of these symptoms [31]. In this regard, CTRF may be homologous to sickness behavior, a normal response to infection or tissue injury [32]. Total body and localized radiation have been shown to induce the production of inflammatory cytokines both in experimental systems and in clinical populations [33].
Although there is much evidence to implicate a role for these cytokines in CTRF, older studies plotting weekly cytokine levels and symptoms of fatigue during radiotherapy for uterine [34], breast [35] and prostate [36] cancer have also failed to find a correlation. Although we demonstrated a trend toward increasing IL-6 during the course of radiotherapy, we did not find any significant correlation between cytokine levels, sleep characteristics or fatigue levels. However, recent studies evaluating cytokine levels and fatigue in other cancer sites were able to demonstrate a positive correlation. A prospective, longitudinal study was performed at MD Anderson in which cytokine levels were measured weekly during chemoradiotherapy for gastrointestinal cancers; this showed increasing TNF-R1 and IL-6 were positively associated with increasing fatigue severity during treatment [31]. The same group also evaluated cytokine levels in patients undergoing chemoradiotherapy for non-small cell lung cancer and found IL-6 to be positively associated with increasing fatigue as well as increasing severity of pain, disturbed sleep, lack of appetite and sore throat [12]. Investigators from the University of California San Diego reported a positive association between IL-6 and the Multidimensional Fatigue Symptom Inventory-Short form score and between IL-6 and IL-1RA and the Pittsburg Sleep Quality Index score in patients undergoing chemotherapy for breast cancer [13].
The strengths of this study include its prospective, longitudinal design as well as the rigorous collection of several metrics of sleep and fatigue using both patient-reported qualitative scales and the AW-S. This is not the first study to attempt to correlate serum cytokine levels with fatigue in patients receiving cancer therapy [37], but the timing of serum collection in this study reflected a better understanding of cytokine kinetics. Older studies measured cytokine concentrations once a week on a set day, regardless of when the patient actually received EBRT [38]. This is problematic because peak serum levels of IL-1β, TNF-α and IL-6 occur at 1–3 h post immune challenge [39]. The persistence of cytokine-related symptoms in animal models is thought to be due to expression of inflammatory cytokines in the brain, which cannot be measured by serum tests [40]. In an effort to record peak post-radiotherapy cytokine levels for each patient, we took serum samples 1 h after radiotherapy. As fatigue is certainly multifactorial, we attempted to control for as many confounding factors as we could. We collected information regarding demographic information and medical comorbidities and did not find them to be significantly associated with differences in fatigue trajectories. The exclusion of patients receiving ADT and patients with baseline depression was also helpful in focusing in on the effects of EBRT on sleep and fatigue.
One limitation of the study design the failure to detect other factors potentially contributing to fatigue including change in work routine or other activities, daily travel to and from the radiotherapy center, and the development of anxiety and/or depression not present at the baseline evaluation. Additionally, the sample size was small in this pilot study. It is possible that type II error was introduced, and a significant correlation exists between the sleep metrics and cytokines studied and fatigue in PCa patients that went undetected. These results will be used in the power analysis for the forthcoming trial. Nonetheless, these data suggest the mechanism of CTRF in patients undergoing ERBT for PCa is complicated and cannot be explained by sleep disturbance and cytokine levels alone. Radiation-induced gene expression changes associated with CTRF are one area of promising ongoing research [41].
In conclusion, patients undergoing EBRT for early-stage PCa reported significantly increased fatigue across treatment weeks despite similar sleep duration, similar rates of sleep disturbances and increased sleep efficiency. IL-6 tended to increase during the course of radiotherapy, but none of the cytokines studied were significantly associated with fatigue scores or sleep disturbance. Further studies are needed to define the mechanisms more clearly for fatigue during radiotherapy.
Acknowledgments
Supported by RSNA Research Resident Grant No. RR0730 (TLM) and National Institute of Nursing Research project no. 5R211NR010363 (LJW).
Role of the funding source
The study sponsors had no role in the study design, collection, analysis or interpretation of data, the writing of the manuscript or the decision to submit the manuscript for publication.
Footnotes
Conflicts of interest statement
The authors have no conflicts of interest to disclose.
These results were presented in abstract form at the 53rd annual meeting of The American Society of Radiation Oncology in Miami, Florida in 2011.
References
- 1.Irvine D, Vincent L, Graydon JE, Bubela N, Thompson L. The prevalence and correlates of fatigue in patients receiving treatment with chemotherapy and radiotherapy. A comparison with the fatigue experienced by healthy individuals. Cancer Nurs. 1994;17:367–78. [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network Clinical Practice Guidelines Version 2. 2015 Cancer Related Fatigue [Internet]. [Cited 21.03.15]. Available from: < http://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf>.
- 3.Rubin GJ, Hotopf M. Systematic review and meta-analysis of interventions for postoperative fatigue. Br J Surg. 2002;89:971–84. doi: 10.1046/j.1365-2168.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 4.Minton O, Strasser F, Radbruch L, Stone P. Identification of factors associated with fatigue in advanced cancer: a subset analysis of the European palliative care research collaborative computerized symptom assessment data set. J Pain Symptom Manage. 2012;43:226–35. doi: 10.1016/j.jpainsymman.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Wang XS, Janjan NA, Guo H, Johnson BA, Engstrom MC, Crane CH, et al. Fatigue during preoperative chemoradiation for resectable rectal cancer. Cancer. 2001;92:1725–32. doi: 10.1002/1097-0142(20010915)92:6+<1725::aid-cncr1504>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 6.Vogelzang NJ, Breitbart W, Cella D, Curt GA, Groopman JE, Horning SJ, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue Coalition. Semin Hematol. 1997;34:4–12. [PubMed] [Google Scholar]
- 7.von Gunten CF, Gafford E. Treatment of non-pain-related symptoms. Cancer J Sudbury Mass. 2013;19:397–404. doi: 10.1097/PPO.0b013e3182a65ecf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bese NS, Hendry J, Jeremic B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int J Radiat Oncol Biol Phys. 2007;68:654–61. doi: 10.1016/j.ijrobp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Wood LJ, Weymann K. Inflammation and neural signaling: etiologic mechanisms of the cancer treatment-related symptom cluster. Curr Opin Support Palliat Care. 2013;7:54–9. doi: 10.1097/SPC.0b013e32835dabe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz AL, Nail LM, Chen S, Meek P, Barsevick AM, King ME, et al. Fatigue patterns observed in patients receiving chemotherapy and radiotherapy. Cancer Invest. 2000;18:11–9. doi: 10.3109/07357900009023057. [DOI] [PubMed] [Google Scholar]
- 11.Wood LJ, Nail LM, Gilster A, Winters KA, Elsea CR. Cancer chemotherapy-related symptoms: evidence to suggest a role for proinflammatory cytokines. Oncol Nurs Forum. 2006;33:535–42. doi: 10.1188/06.ONF.535-542. [DOI] [PubMed] [Google Scholar]
- 12.Matanić D, Beg-Zec Z, Stojanović D, Matakorić N, Flego V, Milevoj-Ribić F. Cytokines in patients with lung cancer. Scand J Immunol. 2003;57:173–8. doi: 10.1046/j.1365-3083.2003.01205.x. [DOI] [PubMed] [Google Scholar]
- 13.Ferrajoli A, Keating MJ, Manshouri T, Giles FJ, Dey A, Estrov Z, et al. The clinical significance of tumor necrosis factor-alpha plasma level in patients having chronic lymphocytic leukemia. Blood. 2002;100:1215–9. [PubMed] [Google Scholar]
- 14.Tang JT, Yamazaki H, Nishimoto N, Inoue T, Nose T, Koizumi M, et al. Effect of radiotherapy on serum level of interleukin 6 in patients with cervical carcinoma. Anticancer Res. 1996;16:2005–8. [PubMed] [Google Scholar]
- 15.Ho S-Y, Rohan KJ, Parent J, Tager FA, McKinley PS. A longitudinal study of depression, fatigue, and sleep disturbances as a symptom cluster in women with breast cancer. J Pain Symptom Manage. 2014 doi: 10.1016/j.jpainsymman.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin S, Chen Y, Yang L, Zhou J. Pain, fatigue, disturbed sleep and distress comprised a symptom cluster that related to quality of life and functional status of lung cancer surgery patients. J Clin Nurs. 2013;22:1281–90. doi: 10.1111/jocn.12228. [DOI] [PubMed] [Google Scholar]
- 17.Budäus L, Bolla M, Bossi A, Cozzarini C, Crook J, Widmark A, et al. Functional outcomes and complications following radiation therapy for prostate cancer: a critical analysis of the literature. Eur Urol. 2012;61:112–27. doi: 10.1016/j.eururo.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 18.Truong PT, Berthelet E, Lee JC, Petersen R, Lim JTW, Gaul CA, et al. Prospective evaluation of the prevalence and severity of fatigue in patients with prostate cancer undergoing radical external beam radiotherapy and neoadjuvant hormone therapy. Can J Urol. 2006;13:3139–46. [PubMed] [Google Scholar]
- 19.Savard J, Hervouet S, Ivers H. Prostate cancer treatments and their side effects are associated with increased insomnia. Psychooncology. 2013;22:1381–8. doi: 10.1002/pon.3150. [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 21.Barry MJ, Fowler FJ, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–57. doi: 10.1016/s0022-5347(17)36966-5. [discussion 1564] [DOI] [PubMed] [Google Scholar]
- 22.Interpreting SF-36 Summary Health Measures: A response [Internet] [Cited 22.03.15]. Available from: < http://www.sf-36.org/news/qolrsupplement.pdf>.
- 23.Jean-Louis G, Von-Gizycki H, Zizi F, Spielman A, Hauri P, Taub H. The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep-wake activity. Percept Mot Skills. 1997;85:207–16. doi: 10.2466/pms.1997.85.1.207. [DOI] [PubMed] [Google Scholar]
- 24.Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep. 2004;27:158–65. doi: 10.1093/sleep/27.1.158. [DOI] [PubMed] [Google Scholar]
- 25.Geinitz H, Thamm R, Scholz C, Heinrich C, Prause N, Kerndl S, et al. Longitudinal analysis of quality of life in patients receiving conformal radiation therapy for prostate cancer. Strahlenther Onkol Organ Dtsch Röntgenges Al. 2010;186:46–52. doi: 10.1007/s00066-009-2023-7. [DOI] [PubMed] [Google Scholar]
- 26.Lee TK, Breau RH, Mallick R, Eapen L. A systematic review of expanded prostate cancer index composite (EPIC) quality of life after surgery or radiation treatment. Can J Urol. 2015;22:7599–606. [PubMed] [Google Scholar]
- 27.Gracey JH, Watson M, Payne C, Rankin J, Dunwoody L. Translation research: “Back on Track”, a multiprofessional rehabilitation service for cancer-related fatigue. BMJ Support Palliat Care. 2014 doi: 10.1136/bmjspcare-2014-000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jim HSL, Jacobsen PB, Phillips KM, Wenham RM, Roberts W, Small BJ. Lagged relationships among sleep disturbance, fatigue, and depressed mood during chemotherapy. Health Psychol Off J Div Health Psychol Am Psychol Assoc. 2013;32:768–74. doi: 10.1037/a0031322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickerson SS, Connors LM, Fayad A, Dean GE. Sleep-wake disturbances in cancer patients: narrative review of literature focusing on improving quality of life outcomes. Nat Sci Sleep. 2014;6:85–100. doi: 10.2147/NSS.S34846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. NeuroImmunoModulation. 2005;12:255–69. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 31.Wang XS, Williams LA, Krishnan S, Liao Z, Liu P, Mao L, et al. Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain Behav Immun. 2012;26:699–705. doi: 10.1016/j.bbi.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chao CC, DeLaHunt M, Hu S, Close K, Peterson PK. Immunologically mediated fatigue: a murine model. Clin Immunol Immunopathol. 1992;64:161–5. doi: 10.1016/0090-1229(92)90194-s. [DOI] [PubMed] [Google Scholar]
- 33.Johnston CJ, Piedboeuf B, Rubin P, Williams JP, Baggs R, Finkelstein JN. Early and persistent alterations in the expression of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiat Res. 1996;145:762–7. [PubMed] [Google Scholar]
- 34.Ahlberg K, Ekman T, Gaston-Johansson F. Levels of fatigue compared to levels of cytokines and hemoglobin during pelvic radiotherapy: a pilot study. Biol Res Nurs. 2004;5:203–10. doi: 10.1177/1099800403259500. [DOI] [PubMed] [Google Scholar]
- 35.Geinitz H, Zimmermann FB, Stoll P, Thamm R, Kaffenberger W, Ansorg K, et al. Fatigue, serum cytokine levels, and blood cell counts during radiotherapy of patients with breast cancer. Int J Radiat Oncol Biol Phys. 2001;51:691–8. doi: 10.1016/s0360-3016(01)01657-1. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg DB, Gray JL, Mannix CM, Eisenthal S, Carey M. Treatment-related fatigue and serum interleukin-1 levels in patients during external beam irradiation for prostate cancer. J Pain Symptom Manage. 1993;8:196–200. doi: 10.1016/0885-3924(93)90127-h. [DOI] [PubMed] [Google Scholar]
- 37.Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun. 2010;24:968–74. doi: 10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu L, Mills PJ, Rissling M, Fiorentino L, Natarajan L, Dimsdale JE, et al. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav Immun. 2012;26:706–13. doi: 10.1016/j.bbi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purswani MU, Eckert SJ, Arora HK, Noel GJ. Effect of ciprofloxacin on lethal and sublethal challenge with endotoxin and on early cytokine responses in a murine in vivo model. J Antimicrob Chemother. 2002;50:51–8. doi: 10.1093/jac/dkf091. [DOI] [PubMed] [Google Scholar]
- 40.Sheng WS, Hu S, Lamkin A, Peterson PK, Chao CC. Susceptibility to immunologically mediated fatigue in C57BL/6 versus Balb/c mice. Clin Immunol Immunopathol. 1996;81:161–7. doi: 10.1006/clin.1996.0172. [DOI] [PubMed] [Google Scholar]
- 41.Saligan LN, Fernández-Martínez JL, deAndrés-Galiana EJ, Sonis S. Supervised classification by filter methods and recursive feature elimination predicts risk of radiotherapy-related fatigue in patients with prostate cancer. Cancer Inform. 2014;13:141–52. doi: 10.4137/CIN.S19745. [DOI] [PMC free article] [PubMed] [Google Scholar]


