Abstract
Objective
To evaluate the effect of chlorogenic acids (CGAs) intake on cognitive function.
Methods
In this pilot study, the Cogstate and CNS Vital Signs test batteries were used to evaluate cognitive function in 8 healthy elderly men and women complaining of subjective memory loss after a 6-month intake of a test beverage containing 330 mg of CGAs just before bedtime.
Results
After a 6-month CGA intake period, significant improvement was observed in the One Back Test of the Cogstate, the Shifting Attention Test, and Finger Tapping Test as well as in the composite memory, verbal memory, complex attention, cognitive flexibility, executive function, and motor speed domains of the CNS Vital Signs test battery.
Conclusion
A 6-month intake of CGAs may improve attentional, executive, and memory functions in the elderly with complaints of subjective memory loss.
1. Introduction
Aging is a biological process that occurs in all life forms and is accompanied by adverse effects on physical and cognitive functions in humans. The incidence of dementia, a disease strongly associated with cognitive impairment, is on the rise globally. Alzheimer's Disease International, a worldwide federation of Alzheimer's associations, estimates that the number of patients with dementia is expected to double every 20 years [1]. Early prevention of dementia is critical because no definitive therapy has been established. One study reported an association between lifestyle factors and the onset of dementia [2]. Other studies have proposed that certain foods, a key lifestyle factor, contain nutrients that prevent dementia [3, 4].
High intake of fruits, vegetables, fish, nuts, and legumes and low intake of meat, high fat dairy, and sweets have been shown to delay aging-related cognitive impairment and reduce the risk of Alzheimer's disease (AD) [5]. The mechanism underlying the amelioration of cognitive impairment and dementia via fruits and vegetable intake is likely mediated by antioxidants and antioxidant vitamins [4]. In addition, the intake of n-3 polyunsaturated fatty acids (PUFAs), which are abundant in fish, has been reported to reduce the incidence of cognitive impairment and dementia [3]. Docosahexaenoic acid was found to reduce amyloid β (Aβ) deposition and to exert neuroprotective and anti-inflammatory effects at synapses in a mouse model of AD [6]. Additionally, a cross-sectional study involving dementia-free participants in the Framingham Study demonstrated that middle-aged and elderly participants who had a low red blood cell concentration of n-3 PUFAs also had small brain volumes and low cognitive function [7]. Studies have also reported an association between consumption of large amounts of green tea or coffee and reduced risk of dementia [8, 9].
Improved cognitive function as a result of drinking coffee is thought to be mediated by caffeine and chlorogenic acids (CGAs). A study conducted in rats showed that caffeine mediates long-term potentiation in the Cornu Ammonis 1 area of the hippocampus with suppression of Aβ aggregation [10]. In addition to caffeine, coffee contains CGAs and has antioxidant properties [11]. The CGAs in coffee beans are composed of 9 major compounds: 3 caffeoylquinic acids (5-CQA, 3-CQA, and 4-CQA); 3 dicaffeoylquinic acids (3,4-diCQA, 3,5-diCQA, and 4,5-diCQA); and 3 feruloylquinic acids (3-FQA, 4-FQA, and 5-FQA). 5-CQA (formerly called 3-CQA or chlorogenic acid) is the main component of CGAs in roasted and green coffee beans.
Studies using mice and cultured neurons have shown that CGAs protect neurons and suppress the aggregation of Aβ through antioxidative effects [12, 13]. Furthermore, caffeic acid, coumaric acid, ferulic acid, and sinapic acid, all of which are metabolites of CGAs, have been shown to improve cognitive function [13–15]. These findings suggest that CGA intake contributes to improved cognitive function, but the mechanism by which CGA intake affects cognitive function in humans is currently unknown.
In this study, we hypothesized that CGAs improve human cognitive function, especially in the elderly, and sought to elucidate the association between CGAs and cognitive function from the perspective of preventing dementia, the incidence of which increases with aging in this population. Therefore, in this study, we enrolled community-dwelling elderly individuals to examine the effects of a 6-month intake of CGAs on cognitive function and the association with biological data.
2. Methods
2.1. Participants
For recruitment of the participants, an explanatory meeting for this study was held at a regional community center in Yonezawa city, Yamagata, Japan, and those who voluntarily agree to participate in the study were checked for eligibility. Participants' recruitment was carried out by distribution of public relations to local residents and 42 people participated in explanatory meeting at the community center. One of the authors (K. M.) provided for participants in the explanatory meeting a 30-minute lecture on nutrition and prevention of cognitive function decline. The lecture presented previous studies which had examined the effects of CGAs intake on endothelial function [16] and body fat [17]. A beverage with the same components had been used, although the amount consumed was different from the amount in this study. Also presented was the possibility of improvement in cognitive function, as demonstrated by animal experiment [12, 15]. After the lecture was over, we also explained the content of the experiment to be conducted in this study. In the explanation of the experiment content, the participants in the explanatory meeting were informed that it was uncertain whether all applicants would be able to become participants in the experiment, even if they were willing. After explaining the content of the experiment, written informed consent for participation was obtained, to apply for becoming subjects in the experiment. 28 participants agreed to participate in this study. Exclusion criteria were physical or mental disorder, visual impairment, and movement-related pain. In addition, for screening, participants completed Kihon Checklist, a self-administered questionnaire consisting of 25 questions in seven categories: instrumental activities of daily living, physical strength, nutritional status, oral function, cognitive function, and depression risk [18]. And those who checked at least one question in the cognitive function category (“Do others point out your forgetfulness or tell you ‘You always ask the same thing'?,” “When you want to make a call, do you usually search for the telephone number and call on your own?,” or “Do you sometimes not know what the date is?”) were considered eligible for enrollment. Finally, 8 participants (2 men, 6 women) were enrolled in this study and those who completed the 6-month trials were included in the analysis.
2.2. Materials
The test beverage for this study consisted of 330 mg CGAs dissolved in 100 mL of water and contained less than 10 kcal of energy. The CGAs were extracted from green coffee beans using a hot water extraction method and decaffeinated by activated carbon; then, a dry powder was obtained by spray drying [16]. The caffeine level of the test beverage was below the limit of quantification (<1 mg/100 g) to avoid its potential effects on cognitive functions and sleep qualities [10, 19]. The CGAs comprised 5-CQA, 3-CQA, 4-CQA, 3,4-diCQA, 3,5-diCQA, 4,5-diCQA, 3-FQA, 4-FQA, and 5-FQA and was assessed using high-performance liquid chromatography. In terms of composition, the CGAs consisted of 58.3% CQA (total of 3-CQA, 4-CQA, and 5-CQA), 19.9% feruloylquinic acid (total of 3-FQA, 4-FQA, and 5-FQA), and 21.8% dicaffeoylquinic acid (3,4-diCQA, 3,5-diCQA, and 4,5-diCQA).
2.3. Study Design
This study was approved by the Ethics Committee of Yonezawa University of Nutrition Sciences (Approval Number 26-3, May 28, 2014) and was conducted in accordance with the Declaration of Helsinki. In this single-arm study, participants ingested one 100 mL bottle of test beverage containing 330 mg of CGAs daily before bedtime during a 6-month period between August 2014 and May 2015. A 1-month supply of test beverages was delivered to the participants' homes every month by the research staff. A diary for recording the details of test beverage consumption and daily activities was collected for evaluation every 2 months, and neurocognitive tests were performed before and after the nutritional intervention period.
2.4. Measurement Items
2.4.1. Participant Attributes
Participant attributes included physical characteristics (height, weight), education history, and Mini-Mental State Examination (MMSE) scores.
2.4.2. Assessment of Cognitive Function
Cognitive function was assessed by using two types of computerized neurocognitive test batteries: Cogstate (Cogstate Ltd., Melbourne VIC, Australia) [20] and CNS Vital Signs (CNS Vital Signs LLC, Morrisville, NC) [21]. A practice test was run with each battery 1 week before the actual test.
(1) Cogstate. The Cogstate test battery consisted of 8 tests for different domains of cognitive function: the Groton Maze Chase Test for assessing visual motor function, Groton Maze Learning Test for executive function, Detection Test for psychomotor function, Identification Test for attention, One Back Test (OBT) and Two Back Test for working memory, One Card Learning Test for visual learning, and Continuous Paired Associate Learning Test for paired associate learning.
(2) CNS Vital Signs. The CNS Vital Signs test battery (basic package) consists of 7 tests for neurocognitive function: Verbal Memory Test (VBM), Visual Memory Test (VIM), Finger Tapping Test (FTT), Symbol Digit Coding (SDC), Stroop Test (ST), Shifting Attention Test (SAT), and Continuous Performance Test (CPT). As shown in Table 1, the scores on the 7 tests generate 11 domain scores: composite memory, verbal memory, visual memory, psychomotor speed, reaction time, complex attention, cognitive flexibility, processing speed, executive function, simple attention, and motor speed. For example, composite memory is calculated from the total number of correct hits and passes in the Verbal Memory Test and Visual Memory Test. The detailed calculation methods of the other domain scores are described in the previous study [21]. All scores are age-adjusted and standardized by setting the mean score to 100 and the standard deviation (SD) to 15. High scores indicate superior neurocognitive function.
Table 1.
Cognitive domain scores in CNS Vital Signs.
| Cognitive domains | Tests for score calculations |
|---|---|
| Composite memory | VBM + VIM |
| Verbal memory | VBM |
| Visual memory | VIM |
| Psychomotor speed | FTT + SDC |
| Reaction time | ST |
| Complex attention | ST + SAT + CPT |
| Cognitive flexibility | ST + SAT |
| Processing speed | SDC |
| Executive function | SAT |
| Simple attention | CPT |
| Motor speed | FTT |
VBM: Verbal Memory Test, VIM: Visual Memory Test, FTT: Finger Tapping Test, SDC: Symbol Digit Cording, ST: Stroop Test, SAT: Shifting Attention Test, and CPT: Continuous Performance Test.
2.4.3. Blood Parameters
Blood was collected early in the morning during fasting. Measurements of cortisol, dehydroepiandrosterone sulfate (DHEA-S), brain-derived neurotrophic factor (BDNF), and Aβ40 and Aβ42 levels were performed by LSI Medience Corp. (Tokyo, Japan) and Cosmo Bio Co., Ltd. (Tokyo, Japan) according to conventional methods.
2.5. Statistical Analysis
Measurement data are expressed as mean and standard deviation (SD). Data obtained before and after the nutritional intervention were compared using the paired t-test, and effect size was calculated using Cohen's d (d). In addition, parameters showing a significant difference after intervention were further analyzed to quantify the change and subjected to Pearson correlation analysis. Statistical analysis was performed using SPSS for Windows (version 22.0J; Tokyo, Japan), and significance was set at p < 0.05.
3. Results
3.1. Participants
Eight community-dwelling elderly individuals aged 71.5 ± 4.2 years fulfilled the inclusion criteria of this study. They had 12.6 ± 2.3 years of education and scored 26 points or higher on the MMSE (mean ± SD, 28.4 ± 1.4). The rate of ingestion of the test beverage was 88.6 ± 7.1%. Analysis of participant attributes revealed that mean preintervention and postintervention heights were 154.3 ± 7.8 cm and 153.7 ± 7.7 cm and preintervention and postintervention were 53.0 ± 8.3 kg and 53.4 ± 9.0 kg, respectively, with no significant change in height (t(7) = 2.291, p = 0.56, and d = 0.039) or weight (t(7) = −0.667, p = 0.526, and d = 0.050). Body mass index was 22.4 ± 3.4 kg/m2 preintervention and 22.6 ± 3.6 kg/m2 postintervention, with no significant difference (t(7) = −0.941, p = 0.378, and d = 0.075).
3.2. Cognitive Function
3.2.1. Cogstate
The computerized neurocognitive Cogstate test battery consists of 8 tests of cognitive function. Table 2 shows scores from the tests conducted before and after a 6-month nutritional intervention. In OBT, the total number of errors decreased significantly from 4.0 ± 4.3 preintervention to 1.1 ± 1.9 postintervention, while the accuracy increased significantly from 89.6 ± 10.2% to 96.7 ± 5.2%, respectively.
Table 2.
Neurocognitive function (Cogstate).
| Task | Pre | Post | t | p value | ES (d) |
|---|---|---|---|---|---|
| Groton Maze Chase Test | |||||
| Number of correct responses | 23.0 ± 11.2 | 25.7 ± 8.6 | 0.797 | 0.456 | 0.271 |
| Number of total errors | 1.1 ± 1.5 | 2.7 ± 3.0 | 1.220 | 0.268 | 0.669 |
| Correct responses per second | 0.77 ± 0.37 | 0.86 ± 0.29 | 0.796 | 0.456 | 0.271 |
| Groton Maze Learning Test | |||||
| Number of total errors | 80.9 ± 25.0 | 89.7 ± 21.0 | 0.936 | 0.385 | 0.383 |
| Correct responses per second | 0.45 ± 0.12 | 0.41 ± 0.09 | 1.346 | 0.227 | 0.371 |
| Detection Test | |||||
| Number of total errors | 0.71 ± 1.10 | 0.86 ± 1.80 | 0.157 | 0.881 | 0.093 |
| Reaction times for correct responses | 433 ± 54 | 535 ± 142 | 2.248 | 0.066 | 0.951 |
| Accuracy (%) | 98.1 ± 2.9 | 97.8 ± 4.0 | 0.113 | 0.914 | 0.067 |
| Identification Test | |||||
| Number of total errors | 1.29 ± 1.25 | 1.86 ± 2.79 | 0.603 | 0.603 | 0.264 |
| Reaction times for correct responses | 698 ± 112 | 705 ± 79 | 0.219 | 0.834 | 0.072 |
| Accuracy (%) | 96.1 ± 3.8 | 94.7 ± 7.3 | 0.555 | 0.599 | 0.229 |
| One Card Learning Test | |||||
| Number of total errors | 34.0 ± 8.6 | 35.6 ± 11.3 | 1.010 | 0.352 | 0.157 |
| Reaction times for correct responses | 1094 ± 263 | 1073 ± 165 | 0.238 | 0.820 | 0.096 |
| Accuracy (%) | 61.6 ± 9.6 | 60.3 ± 12.1 | 0.763 | 0.474 | 0.118 |
| One Back Test | |||||
| Number of total errors | 4.0 ± 4.3 | 1.1 ± 1.9 | 2.547 | 0.044 | 0.865 |
| Reaction times for correct responses | 968 ± 171 | 925 ± 82 | 0.712 | 0.503 | 0.325 |
| Accuracy (%) | 89.6 ± 10.2 | 96.7 ± 5.2 | 2.765 | 0.033 | 0.871 |
| Two Back Test | |||||
| Number of total errors | 6.57 ± 4.43 | 5.57 ± 3.21 | 0.491 | 0.641 | 0.259 |
| Reaction times for correct responses | 1049 ± 251 | 1001 ± 161 | 0.618 | 0.559 | 0.227 |
| Accuracy (%) | 83.9 ± 9.4 | 85.7 ± 7.2 | 0.416 | 0.692 | 0.218 |
| Continuous Paired Associate Learning Test | |||||
| Number of total errors | 131 ± 60 | 125 ± 94 | 0.184 | 0.860 | 0.080 |
| Reaction times for correct responses | 5287.6 ± 1422.0 | 4620.7 ± 2222.7 | 0.874 | 0.416 | 0.431 |
| Accuracy (%) | 32.6 ± 10.1 | 39.1 ± 18.7 | 1.047 | 0.335 | 0.436 |
Pre: preintervention; post: postintervention; ES: effect size; the measurement unit of reaction time is millisecond.
3.2.2. CNS Vital Signs
(1) Neurocognitive Tests. Table 3 shows the results of another computerized neurocognitive test battery, CNS Vital Signs, conducted before and after the 6-month intervention period. The number of right taps during the FTT increased significantly from 47.6 ± 2.3 preintervention to 50.3 ± 3.7 postintervention, but the number of left taps tended to increase from 44.8 ± 5.2 to 46.9 ± 4.2, respectively. There were 25.4 ± 11.4 and 35.1 ± 13.1 correct responses to the SAT before and after intervention, respectively, with a significant increase after intervention. Conversely, the number of errors decreased significantly from 17.1 ± 8.4 preintervention to 9.6 ± 8.1 postintervention.
Table 3.
Neurocognitive function (CNS Vital Signs).
| Task | Pre | Post | t | p value | ES (d) |
|---|---|---|---|---|---|
| Verbal Memory Test | |||||
| Correct hit immediate | 11.4 ± 3.2 | 13.6 ± 0.9 | 1.913 | 0.097 | 0.967 |
| Correct passes immediate | 12.1 ± 3.2 | 12.6 ± 2.60 | 0.661 | 0.529 | 0.172 |
| Correct hits delay | 8.5 ± 5.6 | 11.9 ± 1.6 | 1.478 | 0.183 | 0.820 |
| Correct passes delay | 11.5 ± 3.3 | 12.1 ± 2.7 | 0.615 | 0.558 | 0.206 |
| Visual Memory Test | |||||
| Correct hit immediate | 11.1 ± 2.5 | 11.9 ± 3.2 | 0.622 | 0.554 | 0.263 |
| Correct passes immediate | 7.6 ± 3.8 | 9.1 ± 2.5 | 1.587 | 0.156 | 0.466 |
| Correct hits delay | 9.8 ± 4.5 | 9.6 ± 2.2 | 0.072 | 0.945 | 0.035 |
| Correct passes delay | 6.9 ± 3.8 | 8.0 ± 2.3 | 0.709 | 0.501 | 0.365 |
| Finger Tapping Test | |||||
| Right taps average | 47.6 ± 2.3 | 50.3 ± 3.7 | 2.420 | 0.046 | 0.844 |
| Left taps average | 44.8 ± 5.2 | 46.9 ± 4.2 | 2.323 | 0.053 | 0.451 |
| Symbol Digit Coding | |||||
| Correct hits | 36.0 ± 7.6 | 35.3 ± 9.2 | 0.281 | 0.787 | 0.089 |
| Errors | 1.8 ± 1.7 | 3.8 ± 3.2 | 1.313 | 0.231 | 0.784 |
| Stroop Test | |||||
| Simple reaction time | 566 ± 326 | 464 ± 93 | 1.072 | 0.319 | 0.430 |
| Complex reaction time Correct | 739 ± 203 | 886 ± 128 | 1.623 | 0.149 | 0.865 |
| Stroop reaction time correct | 1131 ± 289 | 1005 ± 138 | 1.367 | 0.214 | 0.557 |
| Stroop commission errors | 1.5 ± 0.5 | 1.9 ± 1.7 | 0.753 | 0.476 | 0.293 |
| Shift Attention Test | |||||
| Correct responses | 25.4 ± 11.4 | 35.1 ± 13.1 | 2.654 | 0.033 | 0.794 |
| Errors | 17.1 ± 8.4 | 9.6 ± 8.1 | 2.772 | 0.028 | 0.906 |
| Correct reaction time | 1311 ± 152 | 1241 ± 167 | 1.197 | 0.270 | 0.437 |
| Continuous Performance Test | |||||
| Correct responses | 39.9 ± 0.4 | 39.6 ± 0.7 | 0.798 | 0.451 | 0.429 |
| Omission errors | 0.13 ± 0.35 | 0.38 ± 0.74 | 0.798 | 0.451 | 0.429 |
| Commission errors | 0.75 ± 0.89 | 0.25 ± 0.71 | 1.080 | 0.316 | 0.624 |
| Choice reaction time correct | 535 ± 78 | 541 ± 44 | 0.268 | 0.796 | 0.087 |
Pre-: preintervention; post: postintervention; ES: effect size.
(2) Neurocognitive Domains. Table 4 shows the scores for 11 neurocognitive domains from the 7 CNS Vital Signs tests. The score for composite memory increased significantly from 77.4 ± 11.0 preintervention to 93.0 ± 18.8 postintervention. Similarly, the score for verbal memory increased significantly from 84.0 ± 10.6 preintervention to 103.4 ± 19.4 postintervention. In terms of complex attention, the score increased significantly from 88.5 ± 13.7 preintervention to 101.6 ± 13.7 postintervention. The score for cognitive flexibility was 82.3 ± 13.3 preintervention and 96.1 ± 14.2 postintervention, showing a significant increase after the 6-month intervention. Additionally, a significant postintervention increase was observed in executive function (82.8 ± 13.0 to 96.3 ± 13.7) and motor speed (92.5 ± 5.2 and 98.4 ± 7.7).
Table 4.
Neurocognitive function (CNS Vital Signs).
| Domain | Pre | Post | t | p value | ES (d) |
|---|---|---|---|---|---|
| Composite memory | 77.4 ± 11.0 | 93.0 ± 18.8 | 2.434 | 0.045 | 1.016 |
| Verbal memory | 84.0 ± 10.6 | 103.4 ± 19.4 | 3.221 | 0.015 | 1.239 |
| Visual memory | 78.1 ± 12.2 | 84.3 ± 19.0 | 0.819 | 0.440 | 0.384 |
| Psychomotor speed | 90.3 ± 8.2 | 95.4 ± 9.4 | 1.323 | 0.228 | 0.581 |
| Reaction time | 76.1 ± 13.7 | 80.1 ± 12.0 | 0.933 | 0.382 | 0.311 |
| Complex attention | 88.5 ± 13.7 | 101.6 ± 13.7 | 2.918 | 0.022 | 0.957 |
| Cognitive flexibility | 82.3 ± 13.3 | 96.1 ± 14.2 | 2.943 | 0.022 | 1.009 |
| Processing speed | 92.4 ± 12.5 | 91.4 ± 10.1 | 0.164 | 0.874 | 0.088 |
| Executive function | 82.8 ± 13.0 | 96.3 ± 13.7 | 2.766 | 0.028 | 1.011 |
| Simple attention | 99.3 ± 8.4 | 104.3 ± 9.2 | 1.228 | 0.259 | 0.570 |
| Motor speed | 92.5 ± 5.2 | 98.4 ± 7.7 | 2.552 | 0.038 | 0.896 |
Pre: preintervention; post: postintervention; t: test; ES: effect size.
3.3. Blood Parameters
Table 5 shows the changes in blood parameters. After the intervention, there was a significant increase in serum DHEA-S levels and a significant decrease in plasma Aβ (1–42) and Aβ42/Aβ40 levels.
Table 5.
Blood parameters.
| Pre | Post | t | p value | ES (d) | |
|---|---|---|---|---|---|
| BDNF (ng/mL) | 22.96 ± 5.12 | 21.90 ± 5.38 | 0.774 | 0.464 | 0.201 |
| DHEA-S (µg/dL) | 55.88 ± 29.53 | 68.50 ± 39.89 | 2.583 | 0.036 | 0.360 |
| Cortisol (µg/dL) | 9.15 ± 0.37 | 10.29 ± 3.79 | 0.846 | 0.426 | 0.344 |
| Amyloid-β 40 | 360.00 ± 42.35 | 352.63 ± 43.50 | 0.768 | 0.468 | 0.172 |
| Amyloid-β 42 | 52.90 ± 6.80 | 38.21 ± 4.49 | 6.762 | <0.001 | 2.549 |
| Amyloid-β 42/40 | 0.149 ± 0.025 | 0.109 ± 0.012 | 6.294 | <0.001 | 1.973 |
Pre: preintervention; post: postintervention; ES: effect size.
3.4. Correlation between Cognitive Function and Blood Parameters
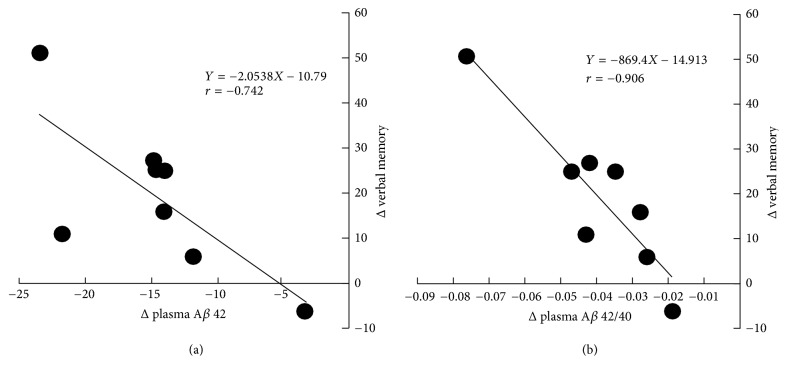
A correlation analysis conducted between biological data and neurocognitive test items showed a significant change due to the intervention: a significant negative correlation between verbal memory and plasma Aβ (1–42) (r = −0.742, p = 0.035) and plasma Aβ42/Aβ40 (r = −0.906, p = 0.002) levels (Figure 1).
Figure 1.
Correlation between Δverbal memory and Δplasma Aβ 42 (a) and plasma Aβ 42/40 (b).
4. Discussion
To our knowledge, this is the first study to show that a 6-month intake of CGAs improves cognitive function in the elderly. The CGA-induced improvement in cognitive function was reflected in the computerized neurocognitive tests OBT, SAT, and FTT. Also, a significant improvement in composite and verbal memory, complex attention, cognitive flexibility, executive function, and motor speed was identified by combining scores from the individual CNS Vital Signs tests. Among the biological parameters, plasma Aβ42 and Aβ42/Aβ40 levels decreased significantly, whereas DHEA-S levels increased significantly. In particular, plasma Aβ42 and Aβ42/Aβ40 decreased to levels where improvement of verbal memory was apparent.
Using 2 computerized test batteries, 15 different neurocognitive tests were conducted in this study. The results showed an improvement in the OBT error response and accuracy, in the SAT correct and error responses, and in the FTT tap average. Previous studies have shown an association between OBT response and working memory [22] and between SAT/FTT performance and attentional and executive functions. Furthermore, cognitive function is associated with working memory and attentional and executive functions in the prefrontal region [23]. Therefore, CGAs are presumed to improve brain function in the prefrontal region.
Previous studies have demonstrated the antioxidant effects of CGAs [24] and the improvement in cognitive function mediated by CGAs through their antioxidative and neuroprotective effects. The antioxidant effects are considered one of the most important factors to prevent the age-related neurodegenerative diseases as recent studies show that the oxidative stress links to Aβ aggregation and following dementia due to AD [25]. In a study using mice with scopolamine-induced amnesia, administration of 5-CQA, the main component of CGAs, inhibited acetylcholinesterase activity in the prefrontal cortex [12]. Whether the results of the animal study can be applied to humans is unknown; nevertheless, the findings of the present study suggest that CGAs improve brain function in the prefrontal region in humans.
The metabolism of CGAs in the body generates gallic acid, caffeic acid, coumaric acid, ferulic acid, and sinapic acid [14], all of which have been shown to suppress the breakdown of the amyloid-precursor protein, which is pathologically related to the Aβ (1–42) protein, lipid peroxidation, and neurite extension of hippocampal neurons [13, 15]. These functions are likely involved in the improved memory following CGA intake.
We also analyzed the correlation between neurocognitive test scores and biological data and confirmed a significant decrease in plasma Aβ42 and Aβ42/Aβ40 levels and a significant increase in DHEA-S levels after a 6-month CGA intake period. Specifically, the change in plasma Aβ42 or Aβ42/Aβ40 levels was negatively correlated with an improvement in verbal memory (Figure 1), indicating that verbal memory improves as the level of Aβ42 or Aβ42/Aβ40 decreases. In previous cohort studies, the level of Aβ42 increased before the onset of AD or in the early stage of AD but decreased gradually thereafter [26–28]. Thus, the level of plasma Aβ is a predictor of AD and cognitive impairment. However, no study has investigated the effect of food or dietary intervention using plasma Aβ as an indicator. In a cross-sectional study of healthy elderly individuals aged ≥65 years with normal cognitive function, Gu et al. investigated the association between nutrient intake and plasma Aβ levels and reported that higher dietary intake of ω-3 PUFAs was associated with lower plasma levels of Aβ42 [29]. In another study of healthy elderly individuals aged ≥65 years, exercise intervention led to an improvement in cognitive function and a significant reduction in plasma Aβ42/Aβ40 levels. However, no correlation with improvement in cognitive function and no degree of change in Aβ42/Aβ40 levels was observed [30]. These findings suggest that although it is too soon to draw conclusions about plasma Aβ42/Aβ40 levels, the correlation between the improvement in verbal memory and the change in Aβ42/Aβ40 levels in this study warrants further investigation.
In this study, attentional, executive, and memory functions improved significantly after a 6-month CGA intake period in community-dwelling elderly individuals with complaints of subjective memory loss. Previous studies have shown that CGAs improve blood pressure and vascular endothelial functions, both of which are associated with the onset of dementia [17, 31, 32]. Because hypertension in middle age is a risk factor for dementia and cognitive impairment in old age [33], continuous consumption of CGAs may delay the onset of dementia.
This study has limitations as follows. First, this was a single-arm study with no control. Therefore, the beneficial effect observed in this study might have been due, at least in part, to placebo effects. The influences of the participants' anticipation and activities other than CGA intake cannot completely be ruled out although there seemed to be no great changes in their activities during the 6-month trial according to the diary they submitted. Second, multiple cognitive functions were carefully investigated in this study, but because of the small number of participants, further study with a larger number of participants and a control group is needed to verify the effect of CGA intake on cognitive function.
5. Conclusion
The findings of this study suggest that CGAs may improve cognitive function, especially attentional, executive, and memory functions in the elderly.
Acknowledgments
The authors thank the participants and professional physicians and students of Yonezawa University of Nutrition Sciences for their cooperation. This study was supported partly by the Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (16K01600).
Conflicts of Interest
This study was supported financially by Kao Corporation. Ryuji Ochiai, Kazuya Kozuma, Hirotaka Sato, and Yoshihisa Katsuragi are Kao Corporation employees.
References
- 1.Prince M., Wimo A., Guerchet M., Ali G. C., Wu Y. T., Prina M. Proceedings of the World Alzheimer Report, 2015., The Global Impact of Dementia: an Analysis of Prevalence, Incidence, Cost and Trends; 2015; London, UK. [Google Scholar]
- 2.Wang H.-X., Jin Y., Hendrie H. C., et al. Late life leisure activities and risk of cognitive decline. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2013;68(2):205–213. doi: 10.1093/gerona/gls153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devore E. E., Grodstein F., Van Rooij F. J. A., et al. Dietary intake of fish and omega-3 fatty acids in relation to long-term dementia risk. American Journal of Clinical Nutrition. 2009;90(1):170–176. doi: 10.3945/ajcn.2008.27037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devore E. E., Grodstein F., van Rooij F. J. A., et al. Dietary antioxidants and long-term risk of dementia. JAMA Neurology. 2010;67(7):819–825. doi: 10.1001/archneurol.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu Y., Scarmeas N. Dietary patterns in Alzheimer's disease and cognitive aging. Current Alzheimer Research. 2011;8(5):510–519. doi: 10.2174/156720511796391836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calon F., Lim G. P., Yang F., et al. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43(5):633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan Z. S., Harris W. S., Beiser A. S., et al. Red blood cell omega-3 fatty acid levels and markers of accelerated brain aging. Neurology. 2012;78(9):658–664. doi: 10.1212/wnl.0b013e318249f6a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuriyama S., Hozaka A., Ohmori K., et al. Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project. American Journal of Clinical Nutrition. 2006;83:355–361. doi: 10.1093/ajcn/83.2.355. [DOI] [PubMed] [Google Scholar]
- 9.van Gelder B. M., Buijsse B., Tijhuis M., et al. Coffee consumption is inversely associated with cognitive decline in elderly European men: The FINE Study. European Journal of Clinical Nutrition. 2007;61(2):226–232. doi: 10.1038/sj.ejcn.1602495. [DOI] [PubMed] [Google Scholar]
- 10.Martín E. D., Buño W. Caffeine-mediated presynaptic long-term potentiation in hippocampal CA1 pyramidal neurons. Journal of Neurophysiology. 2003;89(6):3029–3038. doi: 10.1152/jn.00601.2002. [DOI] [PubMed] [Google Scholar]
- 11.Clifford M. N. Chlorogenic acids and other cinnamates —nature, occurrence and dietary burden. Journal of the Science of Food and Agriculture. 1999;79(3):362–372. doi: 10.1002/(SICI)1097-0010(19990301)79:3<362::AID-JSFA256>3.0.CO;2-D. [DOI] [Google Scholar]
- 12.Kwon S.-H., Lee H.-K., Kim J.-A., et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. European Journal of Pharmacology. 2010;649(1–3):210–217. doi: 10.1016/j.ejphar.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Ito H., Sun X.-L., Watanabe M., Okamoto M., Hatano T. Chlorogenic acid and its metabolite m-coumaric acid evoke neurite outgrowth in hippocampal neuronal cells. Bioscience, Biotechnology, and Biochemistry. 2008;72(3):885–888. doi: 10.1271/bbb.70670. [DOI] [PubMed] [Google Scholar]
- 14.Farah A., Monteiro M., Donangelo C. M., Lafay S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. Journal of Nutrition. 2008;138(12):2309–2315. doi: 10.3945/jn.108.095554. [DOI] [PubMed] [Google Scholar]
- 15.Lee H. E., Kim D. H., Park S. J., et al. Neuroprotective effect of sinapic acid in a mouse model of amyloid β1-42 protein-induced Alzheimer's disease. Pharmacology Biochemistry & Behavior. 2012;103(2):260–266. doi: 10.1016/j.pbb.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Ochiai R., Sugiura Y., Shioya Y., Otsuka K., Katsuragi Y., Hashiguchi T. Coffee polyphenols improve peripheral endothelial function after glucose loading in healthy male adults. Nutrition Research. 2014;34(2):155–159. doi: 10.1016/j.nutres.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Ota N., Soga S., Murase T., Shimotoyodome A., Hase T. Consumption of coffee polyphenols increases fat utilization in humans. Journal of Health Science. 2010;56(6):745–751. doi: 10.1248/jhs.56.745. [DOI] [Google Scholar]
- 18.Satake S., Senda K., Hong Y.-J., et al. Validity of the Kihon Checklist for assessing frailty status. Geriatrics & Gerontology International. 2016;16(6):709–715. doi: 10.1111/ggi.12543. [DOI] [PubMed] [Google Scholar]
- 19.Roehrs T., Roth T. Caffeine: sleep and daytime sleepiness. Sleep Medicine Reviews. 2008;12(2):153–162. doi: 10.1016/j.smrv.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Lewis M. S., Dingwall K. M., Berkhout N., Sayers S., Maruff P., Cairney S. Assessment of cognition in an adolescent Indigenous population. Australian Psychologist. 2010;45(2):123–131. doi: 10.1080/00050060903352998. [DOI] [Google Scholar]
- 21.Gualtieri C. T., Johnson L. G. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Archives of Clinical Neuropsychology. 2006;21(7):623–643. doi: 10.1016/j.acn.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Maruff P., Lim Y. Y., Darby D., et al. Clinical utility of the cogstate brief battery in identifying cognitive impairment in mild cognitive impairment and Alzheimer’s disease. BMC Psychology. 2013;1(1) doi: 10.1186/2050-7283-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCabe D. P., Roediger H. L., McDaniel M. A., Balota D. A., Hambrick D. Z. The relationship between working memory capacity and executive functioning: Evidence for a common executive attention construct. Neuropsychology. 2010;24(2):222–243. doi: 10.1037/a0017619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakatani N., Kayano S.-I., Kikuzaki H., Sumino K., Katagiri K., Mitani T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.) Journal of Agricultural and Food Chemistry. 2000;48(11):5512–5516. doi: 10.1021/jf000422s. [DOI] [PubMed] [Google Scholar]
- 25.Cheignon C., Tomas M., Bonnefont-Rousselot D., Faller P., Hureau C., Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biology. 2017;14:450–464. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayeux R., Honig L. S., Tang M.-X., et al. Plasma Aβ40 and Aβ42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology. 2003;61(9):1185–1190. doi: 10.1212/01.wnl.0000091890.32140.8f. [DOI] [PubMed] [Google Scholar]
- 27.Schupf N., Tang M. X., Fukuyama H., et al. Peripheral Aβ subspecies as risk biomarkers of Alzheimer's disease. Proceedings of the National Acadamy of Sciences of the United States of America. 2008;105(37):14052–14057. doi: 10.1073/pnas.0805902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayeux R., Tang M.-X., Jacobs D. M., et al. Plasma amyloid β-peptide 1-42 and incipient Alzheimer's disease. Annals of Neurology. 1999;46(3):412–416. doi: 10.1002/1531-8249(199909)46:3<412::AID-ANA19>3.0.CO;2-A. doi: 10.1002/1531-8249(199909)46:3<412::AID-ANA19>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 29.Gu Y., Schupf N., Cosentino S. A., Luchsinger J. A., Scarmeas N. Nutrient intake and plasma β-amyloid. Neurology. 2012;78(23):1832–1840. doi: 10.1212/wnl.0b013e318258f7c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokoyama H., Okazaki K., Imai D., et al. The effect of cognitive-motor dual-task training on cognitive function and plasma amyloid β peptide 42/40 ratio in healthy elderly persons: A randomized controlled trial. BMC Geriatrics. 2015;15(1, article no. 60) doi: 10.1186/s12877-015-0058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ochiai R., Jokura H., Suzuki A., et al. Green coffee bean extract improves human vasoreactivity. Hypertension Research. 2004;27(10):731–737. doi: 10.1291/hypres.27.731. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe T., Arai Y., Mitsui Y., et al. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clinical and Experimental Hypertension. 2006;28(5):439–449. doi: 10.1080/10641960600798655. [DOI] [PubMed] [Google Scholar]
- 33.Elias M. F., Goodell A. L., Dore G. A. Hypertension and cognitive functioning: A perspective in historical context. Hypertension. 2012;60(2):260–268. doi: 10.1161/HYPERTENSIONAHA.111.186429. [DOI] [PubMed] [Google Scholar]