Abstract
Background
This study is designed to investigate whether vitamin D promotes diabetic wound healing and explore the potential mechanism which may be involved in the healing process.
Material and Methods
Human umbilical vein endothelial cells (HUVECs) were treated with 200 μg/ml of advanced glycation end product-modified human serum albumin (AGE-HSA) and 250 mg/dl of glucose with vitamin D. Cell viability was analyzed using the CCK-8 assay, and the apoptosis rate was measured using flow cytometry. Endogenous markers of ER stress were quantified using Western blot and a real-time polymerase chain reaction. Diabetic mice were treated with vitamin D (100 ng/kg per day) for 14 days. The ulcer area and ulcerative histology were detected dynamically.
Results
Vitamin D administration not only decreased the apoptosis rate but also increased cell viability. Furthermore, the expression of endogenous markers of ER stress was downregulated as a result of vitamin D treatment. Vitamin D supplementation significantly accelerated wound healing of diabetic mice and improved the healing quality. Further studies showed that reduced ER stress was associated with the positive outcome.
Conclusion
These results suggest that vitamin D may ameliorate impaired wound healing in diabetic mice by suppressing ER stress.
1. Introduction
Endothelial dysfunction plays a vital role in the pathogenesis and progression and complications of diabetic vascular diseases. In the past few decades, the effect of high glucose levels and advanced glycation end product on vascular endothelial cells has been widely studied. The mechanisms underlying endothelial dysfunction include accumulation of advanced glycation end products [1], increased oxidative stress [2], and endoplasmic reticulum (ER) stress [3]. Although antioxidants have been promoted and tested in clinical trials, antioxidants have not been shown to be effective in preventing vascular dysfunction. This finding may in part be due to the presence of ER stress, which is not influenced by antioxidant therapy [4].
The ER is a membrane-bound organelle responsible for several cellular functions, such as posttranslational folding of proteins and calcium storage. If ER homeostasis is disrupted, misfolded, or unfolded proteins within this organelle build-up (ER stress) [5]. The cellular response to ER stress is referred to as an unfolded protein response (UPR), which means translation is slowed down, protein degradation is induced, and the folding capacity of the ER is increased [6]. An UPR may act as a survival or apoptotic pathway depending on the severity of ER stress [7].
Vitamin D is known to enhance intestinal calcium absorption and increase the plasma calcium level [8]. According to recent studies, the biological effects of vitamin D extend far beyond calcium metabolism.
Mounting evidence and data suggest a link between vitamin D deficiency and an increased risk of cardiovascular diseases [9–15]. The low vitamin D status may be a contributing factor in peripheral arterial diseases and diabetic vascular diseases [16, 17]. Vitamin D induces biological effects by binding to its receptor (VDR) on target cells and organs. Activation of VDR impacts downstream genes to cause various effects, such as anti-inflammation, antiatherosclerosis, and direct cardioprotective actions [18–21].
This study is designed to investigate whether Vitamin D promotes diabetic wound healing and, furthermore, explore the potential mechanism which may be involved in the healing process.
2. Materials and Methods
2.1. Materials
The 1,25-dihydroxyvitamin D3 was purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum, Dulbecco's modified Eagle's medium (DMEM)/F12, and trypsin were purchased from Hyclone (Logan, UT, USA). The CCK-8 assay was purchased from Dojindo (Japan). Annexin V-FITC/PI was purchased from Yeasen Corporation (Shanghai, China). A PrimeScript RT Reagent Kit and SYBR Premix Ex Taq™ II were purchased from TaKaRa (Tokyo, Japan). TRIzol reagent was purchased from Invitrogen (Carlsbad, CA, USA). Primers targeting glucose-regulated protein (GRP) 78, ATF4, CHOP, VDR, and GAPDH were synthesized by Sangon Biotech (Shanghai, China). Radio immunoprecipitation assay (RIPA) lysis buffer, a bicinchoninic acid (BCA) protein assay kit, antibody solution, and 5x sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) same loading buffer were purchased from Beyotime Biotechnology (Shanghai, China). Antibodies (VDR, PERK P-PERK, eIF-2α, P-eIF-2α, ATF4, CHOP, and GRP78) were purchased from Cell Signaling Technology (Beverly, MA, USA). Polyvinylidene difluoride membranes (0.45 mm) were purchased from Millipore (Billerica, MA, USA). Human serum albumin (HSA; 1.5 mmol/l) was purchased from Sigma-Aldrich.
2.2. Experimental Design
Human umbilical vein endothelial cells (HUVECs) were divided into four groups based on treatment, as follows: 200 μg/ml of HSA and 100 mg/dl of glucose (normal group); 200 μg/ml of AGE-HSA and 250 mg/dl of glucose (diabetic group); 200 μg/ml of AGE-HSA and 250 mg/dl of glucose with vitamin D (50 ng/ml) (VD treatment group); and 200 μg/ml of AGE-HSA, 250 mg/dl of glucose, vitamin D (50 ng/ml), and VDR siRNA (VD antagonist group). Each experiment was repeated at least three independent experiments.
2.3. Cell Culture
HUVECs were obtained from Shanghai Bogoo Biotechnology (Shanghai, China). Cells were incubated in DMEM/F12 containing 5 mM d-glucose at 37.0°C in a humidified atmosphere of 5% CO2 in air until 80% confluence. The cells were exposed to different conditions. Medium was replaced every 2-3 days and 24 h before the end of the experiment.
2.3.1. Inhibition of VDR Expression via siRNA Silencing
To knock out VDR expression, HUVECs were transfected with 10 nM control siRNA or 10 nM VDR-specific siRNA using lipofectamine (Invitrogen, Carlsbad, CA, USA) for 72 h. VDR levels were then measured by Western blotting.
2.4. Cell Viability
Cell viability was measured using the CCK-8 assay, following the manufacturer's protocol. Briefly, HUVECs were placed in 96-well plates at 5000 cells/well. The plates were preincubated at 37.0°C in a humidified atmosphere of 5% CO2 in air until 80% confluence. Then, 10 μl of the CCK-8 solution was added to each well of the plate. The plate was incubated for 1–4 h. The absorbance was measured at 450 nm using a microplate reader.
2.5. Cell Apoptosis
Cell apoptosis was detected by flow cytometry. Cells were collected after treatment and washed twice with cold phosphate-buffered saline. Then, the cells were resuspended at 1 × 106/ml in 500 μl of binding buffer, which was included in the Annexin V-FITC/propidium iodide(PI) apoptosis detection kit and treated with 5 μl of Annexin V-FITC and 5 μl of PI following the manufacturer's protocol. Subsequently, the cells were incubated for 10 min before the apoptosis rate was measured by flow cytometry.
2.6. RNA Extraction and RT-PCR
Total RNA was extracted from HUVECs with TRIzol reagent following the manufacturer's protocol, and a PrimeScript RT Reagent Kit was used to synthesize complementary DNA. RT-PCR was performed to validate the expression pattern of selected genes (VDR, GRP78, CHOP, and ATF4) using SYBR Premix EX Taq II. Data were analyzed using the 2−∆∆Ct method. GAPDH was used as a reference gene. The primers used are listed in Table 1.
Table 1.
The primes of ER stress genes.
| GRP78 | Forward: 5′-TAGCGTATGGTGCTGCTGTC-3′ |
| Reverse: 5′-CCTTGGAATCAGTTTGGTCAT-3′ | |
|
| |
| CHOP | Forward: 5′-GCCTTTCTCCTTTGGGACACTGTCCAGC-3′ |
| Reverse: 5′-CTCGGCGAGTCGCCTCTACTTCCC-3′ | |
|
| |
| ATF4 | Forward: 5′-GGAGGTGGCCAAGCACTTCA-3′ |
| Reverse: 5′-CTTCTGGCGGTACCTAGTGG-3 | |
|
| |
| VDR | Forward: 5′-AGGCGAAGCATGAAGCGGAAG-3′ |
| Reverse: 5′-GCGTCCAGCAGTATGGCAATGA-3′ | |
|
| |
| GAPDH | Forward: 5′-CCAGCAAGAGCACAAGAGGAA-3′ |
| Reverse: 5′-ATGGTACATGACAAGGTGCGG-3′ | |
2.7. Western Blot Analysis
HUVECs were washed three times with cold phosphate-buffered saline after treatment. Cells were collected and lysed for 30 min on ice in RIPA buffer containing a protease inhibitor cocktail before being centrifuged at 12,000 rpm for 15 min at 4°C to obtain the protein-containing supernatant. The protein concentration was then measured with a BCA assay kit. The proteins were separated by 10%–12% SDS-PAGE and transferred to a 0.45 μm polyvinylidene difluoride membrane, which was then blocked in 5% skim milk for 1 h at room temperature, and incubated with primary antibodies at 4°C overnight. The membranes were then washed three times with Tris-buffered saline Tween 20 and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. The expression of various proteins was subsequently visualized by enhanced chemiluminescence.
2.7.1. Animals and Induction of DM
6-week old male ICR mice were purchased from SLAC Laboratory Animal Co. Ltd. (Shanghai, China) and used to establish a mouse model for DM. All mice were lodged in individual cages in a temperature- and humidity-controlled room (22 ± 1°C and 50 ± 1% humidity) with 12-hour light cycle in the animal facility of the Animal Unit of Tongji University. Diabetes was inducted in mice with STZ injected intraperitoneally once at a dose of 100 mg/kg (in 0.01 M sodium citrate, pH 4.3–4.5). Nondiabetic mice were injected with only a saline vehicle. After 7 days, mice with fasting blood glucose levels higher than 250 mg/dl were considered as diabetic. All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Biological Research Ethics Committee of the Chinese Academy of Sciences. Forty-five mice were randomly divided into three groups, as follows: (1) normal group: nondiabetic mice who received a saline vehicle for 14 days; (2) diabetic mellitus group (DM group): diabetic mice were injected with a saline vehicle for 14 days; and (3) vitamin D treatment group (VD treatment group): diabetic mice were treated with vitamin D (100 ng/kg per day) for 14 days.
2.7.2. Wound Biopsy and Measurement of Wound Closure
After 4 weeks of STZ induction, a model for diabetic wound was created in mice as follows: All mice were anesthetized with isoflurane, and their back were shaved and sterilized. Then full thickness excisional wounds were made on the dorsal back by a disposable 6 mm skin biopsy punch and Westcott scissor. Wounds in individual mice were photographed digitally days 0, 3, 6, and 9 until the end (day 14). A digital camera (EOS50; Cannon, Japan) was employed to take pictures, and the ulcer area was analyzed by Image-Pro Plus 4.5 software.
2.7.3. Histological Assessment of Wound Healing
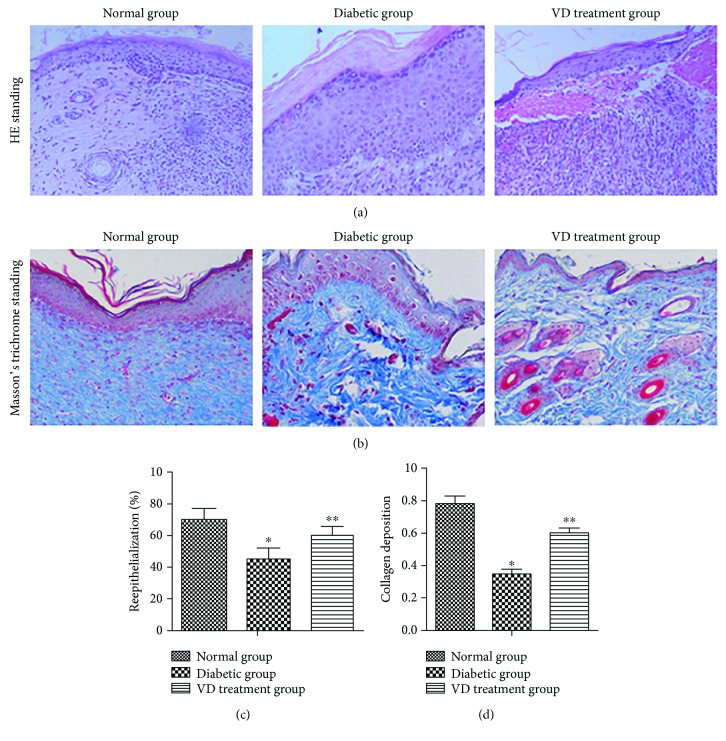
The wounds, together with unwounded skin margins, were collected in both groups, fixed in paraffin, and sectioned at 5.0 μm. The sections were dehydrated with successive concentrations of ethanol and washed twice in distilled water. The sections of the ulcerative tissue at D14 were stained with hematoxylin and eosin (H&E) and with Masson's trichrome in accordance with the protocols of the manufacturer (Cyagen Biosciences Inc.) to detect the reepithelialization/granulation tissue formation and collagen deposition, respectively.
2.7.4. Measurement of ER Stress in Diabetic Wounds
Mouse wounds were harvested and homogenized in cold PBS supplemented with protease inhibitor cocktail (Sigma-Aldrich) by using a Dounce homogenizer and then sonicated and centrifuged at 10000 rpm for 20 minutes at 4°C. Supernatants were used for RNA extraction. RT-PCR was performed to validate the expression pattern of selected genes (GRP78, CHOP, and ATF4) using SYBR Premix EX Taq II. Data were analyzed using the 2−∆∆Ct method. GAPDH was used as a reference gene.
2.8. Statistical Analysis
All cell data are expressed as the mean ± SD of at least three independent experiments. Each in vivo experiment was conducted using at least fifteen mice per group. Results are also expressed as mean ± SD. Differences between the experimental groups were assessed by Student t-test or one-way analysis of variance, followed by Dunnett's test. For all statistical analyses, p values < 0.05 were considered statistically significant.
3. Results
3.1. Cell Experiment
3.1.1. Knockout VDR Expression in HUVECs
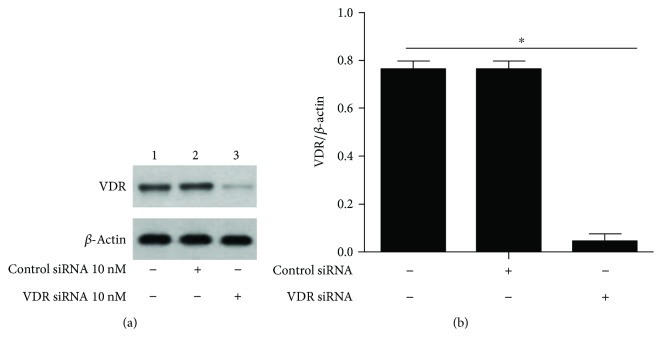
HUVECs were transfected with 10 nM control siRNA or VDR siRNA for 72 h, and then VDR expression was measured by Western blotting (Figure 1). VDR expression decreased by 72.4% compared with the blank and control groups. Based on these results, we confirmed that 10 nM VDR siRNA successfully knocked down VDR expression.
Figure 1.
siRNA-mediated VDR knockout in HUVEC. (a) HUVECs were transfected with either control siRNA (10 nM) or VDR siRNA (10 nM), and VDR expression was measured 72 h later by Western blot. (b) Western blot was quantified, and VDR expression is presented as percentage. ∗p < 0.05, relative to normal cells and cells transfected with control siRNA.
3.1.2. Effect of Vitamin D on Cell Viability and Apoptosis
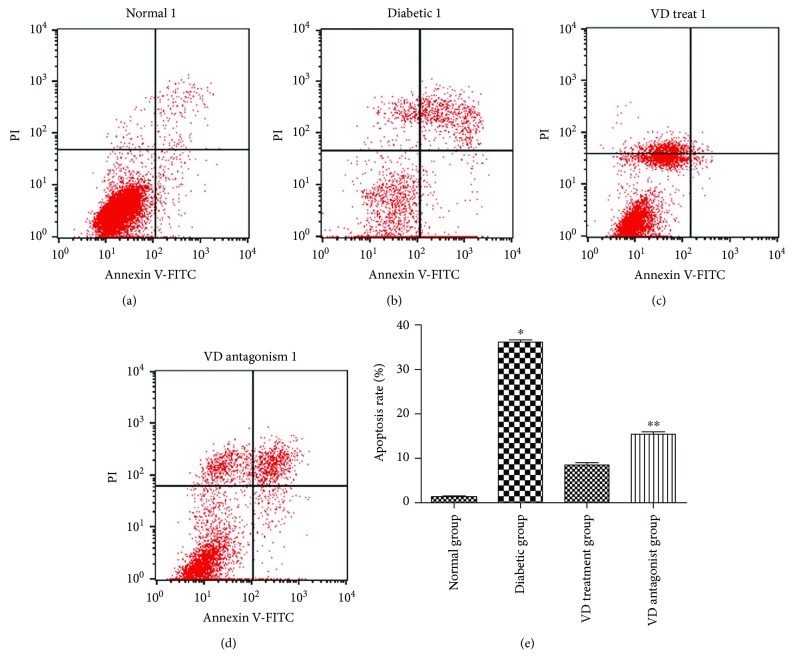
HUVECs were incubated for various lengths of time (0 h, 12 h, and 24 h) under different conditions. The cell viability in each group was measured by the CCK-8 assay. As shown in Figure 2, the cell viability in the diabetic group was sharply diminished compared with the normal group. This reduction was reversed by pretreatment with vitamin D. The cell viability in the vitamin D group was similar to the normal group; thus, vitamin D has the potential to restore endothelial cell dysfunction. Interestingly, coadministration of vitamin D and VDR siRNA abolished the restoration ability; thus, the VDR pathway was involved in the process. A similar outcome was observed by detection of cell apoptosis in different groups (Figure 3). The cell apoptosis rate was measured with an Annexin V-FITC/PI assay using flow cytometry. The apoptosis rate in the diabetic group was significantly higher than the normal group. In the vitamin D group, the endothelial cell apoptosis rate decreased. This positive effect was blocked by the VDR suppressor.
Figure 2.
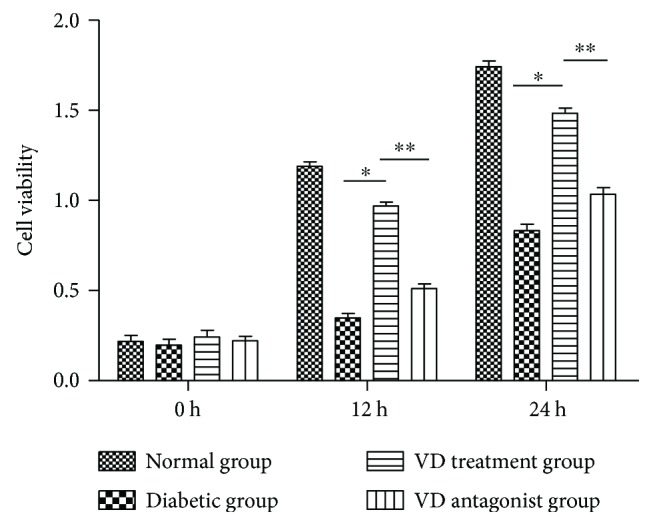
Effect of vitamin D on cell viability: HUVECs were exposed to different conditions for 0, 12, and 24 h. Normal group (200 μg/ml human serum albumin (HAS) and 100 mg/dl glucose); diabetic group (200 μg/ml AGE-HSA and 250 mg/dl glucose); vitamin D treatment group (vitamin D 50 nm, 200 μg/ml AGE-HSA, and 250 mg/dl glucose); and vitamin D antagonist group (vitamin D 50 nm, VDR siRNA 10 nm, 200 μg/ml AGE-HSA, and 250 mg/dl glucose). Cell viability was measured by CCK-8 assay. ∗p < 0.05 relative to the VD treatment group. ∗∗p < 0.05 relative to the VD antagonist group.
Figure 3.
Effect of vitamin D on cell apoptosis. HUVECs were exposed to different conditions. Cell apoptosis rate was measured by flow cytometry. (a) Normal group. (b) Diabetic group. (c) Vitamin D treatment group. (d) Vitamin D antagonist group. (e) Apoptosis rate in each group. ∗p < 0.05 relative to the VD treatment group. ∗∗p < 0.05 relative to the VD treatment group.
3.1.3. The Expression of Endogenous Biomarkers of ER Stress
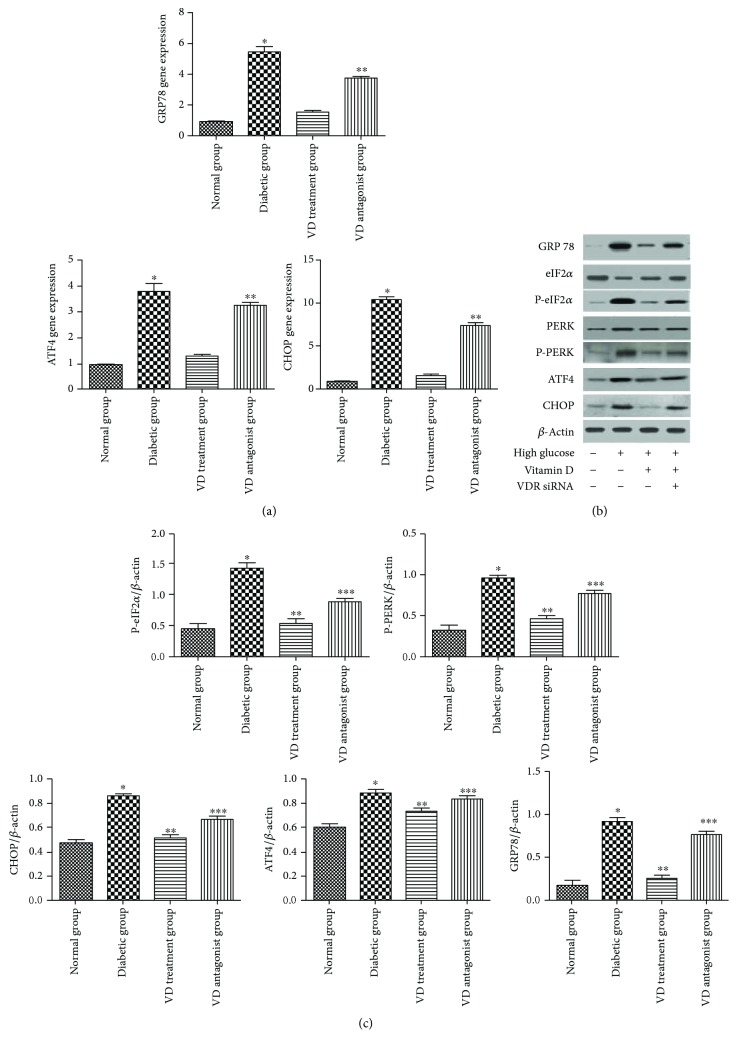
ER stress is a new mechanism underlying the pathogenesis of endothelial dysfunction induced by a diabetic-like environment. In the current study, the endogenous biomarkers of ER stress were detected at the gene and protein levels (Figure 4). The diabetic-like environment induced GRP78 expression relative to the normal group, which was inhibited by treatment with vitamin D for 24 h. Activation of GRP78 enabled dimerization of PERK and its consequent auto-phosphorylation and then phosphorylated downstream eIF2a. Both phosphorylated PERK and eIF2a were inhibited by vitamin D treatment. The diabetic-like environment had no effect on nonphosphorylated PERK and eIF2a. As PERK-eIF2a-ATF4-CHOP represents a classical ER stress pathway, the expression of ATF4 and CHOP was detected by PCR and Western blotting. It was shown that a diabetic-like environment also induced ATF4 and CHOP expression. In the vitamin D group, ATF4 and CHOP expression was decreased. Conversely, the increased expression of ATF4 and CHOP was detected in the VDR antagonist group.
Figure 4.
Effect of vitamin D on endoplasmic reticulum stress. (a) mRNA levels of GRP78, ATF4, and CHOP. ∗p < 0.05, relative to the VD treatment group. ∗∗p < 0.05 relative to the VD treatment group. (b, c) Protein levels of GRP78, PERK, P-PERK, eIF-α, P-eIF-α, ATF4, and CHOP. Expression is presented as percentage. ∗p < 0.05, relative to the normal group. ∗∗p < 0.05, relative to the diabetic group. ∗∗∗p < 0.05, relative to the VD treatment group.
3.1.4. VDR Expression in Different Groups
VDR expression was detected in different groups. RT-PCR was used to analyze the difference in VDR gene expression in each group. The results showed that VDR expression was decreased in the diabetic group compared with the normal group. Vitamin D treatment partly restored VDR expression (Figure 5(a)). There was no statistical significance between the diabetic and VD treatment groups. A similar outcome was confirmed by Western blot analysis (Figure 5(b)).
Figure 5.
VDR expression in different groups: (a) mRNA levels of VDR gene in different groups. (b) Western blot was quantified, and VDR expression is presented as percentage. ∗p < 0.05, relative to the normal group. ∗∗p > 0.05, relative to the diabetic group.
3.1.5. Animal Experiment
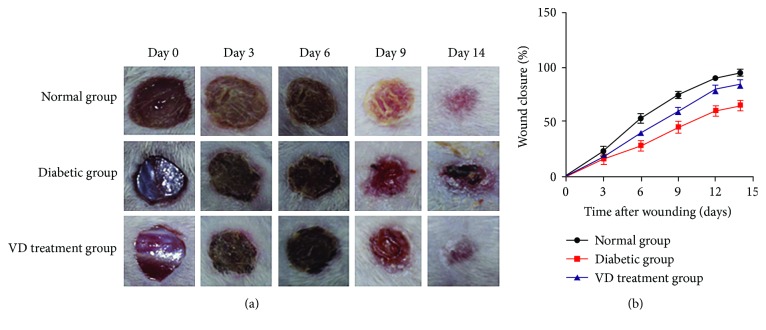
(1) Supplementation of Vitamin D Accelerated Diabetic Wound Healing. The representative ulceration images for the three groups at D0, D3, D6, D9, and D14 posttreatment are presented in Figure 6. Figure 6(b) shows the mean area of ulceration at relevant time points. At D3 posttreatment, incrustation was formed in each group; there was no significant difference to the size of ulcers. By the end of observation (D14), nondiabetic wounds completely healed, while most of the diabetic wounds remained open with a low average closure rate of 65%. Vitamin D significantly improved diabetic wound closure and increased the healing rate of diabetic wound by 20.4% (85.4% versus 65.0%, p < 0.05).
Figure 6.
Vitamin D treatment accelerated diabetic wound healing. (a) 6 mm diameter wounds were created by punch biopsy, and the closure of the wound area was measured by digital camera every 3 days until day 14. (b) Percentage of wound closure (means ± SD). Healing of diabetic wounds significantly delayed compared with normal wounds. Vitamin D began to improve diabetic wound closure on day 6. At the end of the observation (day 14), the VD treatment group exhibited improved wound healing, compared with the diabetic group.
(2) Supplementation of Vitamin D Improved Diabetic Wound Healing. Reepithelialization was measured at day 14 after wounding by the histomorphometric analysis of sections stained with HE. As shown in Figure 7(a), at day 14 after wounding, the wound was not fully reepithelialized in the DM group, while the wound got close to fully reepithelialized in the normal group. In the VD treatment group, the epithelia were significantly longer compared with the DM group. Figure 7(b) shows that collagen formation in the ulcer tissues at D14 was assessed by Masson's trichrome staining using computer-assisted morphometric analysis. Less amount of collagen deposition organized in aligned fibers in the diabetic groups, but relatively more in the normal group (0.35 ± 0.026 versus 0.78 ± 0.045, p < 0.05). Interestingly, vitamin D supplementation improved collagen deposition in ulcer tissues compared with the DM group. The mean value of the VD treatment group was 0.60 ± 0.031, and for the DM group was 0.35 ± 0.026 (p < 0.05).
Figure 7.
Effects of vitamin D on the epithelialization and collagen deposition in ulcerative tissues at D14 after treatment. (a) H&E staining of sections showed better dermal reepithelialization on the diabetic wounds in the VD treatment group compared with the diabetic group. (b) Collagen deposition assessed by Masson's trichrome staining. (c, d) Statistical reepithelialization and thickness of collagen deposition of wounds by computer-assisted morphometric analysis. Data are represented as means ± SD. ∗p < 0.05, compared with the normal group. ∗∗p < 0.05, compared with the diabetic group.
(3) The Expression of Endogenous Biomarkers of ER Stress in Diabetic Wounds. As shown in Figure 8, the markers of ER stress (GRP78, CHOP, and ATF4) were significantly increased in the diabetic group, compared with the normal group. With Vitamin D supplementation, ER stress markers were markedly reduced in diabetic wound tissues, compared with those in the diabetic group.
Figure 8.
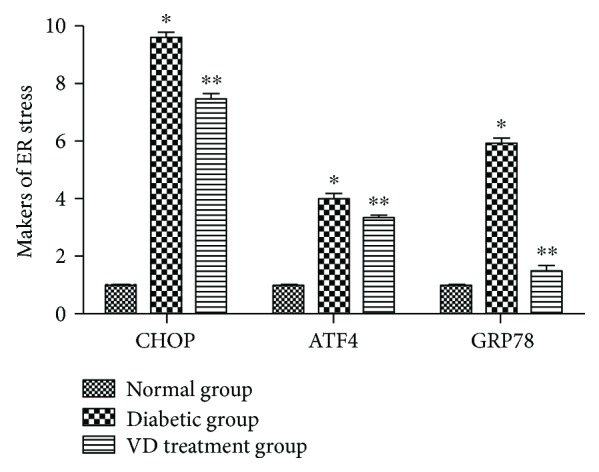
mRNA levels of GRP78, ATF4, and CHOP in diabetic wound tissues. ∗p < 0.05, relative to the normal group. ∗∗p < 0.05 relative to the VD treatment group.
4. Discussion
Vitamin D is well known as a regulator of epidermal and hair follicle differentiation. Tian et al. observed that topical 1,25(OH)2D enhanced wound healing [22]. Luderer et al. observed that in the global vitamin D receptor (VDR) knockout mouse, there was a reduction in TGF-β signaling in the dermis [23]. Oda et al. observed that reepithelialization is impaired when the deletion of VDR is accompanied by a low calcium diet [24]. Besides that, several studies documented an anti-inflammatory effect of vitamin D in variety of cell types, including endothelial cells [25, 26], dentritic cells [27, 28], T cells [29], and macrophages [30], which was in part linked to an inhibition of NF-κB activation and signaling [31]. Mounting researches observed that vitamin D supplementation has positive effect on diabetic wound healing. But the true mechanism is still uncertain, especially the relationship between vitamin D and ER stress.
ER stress promotes endothelial dysfunction and diabetic vascular disease. When ER homeostasis is disrupted, the UPR pathway is activated. The UPR, known to be a crucial defensive mechanism in ER stress, is mediated by three transmembrane proteins (IRE1, protein kinase R-like endoplasmic reticulum kinase, and activating transcription factor 6). Under normal conditions, these proteins are maintained in an inactive state when bound to GRP78, a chaperone considered to be an indicator of ER stress. When homeostasis is disrupted, GRP78 dissociates from membrane proteins, leading to activation of three corresponding pathways [32].
The PERK axis is one of the ER stress pathways. Release of GRP78 upon build-up of misfolded proteins, inadequate glucose levels, or other stressors enable dimerization of PERK and its consequent auto-phosphorylation. Activated PERK then phosphorylates the alpha subunit of eIF2, leading to global translation arrest. Transcripts with alternative upstream open reading frames, such as ATF4, are then preferentially translated. Expression of ATF4 can lead to induction of CHOP. Upon severe and sustained ER stress, CHOP promotes apoptosis.
In the present study, vitamin D supplementation not only partly restores dysfunction of endothelial cells which were exposed to diabetic-like environments but also improves impaired wound healing in streptozotocin-induced diabetic mice. ER stress pathway is involved in both phenomenons. It is reasonable to presume that Vitamin D ameliorates impaired wound healing in streptozotocin-induced diabetic mice by suppressing endoplasmic reticulum stress.
Whether vitamin D could improve other abnormalities leading to diabetic ulcer, such as vascular diseases and neuropathies, will be a next job in our further research work. And whether this drug can be extrapolated to clinical situations still needs further investigation in clinical trials.
Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Janket S. J., Jones J. A., Meurman J. H., Baird A. E., Van Dyke T. E. Oral infection, hyperglycemia, and endothelial dysfunction. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 2008;105(2):173–179. doi: 10.1016/j.tripleo.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cifarelli V., Geng X., Styche A., Lakomy R., Trucco M., Luppi P. C-peptide reduces high-glucose-induced apoptosis of endothelial cells and decreases NADPH-oxidase reactive oxygen species generation in human aortic endothelial cells. Diabetologia. 2011;54(10):2702–2712. doi: 10.1007/s00125-011-2251-0. [DOI] [PubMed] [Google Scholar]
- 3.Schisano B., Harte A. L., Lois K., Saravanan P., AL-Daghri N., AL-Attas O. GLP-1 analogue, Liraglutide protects human umbilical vein endothelial cells against high glucose induced endoplasmic reticulum stress. Regulatory Peptides. 2012;174(1–3):46–52. doi: 10.1016/j.regpep.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Haas M. J., Jafri M., Wehmeier K. R., Onstead-Haas L. M., Mooradian A. D. Inhibition of endoplasmic reticulum stress and oxidative stress by vitamin D in endothelial cells. Free Radical Biology & Medicine. 2016;99:1–10. doi: 10.1016/j.freeradbiomed.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Michalak M., Gye M. C. Endoplasmic reticulum stress in periimplantation embryos. Clinical and Experimental Reproductive Medicine. 2015;42(1):1–7. doi: 10.5653/cerm.2015.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sozen E., Karademir B., Ozer N. K. Basic mechanisms in endoplasmic reticulum stress and relation to cardiovascular diseases. Free Radical Biology & Medicine. 2015;78:30–41. doi: 10.1016/j.freeradbiomed.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Tabas I., Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nature Cell Biology. 2011;13(3):184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams J. S., Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nature Clinical Practice Endocrinology & Metabolism. 2008;4(2):80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foley R. N., Parfrey P. S., Sarnak M. J. Clinical epidemiology of cardiovascular disease in chronic renal disease. American Journal of Kidney Diseases. 1998;32(5) Supplement 3:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 10.Zittermann A., Schleithoff S. S., Tenderich G., Berthold H. K., Körfer R., Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? Journal of the American College of Cardiology. 2003;41(1):105–112. doi: 10.1016/S0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 11.Scragg R., Sowers M., Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. American Journal of Hypertension. 2007;20(7):713–719. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Martins D., Wolf M., Pan D., et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Archives of Internal Medicine. 2007;167(11):1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 13.Forman J. P., Giovannucci E., Holmes M. D., et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E., Liu Y., Hollis B. W., Rimm E. B. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Archives of Internal Medicine. 2008;168(11):1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valdivielso J. M., Coll B., Fernandez E. Vitamin D and the vasculature: can we teach an old drug new tricks? Expert Opinion on Therapeutic Targets. 2009;13(1):29–38. doi: 10.1517/14728220802564390. [DOI] [PubMed] [Google Scholar]
- 16.Brewer L. C., Michos E. D., Reis J. P. Vitamin D in atherosclerosis, vascular disease, and endothelial function. Current Drug Targets. 2011;12(1):54–60. doi: 10.2174/138945011793591617. [DOI] [PubMed] [Google Scholar]
- 17.Riek A. E., Oh J., Sprague J. E., et al. Vitamin D suppression of endoplasmic reticulum stress promotes an antiatherogenic monocyte/macrophage phenotype in type 2 diabetic patients. The Journal of Biological Chemistry. 2012;287(46):38482–38494. doi: 10.1074/jbc.M112.386912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molinari C., Uberti F., Grossini E., et al. 1α,25-dihydroxycholecalciferol induces nitric oxide production in cultured endothelial cells. Cellular Physiology and Biochemistry. 2011;27(6):661–668. doi: 10.1159/000330075. [DOI] [PubMed] [Google Scholar]
- 19.Merke J., Milde P., Lewicka S., et al. Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. The Journal of Clinical Investigation. 1989;83(6):1903–1915. doi: 10.1172/JCI114097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ni W., Watts S. W., Ng M., Chen S., Glenn D. J., Gardner D. G. Elimination of vitamin D receptor in vascular endothelial cells alters vascular function. Hypertension. 2014;64(6):1290–1298. doi: 10.1161/HYPERTENSIONAHA.114.03971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martínez-Miguel P., Valdivielso J. M., Medrano-Andrés D., et al. The active form of vitamin D, calcitriol, induces a complex dual upregulation of endothelin and nitric oxide in cultured endothelial cells. American Journal of Physiology. Endocrinology and Metabolism. 2014;307(12):E1085–E1096. doi: 10.1152/ajpendo.00156.2014. [DOI] [PubMed] [Google Scholar]
- 22.Tian X. Q., Chen T. C., Holick M. F. 1,25-Dihydroxyvitamin D3: a novel agent for enhancing wound healing. Journal of Cellular Biochemistry. 1995;59(1):53–56. doi: 10.1002/jcb.240590107. [DOI] [PubMed] [Google Scholar]
- 23.Luderer H. F., Nazarian R. M., Zhu E. D., Demay M. B. Ligand-dependent actions of the vitamin D receptor are required for activation of TGF-β signaling during the inflammatory response to cutaneous injury. Endocrinology. 2013;154(1):16–24. doi: 10.1210/en.2012-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oda Y., Tu C. L., Menendez A., Nguyen T., Bikle D. D. Vitamin D and calcium regulation of epidermal wound healing. The Journal of Steroid Biochemistry and Molecular Biology. 2015;164:379–385. doi: 10.1016/j.jsbmb.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinesi M., Bruni S., Stio M., Treves C. 1,25-Dihydroxyvitamin D3 inhibits tumor necrosis factor-α-induced adhesion molecule expression in endothelial cells. Cell Biology International. 2006;30(4):365–375. doi: 10.1016/j.cellbi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Equils O., Naiki Y., Shapiro A. M., et al. 1,25-Dihydroxyvitamin D3 inhibits lipopolysaccharide-induced immune activation in human endothelial cells. Clinical & Experimental Immunology. 2006;143(1):58–64. doi: 10.1111/j.1365-2249.2005.02961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shumilina E., Xuan N. T., Matzner N., Bhandaru M., Zemtsova I. M., Lang F. Regulation of calcium signaling in dendritic cells by 1,25-dihydroxyvitamin D3. The FASEB Journal. 2010;24(6):1989–1996. doi: 10.1096/fj.09-142265. [DOI] [PubMed] [Google Scholar]
- 28.Penna G., Adorini L. 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. Journal of Immunology. 2000;164(5):2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 29.Boonstra A., Barrat F. J., Crain C., Heath V. L., Savelkoul H. F., O’Garra A. 1α,25-Dihydroxyvitamin d3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. Journal of Immunology. 2001;167(9):4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 30.Cohen-Lahav M., Douvdevani A., Chaimovitz C., Shany S. The anti-inflammatory activity of 1,25-dihydroxyvitamin D3 in macrophages. The Journal of Steroid Biochemistry and Molecular Biology. 2007;103(3–5):558–562. doi: 10.1016/j.jsbmb.2006.12.093. [DOI] [PubMed] [Google Scholar]
- 31.Wong M. S., Leisegang M. S., Kruse C., et al. Vitamin D promotes vascular regeneration. Circulation. 2014;130(12):976–986. doi: 10.1161/CIRCULATIONAHA.114.010650. [DOI] [PubMed] [Google Scholar]
- 32.Xu J. Z., Chai Y. L., Zhang Y. L. Effect of rosuvastatin on high glucose-induced endoplasmic reticulum stress in human umbilical vein endothelial cells. Genetics and Molecular Research. 2016;15(4) doi: 10.4238/gmr15048935. [DOI] [PubMed] [Google Scholar]